Oxidized LDLs as Signaling Molecules
Abstract
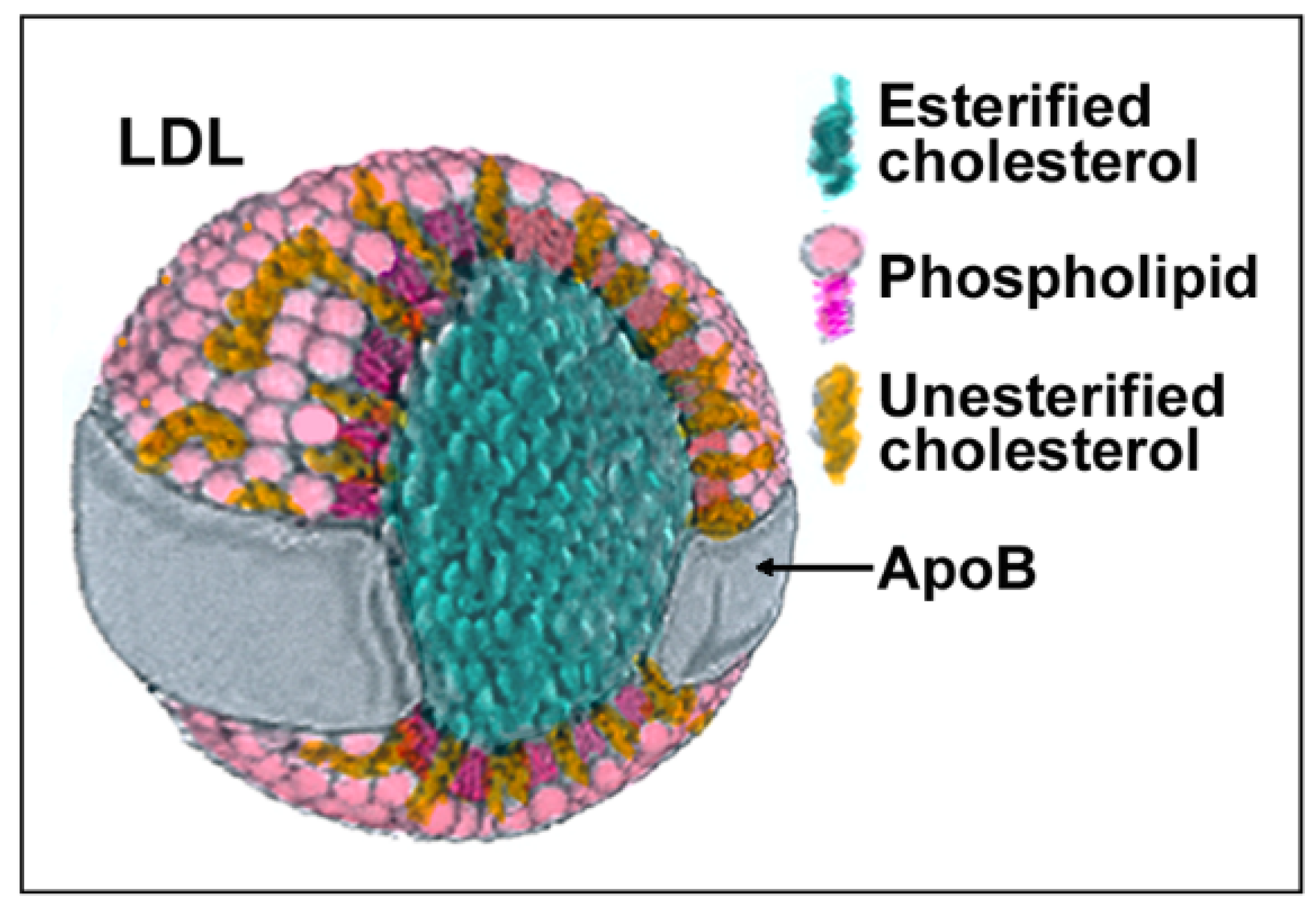
:1. Introduction
2. Formation of oxLDLs In Vitro and In Vivo
3. Removal of oxLDLs from the Circulation
4. Atherogenic Effects of oxLDLs and Their Prevention
4.1. Antioxidant Effects of α-Tocopherol
4.2. Non-Antioxidant Effects of α-Tocopherol
4.3. Prooxidant Effects of α-Tocopherol
5. The oxLDL Signaling
5.1. Signaling by the Oxidized Lipid Content of oxLDLs
5.2. Signaling by oxLDL-Activated Scavenger Receptors
5.3. Signaling of oxLDL to Cells Close to the Atheroma
5.4. Signaling of oxLDLs to Distant Tissues
5.5. Signaling of oxLDLs to Stem Cells
5.6. Signaling of oxLDL to Noncoding RNAs
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Steinberg, D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997, 272, 20963–20966. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, D. Lewis, A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation 1997, 95, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.A.; Heinecke, J.W. The role of oxidized lipoproteins in atherogenesis. Free Radic. Biol. Med. 1996, 20, 707–727. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Auge, N.; Camare, C.; Bacchetti, T.; Ferretti, G.; Salvayre, R. Dual signaling evoked by oxidized LDLs in vascular cells. Free. Radic. Biol. Med. 2017, 106, 118–133. [Google Scholar] [CrossRef]
- Li, D.; Mehta, J.L. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation 2000, 101, 2889–2895. [Google Scholar] [CrossRef] [Green Version]
- Ishigaki, Y.; Oka, Y.; Katagiri, H. Circulating oxidized LDL: A biomarker and a pathogenic factor. Curr. Opin. Lipidol. 2009, 20, 363–369. [Google Scholar] [CrossRef]
- Verhoye, E.; Langlois, M.R. Circulating oxidized low-density lipoprotein: A biomarker of atherosclerosis and cardiovascular risk? Clin. Chem. Lab. Med. 2009, 47, 128–137. [Google Scholar] [CrossRef]
- Requena, J.R.; Fu, M.X.; Ahmed, M.U.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem. J. 1997, 322, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Ferns, G.A.; Lamb, D.J.; Taylor, A. The possible role of copper ions in atherogenesis: The Blue Janus. Atherosclerosis 1997, 133, 139–152. [Google Scholar] [CrossRef]
- Mazur, A.; Gueux, E.; Bureau, I.; Feillet-Coudray, C.; Rock, E.; Rayssiguier, Y. Copper deficiency and lipoprotein oxidation. Atherosclerosis 1998, 137, 443–445. [Google Scholar]
- Sullivan, J.L. Iron in arterial plaque: A modifiable risk factor for atherosclerosis. Biochim. Biophys. Acta 2009, 1790, 718–723. [Google Scholar] [CrossRef] [Green Version]
- Essler, M.; Retzer, M.; Bauer, M.; Heemskerk, J.W.; Aepfelbacher, M.; Siess, W. Mildly oxidized low density lipoprotein induces contraction of human endothelial cells through activation of Rho/Rho kinase and inhibition of myosin light chain phosphatase. J. Biol. Chem. 1999, 274, 30361–30364. [Google Scholar] [CrossRef] [Green Version]
- Retzer, M.; Siess, W.; Essler, M. Mildly oxidised low density lipoprotein induces platelet shape change via Rho-kinase-dependent phosphorylation of myosin light chain and moesin. FEBS Lett. 2000, 466, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Ross, R. Cell biology of atherosclerosis. Annu. Rev. Physiol. 1995, 57, 791–804. [Google Scholar] [CrossRef]
- Kovanen, P.T. Mast cells in human fatty streaks and atheromas: Implications for intimal lipid accumulation. Curr. Opin. Lipidol. 1996, 7, 281–286. [Google Scholar] [CrossRef]
- Kelley, J.L.; Chi, D.S.; Abou-Auda, W.; Smith, J.K.; Krishnaswamy, G. The molecular role of mast cells in atherosclerotic cardiovascular disease. Mol. Med. Today 2000, 6, 304–308. [Google Scholar] [CrossRef]
- Leskinen, M.J.; Kovanen, P.T.; Lindstedt, K.A. Regulation of smooth muscle cell growth, function and death in vitro by activated mast cells--a potential mechanism for the weakening and rupture of atherosclerotic plaques. Biochem. Pharmacol. 2003, 66, 1493–1498. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Negre-Salvayre, A.; Salvayre, R.; Dousset, N.; Curatola, G. Structural modifications of HDL and functional consequences. Atherosclerosis 2006, 184, 1–7. [Google Scholar] [CrossRef]
- Toshima, S.; Hasegawa, A.; Kurabayashi, M.; Itabe, H.; Takano, T.; Sugano, J.; Shimamura, K.; Kimura, J.; Michishita, I.; Suzuki, T.; et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2243–2247. [Google Scholar] [CrossRef] [Green Version]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef] [Green Version]
- Maggi, E.; Bellazzi, R.; Falaschi, F.; Frattoni, A.; Perani, G.; Finardi, G.; Gazo, A.; Nai, M.; Romanini, D.; Bellomo, G. Enhanced LDL oxidation in uremic patients: An additional mechanism for accelerated atherosclerosis? Kidney Int. 1994, 45, 876–883. [Google Scholar] [CrossRef] [Green Version]
- Maggi, E.; Marchesi, E.; Ravetta, V.; Martignoni, A.; Finardi, G.; Bellomo, G. Presence of autoantibodies against oxidatively modified low-density lipoprotein in essential hypertension: A biochemical signature of an enhanced in vivo low-density lipoprotein oxidation. J. Hypertens. 1995, 13, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, G.; Maggi, E.; Poli, M.; Agosta, F.G.; Bollati, P.; Finardi, G. Autoantibodies against oxidatively modified low-density lipoproteins in NIDDM. Diabetes 1995, 44, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, B.; Sozen, E.; Karademir, B.; Ustunsoy, S.; Ince, U.; Zarkovic, N.; Ozer, N.K. CD36 expression in peripheral blood mononuclear cells reflects the onset of atherosclerosis. BioFactors 2018, 44, 588–596. [Google Scholar] [CrossRef]
- Yazgan, B.; Ustunsoy, S.; Karademir, B.; Kartal-Ozer, N. CD36 as a biomarker of atherosclerosis. Free. Radic. Biol. Med. 2014, 75, S10. [Google Scholar] [CrossRef]
- Tsimikas, S. In vivo markers of oxidative stress and therapeutic interventions. Am. J. Cardiol. 2008, 101, 34D–42D. [Google Scholar] [CrossRef]
- Moriel, P.; Okawabata, F.S.; Abdalla, D.S. Oxidized lipoproteins in blood plasma: Possible marker of atherosclerosis progression. IUBMB Life 1999, 48, 413–417. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [Green Version]
- Le, N.A. Lipoprotein-associated oxidative stress: A new twist to the postprandial hypothesis. Int. J. Mol. Sci. 2014, 16, 401–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocker, R. The ambivalence of vitamin E in atherogenesis. Trends Biochem. Sci. 1999, 24, 219–223. [Google Scholar] [CrossRef]
- Di Pietro, N.; Formoso, G.; Pandolfi, A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vasc. Pharmacol. 2016, 84, 1–7. [Google Scholar] [CrossRef]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.; Thomazy, V.A.; Evans, R.M. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, A.; Taddei, M.; Tamburini, I.; Bergamini, E.; Azzi, A.; Zingg, J.M. Antagonistic effects of oxidized low density lipoprotein and alpha-tocopherol on CD36 scavenger receptor expression in monocytes: Involvement of protein kinase B and peroxisome proliferator-activated receptor-gamma. J. Biol. Chem. 2006, 281, 6489–6497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Febbraio, M.; Podrez, E.A.; Smith, J.D.; Hajjar, D.P.; Hazen, S.L.; Hoff, H.F.; Sharma, K.; Silverstein, R.L. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Investig. 2000, 105, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Nozaki, S.; Kashiwagi, H.; Yamashita, S.; Nakagawa, T.; Kostner, B.; Tomiyama, Y.; Nakata, A.; Ishigami, M.; Miyagawa, J.; Kameda-Takemura, K.; et al. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J. Clin. Investig. 1995, 96, 1859–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Febbraio, M.; Abumrad, N.A.; Hajjar, D.P.; Sharma, K.; Cheng, W.; Pearce, S.F.; Silverstein, R.L. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 1999, 274, 19055–19062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janabi, M.; Yamashita, S.; Hirano, K.; Sakai, N.; Hiraoka, H.; Matsumoto, K.; Zhang, Z.; Nozaki, S.; Matsuzawa, Y. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1953–1960. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Kaliora, A.C.; Dedoussis, G.V. Natural antioxidant compounds in risk factors for CVD. Pharmacol. Res. 2007, 56, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Kaliora, A.C.; Dedoussis, G.V.; Schmidt, H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis 2006, 187, 1–17. [Google Scholar] [CrossRef]
- Niki, E. Antioxidants and atherosclerosis. Biochem. Soc. Trans. 2004, 32, 156–159. [Google Scholar] [CrossRef]
- Aviram, M.; Kaplan, M.; Rosenblat, M.; Fuhrman, B. Dietary antioxidants and paraoxonases against LDL oxidation and atherosclerosis development. Handb. Exp. Pharm. 2005, 263–300. [Google Scholar] [CrossRef]
- Terasawa, Y.; Ladha, Z.; Leonard, S.W.; Morrow, J.D.; Newland, D.; Sanan, D.; Packer, L.; Traber, M.G.; Farese, R.V., Jr. Increased atherosclerosis in hyperlipidemic mice deficient in alpha-tocopherol transfer protein and vitamin E. Proc. Natl. Acad. Sci. USA 2000, 97, 13830–13834. [Google Scholar] [CrossRef] [Green Version]
- Suttorp, N.; Toepfer, W.; Roka, L. Antioxidant defense mechanisms of endothelial cells: Glutathione redox cycle versus catalase. Am. J. Physiol. 1986, 251, C671–C680. [Google Scholar] [CrossRef]
- Hennig, B.; Boissonneault, G.A.; Fiscus, L.J.; Marra, M.E. Effect of vitamin E on oxysterol- and fatty acid hydroperoxide-induced changes of repair and permeability properties of cultured endothelial cell monolayers. Int. J. Vitam. Nutr. Res. 1988, 58, 41–47. [Google Scholar]
- Kuzuya, M.; Naito, M.; Funaki, C.; Hayashi, T.; Asai, K.; Kuzuya, F. Probucol prevents oxidative injury to endothelial cells. J. Lipid Res. 1991, 32, 197–204. [Google Scholar] [CrossRef]
- Keaney, J.F., Jr.; Guo, Y.; Cunningham, D.; Shwaery, G.T.; Xu, A.; Vita, J.A. Vascular incorporation of alpha-tocopherol prevents endothelial dysfunction due to oxidized LDL by inhibiting protein kinase C stimulation. J. Clin. Investig. 1996, 98, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Belcher, J.D.; Balla, J.; Balla, G.; Jacobs, D.R., Jr.; Gross, M.; Jacob, H.S.; Vercellotti, G.M. Vitamin E, LDL, and endothelium. Brief oral vitamin supplementation prevents oxidized LDL-mediated vascular injury in vitro. Arterioscler. Thromb. 1993, 13, 1779–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabile, L.; Fitoussi, G.; Periquet, B.; Schmitt, A.; Salvayre, R.; Negre-Salvayre, A. alpha-Tocopherol and trolox block the early intracellular events (TBARS and calcium rises) elicited by oxidized low density lipoproteins in cultured endothelial cells. Free. Radic. Biol. Med. 1995, 19, 177–187. [Google Scholar] [CrossRef]
- Sweetman, L.L.; Zhang, N.Y.; Peterson, H.; Gopalakrishna, R.; Sevanian, A. Effect of linoleic acid hydroperoxide on endothelial cell calcium homeostasis and phospholipid hydrolysis. Arch. Biochem. Biophys. 1995, 323, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, D.; Cantero, A.V.; Therville, N.; Segui, B.; Negre-Salvayre, A.; Thomsen, M.; Benoist, H. Chlamydia pneumoniae alters mildly oxidized low-density lipoprotein-induced cell death in human endothelial cells, leading to necrosis rather than apoptosis. J. Infect. Dis. 2006, 193, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Cominacini, L.; Garbin, U.; Pasini, A.F.; Davoli, A.; Campagnola, M.; Contessi, G.B.; Pastorino, A.M.; Lo Cascio, V. Antioxidants inhibit the expression of intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free. Radic. Biol. Med. 1997, 22, 117–127. [Google Scholar] [CrossRef]
- Chatterjee, S. Role of oxidized human plasma low density lipoproteins in atherosclerosis: Effects on smooth muscle cell proliferation. Mol. Cell. Biochem. 1992, 111, 143–147. [Google Scholar] [CrossRef]
- Chisolm, G.M., 3rd; Chai, Y. Regulation of cell growth by oxidized LDL. Free. Radic. Biol. Med. 2000, 28, 1697–1707. [Google Scholar] [CrossRef]
- Lafont, A.M.; Chai, Y.C.; Cornhill, J.F.; Whitlow, P.L.; Howe, P.H.; Chisolm, G.M. Effect of alpha-tocopherol on restenosis after angioplasty in a model of experimental atherosclerosis. J. Clin. Investig. 1995, 95, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.C.; Howe, P.H.; DiCorleto, P.E.; Chisolm, G.M. Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J. Biol. Chem. 1996, 271, 17791–17797. [Google Scholar] [CrossRef] [Green Version]
- Oinuma, T.; Yamada, T.; Sakurai, I. Effects of copper-zinc type superoxide dismutase on the proliferation and migration of cultured vascular smooth muscle cells induced by oxidized low density lipoprotein. J. Atheroscler. Thromb. 1997, 4, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Stiko, A.; Regnstrom, J.; Shah, P.K.; Cercek, B.; Nilsson, J. Active oxygen species and lysophosphatidylcholine are involved in oxidized low density lipoprotein activation of smooth muscle cell DNA synthesis. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 194–200. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Zingg, J.M.; Azzi, A. Vitamin E: Protective role of a Janus molecule. FASEB J. 2001, 15, 2314–2325. [Google Scholar] [CrossRef]
- Antoniades, C.; Tousoulis, D.; Tentolouris, C.; Toutouzas, P.; Stefanadis, C. Oxidative stress, antioxidant vitamins, and atherosclerosis. From basic research to clinical practice. Herz 2003, 28, 628–638. [Google Scholar] [CrossRef]
- Zingg, J.M.; Azzi, A. Non-antioxidant activities of vitamin E. Curr. Med. Chem. 2004, 11, 1113–1133. [Google Scholar] [CrossRef]
- Munteanu, A.; Zingg, J.M.; Azzi, A. Anti-atherosclerotic effects of vitamin E—Myth or reality? J. Cell. Mol. Med. 2004, 8, 59–76. [Google Scholar] [CrossRef]
- Zingg, J.M. Vitamin E: A role in signal transduction. Annu. Rev. Nutr. 2015, 35, 135–173. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Manabe, H.; Yoshida, N.; Fujita, N.; Ochiai, J.; Matsumoto, N.; Takagi, T.; Naito, Y.; Yoshikawa, T. Alpha-tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur. J. Pharmacol. 2002, 456, 29–37. [Google Scholar] [CrossRef]
- Ozer, N.K.; Azzi, A. Effect of vitamin E on the development of atherosclerosis. Toxicology 2000, 148, 179–185. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Zingg, J.M.; Azzi, A. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation 2000, 102, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Han, J.; Pearce, S.F.; Silverstein, R.L.; Gotto, A.M., Jr.; Hajjar, D.P.; Nicholson, A.C. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J. Lipid Res. 2000, 41, 688–696. [Google Scholar] [CrossRef]
- Lin, C.S.; Lin, F.Y.; Ho, L.J.; Tsai, C.S.; Cheng, S.M.; Wu, W.L.; Huang, C.Y.; Lian, C.H.; Yang, S.P.; Lai, J.H. PKCδ signalling regulates SR-A and CD36 expression and foam cell formation. Cardiovasc. Res. 2012, 95, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Ricciarelli, R.; Tasinato, A.; Clement, S.; Ozer, N.K.; Boscoboinik, D.; Azzi, A. alpha-Tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem. J. 1998, 334, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, S.K.; Devaraj, S.; Yang, T.; Jialal, I. Alpha-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-alpha. Diabetes 2002, 51, 3049–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvester, P.W.; Shah, S.J. Mechanisms mediating the antiproliferative and apoptotic effects of vitamin E in mammary cancer cells. Front. Biosci. 2005, 10, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.; d’Abramo, C.; Traverso, N.; Verzola, D.; Garibotto, G.; Poggi, A.; Odetti, P.; Cottalasso, D.; Marinari, U.M.; Pronzato, M.A.; et al. Central role of PKCdelta in glycoxidation-dependent apoptosis of human neurons. Free. Radic. Biol. Med. 2005, 38, 846–856. [Google Scholar] [CrossRef]
- Cachia, O.; Benna, J.E.; Pedruzzi, E.; Descomps, B.; Gougerot-Pocidalo, M.A.; Leger, C.L. alpha-tocopherol inhibits the respiratory burst in human monocytes. Attenuation of p47(phox) membrane translocation and phosphorylation. J. Biol. Chem. 1998, 273, 32801–32805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boscoboinik, D.; Szewczyk, A.; Hensey, C.; Azzi, A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J. Biol. Chem. 1991, 266, 6188–6194. [Google Scholar] [CrossRef]
- Cook-Mills, J.M. Isoforms of vitamin E differentially regulate PKC α and inflammation: A review. J. Clin. Cell Immunol. 2013, 4, 1000137. [Google Scholar] [CrossRef]
- Thomas, S.R.; Stocker, R. Molecular action of vitamin E in lipoprotein oxidation: Implications for atherosclerosis. Free. Radic. Biol. Med. 2000, 28, 1795–1805. [Google Scholar] [CrossRef]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar]
- Libby, P.; Li, H. Vascular cell adhesion molecule-1 and smooth muscle cell activation during atherogenesis. J. Clin. Investig. 1993, 92, 538–539. [Google Scholar] [CrossRef] [Green Version]
- Zingg, J.M.; Ricciarelli, R.; Azzi, A. Scavenger receptors and modified lipoproteins: Fatal attractions? IUBMB Life 2000, 49, 397–403. [Google Scholar]
- Yamada, Y.; Doi, T.; Hamakubo, T.; Kodama, T. Scavenger receptor family proteins: Roles for atherosclerosis, host defence and disorders of the central nervous system. Cell. Mol. Life Sci. 1998, 54, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Ricciarelli, R.; Azzi, A. Scavenger receptor regulation and atherosclerosis. BioFactors 2000, 11, 189–200. [Google Scholar] [CrossRef]
- Gough, P.J.; Greaves, D.R.; Suzuki, H.; Hakkinen, T.; Hiltunen, M.O.; Turunen, M.; Herttuala, S.Y.; Kodama, T.; Gordon, S. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Hirano, K.; Yamashita, S.; Nakagawa, Y.; Ohya, T.; Matsuura, F.; Tsukamoto, K.; Okamoto, Y.; Matsuyama, A.; Matsumoto, K.; Miyagawa, J.; et al. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ. Res. 1999, 85, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Nakata, A.; Nakagawa, Y.; Nishida, M.; Nozaki, S.; Miyagawa, J.; Nakagawa, T.; Tamura, R.; Matsumoto, K.; Kameda-Takemura, K.; Yamashita, S.; et al. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1333–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajjar, D.P.; Haberland, M.E. Lipoprotein trafficking in vascular cells. Molecular Trojan horses and cellular saboteurs. J. Biol. Chem. 1997, 272, 22975–22978. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, H.; Kume, N.; Miyamoto, S.; Minami, M.; Moriwaki, H.; Murase, T.; Sawamura, T.; Masaki, T.; Hashimoto, N.; Kita, T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation 1999, 99, 3110–3117. [Google Scholar] [CrossRef]
- Zingg, J.M.; Ricciarelli, R.; Andorno, E.; Azzi, A. Novel 5′ exon of scavenger receptor CD36 is expressed in cultured human vascular smooth muscle cells and atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Mulvihill, E.R.; Jaeger, J.; Sengupta, R.; Ruzzo, W.L.; Reimer, C.; Lukito, S.; Schwartz, S.M. Atherosclerotic plaque smooth muscle cells have a distinct phenotype. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1283–1289. [Google Scholar] [CrossRef] [Green Version]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Itoh, K.; Ruiz, E.; Leake, D.S.; Unoki, H.; Yamamoto, M.; Mann, G.E. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: Activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004, 94, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Hajjar, D.P.; Tauras, J.M.; Nicholson, A.C. Cellular cholesterol regulates expression of the macrophage type B scavenger receptor, CD36. J. Lipid Res. 1999, 40, 830–838. [Google Scholar] [CrossRef]
- Fajas, L.; Schoonjans, K.; Gelman, L.; Kim, J.B.; Najib, J.; Martin, G.; Fruchart, J.C.; Briggs, M.; Spiegelman, B.M.; Auwerx, J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: Implications for adipocyte differentiation and metabolism. Mol. Cell. Biol. 1999, 19, 5495–5503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.C.; Glass, C.K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Arkan, M.C.; Basaga, H.; Chiarpotto, E.; Sevanian, A.; Poli, G. Lipid oxidation products in cell signaling. Free. Radic. Biol. Med. 2000, 28, 1370–1378. [Google Scholar] [CrossRef]
- Poli, G.; Sottero, B.; Gargiulo, S.; Leonarduzzi, G. Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol. Asp. Med. 2009, 30, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Vieira, O.; Escargueil-Blanc, I.; Jurgens, G.; Borner, C.; Almeida, L.; Salvayre, R.; Negre-Salvayre, A. Oxidized LDLs alter the activity of the ubiquitin-proteasome pathway: Potential role in oxidized LDL-induced apoptosis. FASEB J. 2000, 14, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Edwards, W.D.; Holmes, D.R.; Jr Shogren, K.L.; Lerman, L.O.; Ciechanover, A.; Lerman, A. Increased ubiquitin immunoreactivity in unstable atherosclerotic plaques associated with acute coronary syndromes. J. Am. Coll. Cardiol. 2002, 40, 1919–1927. [Google Scholar] [CrossRef] [Green Version]
- Versari, D.; Herrmann, J.; Gossl, M.; Mannheim, D.; Sattler, K.; Meyer, F.B.; Lerman, L.O.; Lerman, A. Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2132–2139. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.P.; Han, S.; Okamoto, H.; Carnemolla, R.; Tabas, I.; Accili, D.; Tall, A.R. Increased CD36 protein as a response to defective insulin signaling in macrophages. J. Clin. Investig. 2004, 113, 764–773. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, A.; Ricciarelli, R.; Zingg, J.M. HIV protease inhibitors-induced atherosclerosis: Prevention by alpha-tocopherol. IUBMB Life 2004, 56, 629–631. [Google Scholar] [CrossRef]
- Munteanu, A.; Zingg, J.M.; Ricciarelli, R.; Azzi, A. CD36 overexpression in ritonavir-treated THP-1 cells is reversed by alpha-tocopherol. Free. Radic. Biol. Med. 2005, 38, 1047–1056. [Google Scholar] [CrossRef]
- Stolzing, A.; Widmer, R.; Jung, T.; Voss, P.; Grune, T. Tocopherol-mediated modulation of age-related changes in microglial cells: Turnover of extracellular oxidized protein material. Free. Radic. Biol. Med. 2006, 40, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Azzi, A. Modulation of Cellular Signalling and Gene Expression by Vitamin E. In New Topics in Vitamin E Research; Bendrick, O.H., Ed.; NOVA Publishers: New York, NY, USA, 2006. [Google Scholar]
- Jervis, K.M.; Robaire, B. The effects of long-term vitamin E treatment on gene expression and oxidative stress damage in the aging Brown Norway rat epididymis. Biol. Reprod. 2004, 71, 1088–1095. [Google Scholar] [CrossRef] [Green Version]
- Bardag-Gorce, F.; Li, J.; French, B.A.; French, S.W. The effect of ethanol-induced CYP2E1 on proteasome activity: The role of 4-hydroxynonenal. Exp. Mol. Pathol. 2005, 78, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.; Villacorta, L.; Bonet, B.; Indart, A.; Munteanu, A.; Sanchez-Vera, I.; Azzi, A.; Zingg, J.M. Effects of aldehydes on CD36 expression. Free. Radic. Res. 2005, 39, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Vallve, J.C.; Uliaque, K.; Girona, J.; Cabre, A.; Ribalta, J.; Heras, M.; Masana, L. Unsaturated fatty acids and their oxidation products stimulate CD36 gene expression in human macrophages. Atherosclerosis 2002, 164, 45–56. [Google Scholar] [CrossRef]
- Lizardo, D.Y.; Lin, Y.L.; Gokcumen, O.; Atilla-Gokcumen, G.E. Regulation of lipids is central to replicative senescence. Mol. Biosyst. 2017, 13, 498–509. [Google Scholar] [CrossRef]
- Zingg, J.M.; Hasan, S.T.; Nakagawa, K.; Canepa, E.; Ricciarelli, R.; Villacorta, L.; Azzi, A.; Meydani, M. Modulation of cAMP levels by high-fat diet and curcumin and regulatory effects on CD36/FAT scavenger receptor/fatty acids transporter gene expression. BioFactors 2017, 43, 42–53. [Google Scholar] [CrossRef]
- Riahi, Y.; Kaiser, N.; Cohen, G.; Abd-Elrahman, I.; Blum, G.; Shapira, O.M.; Koler, T.; Simionescu, M.; Sima, A.V.; Zarkovic, N.; et al. Foam cell-derived 4-hydroxynonenal induces endothelial cell senescence in a TXNIP-dependent manner. J. Cell. Mol. Med. 2015, 19, 1887–1899. [Google Scholar] [CrossRef] [Green Version]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Y.; Zhao, X.; Ru, J.; Kang, N.; Tian, T.; Tang, L.; An, Y.; Li, P. oxLDL-mediated cellular senescence is associated with increased NADPH oxidase p47phox recruitment to caveolae. Biosci. Rep. 2018, 38, BSR20180283. [Google Scholar] [CrossRef] [Green Version]
- Yoon, I.K.; Kim, H.K.; Kim, Y.K.; Song, I.H.; Kim, W.; Kim, S.; Baek, S.H.; Kim, J.H.; Kim, J.R. Exploration of replicative senescence-associated genes in human dermal fibroblasts by cDNA microarray technology. Exp. Gerontol. 2004, 39, 1369–1378. [Google Scholar] [CrossRef]
- Chong, M.; Yin, T.; Chen, R.; Xiang, H.; Yuan, L.; Ding, Y.; Pan, C.C.; Tang, Z.; Alexander, P.B.; Li, Q.J.; et al. CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep. 2018, 19, e45274. [Google Scholar] [CrossRef] [PubMed]
- Zani, I.A.; Stephen, S.L.; Mughal, N.A.; Russell, D.; Homer-Vanniasinkam, S.; Wheatcroft, S.B.; Ponnambalam, S. Scavenger receptor structure and function in health and disease. Cells 2015, 4, 178–201. [Google Scholar] [CrossRef] [Green Version]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N. A) Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.; Chevrot, M.; Poirier, H.; Passilly-Degrace, P.; Niot, I.; Besnard, P. CD36 as a lipid sensor. Physiol. Behav. 2011, 105, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Benton, R.; Vannice, K.S.; Vosshall, L.B. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature 2007, 450, 289–293. [Google Scholar] [CrossRef]
- Gomez-Diaz, C.; Bargeton, B.; Abuin, L.; Bukar, N.; Reina, J.H.; Bartoi, T.; Graf, M.; Ong, H.; Ulbrich, M.H.; Masson, J.F.; et al. A CD36 ectodomain mediates insect pheromone detection via a putative tunnelling mechanism. Nat. Commun. 2016, 7, 11866. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Lekli, I.; Das, M.; Azzi, A.; Das, D.K. Cardioprotection with alpha-tocopheryl phosphate: Amelioration of myocardial ischemia reperfusion injury is linked with its ability to generate a survival signal through Akt activation. Biochim. Biophys. Acta 2008, 1782, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Hugou, I.; Jialal, I. Alpha-tocopherol decreases CD36 expression in human monocyte-derived macrophages. J. Lipid Res. 2001, 42, 521–527. [Google Scholar] [CrossRef]
- Munteanu, A.; Zingg, J.M.; Ogru, E.; Libinaki, R.; Gianello, R.; West, S.; Negis, Y.; Azzi, A. Modulation of cell proliferation and gene expression by alpha-tocopheryl phosphates: Relevance to atherosclerosis and inflammation. Biochem. Biophys. Res. Commun. 2004, 318, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Azzi, A.; Meydani, M. α-Tocopheryl phosphate induces VEGF expression via CD36/PI3Kγ in THP-1 monocytes. J. Cell. Biochem. 2017, 118, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Varghese, D.S.; Ali, B.R. Pathological crosstalk between oxidized LDL and ER stress in human diseaseas: A comprehenisve review. Front. Cell Dev. Biol. 2021, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hajjar, D.P.; Febbraio, M.; Nicholson, A.C. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J. Biol. Chem. 1997, 272, 21654–21659. [Google Scholar] [CrossRef] [Green Version]
- Mikita, T.; Porter, G.; Lawn, R.M.; Shiffman, D. Oxidized low density lipoprotein exposure alters the transcriptional response of macrophages to inflammatory stimulus. J. Biol. Chem. 2001, 276, 45729–45739. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, K.; Kinoshita, M.; Kojima, K.; Mikuni, Y.; Kudo, M.; Mori, M.; Fujita, M.; Horie, E.; Shimazu, N.; Teramoto, T. Synergically increased expression of CD36, CLA-1 and CD68, but not of SR-A and LOX-1, with the progression to foam cells from macrophages. J. Atheroscler. Thromb. 2002, 9, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Sugano, R.; Yamamura, T.; Harada-Shiba, M.; Miyake, Y.; Yamamoto, A. Uptake of oxidized low-density lipoprotein in a THP-1 cell line lacking scavenger receptor A. Atherosclerosis 2001, 158, 351–357. [Google Scholar] [CrossRef]
- Serbulea, V.; DeWeese, D.; Leitinger, N. The effect of oxidized phospholipids on phenotypic polarization and function of macrophages. Free. Radic. Biol. Med. 2017, 111, 156–168. [Google Scholar] [CrossRef]
- Febbraio, M.; Guy, E.; Silverstein, R.L. Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Hundal, R.S.; Salh, B.S.; Schrader, J.W.; Gomez-Munoz, A.; Duronio, V.; Steinbrecher, U.P. Oxidized low density lipoprotein inhibits macrophage apoptosis through activation of the PI 3-kinase/PKB pathway. J. Lipid Res. 2001, 42, 1483–1491. [Google Scholar] [CrossRef]
- Chien, M.W.; Chien, C.S.; Hsiao, L.D.; Lin, C.H.; Yang, C.M. OxLDL induces mitogen-activated protein kinase activation mediated via PI3-kinase/Akt in vascular smooth muscle cells. J. Lipid Res. 2003, 44, 1667–1675. [Google Scholar] [CrossRef] [Green Version]
- Kiyan, Y.; Tkachuk, S.; Hilfiker-Kleiner, D.; Haller, H.; Fuhrman, B.; Dumler, I. oxLDL induces inflammatory responses in vascular smooth muscle cells via urokinase receptor association with CD36 and TLR4. J. Mol. Cell. Cardiol. 2014, 66, 72–82. [Google Scholar] [CrossRef]
- Inoue, M.; Itoh, H.; Tanaka, T.; Chun, T.H.; Doi, K.; Fukunaga, Y.; Sawada, N.; Yamshita, J.; Masatsugu, K.; Saito, T.; et al. Oxidized LDL regulates vascular endothelial growth factor expression in human macrophages and endothelial cells through activation of peroxisome proliferator-activated receptor-gamma. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 560–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Luo, N.; Lopes-Virella, M.F. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J. Lipid Res. 2000, 41, 2017–2023. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, N.; Lopes-Virella, M.F.; Garvey, W.T. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 2002, 165, 259–269. [Google Scholar] [CrossRef]
- Chen, L.Y.; Mehta, P.; Mehta, J.L. Oxidized LDL decreases L-arginine uptake and nitric oxide synthase protein expression in human platelets: Relevance of the effect of oxidized LDL on platelet function. Circulation 1996, 93, 1740–1746. [Google Scholar] [CrossRef]
- Singla, B.; Lin, H.P.; Ahn, W.; White, J.; Csanyi, G. Oxidatively modified LDL suppresses lymphangiogenesis via CD36 signaling. Antioxidants 2021, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mehta, J.L. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: Evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Dandapat, A.; Hu, C.; Sun, L.; Mehta, J.L. Small concentrations of oxLDL induce capillary tube formation from endothelial cells via LOX-1-dependent redox-sensitive pathway. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2435–2442. [Google Scholar] [CrossRef] [Green Version]
- Moulton, K.S. Angiogenesis in atherosclerosis: Gathering evidence beyond speculation. Curr. Opin. Lipidol. 2006, 17, 548–555. [Google Scholar] [CrossRef]
- Wu, F.T.; Stefanini, M.O.; Mac Gabhann, F.; Kontos, C.D.; Annex, B.H.; Popel, A.S. A systems biology perspective on sVEGFR1: Its biological function, pathogenic role and therapeutic use. J. Cell. Mol. Med. 2010, 14, 528–552. [Google Scholar] [CrossRef]
- Li, A.C.; Brown, K.K.; Silvestre, M.J.; Willson, T.M.; Palinski, W.; Glass, C.K. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J. Clin. Investig. 2000, 106, 523–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, O.; Thiery, J.; Stein, Y. Is there a genetic basis for resistance to atherosclerosis? Atherosclerosis 2002, 160, 1–10. [Google Scholar] [CrossRef]
- Febbraio, M.; Hajjar, D.P.; Silverstein, R.L. CD36: A class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J. Clin. Investig. 2001, 108, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.A.; el-Maghrabi, M.R.; Amri, E.Z.; Lopez, E.; Grimaldi, P.A. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 1993, 268, 17665–17668. [Google Scholar] [CrossRef]
- Abumrad, N.; Harmon, C.; Ibrahimi, A. Membrane transport of long-chain fatty acids: Evidence for a facilitated process. J. Lipid Res. 1998, 39, 2309–2318. [Google Scholar] [CrossRef]
- Aitman, T.J.; Glazier, A.M.; Wallace, C.A.; Cooper, L.D.; Norsworthy, P.J.; Wahid, F.N.; Al-Majali, K.M.; Trembling, P.M.; Mann, C.J.; Shoulders, C.C.; et al. Identification of CD36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat. Genet. 1999, 21, 76–83. [Google Scholar] [CrossRef]
- Guthmann, F.; Haupt, R.; Looman, A.C.; Spener, F.; Rustow, B. Fatty acid translocase/CD36 mediates the uptake of palmitate by type II pneumocytes. Am. J. Physiol. 1999, 277, L191–L196. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.F.; Mhaidat, N.M.; Ralston, K.J.; Burns, G.F. CD36 is a receptor for oxidized high density lipoprotein: Implications for the development of atherosclerosis. FEBS Lett. 2007, 581, 1227–1232. [Google Scholar] [CrossRef] [Green Version]
- Houben, T.; Brandsma, E.; Walenbergh, S.M.A.; Hofker, M.H.; Shiri-Sverdlov, R. Oxidized LDL at the crossroads of immunity in non-alcoholic steatohepatitis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 416–429. [Google Scholar] [CrossRef]
- Stahlberg, N.; Rico-Bautista, E.; Fisher, R.M.; Wu, X.; Cheung, L.; Flores-Morales, A.; Tybring, G.; Norstedt, G.; Tollet-Egnell, P. Female-predominant expression of fatty acid translocase/CD36 in rat and human liver. Endocrinology 2004, 145, 1972–1979. [Google Scholar] [CrossRef] [Green Version]
- Ulatowski, L.; Dreussi, C.; Noy, N.; Barnholtz-Sloan, J.; Klein, E.; Manor, D. Expression of the alpha-tocopherol transfer protein gene is regulated by oxidative stress and common single-nucleotide polymorphisms. Free. Radic. Biol. Med. 2012, 53, 2318–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, M.; Takitani, K.; Miyazaki, H.; Yamaoka, S.; Tamai, H. Liver X receptor up-regulates alpha-tocopherol transfer protein expression and alpha-tocopherol status. J. Nutr. Biochem. 2013, 24, 2158–2167. [Google Scholar] [CrossRef]
- Finno, C.J.; Bordbari, M.H.; Valberg, S.J.; Lee, D.; Herron, J.; Hines, K.; Monsour, T.; Scott, E.; Bannasch, D.L.; Mickelson, J.; et al. Transcriptome profiling of equine vitamin E deficient neuroaxonal dystrophy identifies upregulation of liver X receptor target genes. Free. Radic. Biol. Med. 2016, 101, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parola, M.; Muraca, R.; Dianzani, I.; Barrera, G.; Leonarduzzi, G.; Bendinelli, P.; Piccoletti, R.; Poli, G. Vitamin E dietary supplementation inhibits transforming growth factor beta 1 gene expression in the rat liver. FEBS Lett. 1992, 308, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Kuniyasu, A.; Hayashi, S.; Nakayama, H. Adipocytes recognize and degrade oxidized low density lipoprotein through CD36. Biochem. Biophys. Res. Commun. 2002, 295, 319–323. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Kuchibhotla, S.; Westfall, K.M.; Silverstein, R.L.; Morton, R.E.; Febbraio, M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc. Res. 2011, 89, 604–613. [Google Scholar] [CrossRef] [Green Version]
- Unno, Y.; Sakai, M.; Sakamoto, Y.; Kuniyasu, A.; Nakayama, H.; Nagai, R.; Horiuchi, S. Advanced glycation end products-modified proteins and oxidized LDL mediate down-regulation of leptin in mouse adipocytes via CD36. Biochem. Biophys. Res. Commun. 2004, 325, 151–156. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Vari, R.; D’Archivio, M.; Santangelo, C.; Filesi, C.; Giovannini, C.; Masella, R. Oxidized LDL impair adipocyte response to insulin by activating serine/threonine kinases. J. Lipid Res. 2009, 50, 832–845. [Google Scholar] [CrossRef] [Green Version]
- Santiago-Fernandez, C.; Martin-Reyes, F.; Tome, M.; Ocana-Wilhelmi, L.; Rivas-Becerra, J.; Tatzber, F.; Pursch, E.; Tinahones, F.J.; Garcia-Fuentes, E.; Garrido-Sanchez, L. Oxidized LDL modify the human adipocyte phenotype to an insulin resistant, proinflamatory and proapoptotic profile. Biomolecules 2020, 10, 534. [Google Scholar] [CrossRef] [Green Version]
- Salabert, A.S.; Mora-Ramirez, E.; Beaurain, M.; Alonso, M.; Fontan, C.; Tahar, H.B.; Boizeau, M.L.; Tafani, M.; Bardies, M.; Payoux, P. Evaluation of [(18)F]FNM biodistribution and dosimetry based on whole-body PET imaging of rats. Nucl. Med. Biol. 2018, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zorn-Pauly, K.; Schaffer, P.; Pelzmann, B.; Bernhart, E.; Wei, G.; Lang, P.; Ledinski, G.; Greilberger, J.; Koidl, B.; Jurgens, G. Oxidized LDL induces ventricular myocyte damage and abnormal electrical activity--role of lipid hydroperoxides. Cardiovasc. Res. 2005, 66, 74–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatnagar, A. Electrophysiological effects of 4-hydroxynonenal, an aldehydic product of lipid peroxidation, on isolated rat ventricular myocytes. Circ. Res. 1995, 76, 293–304. [Google Scholar] [CrossRef]
- Ke, L.Y.; Chan, H.C.; Chen, C.C.; Lu, J.; Marathe, G.K.; Chu, C.S.; Chan, H.C.; Wang, C.Y.; Tung, Y.C.; McIntyre, T.M.; et al. Enhanced sphingomyelinase activity contributes to the apoptotic capacity of electronegative low-density lipoprotein. J. Med. Chem. 2016, 59, 1032–1040. [Google Scholar] [CrossRef]
- Cnop, M.; Hannaert, J.C.; Grupping, A.Y.; Pipeleers, D.G. Low density lipoprotein can cause death of islet beta-cells by its cellular uptake and oxidative modification. Endocrinology 2002, 143, 3449–3453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abderrahmani, A.; Niederhauser, G.; Favre, D.; Abdelli, S.; Ferdaoussi, M.; Yang, J.Y.; Regazzi, R.; Widmann, C.; Waeber, G. Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia 2007, 50, 1304–1314. [Google Scholar] [CrossRef] [Green Version]
- Plaisance, V.; Brajkovic, S.; Tenenbaum, M.; Favre, D.; Ezanno, H.; Bonnefond, A.; Bonner, C.; Gmyr, V.; Kerr-Conte, J.; Gauthier, B.R.; et al. Endoplasmic reticulum stress links oxidative stress to impaired pancreatic beta-cell function caused by human oxidized LDL. PLoS ONE 2016, 11, e0163046. [Google Scholar] [CrossRef] [Green Version]
- Kamanna, V.S.; Bassa, B.V.; Ganji, S.H. Low density lipoproteins transactivate EGF receptor: Role in mesangial cell proliferation. Life Sci. 2008, 83, 595–601. [Google Scholar] [CrossRef]
- Santini, E.; Lupi, R.; Baldi, S.; Madec, S.; Chimenti, D.; Ferrannini, E.; Solini, A. Effects of different LDL particles on inflammatory molecules in human mesangial cells. Diabetologia 2008, 51, 2117–2125. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S. Oxidized LDL, glomerular mesangial cells and collagen. Diabetes Res. Clin. Pract. 1999, 45, 117–122. [Google Scholar] [CrossRef]
- Bussolati, B.; Deregibus, M.C.; Fonsato, V.; Doublier, S.; Spatola, T.; Procida, S.; Di Carlo, F.; Camussi, G. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J. Am. Soc. Nephrol. 2005, 16, 1936–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutwein, P.; Abdel-Bakky, M.S.; Schramme, A.; Doberstein, K.; Kampfer-Kolb, N.; Amann, K.; Hauser, I.A.; Obermuller, N.; Bartel, C.; Abdel-Aziz, A.A.; et al. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am. J. Pathol. 2009, 174, 2061–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawodu, D.; Patecki, M.; Dumler, I.; Haller, H.; Kiyan, Y. oxLDL inhibits differentiation of mesenchymal stem cells into osteoblasts via the CD36 mediated suppression of Wnt signaling pathway. Mol. Biol. Rep. 2019, 46, 3487–3496. [Google Scholar] [CrossRef]
- Parhami, F.; Jackson, S.M.; Tintut, Y.; Le, V.; Balucan, J.P.; Territo, M.; Demer, L.L. Atherogenic diet and minimally oxidized low density lipoprotein inhibit osteogenic and promote adipogenic differentiation of marrow stromal cells. J. Bone Miner. Res. 1999, 14, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Delimaris, I.; Faviou, E.; Antonakos, G.; Stathopoulou, E.; Zachari, A.; Dionyssiou-Asteriou, A. Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin. Biochem. 2007, 40, 1129–1134. [Google Scholar] [CrossRef]
- Khaidakov, M.; Mehta, J.L. Oxidized LDL triggers pro-oncogenic signaling in human breast mammary epithelial cells partly via stimulation of MiR-21. PLoS ONE 2012, 7, e46973. [Google Scholar] [CrossRef]
- Koirala, D.; Beranova-Giorgianni, S.; Giorgianni, F. Early transcriptomic response to OxLDL in human retinal pigment epithelial cells. Int. J. Mol. Sci. 2020, 21, 8818. [Google Scholar] [CrossRef]
- Klein, R.; Lee, K.E.; Tsai, M.Y.; Cruickshanks, K.J.; Gangnon, R.E.; Klein, B.E.K. Oxidized low-density lipoprotein and the incidence of age-related macular degeneration. Ophthalmology 2019, 126, 752–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Willets, R.S.; Polidori, M.C.; Stahl, W.; Nelles, G.; Sies, H.; Griffiths, H.R. Oxidative LDL modification is increased in vascular dementia and is inversely associated with cognitive performance. Free. Radic. Res. 2010, 44, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tang, Z.H.; Peng, J.; Liao, L.; Pan, L.H.; Wu, C.Y.; Jiang, Z.S.; Wang, G.X.; Liu, L.S. The dual behavior of PCSK9 in the regulation of apoptosis is crucial in Alzheimer’s disease progression (Review). Biomed. Rep. 2014, 2, 167–171. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.N.; Hanni, K.B.; Kindy, M.S. Oxidized high-density lipoprotein induces neuron death. Exp. Neurol. 2000, 161, 621–630. [Google Scholar] [CrossRef]
- Keller, J.N.; Hanni, K.B.; Markesbery, W.R. Oxidized low-density lipoprotein induces neuronal death: Implications for calcium, reactive oxygen species, and caspases. J. Neurochem. 1999, 72, 2601–2609. [Google Scholar] [CrossRef]
- Thum, T.; Bauersachs, J. Spotlight on endothelial progenitor cell inhibitors: Short review. Vasc. Med. 2005, 10, S59–S64. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.L.; Yet, S.F.; Hsu, Y.T.; Wang, G.J.; Hung, S.C. Mesenchymal stem cells ameliorate atherosclerotic lesions via restoring endothelial function. Stem cells Transl. Med. 2015, 4, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mohamed, A.S.; Zhou, S.H. Oxidized low density lipoprotein, stem cells, and atherosclerosis. Lipids Health Dis. 2012, 11, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Wu, Y.; Gu, W.; Xu, Q. Response of vascular mesenchymal stem/progenitor cells to hyperlipidemia. Cell. Mol. Life Sci. 2018, 75, 4079–4091. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Jing, S.; Ren, T.; Li, Y.; Cao, Y.; Lin, J. Lectin-like oxidized LDL receptor-1 expresses in mouse bone marrow-derived mesenchymal stem cells and stimulates their proliferation. Exp. Cell Res. 2013, 319, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, C.; Wang, H.; Lu, M.; Li, Y.; Feng, H.; Lin, J.; Yuan, Z.; Wang, X. Ox-LDL promotes migration and adhesion of bone marrow-derived mesenchymal stem cells via regulation of MCP-1 expression. Mediat. Inflamm. 2013, 2013, 691023. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, C.; Lin, J.; Wang, X. Oxidized low-density lipoprotein (ox-LDL) promotes cardiac differentiation of bone marrow mesenchymal stem cells via activating ERK1/2 signaling. Cardiovasc. Ther. 2017, 35. [Google Scholar] [CrossRef]
- Kore, R.A.; Henson, J.C.; Hamzah, R.N.; Griffin, R.J.; Tackett, A.J.; Ding, Z.; Mehta, J.L. Molecular events in MSC exosome mediated cytoprotection in cardiomyocytes. Sci. Rep. 2019, 9, 19276. [Google Scholar] [CrossRef] [PubMed]
- Horgusluoglu, E.; Nudelman, K.; Nho, K.; Saykin, A.J. Adult neurogenesis and neurodegenerative diseases: A systems biology perspective. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 93–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizuka, T.; Nagata, W.; Nomura-Takahashi, S.; Satoh, Y. Effects of oxidized low-density lipoprotein on differentiation of mouse neural progenitor cells into neural cells. Eur. J. Pharmacol. 2020, 888, 173456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Hsi, E.; Cheng, H.Y.; Hsu, S.H.; Liao, Y.C.; Juo, S.H. Let-7g suppresses both canonical and non-canonical NF-kappaB pathways in macrophages leading to anti-atherosclerosis. Oncotarget 2017, 8, 101026–101041. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.C.; Hsieh, I.C.; Hsi, E.; Wang, Y.S.; Dai, C.Y.; Chou, W.W.; Juo, S.H. Negative feedback regulation between microRNA let-7g and the oxLDL receptor LOX-1. J. Cell Sci. 2011, 124, 4115–4124. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Mehta, J.L. LOX-1: A critical player in the genesis and progression of myocardial ischemia. Cardiovasc. Drugs Ther. 2011, 25, 431–440. [Google Scholar] [CrossRef]
- Chen, Y.; Kennedy, D.J.; Ramakrishnan, D.P.; Yang, M.; Huang, W.; Li, Z.; Xie, Z.; Chadwick, A.C.; Sahoo, D.; Silverstein, R.L. Oxidized LDL-bound CD36 recruits an Na(+)/K(+)-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci. Signal. 2015, 8, ra91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaisance, V.; Waeber, G.; Regazzi, R.; Abderrahmani, A. Role of microRNAs in islet beta-cell compensation and failure during diabetes. J. Diabetes Res. 2014, 2014, 618652. [Google Scholar] [CrossRef]
- Huang, R.S.; Hu, G.Q.; Lin, B.; Lin, Z.Y.; Sun, C.C. MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. J. Investig. Med. 2010, 58, 961–967. [Google Scholar] [CrossRef]
- Li, X.; Kong, D.; Chen, H.; Liu, S.; Hu, H.; Wu, T.; Wang, J.; Chen, W.; Ning, Y.; Li, Y.; et al. miR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci. Rep. 2016, 6, 21789. [Google Scholar] [CrossRef] [Green Version]
- Doxaki, C.; Kampranis, S.C.; Eliopoulos, A.G.; Spilianakis, C.; Tsatsanis, C. Coordinated regulation of miR-155 and miR-146a genes during induction of endotoxin tolerance in macrophages. J. Immunol. 2015, 195, 5750–5761. [Google Scholar] [CrossRef] [Green Version]
- Bruen, R.; Fitzsimons, S.; Belton, O. miR-155 in the resolution of atherosclerosis. Front. Pharmacol. 2019, 10, 463. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; He, Y.S.; Wang, X.Q.; Lu, L.; Chen, Q.J.; Liu, J.; Sun, Z.; Shen, W.F. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011, 585, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zhao, Z.; Yang, C.; Song, C. Transfer of microRNA-221 from mesenchymal stem cell-derived extracellular vesicles inhibits atherosclerotic plaque formation. Transl. Res. 2020, 226, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, H.; Zhang, B.; Hu, Q. Exosomal miR-512-3p derived from mesenchymal stem cells inhibits oxidized low-density lipoprotein-induced vascular endothelial cells dysfunction via regulating Keap1. J. Biochem. Mol. Toxicol. 2021, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, C.; Li, Q.; Li, J.; Wu, Y.; Liu, J. MicroRNA-25-5p counteracts oxidized LDL-induced pathological changes by targeting neuronal growth regulator 1 (NEGR1) in human brain micro-vessel endothelial cells. Biochimie 2019, 165, 141–149. [Google Scholar] [CrossRef]
- Pan, Q.; Liao, X.; Liu, H.; Wang, Y.; Chen, Y.; Zhao, B.; Lazartigues, E.; Yang, Y.; Ma, X. MicroRNA-125a-5p alleviates the deleterious effects of ox-LDL on multiple functions of human brain microvessel endothelial cells. Am. J. Physiol. Cell Physiol. 2017, 312, C119–C130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, Q.; Zhao, Y.; He, C.; Bi, K.; Chen, Y.; Zhao, B.; Chen, Y.; Ma, X. MicroRNA-155 regulates ROS production, NO generation, apoptosis and multiple functions of human brain microvessel endothelial cells under physiological and pathological conditions. J. Cell Biochem. 2015, 116, 2870–2881. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, D.; Hang, Y.; Zong, X.; Lv, J.; Bai, X.; Lu, Y.; Zhang, P.; Zhou, M.; Wu, Z.; et al. Downregulation of hsa_circ_0004543 activates oxLDL-induced vascular endothelial cell proliferation and angiogenesis. Front. Genet. 2021, 12, 632164. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jin, W.; Sun, L.; Wu, J.; Hu, H.; Ma, L. Circ_0065149 alleviates oxidized low-density lipoprotein-induced apoptosis and inflammation in atherosclerosis by targeting miR-330-5p. Front. Genet. 2021, 12, 590633. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Ma, L.; Yu, B. Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed. Pharmacother. 2017, 95, 1514–1519. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Z.; Feng, X.; Zang, X.; Ding, W.; Wu, F.; Zhao, Q. circRNA/lncRNA-miRNA-mRNA network in oxidized, low-density, lipoprotein-induced foam cells. DNA Cell Biol. 2019, 38, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, M. CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro. BMC Cardiovasc. Disord. 2021, 21, 51. [Google Scholar] [CrossRef]
- Yang, L.; Yang, F.; Zhao, H.; Wang, M.; Zhang, Y. Circular RNA circCHFR facilitates the proliferation and migration of vascular smooth muscle via miR-370/FOXO1/Cyclin D1 pathway. Mol. Ther. Nucleic Acids 2019, 16, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, L.; Dong, X.; Ding, J.; Ma, H.; Han, W. Circ_GRN promotes the proliferation, migration, and inflammation of vascular smooth muscle cells in atherosclerosis through miR-214-3p/FOXO1 axis. J. Cardiovasc. Pharmacol. 2021, 77, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Song, D.; Wu, J.; Wang, J. Long non-coding RNAs link oxidized low-density lipoprotein with the inflammatory response of macrophages in atherogenesis. Front. Immunol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sun, T.; Shen, S.; Wang, L.; Yan, J. LncRNA DYNLRB2-2 inhibits THP-1 macrophage foam cell formation by enhancing autophagy. Biol. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, W.; Guo, Y.; Chen, W.; Zheng, P.; Zeng, J.; Tong, W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE 2017, 12, e0185406. [Google Scholar] [CrossRef] [PubMed]
- Laguna-Fernandez, A.; Novella, S.; Bueno-Beti, C.; Marrugat, J.; Hermenegildo, C. Endothelial transcriptomic changes induced by oxidized low density lipoprotein disclose an up-regulation of Jak-Stat pathway. Vasc. Pharmacol. 2015, 73, 104–114. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Fedele, E. cAMP, cGMP and amyloid β Three ideal partners for memory formation. Trends Neurosci. 2018, 41, 255–266. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zingg, J.-M.; Vlad, A.; Ricciarelli, R. Oxidized LDLs as Signaling Molecules. Antioxidants 2021, 10, 1184. https://doi.org/10.3390/antiox10081184
Zingg J-M, Vlad A, Ricciarelli R. Oxidized LDLs as Signaling Molecules. Antioxidants. 2021; 10(8):1184. https://doi.org/10.3390/antiox10081184
Chicago/Turabian StyleZingg, Jean-Marc, Adelina Vlad, and Roberta Ricciarelli. 2021. "Oxidized LDLs as Signaling Molecules" Antioxidants 10, no. 8: 1184. https://doi.org/10.3390/antiox10081184
APA StyleZingg, J.-M., Vlad, A., & Ricciarelli, R. (2021). Oxidized LDLs as Signaling Molecules. Antioxidants, 10(8), 1184. https://doi.org/10.3390/antiox10081184






