Measurement of Oxidative Stress Index in Seminal Plasma Can Predict In Vivo Fertility of Liquid-Stored Porcine Artificial Insemination Semen Doses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Media
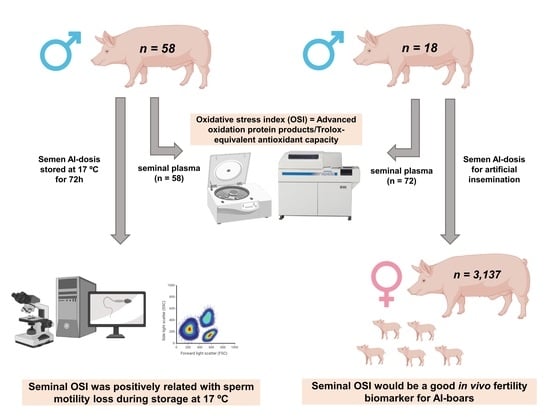
2.2. Boars, Ejaculates and Seminal Plasma Samples
2.3. Oxidative Stress Index Measurement in SP
2.4. Assessment of Sperm Quality
2.5. Experimental Design
2.5.1. Experiment 1: Relationship between Seminal OSI and the Sperm Quality of Semen AI-Doses Stored at 17 °C for 72 h
2.5.2. Experiment 2: Relationship between Seminal OSI and In Vivo Fertility of Semen AI-Doses Stored at 17 °C
2.6. Statistical Analysis
3. Results
3.1. Experiment 1: Relationship between Seminal OSI and Sperm Quality of Semen AI-Doses Stored at 17 °C for 72 h
3.2. Experiment 2: Relationship between Seminal OSI and In Vivo Fertility of Semen AI-Doses Stored at 17 °C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2010, 2010, 686137. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Yeste, M. Oxidative stress in male infertility: Causes, effects in assisted reproductive techniques, and protective support of antioxidants. Biology 2020, 9, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzezek, J.; Lapkiewicz, S.; Lecewicz, M. A note on antioxidant capacity of boar seminal plasma. Anim. Sci. Pap. Rep. 1999, 17, 181–188. [Google Scholar]
- Cerolini, S.; Maldjian, A.; Surai, P.; Noble, R. Viability, susceptibility to peroxidation and fatty acid composition of boar semen during liquid storage. Anim. Reprod. Sci. 2000, 58, 99–111. [Google Scholar] [CrossRef]
- Bathgate, R. Antioxidant mechanisms and their benefit on post-thaw boar sperm quality. Reprod. Domest. Anim. 2011, 46 (Suppl. 2), 23–25. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patiño, C.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. High total antioxidant capacity of the porcine seminal plasma (SP-TAC) relates to sperm survival and fertility. Sci. Rep. 2015, 5, 18538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patino, C.; Alkmin, D.V.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. The activity of paraoxonase type 1 (PON-1) in boar seminal plasma and its relationship with sperm quality, functionality, and in vivo fertility. Andrology 2015, 3, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Barranco, I.; Tvarijonaviciute, A.; Perez-Patino, C.; Vicente-Carrillo, A.; Parrilla, I.; Ceron, J.J.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J. Glutathione Peroxidase 5 is expressed by the entire pig male genital tract and once in the seminal plasma contributes to sperm survival and in vivo fertility. PLoS ONE 2016, 11, e0162958. [Google Scholar] [CrossRef]
- Barranco, I.; Padilla, L.; Tvarijonaviciute, A.; Parrilla, I.; Martínez, E.A.; Rodriguez-Martinez, H.; Yeste, M.; Roca, J. Levels of activity of superoxide dismutase in seminal plasma do not predict fertility of pig AI-semen doses. Theriogenology 2019, 140, 18–24. [Google Scholar] [CrossRef]
- Li, J.; Barranco, I.; Tvarijonaviciute, A.; Molina, M.F.; Martinez, E.A.; Rodriguez-Martinez, H.; Parrilla, I.; Roca, J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology 2018, 107, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Yeste, M. State-of-the-art of boar sperm preservation in liquid and frozen state. Anim. Reprod. 2017, 14, 69–81. [Google Scholar] [CrossRef]
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of preserved boar semen for artificial insemination: Past, present and future challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Robert, K.A.; Sharma, R.; Henkel, R.; Agarwal, A. An update on the techniques used to measure oxidative stress in seminal plasma. Andrologia 2021, 53, e13726. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period. Animal 2013, 7, 1374–1378. [Google Scholar] [CrossRef] [Green Version]
- Kolettis, P.N.; Sharma, R.K.; Pasqualotto, F.F.; Nelson, D.; Thomas, A.J.J.; Agarwal, A. Effect of seminal oxidative stress on fertility after vasectomy reversal. Fertil. Steril. 1999, 71, 249–255. [Google Scholar] [CrossRef]
- Sharma, R.K.; Pasqualotto, F.F.; Nelson, D.R.; Thomas, A.J.J.; Agarwal, A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum. Reprod. 1999, 14, 2801–2807. [Google Scholar] [CrossRef] [Green Version]
- Pasqualotto, F.F.; Sundaram, A.; Sharma, R.K.; Borges, E.J.; Pasqualotto, E.B.; Agarwal, A. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil. Steril. 2008, 89, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. Reprod. Med. Biol. 2021, 20, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio, C.P.; Tvarijonaviciute, A.; Caldin, M.; Hernández-Ruiz, J.; Cerón, J.J.; Martínez-Subiela, S.; Tecles, F. Stability of biomarkers of oxidative stress in canine serum. Res. Vet. Sci. 2018, 121, 85–93. [Google Scholar] [CrossRef]
- Venturini, D.; Simão, A.N.C.; Urbano, M.R.; Dichi, I. Effects of extra virgin olive oil and fish oil on lipid profile and oxidative stress in patients with metabolic syndrome. Nutrition 2015, 31, 834–840. [Google Scholar] [CrossRef]
- Broekhuijse, M.L.W.J.; Sostaric, E.; Feitsma, H.; Gadella, B.M. The value of microscopic semen motility assessment at collection for a commercial artificial insemination center, a retrospective study on factors explaining variation in pig fertility. Theriogenology 2012, 77, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, A.; Kadirvel, G.; Bujarbaruah, K.M.; Bardoloi, R.K.; Das, A.; Kumar, S.; Naskar, S. Preservation of boar semen at 18 degrees C induces lipid peroxidation and apoptosis like changes in spermatozoa. Anim. Reprod. Sci. 2009, 110, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, M.; Wang, N.; Yang, K.; Guo, H.; Wang, J.; Zhang, Y.; Yue, S.; Zhou, J. Effects of L-glutamine on boar sperm quality during liquid storage at 17°C. Anim. Reprod. Sci. 2018, 191, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Barranco, I.; Padilla, L.; Perez-Patino, C.; Vazquez, J.M.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J.; Parrilla, I. Seminal plasma cytokines are predictive of the outcome of boar sperm preservation. Front. Vet. Sci. 2019, 6, 436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Liu, Q.; Wang, L.Q.; Yang, G.S.; Hu, J.H. Effects of glutathione on sperm quality during liquid storage in boars. Anim. Sci. J. 2016, 87, 1195–1201. [Google Scholar] [CrossRef]
- Zhang, X.G.; Li, H.; Wang, L.; Hao, Y.Y.; Liang, G.D.; Ma, Y.H.; Yang, G.S.; Hu, J.H. The effects of different levels of superoxide dismutase in Modena on boar semen quality during liquid preservation at 17 °C. Anim. Sci. J. 2017, 88, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, X.G.; Fang, Q.; Liu, Q.; Du, R.R.; Yang, G.S.; Wang, L.Q.; Hu, J.H. Supplemental effect of different levels of taurine in Modena on boar semen quality during liquid preservation at 17 °C. Anim. Sci. J. 2017, 88, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, N.; Guo, H.T.; Wang, J.R.; Sun, H.H.; Sun, L.Z.; Yue, S.L.; Zhou, J.B. Effect of L-carnitine on sperm quality during liquid storage of boar semen. Asian-Australas. J. Anim. Sci. 2020, 33, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Roychoudhury, S.; Bjugstad, K.B.; Cho, C.-L. Oxidation-reduction potential of semen: What is its role in the treatment of male infertility? Ther. Adv. Urol. 2016, 8, 302–318. [Google Scholar] [CrossRef] [Green Version]
- Barranco, I.; Casao, A.; Perez-Patiño, C.; Parrilla, I.; Muiño-Blanco, T.; Martinez, E.A.; Cebrian-Perez, J.A.; Roca, J. Profile and reproductive roles of seminal plasma melatonin of boar ejaculates used in artificial insemination programs. J. Anim. Sci. 2017, 95, 1660–1668. [Google Scholar] [CrossRef] [Green Version]
- Mateo-Otero, Y.; Fernández-López, P.; Ribas-Maynou, J.; Roca, J.; Miró, J.; Yeste, M.; Barranco, I. Metabolite profiling of pig seminal plasma identifies potential biomarkers for sperm resilience to liquid preservation. Front. Cell Dev. Biol. 2021, 9, 669974. [Google Scholar] [CrossRef]
- Agarwal, A.; Ikemoto, I.; Loughlin, K.R. Relationship of sperm parameters with levels of reactive oxygen species in semen specimens. J. Urol. 1994, 152, 107–110. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [Green Version]
- de Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J. Androl. 1992, 13, 379–386. [Google Scholar]
- Aitken, R.J.; Fisher, H.M.; Fulton, N.; Gomez, E.; Knox, W.; Lewis, B.; Irvine, S. Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol. Reprod. Dev. 1997, 47, 468–482. [Google Scholar] [CrossRef]
- Karunakaran, M.; Chakurkar, E.B.; Ratnakaran, U.; Naik, P.K.; Mondal, M.; Mondal, A.; Singh, N.P. Characteristics of boar semen preserved at liquid state. J. Appl. Anim. Res. 2017, 45, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Baumber, J.; Ball, B.A.; Gravance, C.G.; Medina, V.; Davies-Morel, M.C. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000, 21, 895–902. [Google Scholar] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef]
- Popwell, J.M.; Flowers, W.L. Variability in relationships between semen quality and estimates of in vivo and in vitro fertility in boars. Anim. Reprod. Sci. 2004, 81, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Harkiss, D.; Buckingham, D. Relationship between iron-catalysed lipid peroxidation potential and human sperm function. J. Reprod. Fertil. 1993, 98, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Boe-Hansen, G.B.; Ersbøll, A.K.; Greve, T.; Christensen, P. Increasing storage time of extended boar semen reduces sperm DNA integrity. Theriogenology 2005, 63, 2006–2019. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Broekhuijse, M.L.W.J.; Parrilla, I.; Rodriguez-Martinez, H.; Martinez, E.A.; Bolarin, A. Boar differences in artificial insemination outcomes: Can they be minimized? Reprod. Domest. Anim. 2015, 50 (Suppl. 2), 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barranco, I.; Rubio, C.P.; Tvarijonaviciute, A.; Rodriguez-Martinez, H.; Roca, J. Measurement of Oxidative Stress Index in Seminal Plasma Can Predict In Vivo Fertility of Liquid-Stored Porcine Artificial Insemination Semen Doses. Antioxidants 2021, 10, 1203. https://doi.org/10.3390/antiox10081203
Barranco I, Rubio CP, Tvarijonaviciute A, Rodriguez-Martinez H, Roca J. Measurement of Oxidative Stress Index in Seminal Plasma Can Predict In Vivo Fertility of Liquid-Stored Porcine Artificial Insemination Semen Doses. Antioxidants. 2021; 10(8):1203. https://doi.org/10.3390/antiox10081203
Chicago/Turabian StyleBarranco, Isabel, Camila P. Rubio, Asta Tvarijonaviciute, Heriberto Rodriguez-Martinez, and Jordi Roca. 2021. "Measurement of Oxidative Stress Index in Seminal Plasma Can Predict In Vivo Fertility of Liquid-Stored Porcine Artificial Insemination Semen Doses" Antioxidants 10, no. 8: 1203. https://doi.org/10.3390/antiox10081203
APA StyleBarranco, I., Rubio, C. P., Tvarijonaviciute, A., Rodriguez-Martinez, H., & Roca, J. (2021). Measurement of Oxidative Stress Index in Seminal Plasma Can Predict In Vivo Fertility of Liquid-Stored Porcine Artificial Insemination Semen Doses. Antioxidants, 10(8), 1203. https://doi.org/10.3390/antiox10081203