Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Excluded Studies
2.4. Data Extraction
2.5. Assessment of Study Quality
2.6. Statistical Analysis
2.7. Certainty Assessment
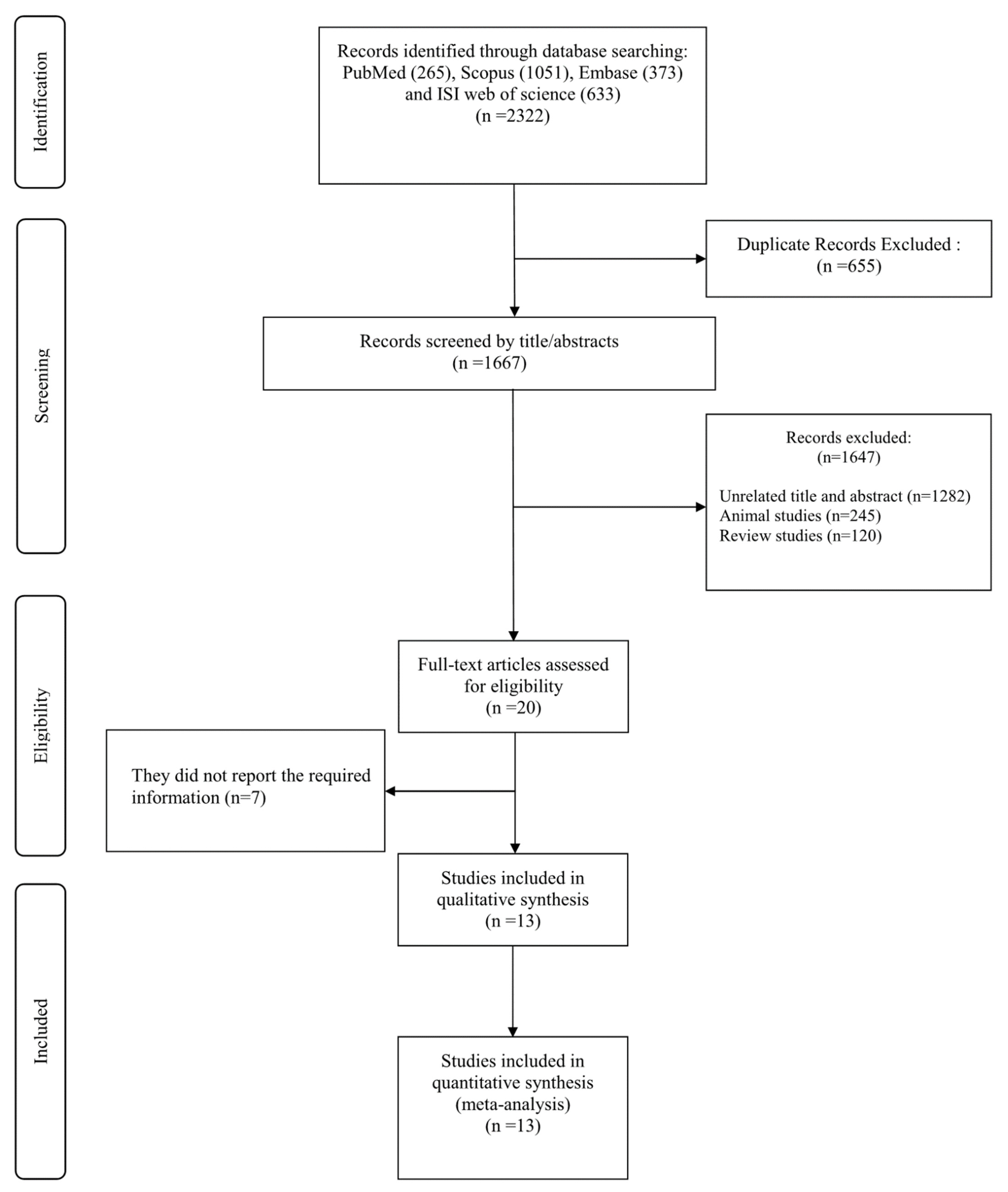
3. Results
3.1. Findings from the Systematic Review
3.2. Findings from the Meta-Analysis
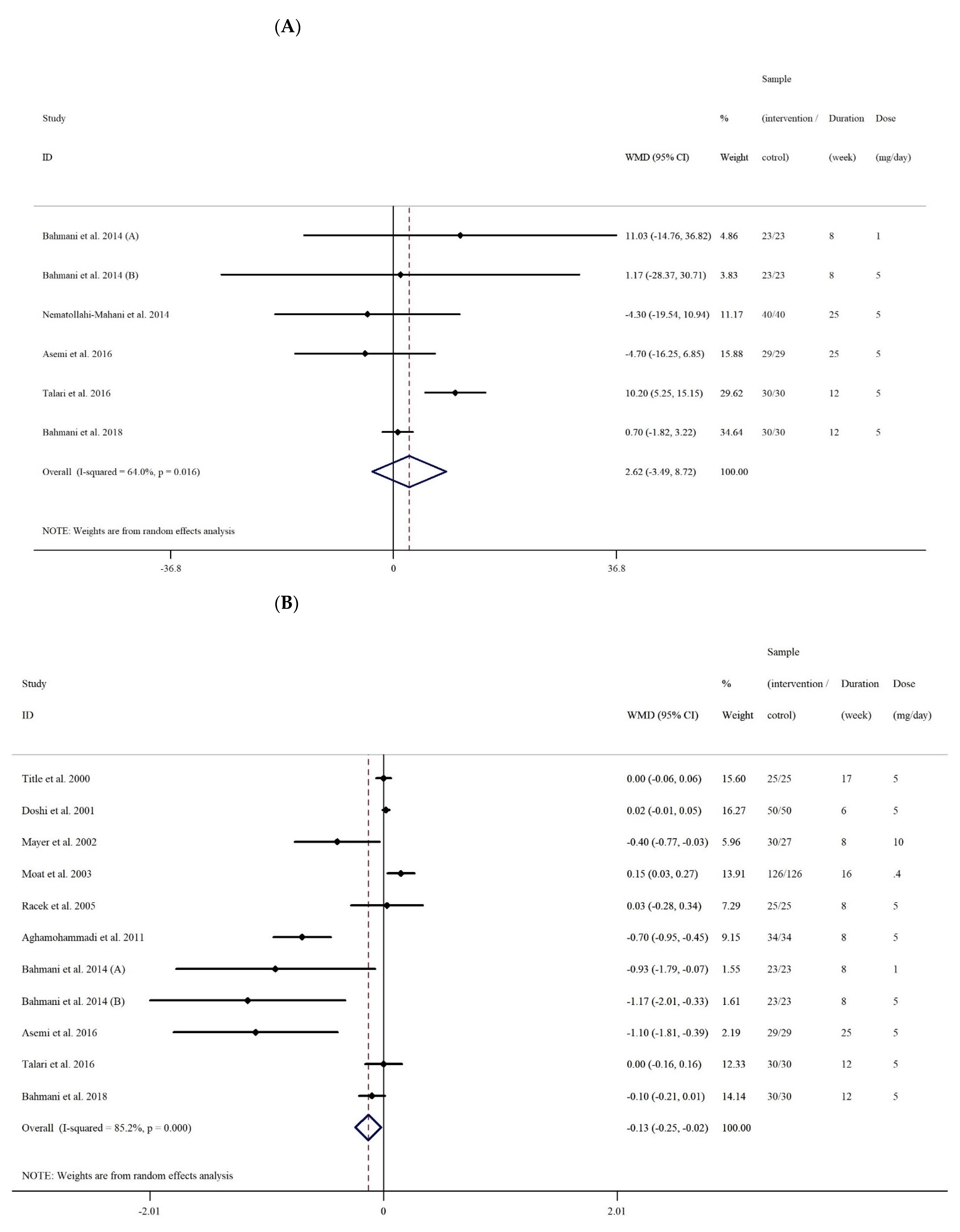
3.2.1. The Effect of Folic Acid Supplementation on Serum Concentrations of NO
3.2.2. The Effect of Folic Acid Supplementation on Serum Concentrations of MDA
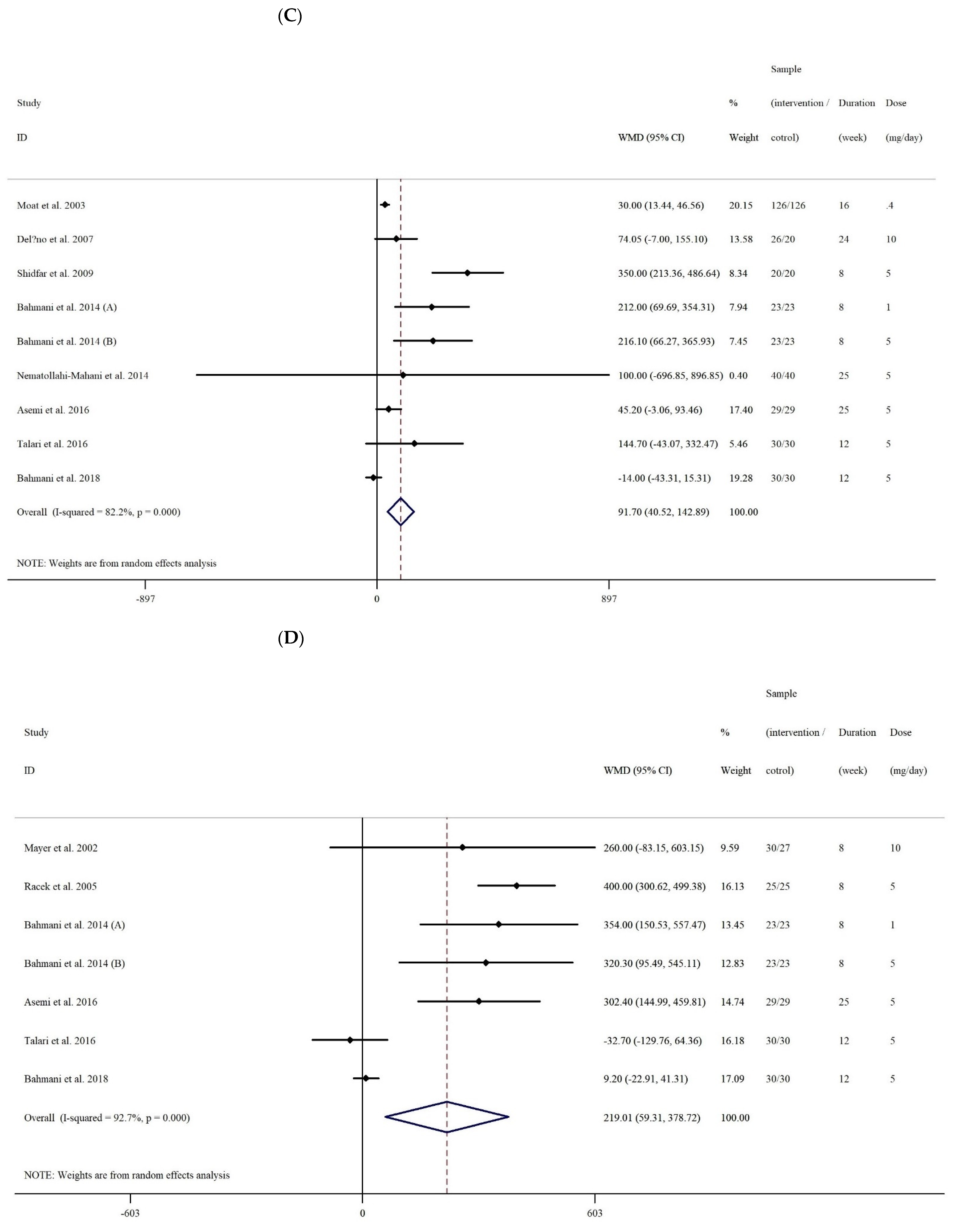
3.2.3. The Effect of Folic Acid Supplementation on Serum Concentrations of TAC
3.2.4. The Effect of Folic Acid Supplementation on Serum Concentrations of GSH
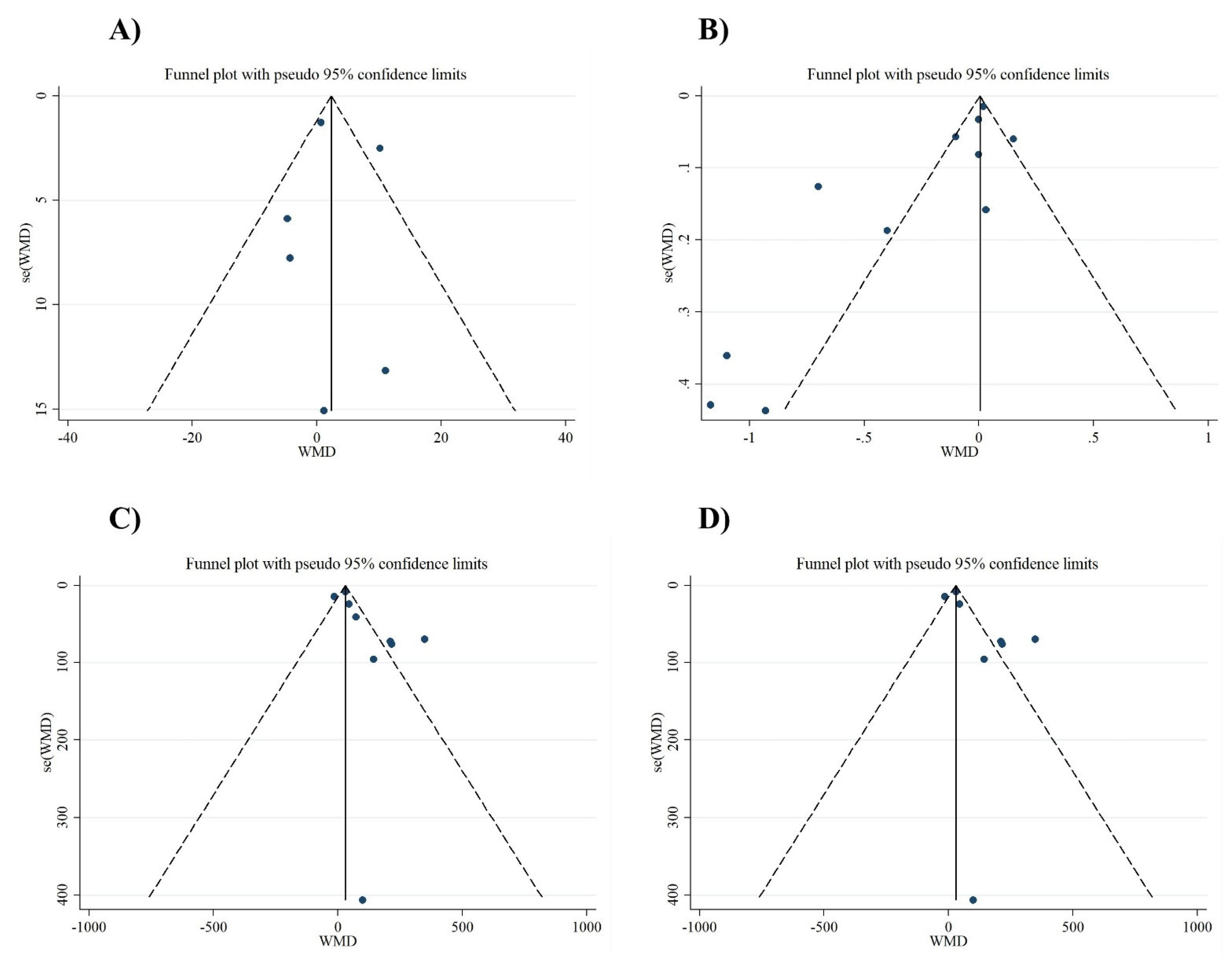
3.2.5. Publication Bias and Sensitivity Analyses
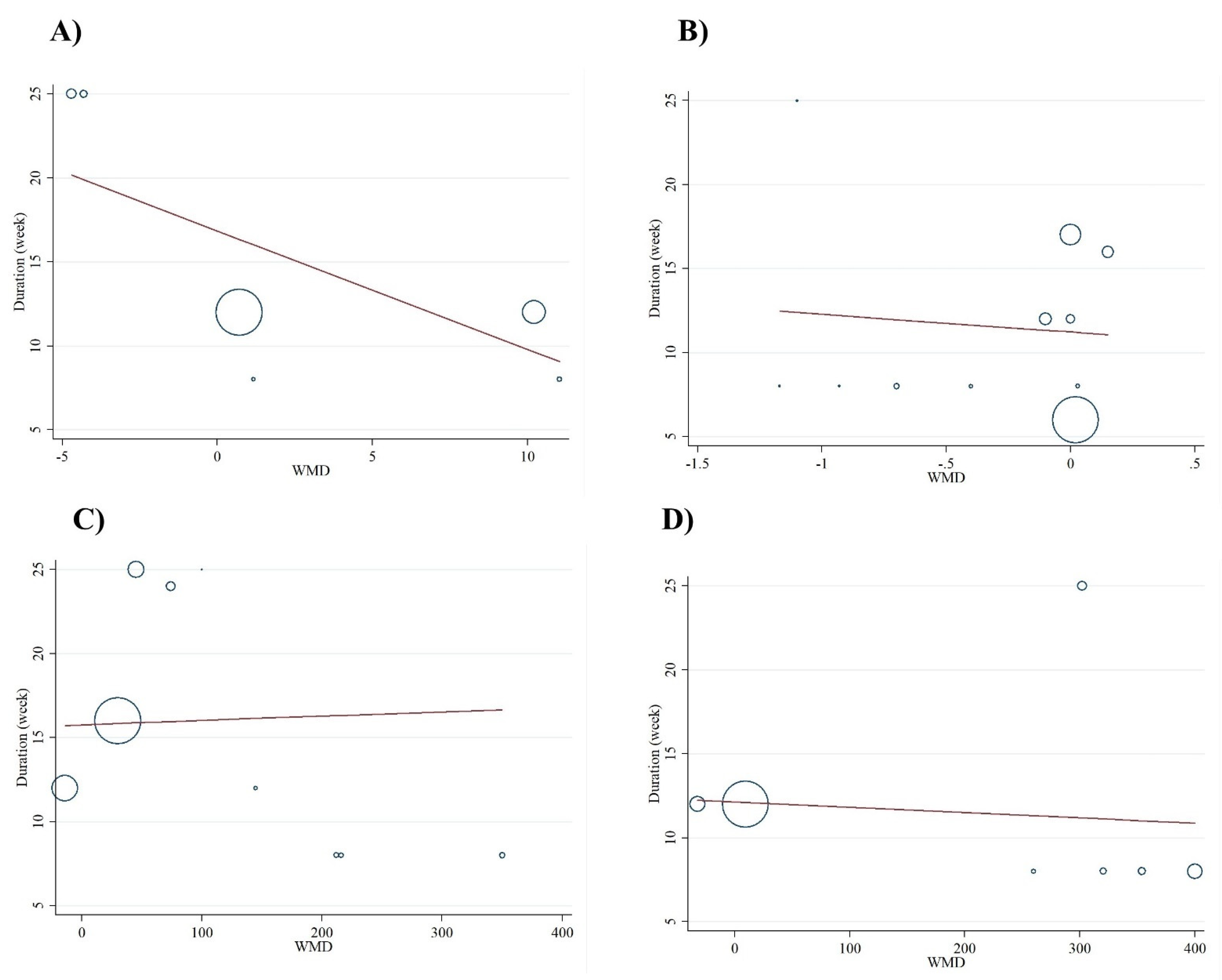
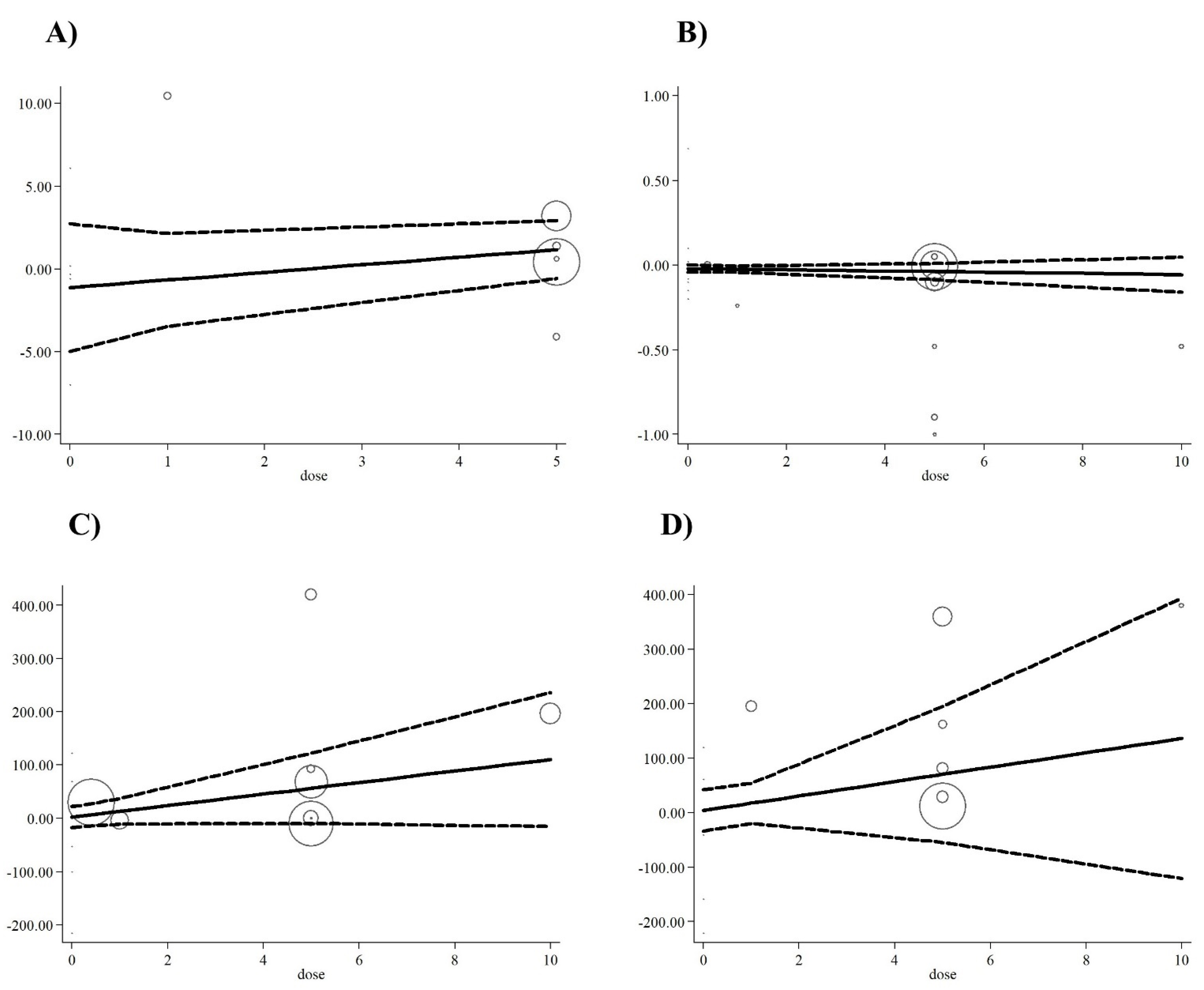
3.3. Dose-Response Analyses
Grading of Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F.J.V. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Paknahad, Z.; Habibagahi, Z. A randomized, double-blind, placebo-controlled clinical trial, evaluating the garlic supplement effects on some serum biomarkers of oxidative stress, and quality of life in women with rheumatoid arthritis. Int. J. Clin. Pract. 2020, 74, e13498. [Google Scholar] [CrossRef]
- Gill, J.G.; Piskounova, E.; Morrison, S.J. Cancer, oxidative stress, and metastasis. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejhad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Moosavian, S.P.; Arab, A.; Paknahad, Z.; Moradi, S. The effects of garlic supplementation on oxidative stress markers: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 50, 102385. [Google Scholar] [CrossRef] [PubMed]
- Mutavdzin, S.; Gopcevic, K.; Stankovic, S.; Uzelac, J.J.; Borovic, M.L.; Djuric, D. The effects of folic acid administration on cardiac oxidative stress and cardiovascular biomarkers in diabetic rats. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sijilmassi, O. Folic acid deficiency and vision: A review. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie 2019, 257, 1573–1580. [Google Scholar]
- Asbaghi, O.; Ashtary-Larky, D.; Bagheri, R.; Nazarian, B.; Olyaei, H.P.; Kelishadi, M.R.; Nordvall, M.; Wong, A.; Dutheil, F.; Naeini, A.A. Beneficial effects of folic acid supplementation on lipid markers in adults: A GRADE-assessed systematic review and dose-response meta-analysis of data from 21,787 participants in 34 randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 42, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. Journal of inherited metabolic disease. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Bahmani, F.; Karamali, M.; Shakeri, H.; Asemi, Z. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled clinical trial. Clin. Endocrinol. 2014, 81, 582–587. [Google Scholar] [CrossRef]
- Wotherspoon, F.; Laight, D.W.; Turner, C.H.; Meeking, D.R.; Allard, S.E.; Munday, L.J.; Shaw, K.M.; Cummings, M.H. The effect of oral folic acid upon plasma homocysteine, endothelial function and oxidative stress in patients with type 1 diabetes and microalbuminuria. Int. J. Clin. Pract. 2008, 62, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother. Res. 2014, 28, 633–642. [Google Scholar] [CrossRef]
- Xu, C.; Doi, S.A. The robust error meta-regression method for dose–response meta-analysis. JBI Evid. Implement. 2018, 16, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Oxman, A.; Vist, G.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. Rating quality of evidence and strength of recommendations: GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar]
- Title, L.M.; Cummings, P.M.; Giddens, K.; Genest, J.J.; Jr Nassar, B.A. Effect of folic acid and antioxidant vitamins on endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Cardiol. 2000, 36, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Doshi, S.N.; McDowell, I.F.; Moat, S.J.; Lang, D.; Newcombe, R.G.; Kredan, M.B.; Lewis, M.J.; Goodfellow, J. Folate improves endothelial function in coronary artery disease: An effect mediated by reduction of intracellular superoxide? Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1196–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moat, S.J.; Hill, M.H.; McDowell, I.F.W.; Pullin, C.H.; Ashfield-Watt, P.A.L.; Clark, Z.; Whiting, J.M.; Newcombe, R.G.; Lewis, M.J.; Powers, H.J. Reduction in plasma total homocysteine through increasing folate intake in healthy individuals is not associated with changes in measures of antioxidant activity or oxidant damage. Eur. J. Clin. Nutr. 2003, 57, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Racek, J.; Rusnakova, H.; Trefil, L.; Siala, K.K. The influence of folate and antioxidants on homocysteine levels and oxidative stress in patients with hyperlipidemia and hyperhomocysteinemia. Physiol. Res. 2005, 54, 87–95. [Google Scholar]
- Asemi, Z.; Vahedpoor, Z.; Jamilian, M.; Bahmani, F.; Esmaillzadeh, A. Effects of long-term folate supplementation on metabolic status and regression of cervical intraepithelial neoplasia: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 681–686. [Google Scholar] [CrossRef]
- Talari, H.R.; Rafiee, M.; Farrokhian, A.; Raygan, F.; Bahmani, F.; Mofrad, M.D.; Hamidian, Y.; Tamtaji, O.R.; Karamali, F.; Asemi, Z. The effects of folate supplementation on carotid intima-media thickness and metabolic status in patients with metabolic syndrome. Ann. Nutr. Metab. 2016, 69, 41–50. [Google Scholar] [CrossRef]
- Bahmani, F.; Galougahi, F.R.; Vahedpoor, Z.; Jamilian, M.; Mahmoodi, S.; Baghban, R.; Bagherian, T.; Mehrizi, M.Z.; Asemi, Z. The effects of folic acid supplementation on recurrence and metabolic status in endometrial hyperplasia: A randomized, double-blind, placebo-controlled trial. Arch. Iran. Med. 2018, 21, 452–459. [Google Scholar]
- Shidfar, F.; Homayounfar, R.; Fereshtehnejad, S.M.; Kalani, A. Effect of folate supplementation on serum homocysteine and plasma total antioxidant capacity in hypercholesterolemic adults under lovastatin treatment: A double-blind randomized controlled clinical trial. Arch. Med Res. 2009, 40, 380–386. [Google Scholar] [CrossRef]
- Nematollahi-Mahani, S.N.; Azizollahi, G.H.; Baneshi, M.R.; Safari, Z.; Azizollahi, S. Effect of folic acid and zinc sulphate on endocrine parameters and seminal antioxidant level after varicocelectomy. Andrologia 2014, 46, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, V.; Gargari, B.P.; Aliasgharzadeh, A. Effect of folic acid supplementation on homocysteine, serum total antioxidant capacity, and malondialdehyde in patients with type 2 diabetes mellitus. J. Am. Coll. Nutr. 2011, 30, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O.; Simon, J.; Rosolova, H.; Hromadka, M.; Subrt, I.; Vobrubova, I. The effects of folate supplementation on some coagulation parameters and oxidative status surrogates. Eur. J. Clin. Pharmacol. 2002, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Delfino, V.D.A.; de Andrade Vianna, A.C.; Mocelin, A.J.; Barbosa, D.S.; Mise, R.A.; Matsuo, T.J.N. Folic acid therapy reduces plasma homocysteine levels and improves plasma antioxidant capacity in hemodialysis patients. Nutrition 2007, 23, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Frijhoff, J.; Winyard, P.; Zarkovic, N.; Davies, S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.R.; Tyagi, S.C. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: The pleiotropic effects of folate supplementation. Nutr. J. 2004, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Stanger, O.; Wonisch, W. Enzymatic and non-enzymatic antioxidative effects of folic acid and its reduced derivates. Sub. Cell. Biochem. 2012, 56, 131–161. [Google Scholar]
- Rogers, L.M.; Cordero, A.M.; Pfeiffer, C.M.; Hausman, D.B.; Tsang, B.L.; De-Regil, L.M.; Rosenthal, J.; Razzaghi, H.; Wong, E.C.; Weakland, A.P.; et al. Global folate status in women of reproductive age: A systematic review with emphasis on methodological issues. Ann. N. Y. Acad. Sci. 2018, 1431, 35–57. [Google Scholar] [CrossRef] [PubMed]
- White, R.E.; Gerrity, R.; Barman, S.A.; Han, G. Estrogen and oxidative stress: A novel mechanism that may increase the risk for cardiovascular disease in women. Steroids 2010, 75, 788–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ericson, U.; Borgquist, S.; Ivarsson, M.I.; Sonestedt, E.; Gullberg, B.; Carlson, J.; Olsson, H.; Jirstrom, K.; Wirfalt, E. Plasma folate concentrations are positively associated with risk of estrogen receptor beta negative breast cancer in a Swedish nested case control study. J. Nutr. 2010, 140, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Sütterlin, M.W.; Bussen, S.S.; Rieger, L.; Dietl, J.; Steck, T. Serum folate and Vitamin B12 levels in women using modern oral contraceptives (OC) containing 20 microg ethinyl estradiol. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 107, 57–61. [Google Scholar] [CrossRef]
- Fatahi, S.; Pezeshki, M.; Mousavi, S.; Teymouri, A.; Rahmani, J.; Varkaneh, H.K.; Ghaedi, E. Effects of folic acid supplementation on C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, I.A.; van Dusseldorp, M.; West, C.E.; Steegers-Theunissen, R.P. Bioavailability and bioefficacy of folate and folic acid in man. Nutr. Res. Rev. 2001, 14, 267–294. [Google Scholar] [CrossRef]
- Clarke, R.; Frost, J.; Sherliker, P.; Lewington, S.; Collins, R.; Brattstrom, L.; Brouwer, I.; Van Dusseldorp, M.; Steegers-Theunissen, R.P.M.; Cuskelly, G.; et al. Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials. Am. J. Clin. Nutr. 2005, 82, 806–812. [Google Scholar]
- Monserrat, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic syndrome is associated with oxidative stress and proinflammatory state. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M. Oxidative Stress and Polycystic Ovary Syndrome: A Brief Review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef]
- Moens, A.L.; Vrints, C.J.; Claeys, M.J.; Timmermans, J.-P.; Champion, H.C.; Kass, D.A. Mechanisms and potential therapeutic targets for folic acid in cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1971–H1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boushey, C.J.; Beresford, S.A.; Omenn, G.S.; Motulsky, A.G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 1995, 274, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, B.; Qi, J.; Shen, B. Meta-analysis of clinical trials of folic acid, vitamin B12 and B6 supplementation on plasma homocysteine level and risk of cardiovascular disease. Zhonghua Xin Xue Guan Bing Za Zhi 2015, 43, 554–561. [Google Scholar] [PubMed]
- Qin, X.; Huo, Y.; Xie, D.; Hou, F.; Xu, X.; Wang, X.J.C. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: A meta-analysis of randomized controlled trials. Clin. Nutr. 2013, 32, 722–727. [Google Scholar] [CrossRef]
- Tyagi, N.; Sedoris, K.C.; Steed, M.; Ovechkin, A.V.; Moshal, K.S.; Tyagi, S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2649–H2656. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Sarna, L.K.; Wu, N.; Wang, P.; Hwang, S.Y.; Siow, Y.L.; O, K. Folic acid supplementation attenuates high fat diet induced hepatic oxidative stress via regulation of NADPH oxidase. Can. J. Physiol. Pharmacol. 2012, 90, 155–165. [Google Scholar] [CrossRef]
- Joshi, R.; Adhikari, S.; Patro, B.S.; Chattopadhyay, S.; Mukherjee, T. Free radical scavenging behavior of folic acid: Evidence for possible antioxidant activity. Free Radic. Biol. Med. 2001, 30, 1390–1399. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Kenney, W.L. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr. Rev. 2017, 75, 61–70. [Google Scholar] [CrossRef]

| Study Design | Participant | Sample Size and Sex | Sample Size | Trial Duration (Week) | Means Age | Means BMI | Intervention | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IG | CG | IG | CG | IG | CG | Folic Acid Dose (mg/d) | Control Group | ||||
| RA/DB/PC (parallel) | Coronary Artery Disease | 50: 40M, 10F | 25 | 25 | 17 | 57.2 ± 9.8 | 60.6 ± 8.6 | NR | NR | 5 | Placebo |
| RA/PC (parallel) | Coronary Artery Disease | 50: 44M, 6F | 50 | 50 | 6 | 57 ± 8 | 57 ± 8 | 28.5 ± 4.4 | 28.5 ± 4.4 | 5 | Placebo |
| RA/PC (parallel) | high coronary risk | 57: 30M,27F | 30 | 27 | 8 | 61.2 ± 24.46 | 61.2 ± 24.46 | 28.2 ± 16.53 | 28.2 ± 16.53 | 10 | Placebo |
| RA/DB/PC (parallel) | healthy individuals | 126 M/F | 126 | 126 | 16 | 18-65 | 18-65 | NR | NR | 0.4 | Placebo |
| RA (parallel) | Hyperlipidemia and Hyperhomocysteinemia | 50: 37M, 13F | 25 | 25 | 8 | 59 ± 9.75 | 56.4 ± 9.75 | NR | NR | 5 | No intervention |
| RA/DB/PC (parallel) | Hemodialysis patients | 46: NR | 26 | 20 | 24 | 51.6 ± 10.7 | 52.3 ± 15 | NR | NR | 10 | Placebo |
| RA/DB/PC (parallel) | Hypercholesterolemic Adults | 40: 16M, 24F | 20 | 20 | 8 | 44 ± 7.06 | 45 ± 7.78 | 27.06 ± 2.64 | 26.05 ± 2.17 | 5 | Placebo |
| RA/DB/PC (parallel) | Type 2 diabetes | 68 M/F | 34 | 34 | 8 | 58.72 ± 6 7.2 | 55.6 ± 6 9.3 | 27.4 ± 6 3.2 | 27.8 ± 6 4 | 5 | Placebo |
| RA/DB/PC (parallel) | overweight and obese women with polycystic ovary syndrome | 46: 46F | 23 | 23 | 8 | 24.1 ± 5.4 | 24.9 ± 5.9 | 26.1 ± 6.2 | 27.6 ± 5.7 | 1 | Placebo |
| RA/DB/PC (parallel) | overweight and obese women with polycystic ovary syndrome | 46: 46F | 23 | 23 | 8 | 25.1 ± 4.9 | 24.9 ± 5.9 | 29 ± 5.9 | 27.6 ± 5.7 | 5 | Placebo |
| RA/PC (parallel) | varicocelectomy | 80: 80M | 40 | 40 | 25 | NR | NR | NR | NR | 5 | Placebo |
| RA/DB/PC (parallel) | cervical intraepithelial neoplasia grade 1 | 58: 58F | 29 | 29 | 25 | 36.8 ± 8.8 | 39.1 ± 9.1 | 28.2 ± 3.5 | 29.8 ± 6.4 | 5 | Placebo |
| RA/DB/PC (parallel) | Metabolic Syndrome | 60: 26M, 34F | 30 | 30 | 12 | 62.1 ± 9.6 | 65.4 ± 11.5 | 29.8 ± 3.8 | 29.8 ± 4.4 | 5 | Placebo |
| RA/DB/PC (parallel) | Endometrial Hyperplasia | 60: 60F | 30 | 30 | 12 | 44.4 ± 6.5 | 44.7 ± 3.1 | 30.7 ± 4.6 | 30.5 ± 3.8 | 5 | Placebo |
| Studies | Random Sequence Generation | Allocation Concealment | Selective Reporting | Other Sources of Bias | Blinding (Participants and Personnel) | Blinding (Outcome Assessment) | Incomplete Outcome Data | Overall Quality |
|---|---|---|---|---|---|---|---|---|
| Title et al., 2000 | L | H | H | H | L | H | L | Good |
| Doshi et al., 2001 | L | H | H | H | H | H | L | Fair |
| Mayer et al., 2002 | L | H | H | H | H | H | L | Fair |
| Moat et al., 2003 | L | H | H | H | L | H | L | Good |
| Racek et al., 2005 | L | H | H | H | H | H | L | Fair |
| Delfino et al., 2007 | L | L | L | H | L | L | H | Good |
| Shidfar et al., 2009 | L | H | H | H | L | H | L | Good |
| Agha mohammadi et al., 2001 | L | U | H | H | L | U | L | Good |
| Bahmani et al., 2014 | L | H | L | H | L | H | L | Good |
| Nematollahi-Mahani et al., 2014 | L | H | H | H | H | H | L | Fair |
| Asemi et al., 2016 | L | H | L | H | L | H | L | Good |
| Talari et al., 2016 | L | H | L | H | L | H | L | Good |
| Bahmani et al., 2018 | L | H | L | H | L | H | L | Good |
| Number of Effect Sizes | WMD (95%CI) | P within Group | Heterogeneity | |||
|---|---|---|---|---|---|---|
| P Heterogeneity | I2 | P between Sub-Groups | ||||
| Subgroup analyses of folic acid supplementation on NO | ||||||
| Overall effect | 6 | 2.61 (−3.48, 8.72) | 0.400 | 0.016 | 64.0% | |
| Trial duration (week) | ||||||
| ≤8 | 2 | 6.76 (−12.66, 26.19) | 0.495 | 0.622 | 0.0% | 0.650 |
| >8 | 4 | 2.06 (−4.83, 8.96) | 0.557 | 0.004 | 77.7% | |
| Intervention dose (mg/d) | ||||||
| <5 | 1 | 11.03 (−14.75, 36.81) | 0.402 | - | - | 0.506 |
| ≥5 | 5 | 2.12 (−4.33, 8.57) | 0.520 | 0.009 | 70.3% | |
| Sex | ||||||
| Both sexes | 1 | 10.20 (5.24, 15.15) | <0.001 | - | - | 0.002 |
| Female | 4 | 0.55 (−1.88, 2.99) | 0.656 | 0.695 | 0.0% | |
| Male | 1 | −4.30 (−19.53, 10.93) | 0.580 | - | - | |
| Subgroup analyses of folic acid supplementation on MDA | ||||||
| Overall effect | 11 | −0.13 (−0.24, −0.02) | 0.020 | <0.001 | 85.2% | |
| Trial duration (week) | ||||||
| ≤8 | 6 | −0.41 (−0.78, −0.05) | 0.026 | <0.001 | 89.8% | 0.902 |
| >8 | 5 | −0.02 (−0.14, 0.10) | 0.747 | 0.001 | 78.4% | |
| Intervention dose (mg/d) | ||||||
| <5 | 2 | −0.30 (−1.34, 0.74) | 0.569 | 0.014 | 83.3% | 0.031 |
| ≥5 | 9 | −0.16 (−0.28, −0.04) | 0.008 | <0.001 | 86.0% | |
| Health status | ||||||
| CVD | 3 | 0.01 (−0.01, 0.04) | 0.225 | 0.857 | 0.0% | 0.012 |
| non-CVD | 8 | −0.35 (−0.60, −0.10) | 0.005 | <0.001 | 88.5% | |
| Sex | ||||||
| Both sexes | 6 | 0.06 (−0.18, 0.04) | 0.239 | <0.001 | 85.9% | 0.003 |
| Female | 4 | −0.75 (−1.44, −0.06) | 0.032 | 0.001 | 81.9% | |
| Subgroup analyses of folic acid supplementation on TAC | ||||||
| Overall effect | 9 | 91.70 (40.52, 142.88) | <0.001 | <0.001 | 82.2% | |
| Trial duration (week) | ||||||
| ≤8 | 3 | 262.63 (171.87, 353.40) | <0.001 | 0.297 | 17.6% | <0.001 |
| >8 | 6 | 27.90 (−2.72, 57.35) | 0.075 | 0.056 | 53.7% | |
| Intervention dose (mg/d) | ||||||
| <5 | 2 | 106.71 (−69.43, 282.85) | 0.235 | 0.013 | 83.9% | 0.612 |
| ≥5 | 7 | 113.87 (30.06, 197.68) | 0.008 | <0.001 | 84.4% | |
| Health status | ||||||
| CVD | 1 | 350.00 (213.36, 486.63) | <0.001 | - | - | <0.001 |
| non-CVD | 8 | 55.01 (14.56, 95.46) | 0.008 | 0.001 | 70.4% | |
| Sex | ||||||
| Both sexes | 4 | 134.81 (15.51, 254.11) | 0.027 | <0.001 | 86.9% | 0.344 |
| Female | 4 | 84.36 (−2.07, 170.80) | 0.056 | <0.001 | 84.6% | |
| Male | 1 | 100.00 (−696.84, 896.84) | 0.806 | - | - | |
| Subgroup analyses of folic acid supplementation on GSH | ||||||
| Overall effect | 7 | 219.01 (59.30, 378.71) | 0.007 | <0.001 | 92.7% | |
| Trial duration (week) | ||||||
| ≤8 | 4 | 374.77 (294.10, 455.43) | <0.001 | 0.815 | 0.0% | <0.001 |
| >8 | 3 | 72.32 (−63.49, 208.13) | 0.297 | 0.001 | 85.6% | |
| Intervention dose (mg/d) | ||||||
| <5 | 1 | 354.00 (150.53, 557.46) | 0.001 | - | - | 0.004 |
| ≥5 | 6 | 197.82 (27.78, 367.86) | 0.023 | <0.001 | 93.2% | |
| Health status | ||||||
| CVD | 1 | 400.00 (300.61, 499.38) | <0.001 | - | - | <0.001 |
| non-CVD | 6 | 172.01 (37.03, 306.99) | 0.012 | <0.001 | 84.7% | |
| Sex | ||||||
| Both sexes | 3 | 204.25 (−134.93, 543.45) | 0.238 | <0.001 | 94.7% | <0.001 |
| Quality Assessment | Summary of Findings | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Number of Intervention/Control | WMD (95%CI) |
| NO | No serious limitations | serious limitations a | No serious limitations | Serious Limitations e | No serious limitations | 175/175 | 2.61 (−3.48, 8.72) |
| MDA | No serious limitations | Very serious b | No serious limitations | No serious limitations | No serious limitations | 425/422 | −0.13 (−0.24, −0.02) |
| TAC | No serious limitations | Very serious c | No serious limitations | No Serious Limitations | No serious limitations | 347/341 | 91.70 (40.52, 142.88) |
| GSH | No serious limitations | Very serious d | No serious limitations | No serious limitations | No serious limitations | 190/187 | 219.01 (59.30, 378.71) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asbaghi, O.; Ghanavati, M.; Ashtary-Larky, D.; Bagheri, R.; Rezaei Kelishadi, M.; Nazarian, B.; Nordvall, M.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2021, 10, 871. https://doi.org/10.3390/antiox10060871
Asbaghi O, Ghanavati M, Ashtary-Larky D, Bagheri R, Rezaei Kelishadi M, Nazarian B, Nordvall M, Wong A, Dutheil F, Suzuki K, et al. Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants. 2021; 10(6):871. https://doi.org/10.3390/antiox10060871
Chicago/Turabian StyleAsbaghi, Omid, Matin Ghanavati, Damoon Ashtary-Larky, Reza Bagheri, Mahnaz Rezaei Kelishadi, Behzad Nazarian, Michael Nordvall, Alexei Wong, Frédéric Dutheil, Katsuhiko Suzuki, and et al. 2021. "Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Antioxidants 10, no. 6: 871. https://doi.org/10.3390/antiox10060871
APA StyleAsbaghi, O., Ghanavati, M., Ashtary-Larky, D., Bagheri, R., Rezaei Kelishadi, M., Nazarian, B., Nordvall, M., Wong, A., Dutheil, F., Suzuki, K., & Alavi Naeini, A. (2021). Effects of Folic Acid Supplementation on Oxidative Stress Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants, 10(6), 871. https://doi.org/10.3390/antiox10060871









