Neonatal Vitamin C and Cysteine Deficiencies Program Adult Hepatic Glutathione and Specific Activities of Glucokinase, Phosphofructokinase, and Acetyl-CoA Carboxylase in Guinea Pigs’ Livers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Model
3. Results
3.1. Diet Type and Phenotypical Changes
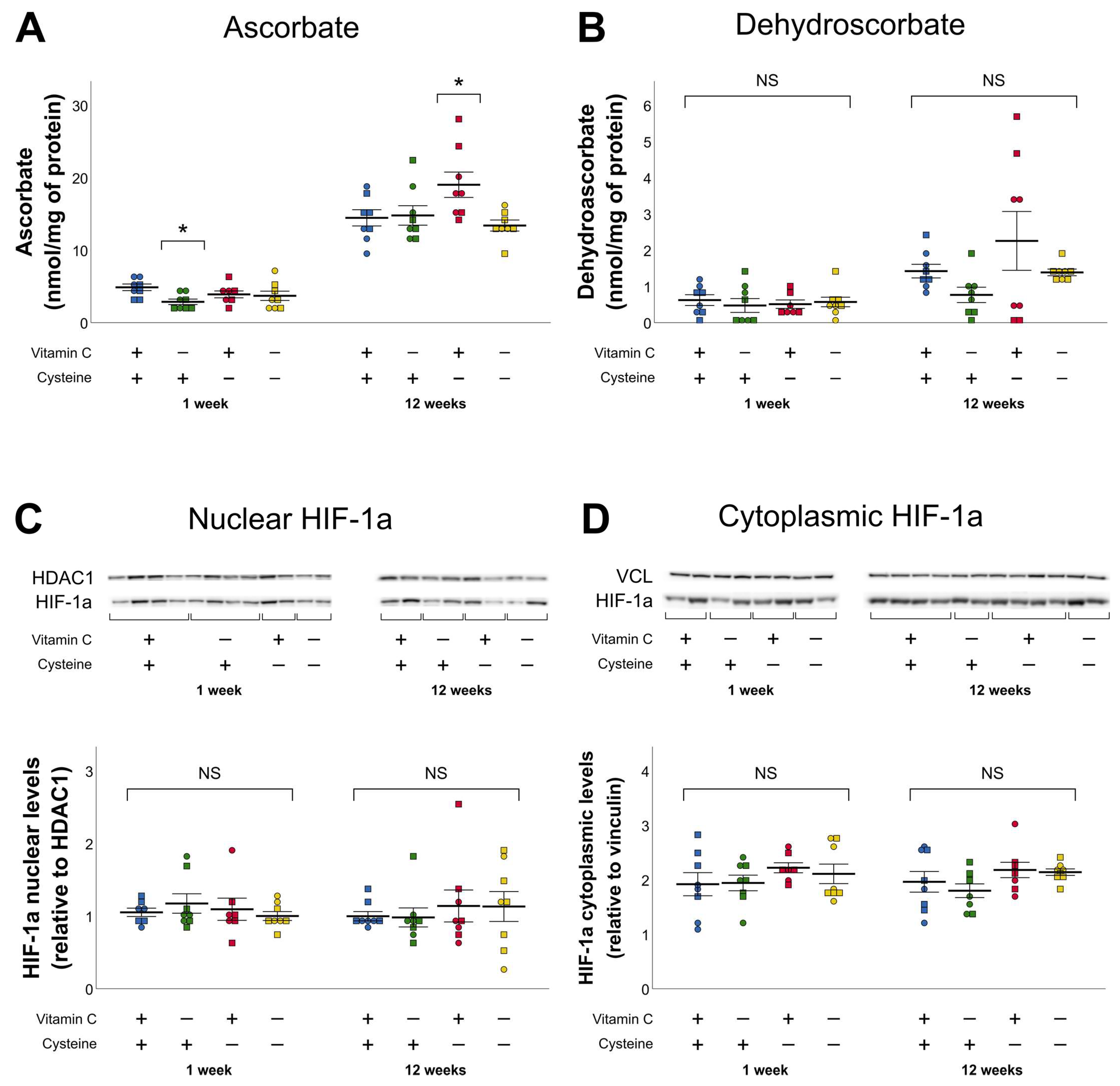
3.2. Ascorbate and Dehydroascorbate (DHA)
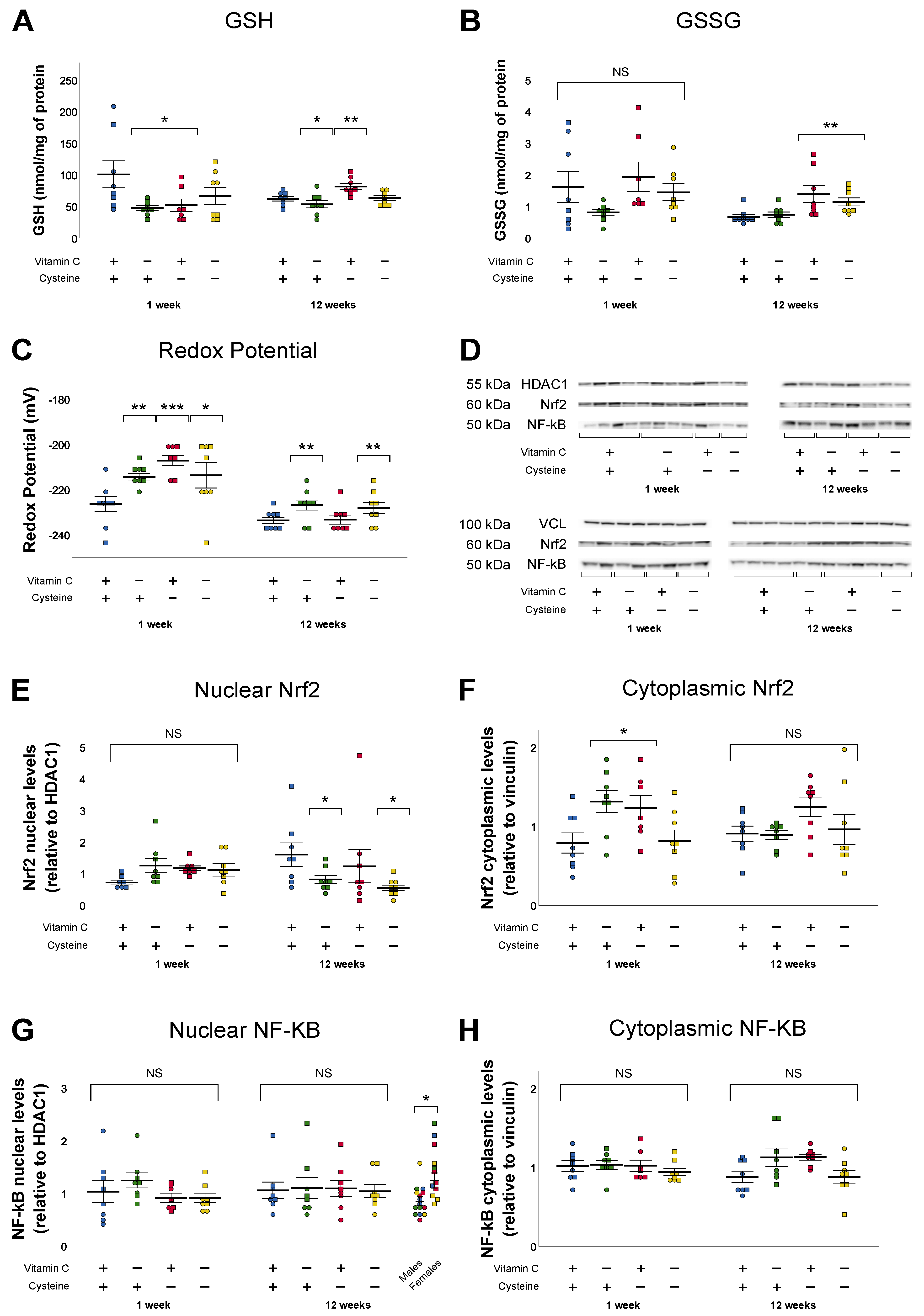
3.3. Glutathione and Oxidative Stress
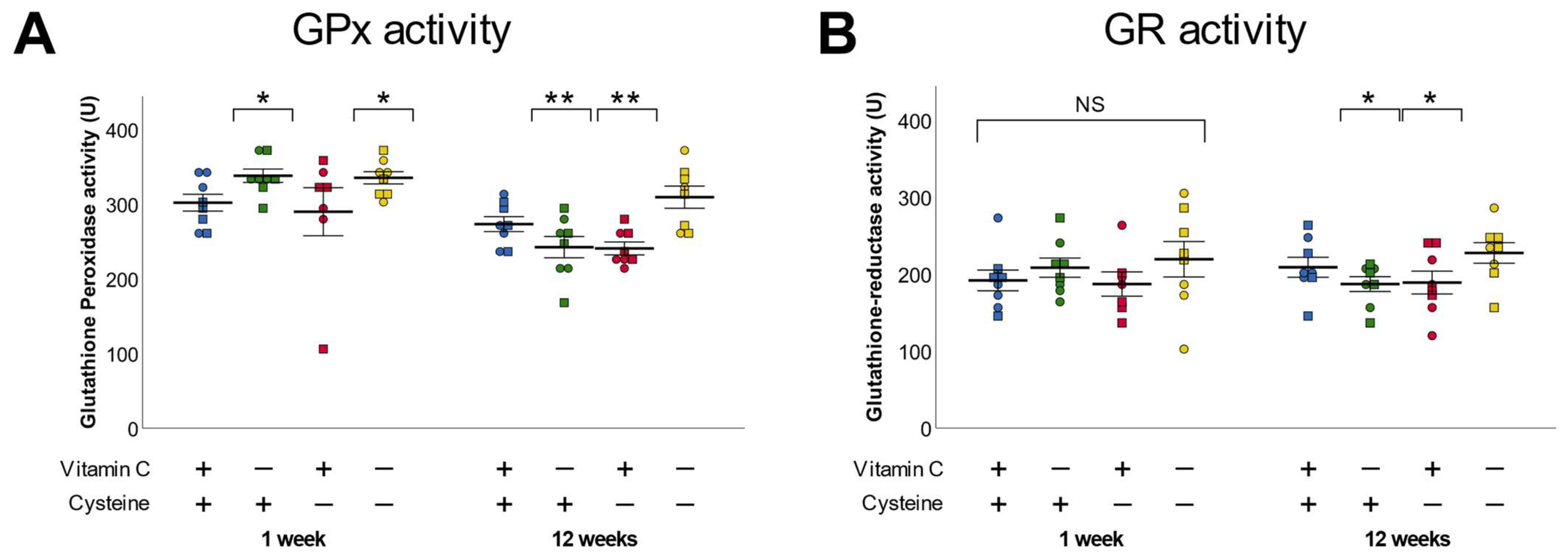
3.4. Glutathione Peroxidase and Reductase
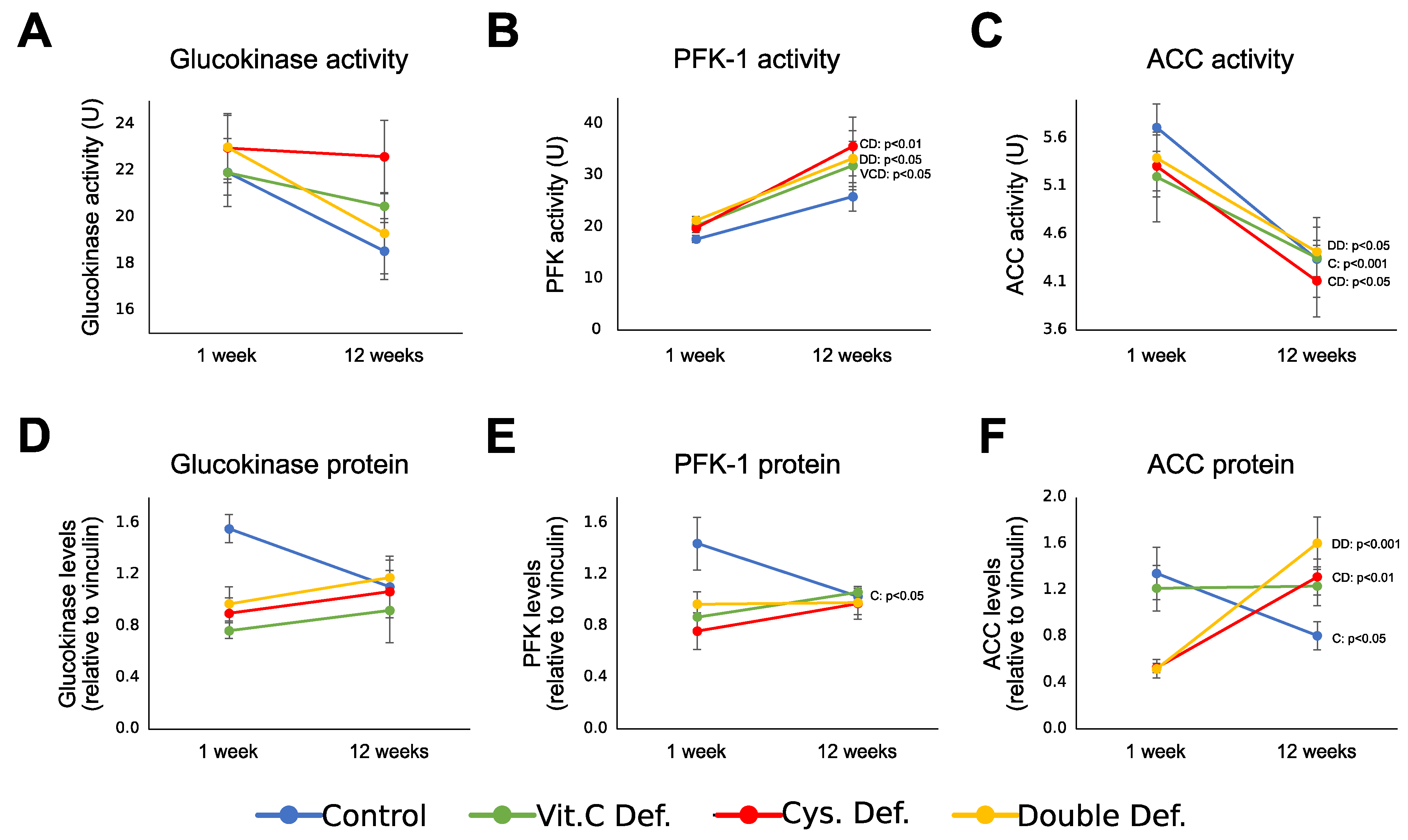
3.5. Energy Metabolism Enzymes
3.6. Developmental Change
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hitchler, M.J.; Domann, F.E. The epigenetic and morphogenetic effects of molecular oxygen and its derived reactive species in development. Free Radic. Biol. Med. 2021. [Google Scholar] [CrossRef]
- Martin, A.; Faes, C.; Debevec, T.; Rytz, C.; Millet, G.; Pialoux, V. Preterm birth and oxidative stress: Effects of acute physical exercise and hypoxia physiological responses. Redox Biol. 2018, 17, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Saphier, O.; Schneid-Kofman, N.; Silberstein, E.; Silberstein, T. Does mode of delivery affect neonate oxidative stress in parturition? Review of literature. Arch. Gynecol. Obstet. 2012, 287, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.D.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.; Elremaly, W.; Rouleau, T.; Lavoie, J.-C. Oxygen and parenteral nutrition two main oxidants for extremely preterm infants: “It all adds up”. J. Neonatal Perinat. Med. 2015, 8, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Repa, A.; Binder, C.; Thanhaeuser, M.; Kreissl, A.; Pablik, E.; Huber-Dangl, M. A mixed lipid emulsion for prevention of parenteral nutrition associated cholestasis in extremely low birth weight infants: A randomized clinical trial. J. Pediatr. 2018, 194, 87–93.e1. [Google Scholar] [CrossRef] [PubMed]
- Ozsurekci, Y.; Aykac, K. Oxidative stress related diseases in newborns. Oxid. Med. Cell. Longev. 2016, 2016, 2768365. [Google Scholar] [CrossRef]
- Flahault, A.; Paquette, K.; Fernandes, R.O.; Delfrate, J.; Cloutier, A.; Henderson, M. Increased incidence but lack of association between cardiovascular risk factors in adults born preterm. Hypertension 2020, 75, 796–805. [Google Scholar] [CrossRef]
- South, A.M.; Nixon, P.A.; Chappell, M.C.; Diz, D.I.; Russell, G.B.; Jensen, E.T. Renal function and blood pressure are altered in adolescents born preterm. Pediatr. Nephrol. 2018, 34, 137–144. [Google Scholar] [CrossRef]
- Parkinson, J.R.; Hyde, M.J.; Gale, C.; Santhakumaran, S.; Modi, N. Preterm birth and the metabolic syndrome in adult life: A systematic review and meta-analysis. Pediatrics 2013, 131, e1240–e1263. [Google Scholar] [CrossRef] [PubMed]
- Breij, L.M.; Kerkhof, G.F.; Hokken-Koelega, A.C. Risk for nonalcoholic fatty liver disease in young adults born preterm. Horm. Res. Paediatr. 2015, 84, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Vohr, B.R.; Heyne, R.; Bann, C.; Das, A.; Higgins, R.D.; Hintz, S.R.; Jobe, A.H.; Caplan, M.S.; Polin, R.A.; Laptook, A.R.; et al. Extreme preterm infant rates of overweight and obesity at school age in the SUPPORT neuroimaging and neurodevelopmental outcomes cohort. J. Pediatr. 2018, 200, 132–139.e3. [Google Scholar] [CrossRef] [PubMed]
- Knafo, L.; Chessex, P.; Rouleau, T.; Lavoie, J.C. Association between hydrogen peroxide-dependent byproducts of ascorbic acid and increased hepatic acetyl-CoA carboxylase activity. Clin. Chem. 2005, 8, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.-C.; Bélanger, S.; Spalinger, M.; Chessex, P. Admixture of a multivitamin preparation to parenteral nutrition: The major contributor to in vitro generation of peroxides. Pediatrics 1997, 99, e6. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.-C.; Chessex, P.; Rouleau, T.; Tsopmo, A.; Friel, J. Shielding parenteral multivitamins from light increases vitamin A and E concentration in lung of newborn guinea pigs. Clin. Nutr. 2007, 26, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Guiraut, C.; Mohamed, I.; Lavoie, J.-C. Neonatal parenteral nutrition affects the metabolic flow of glucose in newborn and adult male Hartley guinea pigs’ liver. J. Dev. Orig. Health Dis. 2020, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kleiber, N.; Chessex, P.; Rouleau, T.; Nuyt, A.-M.; Perreault, M.; Lavoie, J.-C. Neonatal exposure to oxidants induces later in life a metabolic response associated to a phenotype of energy deficiency in an animal model of total parenteral nutrition. Pediatr. Res. 2010, 68, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.J. Missing step in man, monkey and guinea pig required for the biosynthesis of L-ascorbic acid. Nature 1957, 4585, 553. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.-C.; Mendiratta, S. Protection and recycling of α-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch. Biochem. Biophys. 1998, 349, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, K.; Zeng, X.; Yang, J.; Wu, Y.; Shi, X.; Qin, B.; Zeng, L.; Esteban, M.A.; Pan, G.; et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell 2011, 9, 575–587. [Google Scholar] [CrossRef]
- Minor, E.A.; Court, B.L.; Young, J.I.; Wang, G. Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013, 288, 13669–13674. [Google Scholar] [CrossRef]
- Hirsilä, M.; Koivunen, P.; Günzler, V.; Kivirikko, K.I.; Myllyharju, J. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 2003, 278, 30772–30780. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Huseby, N.-E.; Sundkvist, E.; Svineng, G. Glutathione and sulfur containing amino acids: Antioxidant and conjugation activities. In Glutathione and Sulfur Amino Acids in Human Health and Disease; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 93–120. [Google Scholar]
- Pallardó, F.V.; Markovic, J.; Viña, J. Cellular compartmentalization of glutathione. In R MMazza Geditors Glutathione and Sulfur Amino Acids in Human Health and Disease; Wiley: Hoboken, NJ, USA, 2008; pp. 35–45. [Google Scholar]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, H.; Sun, H.M.; Sue, G.R.; Jeong, W. Dynein light chain LC8 negatively regulates NF-κB through the redox-dependent interaction with IκBα. J. Biol. Chem. 2008, 35, 23863–23871. [Google Scholar] [CrossRef]
- Lavoie, J.-C.; Rouleau, T.; Truttmann, A.C.; Chessex, P. Postnatal gender-dependent maturation of cellular cysteine uptake. Free Radic. Res. 2002, 36, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Martensson, J.; Han, J.; Griffith, O.W.; Meister, A. Glutathione ester delays the onset of scurvy in ascorbate-deficient guinea pigs. Proc. Natl. Acad. Sci. USA 1993, 90, 317–321. [Google Scholar] [CrossRef]
- Mrtensson, J.; Meister, A. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: Ascorbate spares glutathione and protects. Proc. Natl. Acad. Sci. USA 1991, 88, 4656–4660. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Laboratory Animals; National Academies Press: Washington, DC, USA, 1995; p. 192. [Google Scholar]
- Dresler, S.; Maksymiec, W. Capillary zone electrophoresis for determination of reduced and oxidised ascorbate and glutathione in roots and leaf segments of Zea mays plants exposed to Cd and Cu. Acta. Sci. Pol. Hortorum Cultus 2013, 12, 143–155. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 1–2, 248–254. [Google Scholar] [CrossRef]
- Kudo, N.; Barr, A.J.; Barr, R.L.; Desai, S.; Lopaschuk, G.D. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J. Biol. Chem. 1995, 29, 17513–17520. [Google Scholar] [CrossRef] [PubMed]
- Maghdessian, R.; Côté, F.; Rouleau, T.; Ouadda, A.B.D.; Levy, É.; Lavoie, J.-C. Ascorbylperoxide contaminating parenteral nutrition perturbs the lipid metabolism in newborn guinea pig. J. Pharmacol. Exp. Ther. 2010, 334, 278–284. [Google Scholar] [CrossRef]
- Turcot, V.; Rouleau, T.; Tsopmo, A.; Germain, N.; Potvin, L.; Nuyt, A.-M.; Lavoie, J.-C. Long-term impact of an antioxidant-deficient neonatal diet on lipid and glucose metabolism. Free Radic. Biol. Med. 2009, 47, 275–282. [Google Scholar] [CrossRef]
- Mustafi, S.; Camarena, V.; Qureshi, R.; Yoon, H.; Volmar, C.-H.; Huff, T.C.; Sant, D.W.; Zheng, L.; Brothers, S.P.; Wahlestedt, C.; et al. Vitamin C supplementation expands the therapeutic window of BETi for triple negative breast cancer. EBioMedicine 2019, 43, 201–210. [Google Scholar] [CrossRef]
- CUI, Y.; OTSUKA, M.; FUJIWARA, Y. Reduction of dehydroerythorbic acid in vitamin C-deficient guinea pigs. J. Nutr. Sci. Vitaminol. (Tokyo) 2001, 47, 316–320. [Google Scholar] [CrossRef]
- Frikke-Schmidt, H.; Tveden-Nyborg, P.; Lykkesfeldt, J. L-dehydroascorbic acid can substitute l-ascorbic acid as dietary vitamin C source in guinea pigs. Redox Biol. 2016, 7, 8–13. [Google Scholar] [CrossRef]
- Beatty, P.; Reed, D.J. Influence of cysteine upon the glutathione status of isolated rat hepatocytes. Biochem. Pharmacol. 1981, 30, 1227–1230. [Google Scholar] [CrossRef]
- Wells, W.W.; Xu, D.P.; Yang, Y.F.; Rocque, P.A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J. Biol. Chem. 1990, 26, 15361–15364. [Google Scholar] [CrossRef]
- Paolicchi, A.; Pezzini, A.; Saviozzi, M.; Piaggi, S.; Andreuccetti, M.; Chieli, E.; Malvaldi, G.; Casini, A.F. Localization of a GSH-dependent dehydroascorbate reductase in rat tissues and subcellular fractions. Arch. Biochem. Biophys. 1996, 333, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.; Corey, P.N.; El-Sohemy, A. Vitamin C deficiency in a population of young canadian adults. Am. J. Epidemiol. 2009, 170, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.; Carr, A.C. Global vitamin c status and prevalence of deficiency: A cause for concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef]
- De Oliveira, A.M.; Rondo, P.R.D.C.; Mastroeni, S.S.; Oliveira, J.M. Plasma concentrations of ascorbic acid in parturients from a hospital in Southeast Brazil. Clin. Nutr. 2008, 27, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.D.; Vashist, P.; Gupta, S.K.; Young, I.; Maraini, G.; Camparini, M.; Jayanthi, R.; John, N.; Fitzpatrick, K.E.; Chakravarthy, U.; et al. Prevalence and risk factors for vitamin C deficiency in North and South India: A two centre population based study in people aged 60 years and over. PLoS ONE 2011, 6, e28588. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990, 5, 2327–2334. [Google Scholar] [CrossRef]
- Sica, A.; Tan, T.H.; Rice, N.; Kretzschmar, M.; Ghosh, P.; Young, H.A. The c-rel protooncogene product c-Rel but not NF-κB binds to the intronic region of the human interferon-γ gene at a site related to an interferon- stimulable response element. Proc. Natl. Acad. Sci. USA 1992, 5, 1740–1744. [Google Scholar] [CrossRef]
- Arima, N.; Matsushita, K.; Obata, H.; Ohtsubo, H.; Fujiwara, H.; Arimura, K. NF-κB involvement in the activation of primary adult T-cell leukemia cells and its clinical implications. Exp. Hematol. 1999, 7, 1168–1175. [Google Scholar] [CrossRef]
- Banning, A.; Deubel, S.; Kluth, D.; Zhou, Z.; Brigelius-Flohé, R. The GI-GPx gene is a target for Nrf2. Mol. Cell Biol. 2005, 12, 4914–4923. [Google Scholar] [CrossRef]
- Agyeman, A.S.; Chaerkady, R.; Shaw, P.G.; Davidson, N.E.; Visvanathan, K.; Pandey, A. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res. Treat. 2012, 1, 175–187. [Google Scholar] [CrossRef]
- Tippett, P.S.; Neet, K.E. Interconversions between different sulfhydryl-related kinetic states in glucokinase. Arch. Biochem. Biophys. 1983, 222, 285–298. [Google Scholar] [CrossRef]
- Gilbert, H.F. Biological disulfides: The third messenger? Modulation of phosphofructokinase activity by thiol/disulfide exchange. J. Biol. Chem. 1982, 20, 12086–12091. [Google Scholar] [CrossRef]
- Rao, R.K.; Clayton, L.W. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem. Biophys. Res. Commun. 2002, 293, 610–616. [Google Scholar] [CrossRef]
- Ballard, F.J.; Hanson, R.W. Changes in lipid synthesis in rat liver during development. Biochem. J. 1967, 102, 952–958. [Google Scholar] [CrossRef]
- Burch, H.B.; Lowry, O.H.; Kuhlman, A.M.; Slerjance, J.; Diamant, E.J.; Lowry, S.R. Changes in patterns of enzymes of carbohydrate metabolism in the developing rat liver. J. Biol. Chem. 1963, 7, 2267–2273. [Google Scholar] [CrossRef]
- Lengo, A.M.; Guiraut, C.; Mohamed, I.; Lavoie, J.-C. Relationship between redox potential of glutathione and DNA methylation level in liver of newborn guinea pigs. Epigenetics 2020, 15, 1348–1360. [Google Scholar] [CrossRef]
- Yara, S.; Levy, E.; Elremaly, W.; Rouleau, T.; Lavoie, J.-C. Total parenteral nutrition induces sustained hypomethylation of DNA in newborn guinea pigs. Pediatr. Res. 2013, 73, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Fei, J.; Lan, M.S.; Lu, Z.P.; Liu, M.; Fan, W.W.; Gao, X.; Lu, D.R. Hypermethylation of hepatic Gck promoter in ageing rats contributes to diabetogenic potential. Diabetologia 2008, 51, 1525–1533. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, Y.; Liu, M.; Lan, M.S.; Fei, J.; Fan, W.; Gao, X.; Lu, D.R. Hypermethylation of hepatic glucokinase and L-type pyruvate kinase promoters in high-fat diet-induced obese rats. Endocrinology 2011, 152, 1284–1289. [Google Scholar] [CrossRef]
- Mao, J.; DeMayo, F.J.; Li, H.; Abu-Elheiga, L.; Gu, Z.; Shaikenov, T.E. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc. Natl. Acad. Sci. USA 2006, 22, 8552–8557. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Roncal-Jimenez, C.A.; Orlicky, D.J.; Cicerchi, C.; Mcmahan, R.H.; Abdelmalek, M.F.; Rosen, H.R.; Jackman, M.R.; et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013, 58, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Elremaly, W.; Rouleau, T.; Lavoie, J.C. Inhibition of hepatic methionine adenosyltransferase by peroxides contaminating parenteral nutrition leads to a lower level of glutathione in newborn Guinea pigs. Free Radic. Biol. Med. 2012, 53, 2250–2255. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Dunn, S.E.; Ousman, S.S.; Sobel, R.A.; Zuniga, L.; Baranzini, S.E.; Youssef, S. Peroxisome proliferator-activated receptor (PPAR) α expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J. Exp. Med. 2007, 2, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.L.; Botting, K.J.; Darby, J.R.T.; David, A.L.; Dyson, R.M.; Gatford, K.L.; Gray, C.; Herrera, E.A.; Hirst, J.J.; Kim, B.; et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J. Physiol. 2018, 596, 5535–5569. [Google Scholar] [CrossRef] [PubMed]
- Morin, G.; Guiraut, C.; Marcogliese, M.P.; Mohamed, I.; Lavoie, J.-C. Glutathione supplementation of parenteral nutrition prevents oxidative stress and sustains protein synthesis in guinea pig model. Nutrients 2019, 11, 2063. [Google Scholar] [CrossRef] [PubMed]
- Elremaly, W.; Mohamed, I.; Rouleau, T.; Lavoie, J.C. Adding glutathione to parenteral nutrition prevents alveolar loss in newborn Guinea pig. Free Radic. Biol. Med. 2015, 87, 274–281. [Google Scholar] [CrossRef]
- Cisternas, P.; Martínez, F.; Fernandez, E.; Ferrada, L.; Oyarce, K.; Salazar, K.; Bolanos, J.P.; Nualart, F.; Silva-Alvarez, C. The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 2014, 129, 663–671. [Google Scholar] [CrossRef]
- Roudot-Algaron, F. Le goût des acides aminés, des peptides et des protéines: Exemple de peptides sapides dans les hydrolysats de caséines. Lait 1996, 4, 313–348. [Google Scholar] [CrossRef]
- Thorn, S.L.; Young, G.S.; Kirkland, J.B. The guinea-pig is a poor animal model for studies of niacin deficiency and presents challenges in any study using purified diets. Br. J. Nutr. 2007, 98, 78–85. [Google Scholar] [CrossRef][Green Version]
- Reid, M.E.; Mickelsen, O. Nutritional studies with the guinea pig. VII. Effect of different proteins, with and without amino acid supplements, on growth. J. Nutr. 1963, 80, 25–32. [Google Scholar] [PubMed]
- Burk, R.F.; Christensen, J.M.; Maguire, M.J.; Austin, L.M.; Whetsell, W.O.; May, J.M.; Hill, K.E.; Ebner, F.F. A combined deficiency of vitamins E and C causes severe central nervous system damage in guinea pigs. J. Nutr. 2006, 136, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Motley, A.K.; May, J.M.; Burk, R.F. Combined selenium and vitamin C deficiency causes cell death in guinea pig skeletal muscle. Nutr. Res. 2009, 3, 213–219. [Google Scholar] [CrossRef]
- Barja, G.; López-Torres, M.; Pérez-Campo, R.; Rojas, C.; Cadenas, S.; Prat, J.; Pamplona, R. Dietary vitamin C decreases endogenous protein oxidative damage, malondialdehyde, and lipid peroxidation and maintains fatty acid unsaturation in the guinea pig liver. Free Radic. Biol. Med. 1994, 17, 105–115. [Google Scholar] [CrossRef]
- Schjoldager, J.G.; Paidi, M.D.; Lindblad, M.M.; Birck, M.M.; Kjærgaard, A.B.; Dantzer, V. Maternal vitamin C deficiency during pregnancy results in transient fetal and placental growth retardation in guinea pigs. Eur. J. Nutr. 2015, 4, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Paidi, M.D.; Schjoldager, J.G.; Lykkesfeldt, J.; Tveden-Nyborg, P. Prenatal vitamin C deficiency results in differential levels of oxidative stress during late gestation in foetal guinea pig brains. Redox Biol. 2014, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]


| Control Diet (C) | Vitamin C Deficient Diet (VCD) | Cysteine Deficient Diet (CD) | Double Deficiency Diet (DD) | Long-Term Standard Diet | |
|---|---|---|---|---|---|
| Energy (kcal/g) | 3.5 | 3.5 | 3.5 | 3.5 | 2.4 |
| Protein (% kcal) | 18.4 | 18.4 | 18.4 | 18.4 | 32 |
| Carbohydrate (% kcal) | 55.7 | 55.7 | 55.7 | 55.7 | 18 |
| Fat (% kcal) | 25.9 | 25.9 | 25.9 | 25.9 | 50 |
| L-alanine (g/kg) | 3.65 | 3.65 | 4.01 | 4.01 | 9 |
| L-aspartic acid (g/kg) | 3.65 | 3.65 | 4.19 | 4.19 | 17 |
| L-cystine (g/kg) | 2.70 | 2.70 | - | - | 3 |
| L-glutamic acid (g/kg) | 41.67 | 41.67 | 42.27 | 42.27 | 27 |
| Glycine (g/kg) | 24.45 | 24.45 | 24.76 | 24.76 | 10 |
| L-methionine (g/kg) | 2.90 | 2.90 | 2.00 | 2.00 | 3 |
| L-proline (g/kg) | 3.65 | 3.65 | 4.12 | 4.12 | 11 |
| L-serine (g/kg) | 3.65 | 3.65 | 4.08 | 4.08 | 10 |
| Vitamin C (mg/kg) | 203 | - | 203 | - | 1050 |
| 1-Week-Old Animals | 12-Week-Old Animals | |||||||
|---|---|---|---|---|---|---|---|---|
| Control (C) | Vitamin C Deficient (VCD) | Cysteine Deficient (CD) | Double Deficiency (DD) | Control (C) | Vitamin C Deficient (VCD) | Cysteine Deficient (CD) | Double Deficiency (DD) | |
| Bodyweight at day 3 (g) | 113 ± 4 | 100 ± 3 | 107 ± 3 | 107 ± 3 | 107 ± 4 | 100 ± 3 | 105 ± 3 | 99 ± 4 |
| Bodyweight at sacrifice (g) | 124 ± 4 | 107 ± 4 ** | 104 ± 2 ** | 101 ± 3 ** | 577 ± 25 | 588 ± 34 | 599 ± 29 | 558 ± 21 |
| Bodyweight change from day 3 to sacrifice (%) | 110 ± 3 | 107 ± 1 | 97 ± 1 *** | 94 ± 1 *** | 542 ± 21 | 594 ± 40 | 575 ± 37 | 572 ± 31 |
| Liver weight at sacrifice (g) | 3.8 ± 0.4 | 3.4 ± 0.1 | 3.2 ± 0.1 | 3.3 ± 0.1 | 18 ± 1.1 | 20 ± 1.7 | 18 ± 1.1 | 16 ± 0.7 |
| Liver weight/bodyweight (%) | 30 ± 2 | 32 ± 1 | 31 ± 1 | 33 ± 1 | 31 ± 1 | 34 ± 2 | 29 ± 1 ** | 30 ± 1 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, V.; Mohamed, I.; Lavoie, J.-C. Neonatal Vitamin C and Cysteine Deficiencies Program Adult Hepatic Glutathione and Specific Activities of Glucokinase, Phosphofructokinase, and Acetyl-CoA Carboxylase in Guinea Pigs’ Livers. Antioxidants 2021, 10, 953. https://doi.org/10.3390/antiox10060953
Teixeira V, Mohamed I, Lavoie J-C. Neonatal Vitamin C and Cysteine Deficiencies Program Adult Hepatic Glutathione and Specific Activities of Glucokinase, Phosphofructokinase, and Acetyl-CoA Carboxylase in Guinea Pigs’ Livers. Antioxidants. 2021; 10(6):953. https://doi.org/10.3390/antiox10060953
Chicago/Turabian StyleTeixeira, Vitor, Ibrahim Mohamed, and Jean-Claude Lavoie. 2021. "Neonatal Vitamin C and Cysteine Deficiencies Program Adult Hepatic Glutathione and Specific Activities of Glucokinase, Phosphofructokinase, and Acetyl-CoA Carboxylase in Guinea Pigs’ Livers" Antioxidants 10, no. 6: 953. https://doi.org/10.3390/antiox10060953
APA StyleTeixeira, V., Mohamed, I., & Lavoie, J.-C. (2021). Neonatal Vitamin C and Cysteine Deficiencies Program Adult Hepatic Glutathione and Specific Activities of Glucokinase, Phosphofructokinase, and Acetyl-CoA Carboxylase in Guinea Pigs’ Livers. Antioxidants, 10(6), 953. https://doi.org/10.3390/antiox10060953








