Eupatilin Impacts on the Progression of Colon Cancer by Mitochondria Dysfunction and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
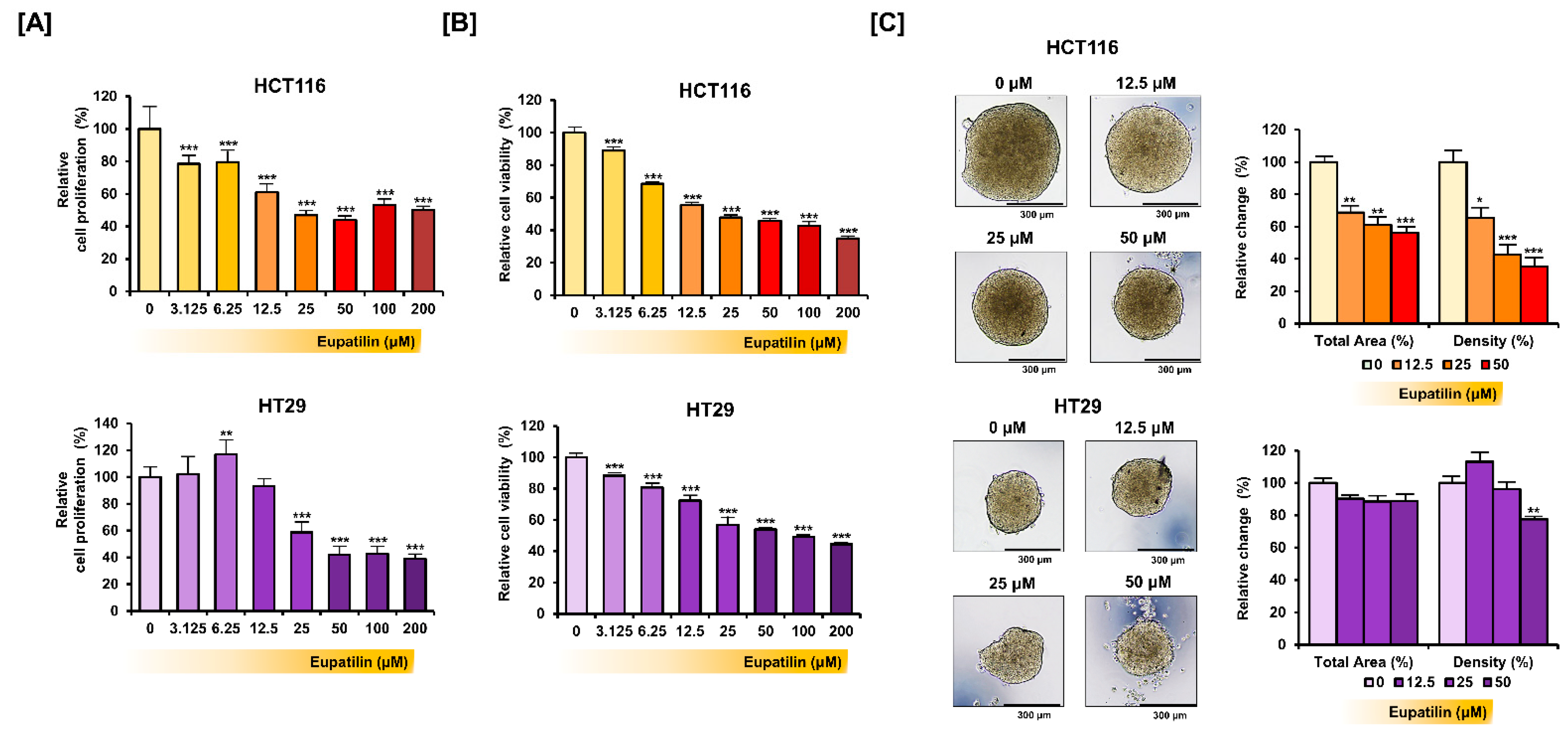
2.3. Proliferation Assay and Cell Viability Test
2.4. Spheroid Culture
2.5. Annexin V and Propidium Iodide Staining
2.6. Terminal Deoxynucleotidyl Transferase 2’-Deoxyuridine-5’-Triphosphate (dUTP) Nick end Labeling (TUNEL) Assay
2.7. JC-1 Staining
2.8. Western Blot Analysis
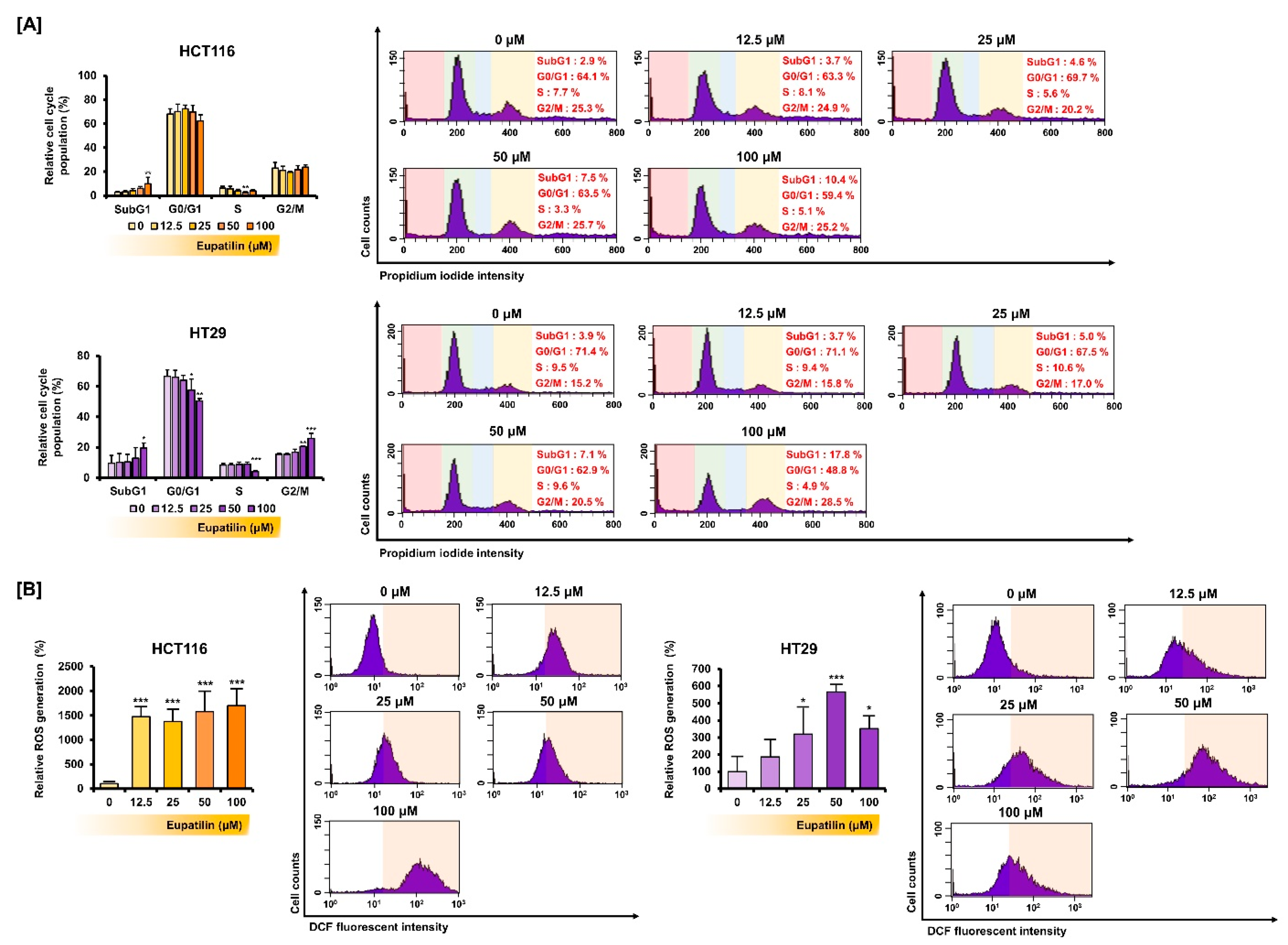
2.9. Cell Cycle Analysis
2.10. Reactive Oxygen Species (ROS) Analysis
2.11. Transwell Invasion Assay and Migration Assay
2.12. Statistics
3. Results
3.1. Eupatilin Inhibits Colon Cancer Cell Growth
3.2. Eupatilin Induces Apoptotic Processes in Colon Cancer Cells
3.3. Eupatilin Regulates the Cell Cycle Phases and Promotes ROS Production in Colon Cancer
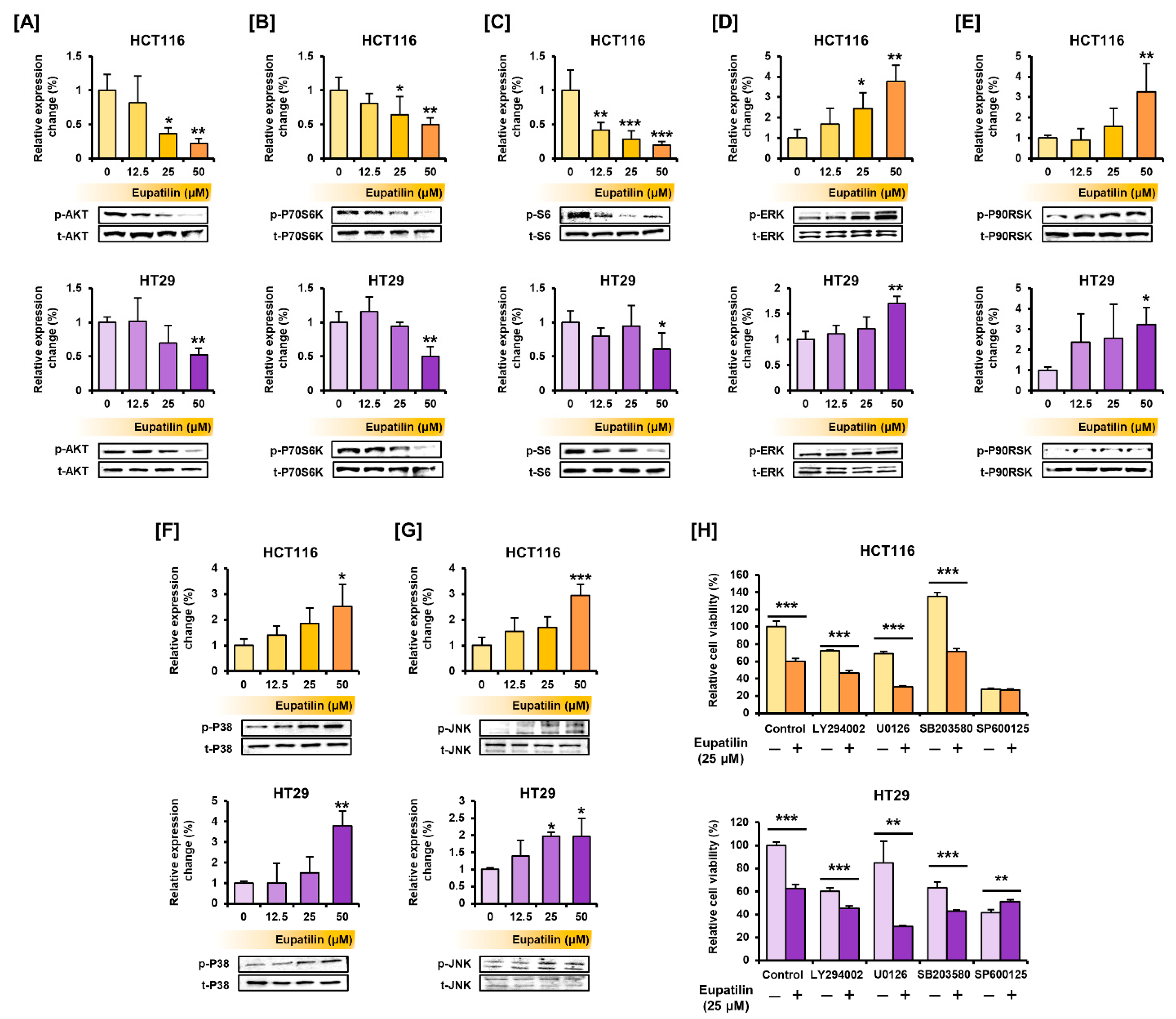
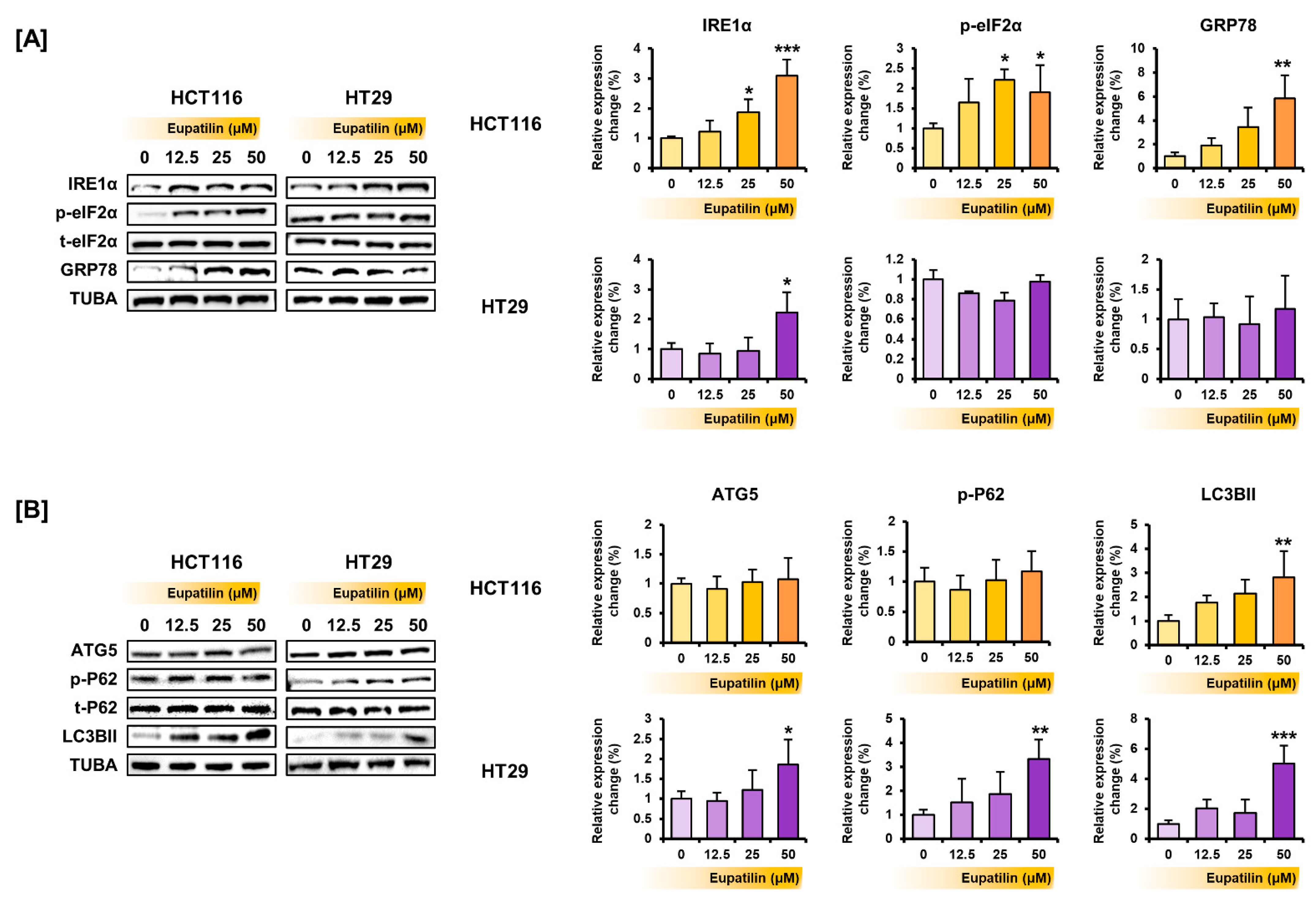
3.4. Eupatilin Regulates the Proteins Involved in the PI3K/AKT and MAPK Pathways, Endoplasmic Reticulum (ER) Stress, and Autophagy in Colon Cancer Cells
3.5. Eupatilin Inhibits Invasion and Migration in Colon Cancer Cells
3.6. Eupatilin Has a Synergistic Effect with Standard Anticancer Drugs on Colon Cancer Cells
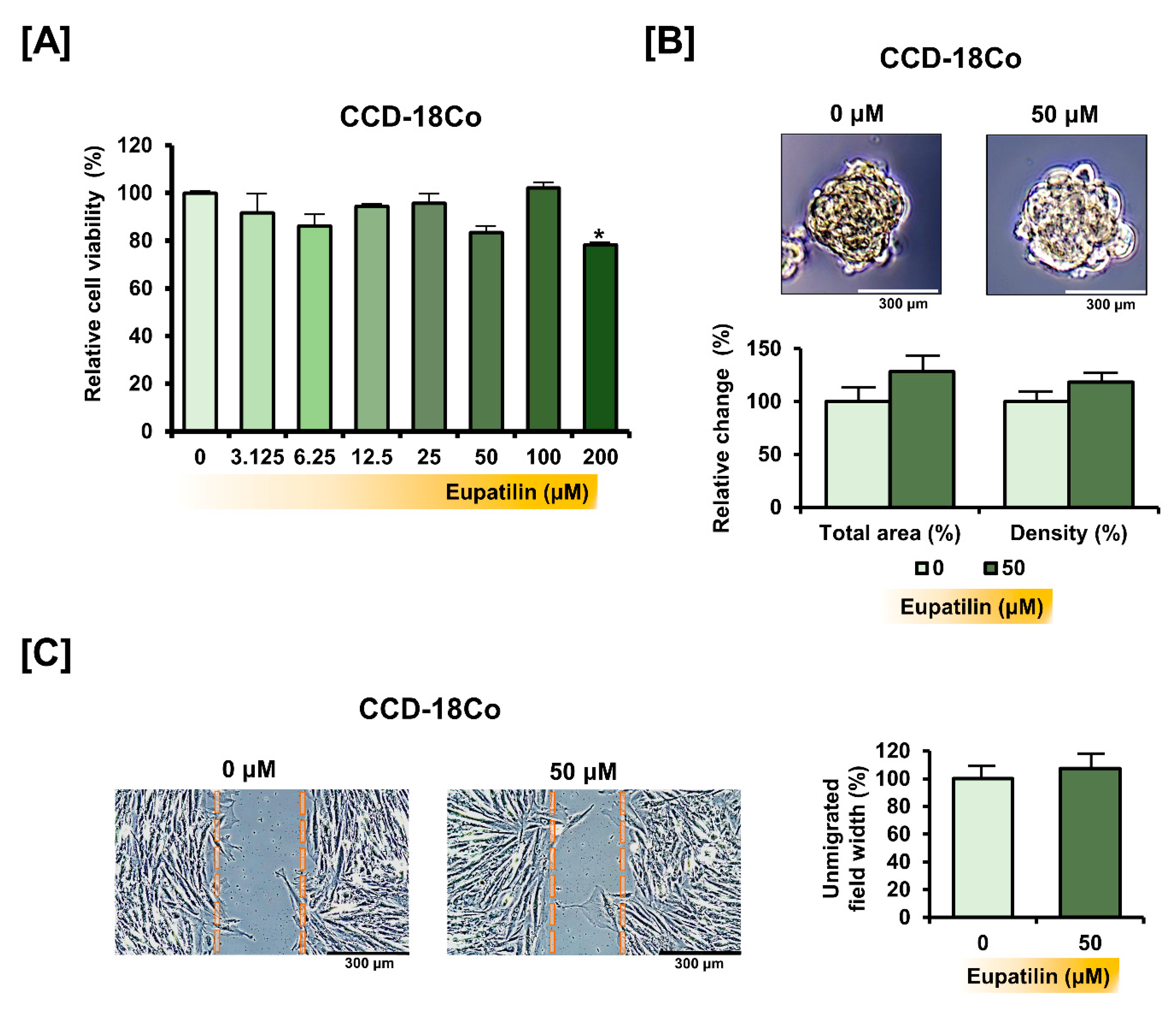
3.7. Eupatilin Does Not Cause Changes in the Properties of Normal Colon Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Landre, T.; Uzzan, B.; Nicolas, P.; Aparicio, T.; Zelek, L.; Mary, F.; Taleb, C.; Guetz, G.D. Doublet chemotherapy vs. single-agent therapy with 5FU in elderly patients with metastatic colorectal cancer. a meta-analysis. Int. J. Color. Dis. 2015, 30, 1305–1310. [Google Scholar] [CrossRef]
- Xie, Q.; Wu, M.-Y.; Zhang, D.-X.; Yang, Y.-M.; Wang, B.-S.; Zhang, J.; Xu, J.; Zhong, W.-D.; Hu, J.-N. Synergistic anticancer effect of exogenous wild-typep53gene combined with 5-FU in human colon cancer resistant to 5-FUin vivo. World J. Gastroenterol. 2016, 22, 7342–7352. [Google Scholar] [CrossRef] [PubMed]
- Webber, E.M.; Kauffman, T.L.; O’Connor, E.; Goddard, K.A.B. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer 2015, 15, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touil, Y.; Igoudjil, W.; Corvaisier, M.; Dessein, A.-F.; Vandomme, J.; Monte, D.; Stechly, L.; Skrypek, N.; Langlois, C.; Grard, G.; et al. Colon Cancer Cells Escape 5FU Chemotherapy-Induced Cell Death by Entering Stemness and Quiescence Associated with the c-Yes/YAP Axis. Clin. Cancer Res. 2014, 20, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jegal, K.H.; Ko, H.L.; Park, S.M.; Byun, S.H.; Kang, K.W.; Cho, I.J.; Kim, S.C. Eupatilin induces Sestrin2-dependent autophagy to prevent oxidative stress. Apoptosis 2016, 21, 642–656. [Google Scholar] [CrossRef]
- Choi, E.-J.; Lee, S.; Chae, J.-R.; Lee, H.-S.; Jun, C.-D.; Kim, S.-H. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 2011, 88, 1121–1126. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, D.-H.; Na, H.-K.; Oh, T.Y.; Shin, C.-Y.; Surh, Y.-J. Eupatilin, a Pharmacologically Active Flavone Derived from Artemisia Plants, Induces Apoptosis in Human Gastric Cancer (AGS) Cells. J. Environ. Pathol. Toxicol. Oncol. 2005, 24, 261–270. [Google Scholar] [CrossRef]
- Koroglu, C.; Erdogan, S. Eupatilin Inhibits the Proliferation and Migration of Prostate Cancer Cells through Modulation of PTEN and NF-κB Signaling. Anti-Cancer Agents Med. Chem. 2021, 21, 372–382. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Zhu, L.; Chen, X.; Xu, Y.; Zhao, Y.; Shao, Y.; Li, F.; Jiang, Y.; Lu, J.; et al. Eupatilin inhibits the proliferation of human esophageal cancer TE1 cells by targeting the Akt-GSK3β and MAPK/ERK signaling cascades. Oncol. Rep. 2018, 39, 2942–2950. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hou, H.; Li, M.; Yang, Y.; Sun, L. Anticancer effect of eupatilin on glioma cells through inhibition of the Notch-1 signaling pathway. Mol. Med. Rep. 2016, 13, 1141–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, H.-J.; Surh, Y.-J. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human promyelocytic leukemia cells. Mutat. Res. Toxicol. Environ. Mutagen. 2001, 496, 191–198. [Google Scholar] [CrossRef]
- Cho, J.-H.; Lee, J.-G.; Yang, Y.-I.; Kim, J.-H.; Ahn, J.-H.; Baek, N.-I.; Lee, K.-T.; Choi, J.-H. Eupatilin, a dietary flavonoid, induces G2/M cell cycle arrest in human endometrial cancer cells. Food Chem. Toxicol. 2011, 49, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Bae, H.; Yang, C.; Park, S.; Youn, B.-S.; Kim, H.-S.; Song, G.; Lim, W. Eupatilin Promotes Cell Death by Calcium Influx through ER-Mitochondria Axis with SERPINB11 Inhibition in Epithelial Ovarian Cancer. Cancers 2020, 12, 1459. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.Y.; Kim, J.S.; Kim, J.M.; Lee, J.Y.; Kim, N.; Jung, H.C.; Song, I.S. DA-6034, a Derivative of Flavonoid, Prevents and Ameliorates Dextran Sulfate Sodium–Induced Colitis and Inhibits Colon Carcinogenesis. Exp. Biol. Med. 2008, 233, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Foty, R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. J. Vis. Exp. 2011, e2720. [Google Scholar] [CrossRef]
- Lim, W.; Yang, C.; Bazer, F.W.; Song, G. Chrysophanol Induces Apoptosis of Choriocarcinoma Through Regulation of ROS and the AKT and ERK1/2 Pathways. J. Cell. Physiol. 2016, 232, 331–339. [Google Scholar] [CrossRef]
- Rosa, A.; Isola, R.; Pollastro, F.; Caria, P.; Appendino, G.; Nieddu, M. The dietary flavonoid eupatilin attenuates in vitro lipid peroxidation and targets lipid profile in cancer HeLa cells. Food Funct. 2020, 11, 5179–5191. [Google Scholar] [CrossRef] [PubMed]
- Storey, S. Targeting apoptosis: Selected anticancer strategies. Nat. Rev. Drug Discov. 2008, 7, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive Oxygen Species as Intracellular Messengers During Cell Growth and Differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Gheytanchi, E.; Naseri, M.; Karimi-Busheri, F.; Atyabi, F.; Mirsharif, E.S.; Bozorgmehr, M.; Ghods, R.; Madjd, Z. Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int. 2021, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Jeong, W.-J.; Ro, E.J.; Choi, K.-Y. Interaction between Wnt/β-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of β-catenin and RAS by targeting the Wnt/β-catenin pathway. NPJ Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siraj, A.K.; Parvathareddy, S.K.; Pratheeshkumar, P.; Divya, S.P.; Ahmed, S.O.; Melosantos, R.; Begum, R.; Concepcion, R.M.J.; Al-Sanea, N.; Ashari, L.H.; et al. APC truncating mutations in Middle Eastern Population: Tankyrase inhibitor is an effective strategy to sensitize APC mutant CRC To 5-FU chemotherapy. Biomed. Pharmacother. 2020, 121, 109572. [Google Scholar] [CrossRef]
- Teimoori-Toolabi, L.; Hashemi, S.; Azadmanesh, K.; Eghbalpour, F.; Safavifar, F.; Khorramizadeh, M.R. Silencing the wild-type and mutant K-ras increases the resistance to 5-flurouracil in HCT-116 as a colorectal cancer cell line. Anti-Cancer Drugs 2015, 26, 187–196. [Google Scholar] [CrossRef]
- Chun, S.Y.; Johnson, C.; Washburn, J.G.; Cruz-Correa, M.R.; Dang, D.T.; Dang, L.H. Oncogenic KRAS modulates mitochondrial metabolism in human colon cancer cells by inducing HIF-1a and HIF-2a target genes. Mol. Cancer 2010, 9, 293. [Google Scholar] [CrossRef] [Green Version]
- Cristofaro, M.; Contursi, A.; D’Amore, S.; Martelli, N.; Spaziante, A.F.; Moschetta, A.; Villani, G. Adenomatous polyposis coli (APC)-induced apoptosis of HT29 colorectal cancer cells depends on mitochondrial oxidative metabolism. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1719–1728. [Google Scholar] [CrossRef] [Green Version]
- Fei, X.; Wang, J.; Chen, C.; Ding, B.; Fu, X.; Chen, W.; Wang, C.; Xu, R. Eupatilin inhibits glioma proliferation, migration, and invasion by arresting cell cycle at G1/S phase and disrupting the cytoskeletal structure. Cancer Manag. Res. 2019, 11, 4781–4796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, W.-F.; Wang, X.-H.; Pan, B.; Li, F.; Kuang, L.; Su, Z.-X. Eupatilin induces human renal cancer cell apoptosis via ROS-mediated MAPK and PI3K/AKT signaling pathways. Oncol. Lett. 2016, 12, 2894–2899. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.-L.; Wu, D.-W.; Huang, C.-C.; He, T.-Y.; Chou, M.-C.; Sheu, G.-T.; Lee, H. MicroRNA-21 promotes tumour malignancy via increased nuclear translocation of β-catenin and predicts poor outcome in APC-mutated but not in APC-wild-type colorectal cancer. Carcinogenesis 2014, 35, 2175–2182. [Google Scholar] [CrossRef] [Green Version]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef] [Green Version]
- Martucciello, S.; Masullo, M.; Cerulli, A.; Piacente, S. Natural Products Targeting ER Stress, and the Functional Link to Mitochondria. Int. J. Mol. Sci. 2020, 21, 1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönthal, A.H. Endoplasmic Reticulum Stress: Its Role in Disease and Novel Prospects for Therapy. Scientifica 2012, 2012, 857516. [Google Scholar] [CrossRef] [Green Version]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, J.; LoGrasso, P.V. Mitochondrial c-Jun N-terminal Kinase (JNK) Signaling Initiates Physiological Changes Resulting in Amplification of Reactive Oxygen Species Generation. J. Biol. Chem. 2011, 286, 16052–16062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, K.H.; Luan, Q.; Croft, A.; Jiang, C.C.; Jin, L.; Zhang, X.D.; Tseng, H.-Y. Sustained IRE1 and ATF6 signaling is important for survival of melanoma cells undergoing ER stress. Cell. Signal. 2014, 26, 287–294. [Google Scholar] [CrossRef]
- Swart, C.; Du Toit, A.; Loos, B. Autophagy and the invisible line between life and death. Eur. J. Cell Biol. 2016, 95, 598–610. [Google Scholar] [CrossRef]
- Amaravadi, R.; Kimmelman, A.C.; White, E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016, 30, 1913–1930. [Google Scholar] [CrossRef]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y.; et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Qu, X.; Yu, J.; Bhagat, G.; Furuya, N.; Hibshoosh, H.; Troxel, A.; Rosen, J.; Eskelinen, E.-L.; Mizushima, N.; Ohsumi, Y.; et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef] [Green Version]
- Bursch, W.; Ellinger, A.; Gerner, C.; Fröhwein, U.; Schulte-Hermann, R. Programmed Cell Death (PCD): Apoptosis, Autophagic PCD, or Others? Ann. N. Y. Acad. Sci. 2006, 926, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Seto, S.; Tsujimura, K.; Horii, T.; Koide, Y. Autophagy Adaptor Protein p62/SQSTM1 and Autophagy-Related Gene Atg5 Mediate Autophagosome Formation in Response to Mycobacterium tuberculosis Infection in Dendritic Cells. PLoS ONE 2013, 8, e86017. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, D.H.; Jeon, Y.; Kim, Y.-J.; Lee, J.; Shin, M.-S.; Kang, K.S.; Hwang, G.S.; Kim, H.Y.; Yamabe, N. Eupatilin inhibits angiogenesis-mediated human hepatocellular metastasis by reducing MMP-2 and VEGF signaling. Bioorganic Med. Chem. Lett. 2018, 28, 3150–3154. [Google Scholar] [CrossRef]
- Park, B.B.; Yoon, J.S.; Kim, E.S.; Choi, J.; Won, Y.-W.; Choi, J.H.; Lee, Y.Y. Inhibitory effects of eupatilin on tumor invasion of human gastric cancer MKN-1 cells. Tumor Biol. 2013, 34, 875–885. [Google Scholar] [CrossRef] [PubMed]



| Primary Antibodies | Dilution | Supplier | Catalog Number |
|---|---|---|---|
| BCL-xL | 1:1000 | Cell Signaling Technology | 2764 |
| BAK | 1:1000 | Cell Signaling Technology | 12105 |
| Cytochrome C | 1:1000 | Cell Signaling Technology | 11940 |
| TUBA | 1:2000 | Santa Cruz | sc-5286 |
| Phosphor-AKT (Ser473) | 1:1000 | Cell Signaling Technology | 4060 |
| AKT | 1:1000 | Cell Signaling Technology | 9272 |
| Phosphor-P70S6K (Thr421/Ser424) | 1:1000 | Cell Signaling Technology | 9204 |
| P70S6K | 1:1000 | Cell Signaling Technology | 2708 |
| Phosphor-S6 (Ser235/Ser236) | 1:1000 | Cell Signaling Technology | 2211 |
| S6 | 1:1000 | Cell Signaling Technology | 2217 |
| Phosphor-ERK1/2 (Thr202/Tyr204) | 1:1000 | Cell Signaling Technology | 9101 |
| ERK1/2 | 1:1000 | Cell Signaling Technology | 4695 |
| Phosphor-P90RSK (Ser573) | 1:1000 | Cell Signaling Technology | 9346 |
| P90RSK | 1:1000 | Cell Signaling Technology | 9335 |
| Phosphor-P38 (Thr180/Tyr182) | 1:1000 | Cell Signaling Technology | 4511 |
| P38 | 1:1000 | Cell Signaling Technology | 9212 |
| Phosphor-JNK (Thr183/Tyr185) | 1:1000 | Cell Signaling Technology | 4668 |
| JNK | 1:1000 | Cell Signaling Technology | 9252 |
| IRE1α | 1:1000 | Cell Signaling Technology | 3294 |
| Phosphor-eIF2α (Ser51) | 1:1000 | Cell Signaling Technology | 3398 |
| eIF2α | 1:1000 | Cell Signaling Technology | 5324 |
| GRP78 | 1:1000 | Santa Cruz | sc-13968 |
| ATG5 | 1:1000 | Cell Signaling Technology | 12994 |
| Phosphor-P62 (Ser349) | 1:1000 | Cell Signaling Technology | 16177 |
| P62 | 1:1000 | Cell Signaling Technology | 88588 |
| LC3B | 1:1000 | Cell Signaling Technology | 3868 |
| TYMS | 1:1000 | Cell Signaling Technology | 9045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Yang, C.; Song, G.; Lim, W. Eupatilin Impacts on the Progression of Colon Cancer by Mitochondria Dysfunction and Oxidative Stress. Antioxidants 2021, 10, 957. https://doi.org/10.3390/antiox10060957
Lee M, Yang C, Song G, Lim W. Eupatilin Impacts on the Progression of Colon Cancer by Mitochondria Dysfunction and Oxidative Stress. Antioxidants. 2021; 10(6):957. https://doi.org/10.3390/antiox10060957
Chicago/Turabian StyleLee, Minkyeong, Changwon Yang, Gwonhwa Song, and Whasun Lim. 2021. "Eupatilin Impacts on the Progression of Colon Cancer by Mitochondria Dysfunction and Oxidative Stress" Antioxidants 10, no. 6: 957. https://doi.org/10.3390/antiox10060957
APA StyleLee, M., Yang, C., Song, G., & Lim, W. (2021). Eupatilin Impacts on the Progression of Colon Cancer by Mitochondria Dysfunction and Oxidative Stress. Antioxidants, 10(6), 957. https://doi.org/10.3390/antiox10060957







