The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered byAβ1-42 Oligomers in Primary Cortical Neurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. In Vitro Anti-Acetylcholinesterase Activity
2.3. Cell Cultures
2.4. Primary Cortical Neurons
2.5. Aβ Treatment
2.6. MTT Assay
2.7. Assessment of Intracellular ROS Production
2.8. Electrophysiology
2.9. Assessment of Nuclear Morphology
2.10. Western Blotting
2.11. Statistics
3. Results
3.1. Effect of Limonene on Acetylcholinesterase Activity
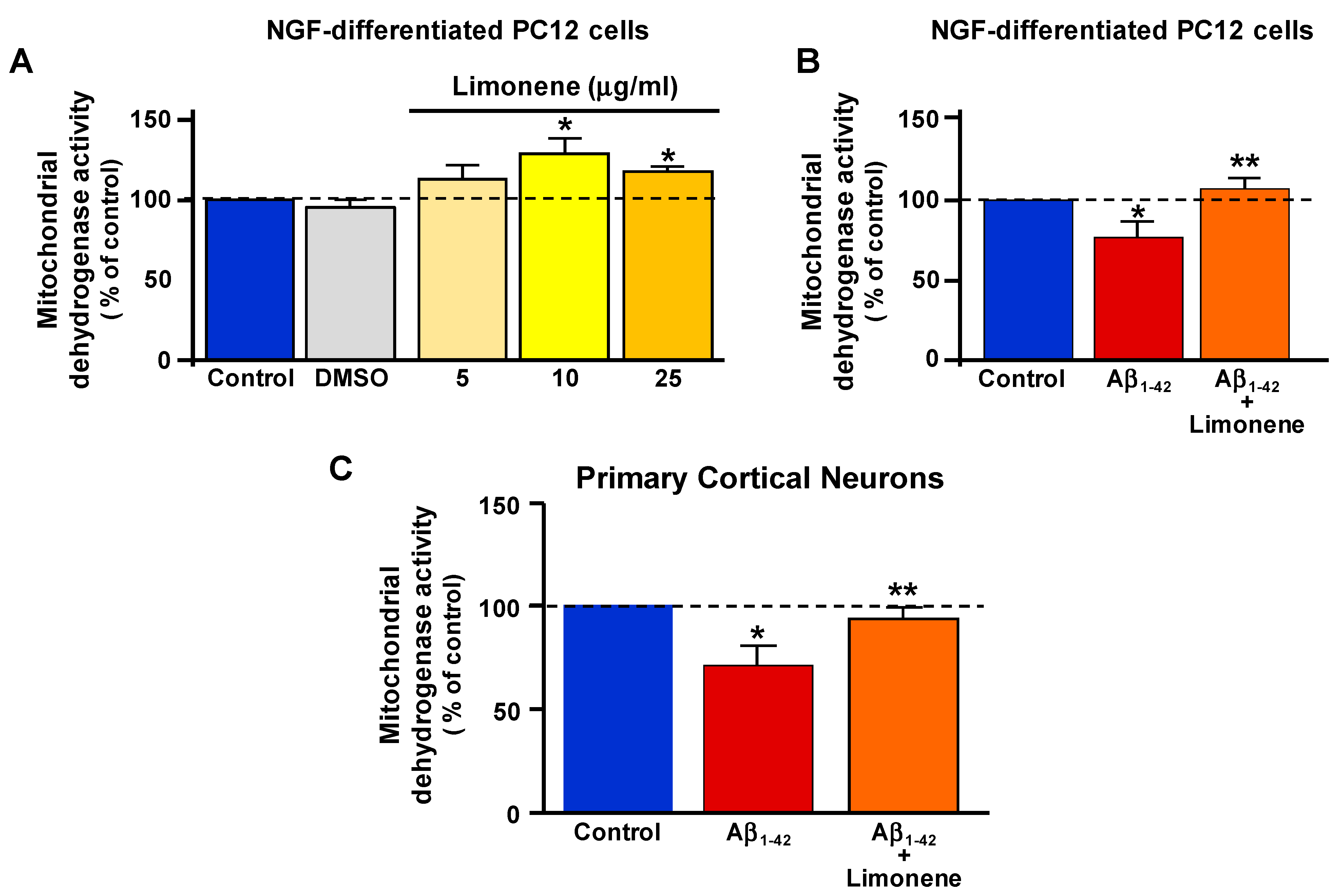
3.2. Effect of Limonene on Mitochondrial Dehydrogenase Activity Reduction Induced by Aβ1-42 Oligomers in Primary Cortical Neurons
3.3. Effect of Limonene on Nuclear Morphology Alteration Induced by Aβ1-42 Oligomers in Primary Cortical Neurons
3.4. Effect of Limonene on ROS Production Induced by Aβ1-42 Oligomers in Primary Cortical Neurons
3.5. Effect of Limonene on the Upregulation of Fast-Inactivating IA Currents Triggered by Aβ1-42 Oligomers in Primary Cortical Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Katsuno, M. Ultrastructure in Transthyretin Amyloidosis: From Pathophysiology to Therapeutic Insights. Biomedicines 2019, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- McCarty, M.F.; Di Nicolantonio, J.J.; Lerner, A.A. Fundamental Role for Oxidants and Intracellular Calcium Signals in Alzheimer’s Pathogenesis-And How a Comprehensive Antioxidant Strategy May Aid Prevention of This Disorder. Int. J. Mol. Sci. 2021, 22, 2140. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of reactive oxygen species in the progression of Alzheimer’s disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Pannaccione, A.; Secondo, A.; Molinaro, P.; D’Avanzo, C.; Cantile, M.; Esposito, A.; Boscia, F.; Scorziello, A.; Sirabella, R.; Sokolow, S.; et al. A new concept: Aβ1-42 generates a hyperfunctional proteolytic NCX3 fragment that delays caspase-12 activation and neuronal death. J. Neurosci. 2012, 32, 10609–10617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteras, N.; Abramov, A.Y. Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology. Cells 2020, 9, 2135. [Google Scholar] [CrossRef]
- Leong, Y.Q.; Ng, K.Y.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metab. Brain Dis. 2020, 35, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Eldufani, J.; Blaise, G. The role of acetylcholinesterase inhibitors such as neostigmine and rivastigmine on chronic pain and cognitive function in aging: A review of recent clinical applications. Alzheimers Dement. 2019, 5, 175–183. [Google Scholar] [CrossRef]
- Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat. Rev. Drug Discov. 2006, 5, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen. Res. 2017, 12, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, J.; Zaldívar-Machorro, V.J.; Villanueva-Porras, D.; Vega-Ávila, E.; Chavarría, A. A Potential Alternative against Neurodegenerative Diseases: Phytodrugs. Oxid. Med. Cell. Longev. 2016, 8378613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliberti, L.; Caputo, L.; De Feo, V.; De Martino, L.; Nazzaro, F.; Souza, L.F. Chemical Composition and in Vitro Antimicrobial, Cytotoxic, and Central Nervous System Activities of the Essential Oils of Citrus medica L. cv.‘Liscia’ and C. medica cv. ‘Rugosa’ Cultivated in Southern Italy. Molecules 2016, 21, 1244. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Seo, S.; Lamichhane, S.; Seo, J.; Hong, J.T.; Cha, H.J.; Yun, J. Limonene has anti-anxiety activity via adenosine A2A receptor-mediated regulation of dopaminergic and GABAergic neuronal function in the striatum. Phytomedicine 2021, 83, 153474. [Google Scholar] [CrossRef]
- Piccinelli, A.C.; Morato, P.N.; Dos Santos Barbosa, M.; Croda, J.; Sampson, J.; Kong, X.; Konkiewitz, E.C.; Ziff, E.B.; Amaya-Farfan, J.; Kassuya, C.A. Limonene reduces hyperalgesia induced by gp120 and cytokines by modulation of IL-1 β and protein expression in spinal cord of mice. Life Sci. 2017, 174, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharmacol. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kummer, R.; Fachini-Queiroz, F.C.; Estevão-Silva, C.F.; Grespan, R.; Silva, E.L.; Bersani-Amado, C.A.; Cuman, R.K. Evaluation of Anti-Inflammatory Activity of Citrus latifolia Tanaka Essential Oil and Limonene in Experimental Mouse Models. Evid. Based Complement. Alternat. Med. 2013, 859083. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, G.; Shen, W. Protective effects of D-limonene against transient cerebral ischemia in stroke-prone spontaneously hypertensive rats. Exp. Ther. Med. 2018, 15, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2013, 715, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinomiya, M.; Kawamura, K.; Tanida, E.; Nagoshi, M.; Motoda, H.; Kasanami, Y.; Hiragami, F.; Kano, Y. Neurite outgrowth of PC12 mutant cells induced by orange oil and d-limonene via the p38 MAPK pathway. Acta Med. Okayama 2012, 66, 111–118. [Google Scholar]
- Soundharrajan, I.; Kim, D.H.; Srisesharam, S.; Kuppusamy, P.; Choi, K.C. R-Limonene Enhances Differentiation and 2-Deoxy-D-Glucose Uptake in 3T3-L1 Preadipocytes by Activating the Akt Signaling Pathway. Evid. Based Complement. Alternat. Med. 2018, 4573254. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Tahir, M.; Khan, A.Q.; Khan, R.; Oday-O-Hamiza, L.A.; Hassan, S.K.; Rashid, S.; Ali, N.; Zeeshan, M.; Sultana, S. D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Exp. Biol. Med. 2014, 239, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Liu, Q.F.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.S.; Koo, B.S.; et al. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Etcheberrigaray, R.; Bhagavan, S. Ionic and signal transduction alterations in Alzheimer’s disease: Relevance of studies on peripheral cells. Mol. Neurobiol. 1999, 20, 93–109. [Google Scholar] [CrossRef]
- Ramsden, M.; Plant, L.D.; Webster, N.J.; Vaughan, P.F.; Henderson, Z.; Pearson, H.A. Differential effects of unaggregated and aggregated amyloid beta protein (1-40) on K(+) channel currents in primary cultures of rat cerebellar granule and cortical neurones. J. Neurochem. 2001, 79, 699–712. [Google Scholar] [CrossRef]
- Angulo, E.; Noe, V.; Casado, V.; Mallol, J.; Gomez-Isla, T.; Lluis, C.; Ferrer, I.; Ciudad, C.J.; Franco, R. Up-regulation of the KV3.4 potassium channel subunit in early stages of Alzheimer’s disease. J. Neurochem. 2004, 91, 547–557. [Google Scholar] [CrossRef]
- Yu, S.P. Regulation and critical role of potassium homeostasis in apoptosis. Prog. Neurobiol. 2003, 70, 363–386. [Google Scholar] [CrossRef]
- Yi, M.; Yu, P.; Lu, Q.; Geller, H.M.; Yu, Z.; Chen, H. KCa3.1 constitutes a pharmacological target for astrogliosis associated with Alzheimer’s disease. Mol. Cell. Neurosci. 2016, 76, 21–32. [Google Scholar] [CrossRef]
- Boscia, F.; Pannaccione, A.; Ciccone, R.; Casamassa, A.; Franco, C.; Piccialli, I.; de Rosa, V.; Vinciguerra, A.; Di Renzo, G.; Annunziato, L. The expression and activity of KV3.4 channel subunits are precociously upregulated in astrocytes exposed to Aβ oligomers and in astrocytes of Alzheimer’s disease Tg2576 mice. Neurobiol. Aging 2017, 54, 187–198. [Google Scholar] [CrossRef]
- Wei, T.; Wang, Y.; Xu, W.; Liu, Y.; Chen, H.; Yu, Z. KCa3.1 deficiency attenuates neuroinflammation by regulating an astrocyte phenotype switch involving the PI3K/AKT/GSK3β pathway. Neurobiol. Dis. 2019, 132, 104588. [Google Scholar] [CrossRef]
- Maqoud, F.; Scala, R.; Hoxha, M.; Zappacosta, B.; Tricarico, D. ATP-sensitive potassium channel subunits in the neuroinflammation: Novel drug targets in neurodegenerative disorders. CNS Neurol. Disord. Drug Targets 2021. [Google Scholar] [CrossRef]
- Pannaccione, A.; Secondo, A.; Scorziello, A.; Calì, G.; Taglialatela, M.; Annunziato, L. Nuclear factor-kappaB activation by reactive oxygen species mediates voltage-gated K+ current enhancement by neurotoxic beta-amyloid peptides in nerve growth factor-differentiated PC-12 cells and hippocampal neurones. J. Neurochem. 2005, 94, 572–586. [Google Scholar] [CrossRef]
- Pannaccione, A.; Boscia, F.; Scorziello, A.; Adornetto, A.; Castaldo, P.; Sirabella, R.; Taglialatela, M.; Di Renzo, G.F.; Annunziato, L. Up-regulation and increased activity of KV3.4 channels and their accessory subunit MinK-related peptide 2 induced by amyloid peptide are involved in apoptotic neuronal death. Mol. Pharmacol. 2007, 72, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boda, E.; Hoxha, E.; Pini, A.; Montarolo, F.; Tempia, F. Brain expression of Kv3 subunits during development, adulthood and aging and in a murine model of Alzheimer’s disease. J. Mol. Neurosci. 2012, 46, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, R.; Piccialli, I.; Grieco, P.; Merlino, F.; Annunziato, L.; Pannaccione, A. Synthesis and Pharmacological Evaluation of a Novel Peptide Based on Anemonia sulcata BDS-I Toxin as a New KV3.4 Inhibitor Exerting a Neuroprotective Effect Against Amyloid-β Peptide. Front. Chem. 2019, 7, 479. [Google Scholar] [CrossRef] [PubMed]
- Piccialli, I.; Ciccone, R.; Pannaccione, A. The new KV3.4 inhibitor BDS-I[1-8] as a potential pharmacological opportunity in Alzheimer’s disease therapy. Neural Regen. Res. 2020, 15, 1255–1256. [Google Scholar] [PubMed]
- Piccialli, I.; Tedeschi, V.; Boscia, F.; Ciccone, R.; Casamassa, A.; de Rosa, V.; Grieco, P.; Secondo, A.; Pannaccione, A. The Anemonia sulcata Toxin BDS-I Protects Astrocytes Exposed to Aβ1-42 Oligomers by Restoring [Ca2+]i Transients and ER Ca2+ Signaling. Toxins 2020, 13, 20. [Google Scholar] [CrossRef]
- Song, M.S.; Ryu, P.D.; Lee, S.Y. Kv3.4 is modulated by HIF-1α to protect SH-SY5Y cells against oxidative stress-induced neural cell death. Sci. Rep. 2017, 7, 2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Caputo, L.; Reguilon, M.D.; Mińarro, J.; De Feo, V.; Rodriguez-Arias, M. Lavandula angustifolia Essential Oil and Linalool Counteract Social Aversion Induced by Social Defeat. Molecules 2018, 23, 2694. [Google Scholar] [CrossRef] [Green Version]
- Secondo, A.; Petrozziello, T.; Tedeschi, V.; Boscia, F.; Vinciguerra, A.; Ciccone, R.; Pannaccione, A.; Molinaro, P.; Pignataro, G.; Annunziato, L. ORAI1/STIM1 Interaction Intervenes in Stroke and in Neuroprotection Induced by Ischemic Preconditioning Through Store-Operated Calcium Entry. Stroke 2019, 50, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Secondo, A.; Staiano, R.I.; Scorziello, A.; Sirabella, R.; Boscia, F.; Adornetto, A.; Valsecchi, V.; Molinaro, P.; Canzoniero, L.M.; Di Renzo, G.; et al. BHK cells transfected with NCX3 are more resistant to hypoxia followed by reoxygenation than those transfected with NCX1 and NCX2: Possible relationship with mitochondrial membrane potential. Cell Calcium 2007, 42, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Petrozziello, T.; Secondo, A.; Tedeschi, V.; Esposito, A.; Sisalli, M.; Scorziello, A.; Di Renzo, G.; Annunziato, L. ApoSOD1 lacking dismutase activity neuroprotects motorneurons exposed to beta-methylamino-L-alanine through the Ca2+/Akt/ERK1/2 prosurvival pathway. Cell Death Differ. 2017, 24, 511–522. [Google Scholar] [CrossRef]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef]
- Tang, X.P.; Guo, X.H.; Geng, D.; Weng, L.J. d-Limonene protects PC12 cells against corticosterone-induced neurotoxicity by activating the AMPK pathway. Environ. Toxicol. Pharmacol. 2019, 70, 103192. [Google Scholar] [CrossRef]
- Diochot, S.; Schweitz, H.; Beress, L.; Lazdunski, M. Sea anemone peptides with a specific blocking activity against the fast inactivating potassium channel KV3.4. J. Biol.Chem. 1998, 273, 6744–6749. [Google Scholar] [CrossRef] [Green Version]
- Berliocchi, L.; Chiappini, C.; Adornetto, A.; Gentile, D.; Cerri, S.; Russo, R.; Bagetta, G.; Corasaniti, M.T. Early LC3 lipidation induced by d-limonene does not rely on mTOR inhibition, ERK activation and ROS production and it is associated with reduced clonogenic capacity of SH-SY5Y neuroblastoma cells. Phytomedicine 2018, 40, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Song, M.S.; Park, S.M.; Park, J.S.; Byun, J.H.; Jin, H.J.; Seo, S.H.; Ryu, P.D.; Lee, S.Y. Kv3.1 and Kv3.4, Are Involved in Cancer Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamagno, E.; Guglielmotto, M.; Giliberto, L.; Vitali, A.; Borghi, R.; Autelli, R.; Danni, O.; Tabaton, M. JNK and ERK1/2 pathways have a dual opposite effect on the expression of BACE1. Neurobiol. Aging 2009, 30, 1563–1573. [Google Scholar] [CrossRef]
- Pei, J.J.; Braak, H.; An, W.-L.; Winblad, B.; Cowburn, R.F.; Iqbal, K.; Grundke-Iqbal, I. Up-regulation of mitogen-activated protein kinases ERK1/2 and MEK1/2 is associated with the progression of neurofibrillary degeneration in Alzheimer’s disease. Brain Res. Mol. Brain Res. 2002, 109, 45–55. [Google Scholar] [CrossRef]
- Wadhwani, A.R.; Affaneh, A.; Van Gulden, S.; Kessler, J.A. Neuronal apolipoprotein E4 increases cell death and phosphorylated tau release in alzheimer disease. Ann. Neurol. 2019, 85, 726–739. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Erdogan Orhan, I.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive Facilitation and Antioxidant Effects of an Essential Oil Mix on Scopolamine-Induced Amnesia in Rats: Molecular Modeling of In Vitro and In Vivo Approaches. Molecules 2020, 25, 1519. [Google Scholar] [CrossRef] [Green Version]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef]
- Jean, L.; Brimijoin, S.; Vaux, D.J. In vivo localization of human acetylcholinesterase-derived species in a β-sheet conformation at the core of senile plaques in Alzheimer’s disease. J. Biol. Chem. 2019, 294, 6253–6272. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.; Dinamarca, M.C.; Pérez-Acle, T.; Colombres, M. Acetylcholinesterase-amyloid-beta-peptide interaction: Effect of Congo Red and the role of the Wnt pathway. Curr. Alzheimer Res. 2005, 2, 301–306. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Dinamarca, M.C.; Alvarez, A. Amyloid-cholinesterase interactions. Implications for Alzheimer’s disease. FEBS J. 2008, 275, 625–632. [Google Scholar] [CrossRef]
- Ismaili, L.; Refouvelet, B.; Benchekroun, M.; Brogi, S.; Brindisi, M.; Gemma, S.; Campiani, G.; Filipic, S.; Agbaba, D.; Esteban, G.; et al. Multitarget compounds bearing tacrine- and donepezil-like structural and functional motifs for the potential treatment of Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 4–34. [Google Scholar] [CrossRef] [PubMed]




| Compound | Concentration (mg/mL) | AChE Inhibition (%) | IC50 (mg/mL) |
|---|---|---|---|
| Limonene | 100 | 87.8 ± 1.4 | |
| 10 | 78.4 ± 1.8 | 7.7 ± 1.2 | |
| 1 | n.a | ||
| Galantamine | 100 | 100.1 ± 1.9 | |
| 10 | 96.2 ± 2.1 | ||
| 1 | 85.0 ± 0.8 | 0.6 ± 0.2 | |
| 0.1 | n.a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered byAβ1-42 Oligomers in Primary Cortical Neurons. Antioxidants 2021, 10, 937. https://doi.org/10.3390/antiox10060937
Piccialli I, Tedeschi V, Caputo L, Amato G, De Martino L, De Feo V, Secondo A, Pannaccione A. The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered byAβ1-42 Oligomers in Primary Cortical Neurons. Antioxidants. 2021; 10(6):937. https://doi.org/10.3390/antiox10060937
Chicago/Turabian StylePiccialli, Ilaria, Valentina Tedeschi, Lucia Caputo, Giuseppe Amato, Laura De Martino, Vincenzo De Feo, Agnese Secondo, and Anna Pannaccione. 2021. "The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered byAβ1-42 Oligomers in Primary Cortical Neurons" Antioxidants 10, no. 6: 937. https://doi.org/10.3390/antiox10060937
APA StylePiccialli, I., Tedeschi, V., Caputo, L., Amato, G., De Martino, L., De Feo, V., Secondo, A., & Pannaccione, A. (2021). The Antioxidant Activity of Limonene Counteracts Neurotoxicity Triggered byAβ1-42 Oligomers in Primary Cortical Neurons. Antioxidants, 10(6), 937. https://doi.org/10.3390/antiox10060937









