Healthberry 865® and Its Related, Specific, Single Anthocyanins Exert a Direct Vascular Action, Modulating Both Endothelial Function and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Vascular Reactivity Studies
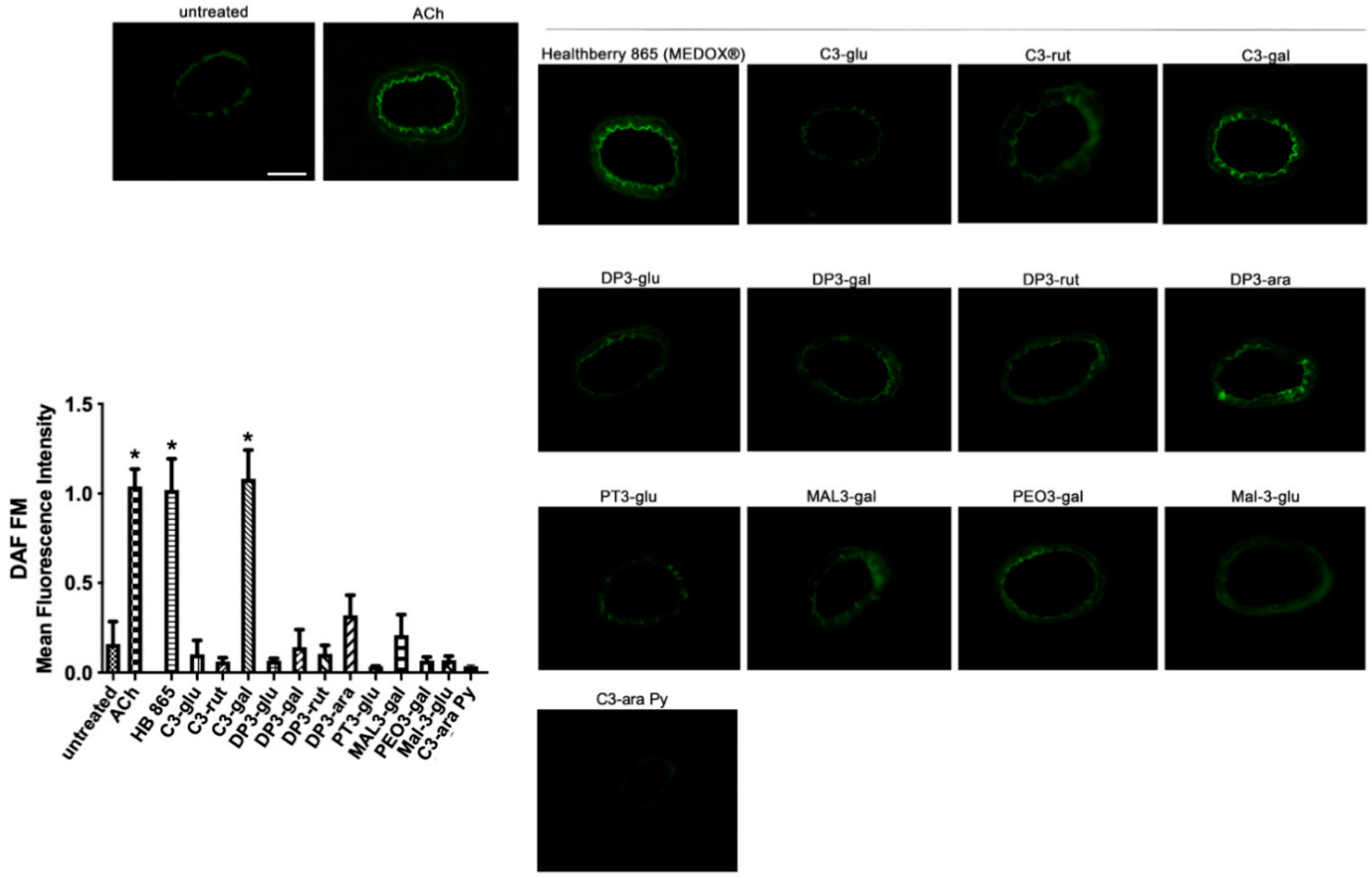
2.3. Evaluation of NO Production by DAF
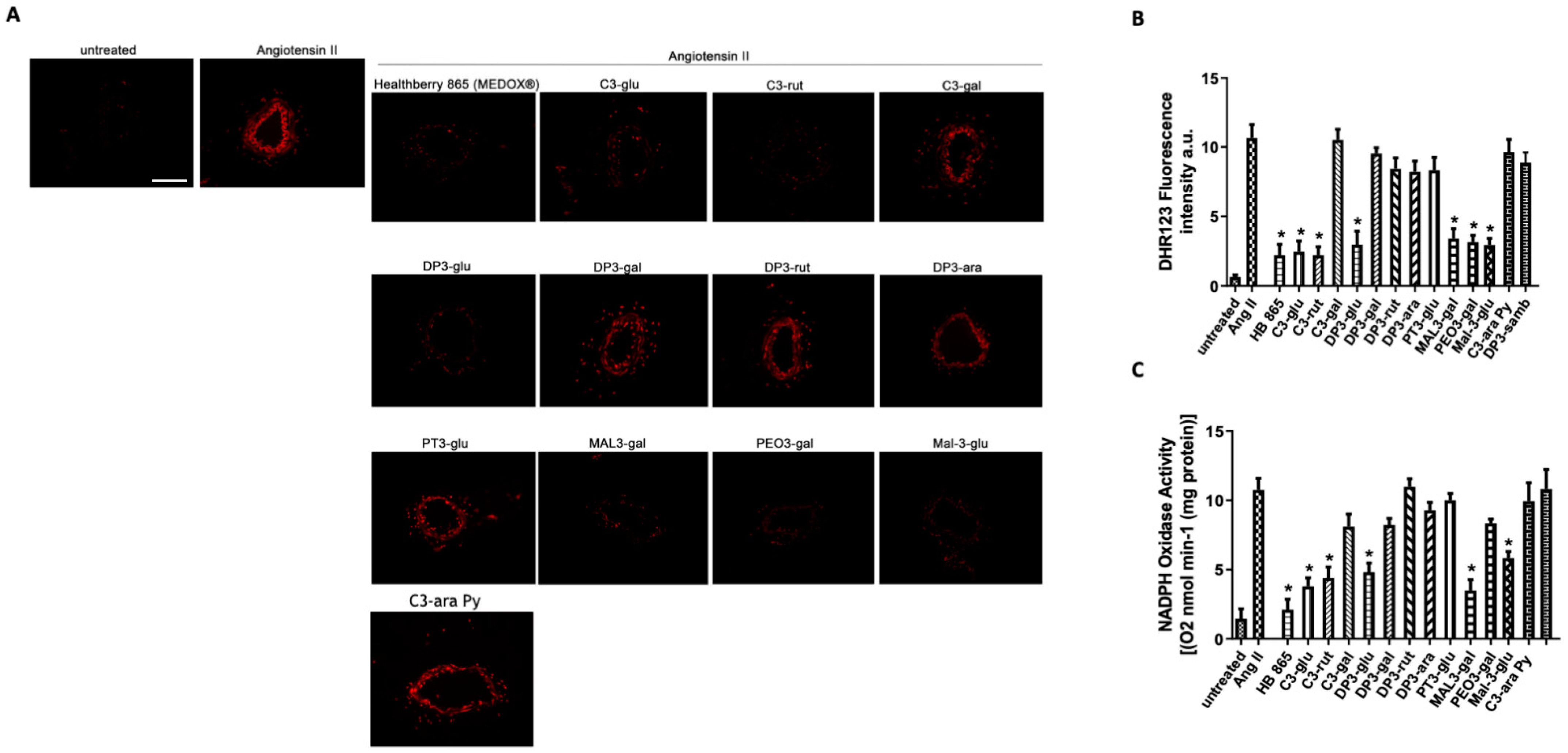
2.4. Analysis of Total ROS Production
2.5. Evaluation of NADPH-mediated O2− Production
2.6. Gel Electrophoresis and Immunoblotting
2.7. Human Vessels Preparation and Ex-Vivo Experiments
2.8. Statistical Analysis
3. Results
3.1. Healthberry 865® Evokes a Direct Vasorelaxant Action of Conduit and Resistance Arteries
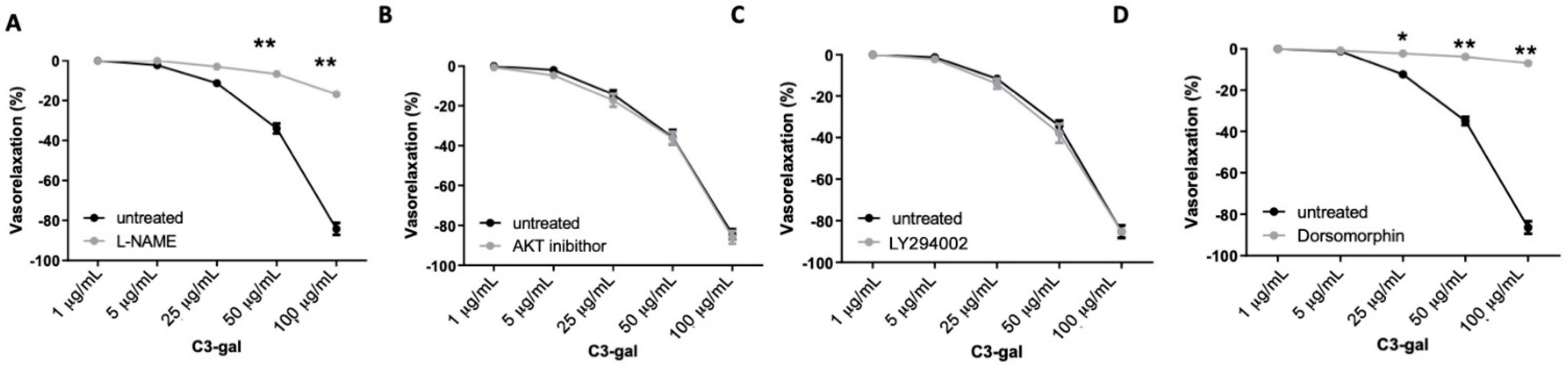
3.2. The Vascular Effect of Healthberry 865® Is Endothelial Nitric Oxide Synthase-Mediated
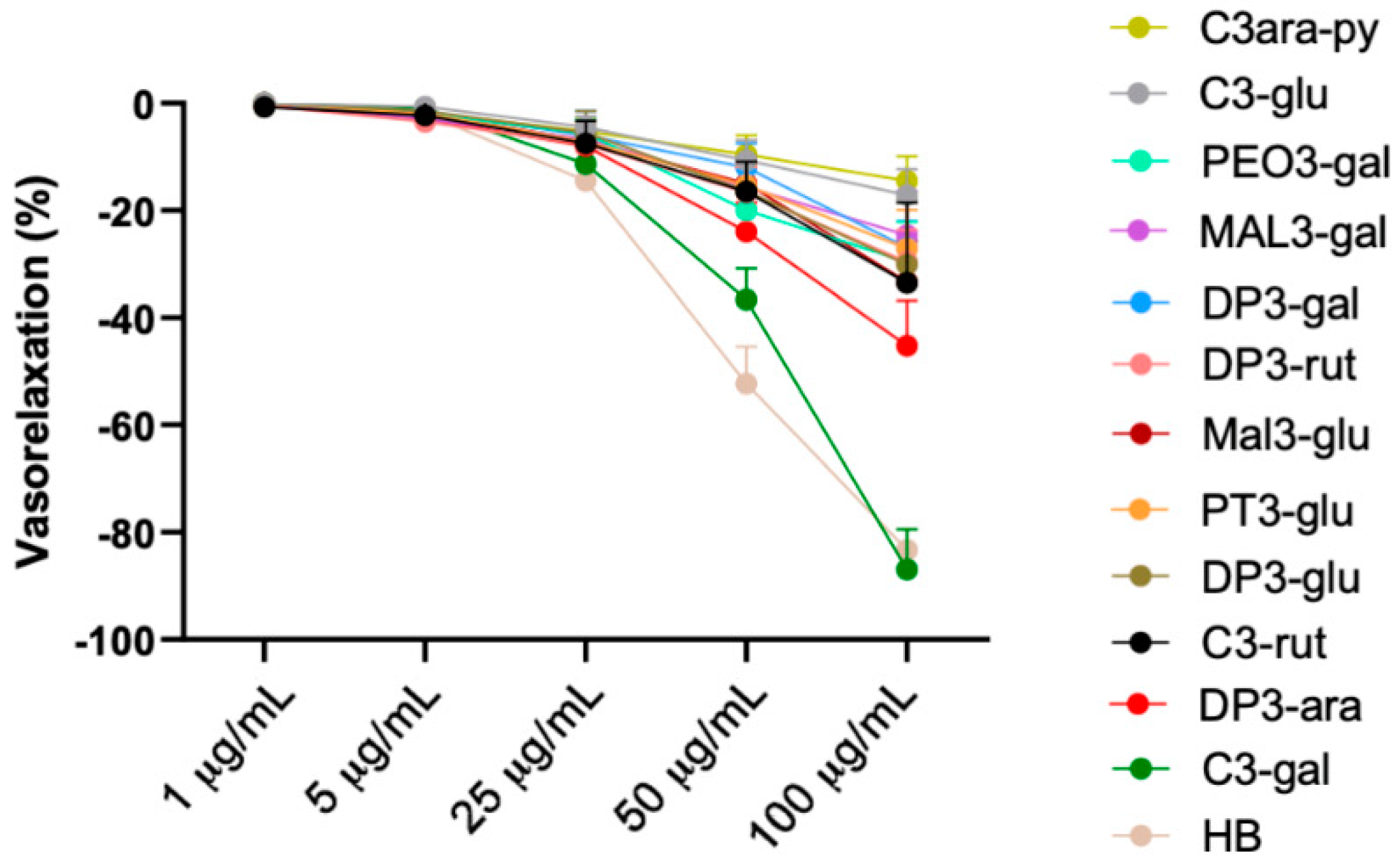
3.3. Vascular Evaluation of Most Abundant Single Anthocyanins Included in Healthberry 865® Composition
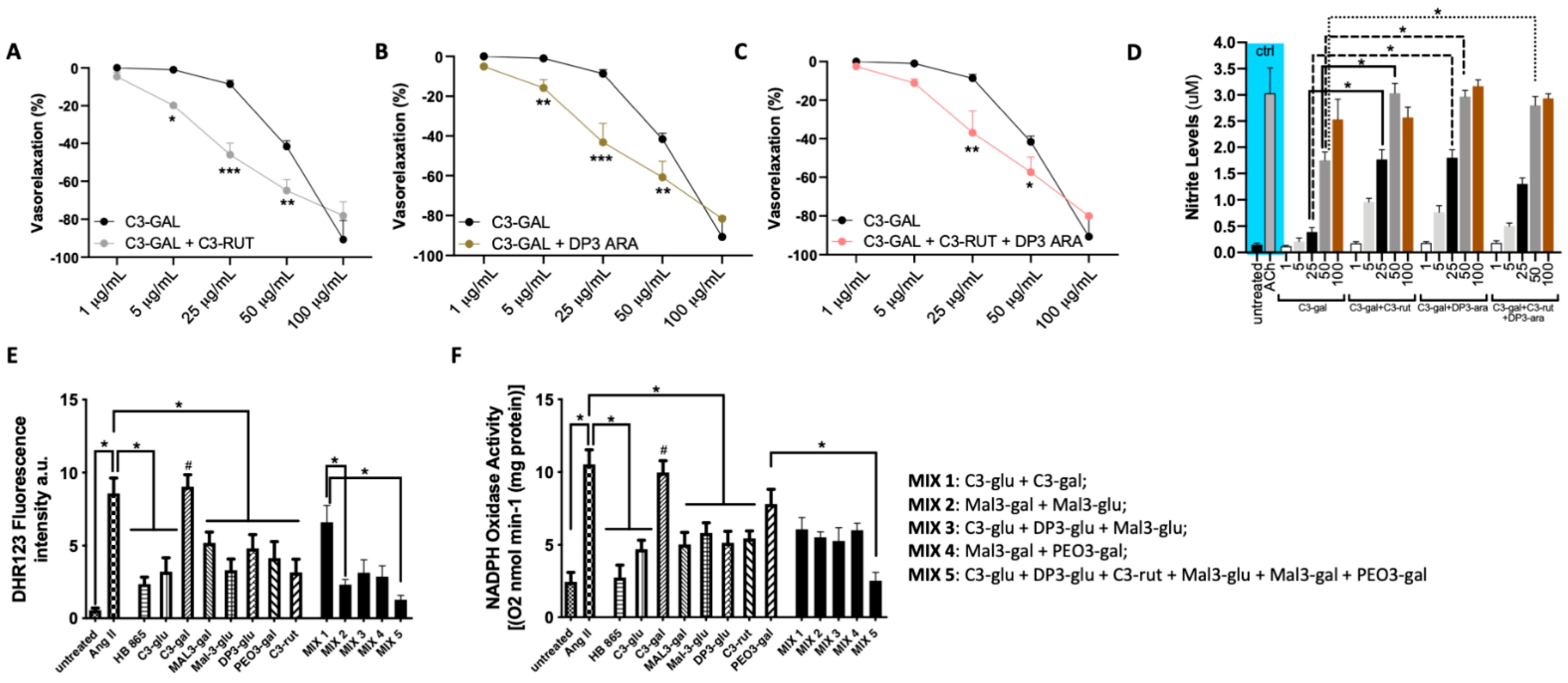
3.4. Cyanidin-3-Galactoside Exerts Vasorelaxant Action Using eNOS Pathway
3.5. The Antioxidant Vascular Action of Healthberry 865® Is Due to the Combination of the Contained Anthocyanins
3.6. A Mix of Specific Healthberry 865®-Anthocyanins Exert a Powerful Vasorelaxant and Antioxidative Action
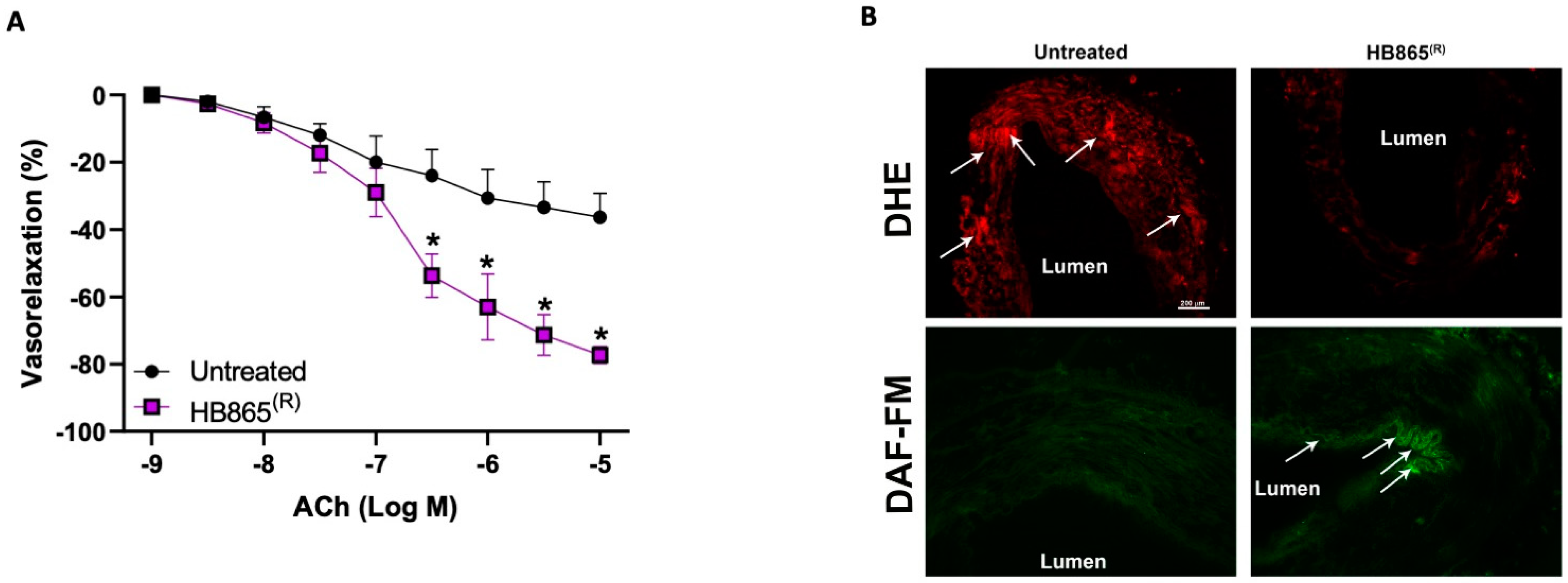
3.7. Healthberry 865® Reduces Oxidative Stress and Improves NO Bioavailability in Human Dysfunctional Vessels
4. Discussion
5. Conclusions
6. Limitation of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raman Mishra, M.; Monica. Determinants of cardiovascular disease and sequential decision-making for treatment among women: A Heckman’s approach. SSM Popul. Health 2019, 7, 100365. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, P.; Pagano, S.; Boccia, G.; De Caro, F.; De Santis, M.; Capunzo, M. Network analysis of drug prescriptions. Pharmacoepidemiol. Drug Saf. 2013, 22, 130–137. [Google Scholar] [CrossRef]
- Imamura, H.; Yamaguchi, T.; Nagayama, D.; Saiki, A.; Shirai, K.; Tatsuno, I. Resveratrol Ameliorates Arterial Stiffness Assessed by Cardio-Ankle Vascular Index in Patients with Type 2 Diabetes Mellitus. Int. Heart J. 2017, 58, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrizzo, A.; Puca, A.; Damato, A.; Marino, M.; Franco, E.; Pompeo, F.; Traficante, A.; Civitillo, F.; Santini, L.; Trimarco, V.; et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 2013, 62, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, J.; Ferro, A. Nutraceuticals: Is there good science behind the hype? Br. J. Clin. Pharmacol. 2013, 75, 585–587. [Google Scholar] [CrossRef]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; De Lucia, M.; Madonna, M.; et al. Novel Potent Decameric Peptide of Spirulina platensis Reduces Blood Pressure Levels Through a PI3K/AKT/eNOS-Dependent Mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, S.; Chen, W.; Gaofeng, Z.; Changcheng, L.; Guoping, T.; Minyan, Z.; Yang, L.; Min, Y.; Luo, J. Cyanidin3Obetaglucoside protects against pulmonary artery hypertension induced by monocrotaline via the TGFbeta1/p38 MAPK/CREB signaling pathway. Mol. Med. Rep. 2021, 23. [Google Scholar] [CrossRef]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.H.; Xu, Z.L.; Dong, D.; He, S.A.; Yu, H. Protective Effect of Anthocyanins Extract from Blueberry on TNBS-Induced IBD Model of Mice. Evid. Based Complement. Alternat. Med. 2011, 2011, 525462. [Google Scholar] [CrossRef]
- Si, X.; Bi, J.; Chen, Q.; Cui, H.; Bao, Y.; Tian, J.; Shu, C.; Wang, Y.; Tan, H.; Zhang, W.; et al. Effect of Blueberry Anthocyanin-Rich Extracts on Peripheral and Hippocampal Antioxidant Defensiveness: The Analysis of the Serum Fatty Acid Species and Gut Microbiota Profile. J. Agric. Food Chem. 2021, 69, 3658–3666. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef]
- Jia, Y.; Wu, C.; Kim, Y.S.; Yang, S.O.; Kim, Y.; Kim, J.S.; Jeong, M.Y.; Lee, J.H.; Kim, B.; Lee, S.; et al. A dietary anthocyanin cyanidin-3-O-glucoside binds to PPARs to regulate glucose metabolism and insulin sensitivity in mice. Commun. Biol. 2020, 3, 514. [Google Scholar] [CrossRef]
- Li, L.; Adams, L.S.; Chen, S.; Killian, C.; Ahmed, A.; Seeram, N.P. Eugenia jambolana Lam. berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J. Agric. Food Chem. 2009, 57, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Istas, G.; Boschek, L.; Feliciano, R.P.; Mills, C.E.; Boby, C.; Gomez-Alonso, S.; Milenkovic, D.; Heiss, C. Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights from Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J. Gerontol. Biol. 2019, 74, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial (vol 109, pg 1535, 2019). Am. J. Clin. Nutr. 2019, 110, 1262. [Google Scholar] [CrossRef]
- Carrizzo, A.; Ambrosio, M.; Damato, A.; Madonna, M.; Storto, M.; Capocci, L.; Campiglia, P.; Sommella, E.; Trimarco, V.; Rozza, F.; et al. Morus alba extract modulates blood pressure homeostasis through eNOS signaling. Mol. Nutr. Food Res. 2016, 60, 2304–2311. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Basilicata, M.G.; Pepe, G.; Sorensen, K.K.; Ciccarelli, M.; Sarno, V.D.; Damato, A.; Venturini, E.; Borrelli, A.; Musella, S.; et al. A Novel Vasoactive Peptide “PG1” from Buffalo Ice-Cream Protects from Angiotensin-Evoked High Blood Pressure. Antioxidants 2021, 10, 441. [Google Scholar] [CrossRef]
- Carrizzo, A.; Moltedo, O.; Damato, A.; Martinello, K.; Di Pietro, P.; Oliveti, M.; Acernese, F.; Giugliano, G.; Izzo, R.; Sommella, E.; et al. New Nutraceutical Combination Reduces Blood Pressure and Improves Exercise Capacity in Hypertensive Patients Via a Nitric Oxide-Dependent Mechanism. J. Am. Heart Assoc. 2020, 9, e014923. [Google Scholar] [CrossRef]
- Bridges, L.E.; Williams, C.L.; Pointer, M.A.; Awumey, E.M. Mesenteric artery contraction and relaxation studies using automated wire myography. J. Vis. Exp. 2011. [Google Scholar] [CrossRef]
- Zacchigna, L.; Vecchione, C.; Notte, A.; Cordenonsi, M.; Dupont, S.; Maretto, S.; Cifelli, G.; Ferrari, A.; Maffei, A.; Fabbro, C.; et al. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell 2006, 124, 929–942. [Google Scholar] [CrossRef]
- Welsh, D.G.; Morielli, A.D.; Nelson, M.T.; Brayden, J.E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002, 90, 248–250. [Google Scholar] [CrossRef]
- Villa, F.; Carrizzo, A.; Spinelli, C.C.; Ferrario, A.; Malovini, A.; Maciag, A.; Damato, A.; Auricchio, A.; Spinetti, G.; Sangalli, E.; et al. Genetic Analysis Reveals a Longevity-Associated Protein Modulating Endothelial Function and Angiogenesis. Circ. Res. 2015, 117, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Karlsen, A.; Retterstol, L.; Laake, P.; Paur, I.; Bohn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Wallace, T.C. Anthocyanins in Cardiovascular Disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Xu, L.; Chang, K.C.; Shin, S.C.; Chung, J.I.; Kang, D.; Kim, S.H.; Hur, J.A.; Choi, T.H.; Kim, S.; et al. Anti-inflammatory effects of anthocyanins from black soybean seed coat on the keratinocytes and ischemia-reperfusion injury in rat skin flaps. Microsurgery 2012, 32, 563–570. [Google Scholar] [CrossRef]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.T.; Liu, J.; Mou, H.; Cao, L.; Ling, W.H. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular Disease Risk Biomarkers and Liver and Kidney Function Are Not Altered in Postmenopausal Women after Ingesting an Elderberry Extract Rich in Anthocyanins for 12 Weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxley, R.R.; Perkovic, V. The modifiable burden of worldwide mortality from cardiovascular diseases. Lancet Diabetes Endocrinol. 2014, 2, 604–606. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J. Nutr. Metab. 2012, 2012, 569486. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.C.; Slavin, M.; Frankenfeld, C.L. Systematic Review of Anthocyanins and Markers of Cardiovascular Disease. Nutrients 2016, 8, 32. [Google Scholar] [CrossRef]
- Lee, G.H.; Hoang, T.H.; Jung, E.S.; Jung, S.J.; Han, S.K.; Chung, M.J.; Chae, S.W.; Chae, H.J. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell 2020, 19, e13279. [Google Scholar] [CrossRef]
- Puca, A.A.; Carrizzo, A.; Spinelli, C.; Damato, A.; Ambrosio, M.; Villa, F.; Ferrario, A.; Maciag, A.; Fornai, F.; Lenzi, P.; et al. Single systemic transfer of a human gene associated with exceptional longevity halts the progression of atherosclerosis and inflammation in ApoE knockout mice through a CXCR4-mediated mechanism. Eur. Heart J. 2020, 41, 2487–2497. [Google Scholar] [CrossRef] [Green Version]
- Langston-Cox, A.; Leo, C.H.; Tare, M.; Wallace, E.M.; Marshall, S.A. Sulforaphane improves vascular reactivity in mouse and human arteries after “preeclamptic-like” injury. Placenta 2020, 101, 242–250. [Google Scholar] [CrossRef]
- Vecchione, C.; Villa, F.; Carrizzo, A.; Spinelli, C.C.; Damato, A.; Ambrosio, M.; Ferrario, A.; Madonna, M.; Uccellatore, A.; Lupini, S.; et al. A rare genetic variant of BPIFB4 predisposes to high blood pressure via impairment of nitric oxide signaling. Sci. Rep. 2017, 7, 9706. [Google Scholar] [CrossRef]
- Lind, L.; Berglund, L.; Larsson, A.; Sundstrom, J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 2011, 123, 1545–1551. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Wang, L.X.; Dong, S.S.; Hong, Y.; Zhou, X.H.; Zheng, W.W.; Zheng, C. Phlorizin Exerts Direct Protective Effects on Palmitic Acid (PA)-Induced Endothelial Dysfunction by Activating the PI3K/AKT/eNOS Signaling Pathway and Increasing the Levels of Nitric Oxide (NO). Med. Sci. Monit. Basic Res. 2018, 24, 1–9. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between Anthocyanins and Gut Microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassenge, E.; Schneider, H.T.; Daiber, A. Oxidative stress and cardiovascular diseases. Deut. Med. Wochenschr. 2005, 130, 2904–2909. [Google Scholar] [CrossRef]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial Function and Oxidative Stress in Cardiovascular Diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puca, A.A.; Carrizzo, A.; Villa, F.; Ferrario, A.; Casaburo, M.; Maciag, A.; Vecchione, C. Vascular ageing: The role of oxidative stress. Int. J. Biochem. Cell B 2013, 45, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, C.; Patrucco, E.; Marino, G.; Barberis, L.; Poulet, R.; Aretini, A.; Maffei, A.; Gentile, M.T.; Storto, M.; Azzolino, O.; et al. Protection from angiotensin II-mediated vasculotoxic and hypertensive response in mice lacking PI3Kgamma. J. Exp. Med. 2005, 201, 1217–1228. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrizzo, A.; Lizio, R.; Di Pietro, P.; Ciccarelli, M.; Damato, A.; Venturini, E.; Iannece, P.; Sommella, E.; Campiglia, P.; Ockermann, P.; et al. Healthberry 865® and Its Related, Specific, Single Anthocyanins Exert a Direct Vascular Action, Modulating Both Endothelial Function and Oxidative Stress. Antioxidants 2021, 10, 1191. https://doi.org/10.3390/antiox10081191
Carrizzo A, Lizio R, Di Pietro P, Ciccarelli M, Damato A, Venturini E, Iannece P, Sommella E, Campiglia P, Ockermann P, et al. Healthberry 865® and Its Related, Specific, Single Anthocyanins Exert a Direct Vascular Action, Modulating Both Endothelial Function and Oxidative Stress. Antioxidants. 2021; 10(8):1191. https://doi.org/10.3390/antiox10081191
Chicago/Turabian StyleCarrizzo, Albino, Rosario Lizio, Paola Di Pietro, Michele Ciccarelli, Antonio Damato, Eleonora Venturini, Patrizia Iannece, Eduardo Sommella, Pietro Campiglia, Philipp Ockermann, and et al. 2021. "Healthberry 865® and Its Related, Specific, Single Anthocyanins Exert a Direct Vascular Action, Modulating Both Endothelial Function and Oxidative Stress" Antioxidants 10, no. 8: 1191. https://doi.org/10.3390/antiox10081191
APA StyleCarrizzo, A., Lizio, R., Di Pietro, P., Ciccarelli, M., Damato, A., Venturini, E., Iannece, P., Sommella, E., Campiglia, P., Ockermann, P., & Vecchione, C. (2021). Healthberry 865® and Its Related, Specific, Single Anthocyanins Exert a Direct Vascular Action, Modulating Both Endothelial Function and Oxidative Stress. Antioxidants, 10(8), 1191. https://doi.org/10.3390/antiox10081191








