1,3-Dicaffeoylquinic Acid as an Active Compound of Arctium lappa Root Extract Ameliorates Depressive-Like Behavior by Regulating Hippocampal Nitric Oxide Synthesis in Ovariectomized Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
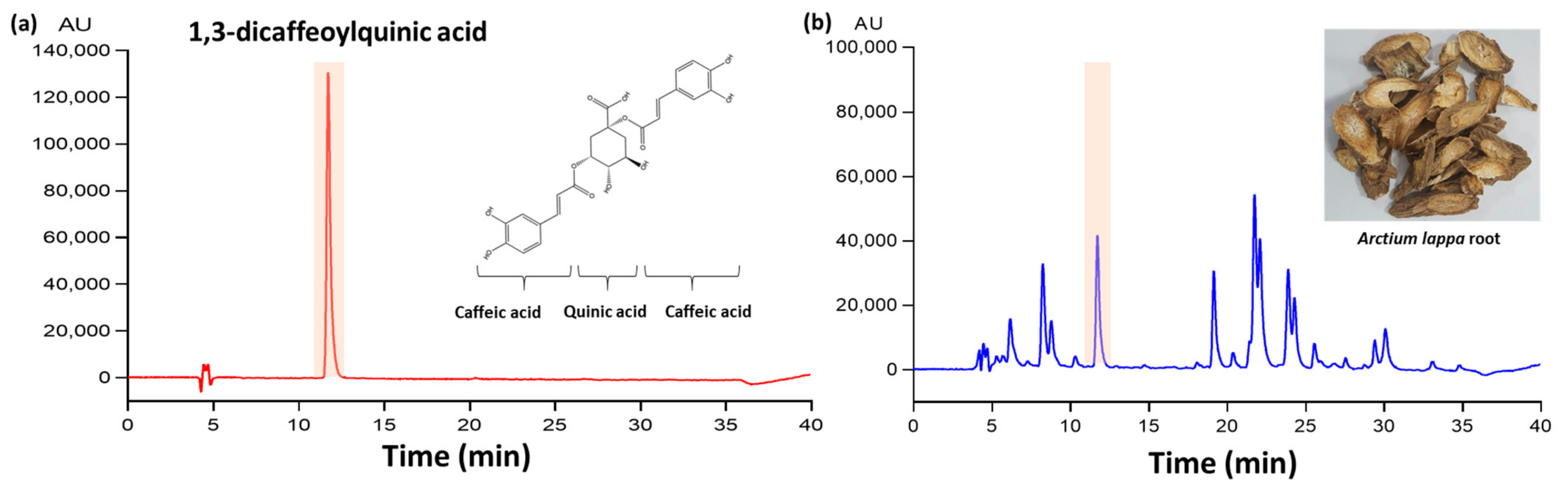
2.2. Sample Preparation and HPLC Analysis
2.3. Animals
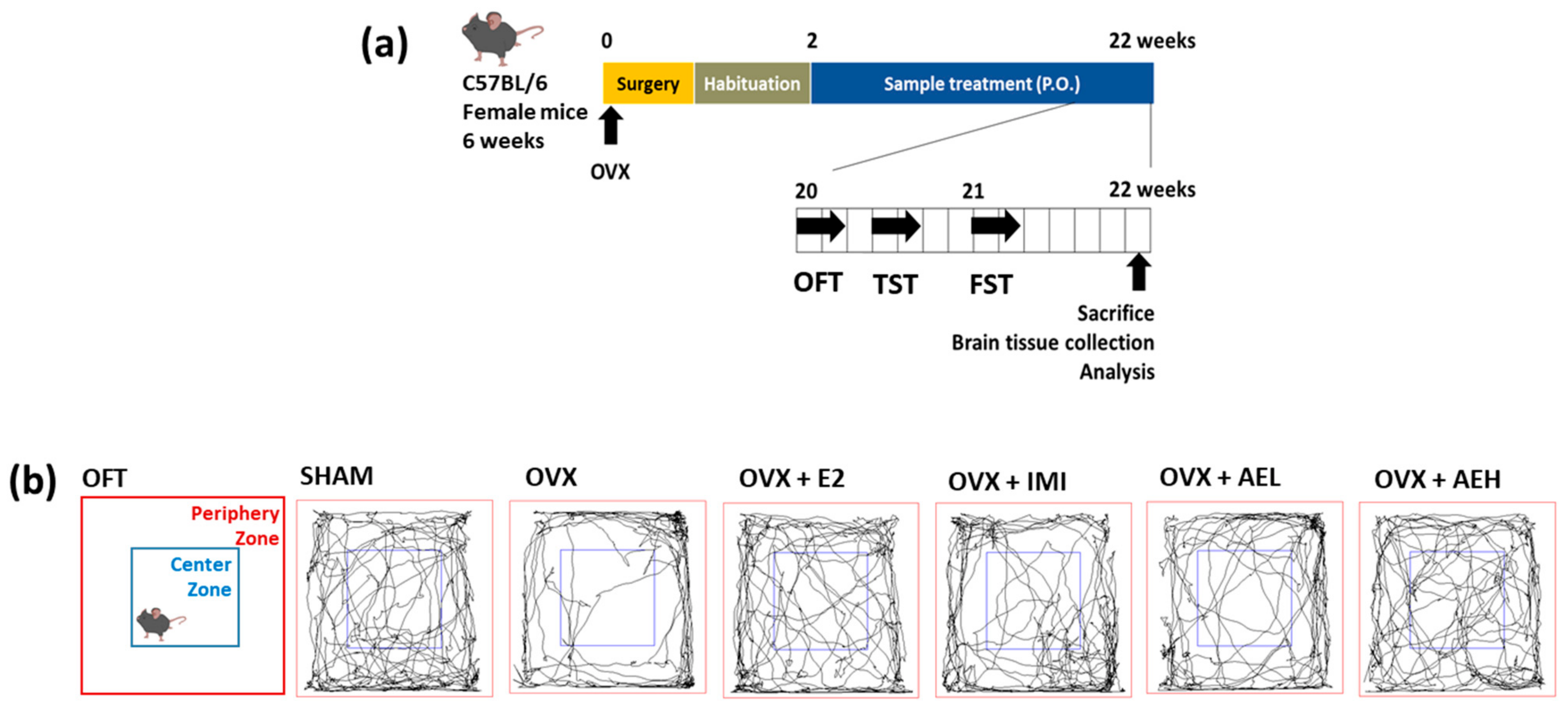
2.4. Ovariectomy and Treatments
2.4.1. Experiment 1
2.4.2. Experiment 2
2.5. Behavior Tests
2.5.1. Open Field Test (OFT)
2.5.2. Tail Suspension Test (TST)
2.5.3. Forced Swimming Test (FST)
2.6. Cell Culture
2.6.1. SH-SY5Y Cell Culture
2.6.2. Primary Hippocampal Neuron Culture
2.7. Measurement of NO Contents
2.8. Immunofluorescence Staining and Imaging
2.9. Immunohistochemistry
2.10. Immunoblotting
2.11. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.12. Statistical Analysis
3. Results
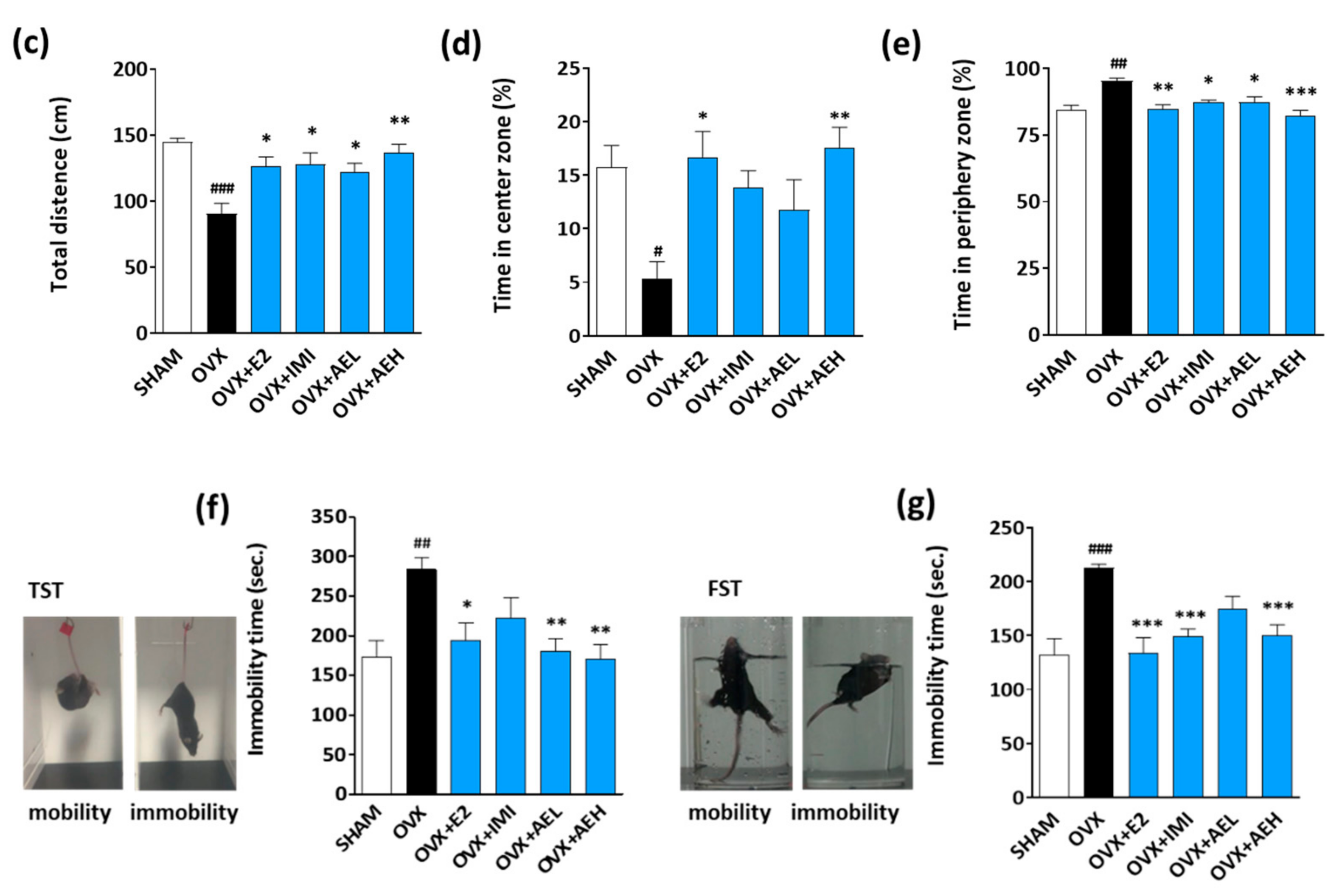
3.1. Effect of AE on Depressant-Like Behaviors Caused by Estrogen Deficiency in OVX Mice
3.2. AE Restores NO Shortage via Regulation of Hippocampal nNOS Expression in OVX Mice
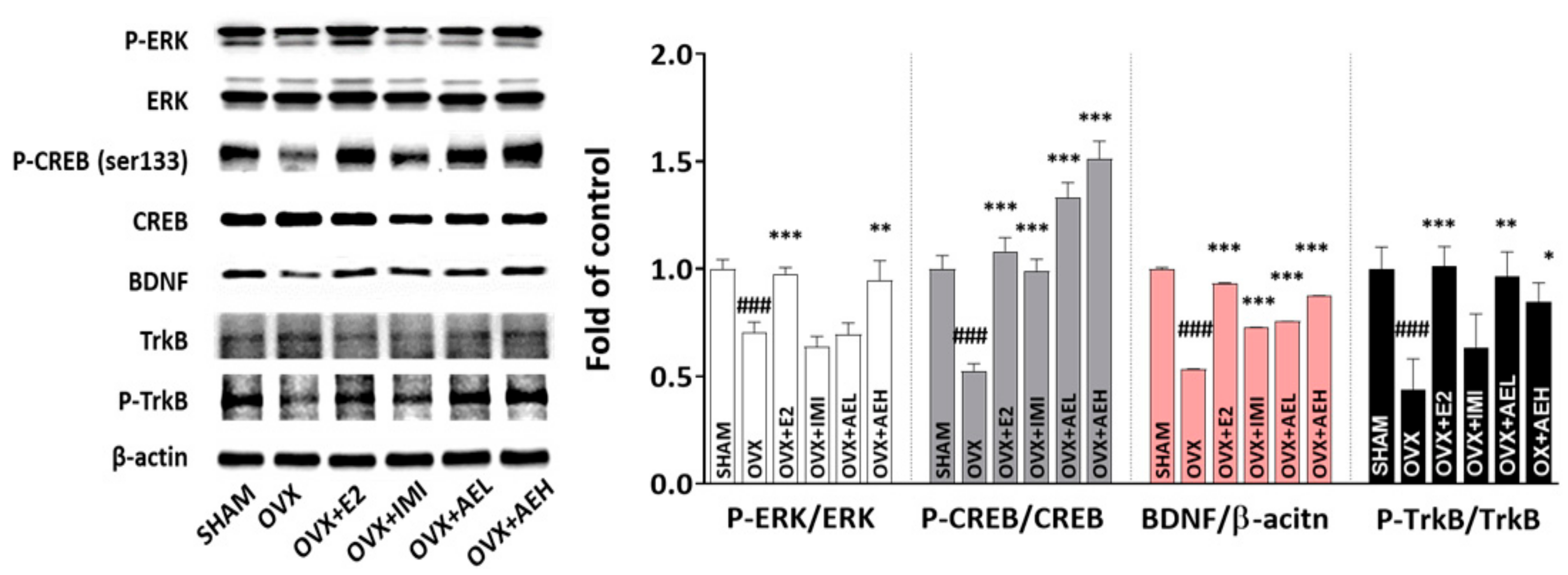
3.3. AE Restores Hippocampal ERK–CREB–BDNF Signaling Networks in OVX Mice
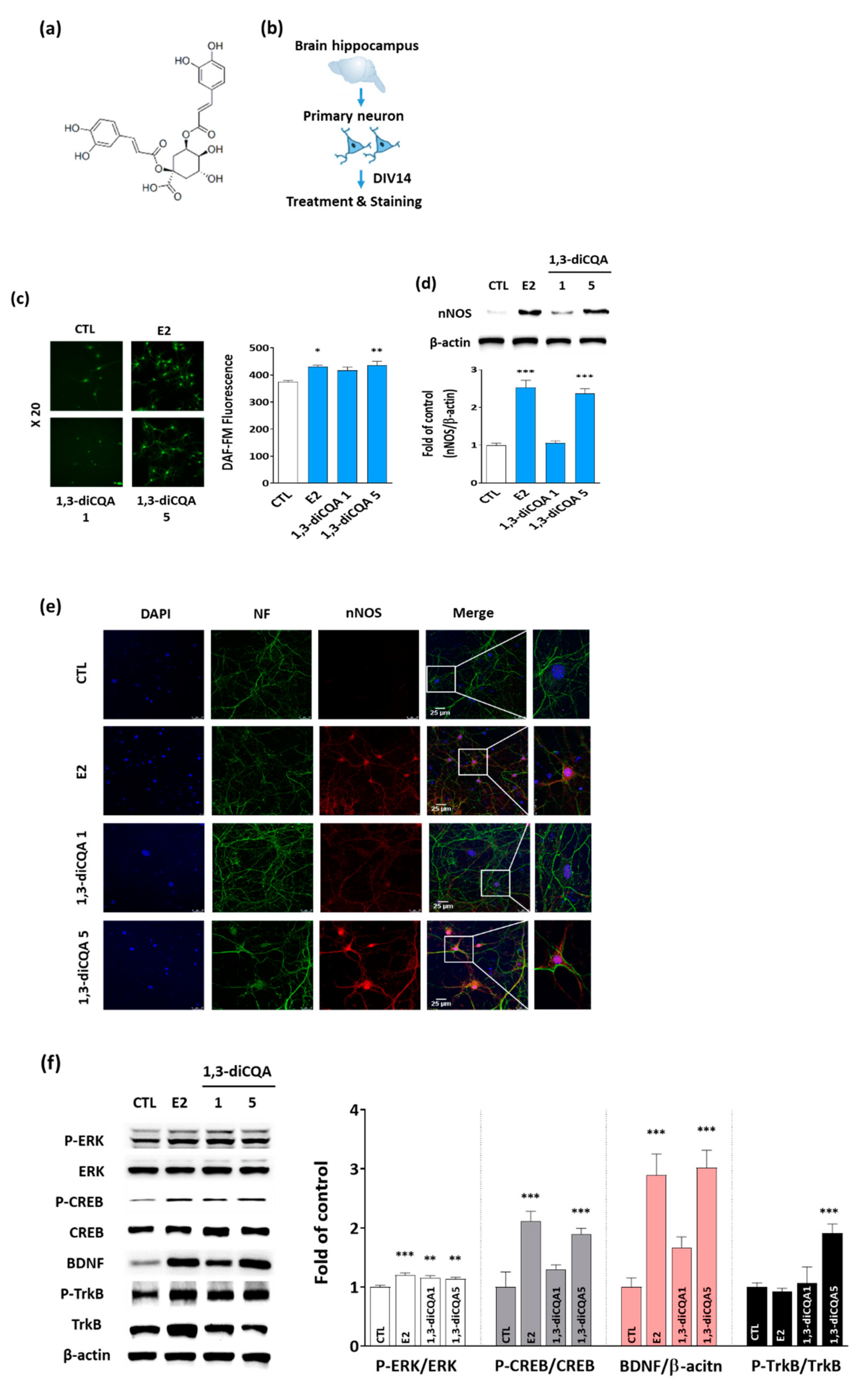
3.4. 1,3-diCQA Restores NO Shortage via Regulation of nNOS Expression and ERK–CREB–BDNF Signaling Networks in Primary Hippocampal Neurons
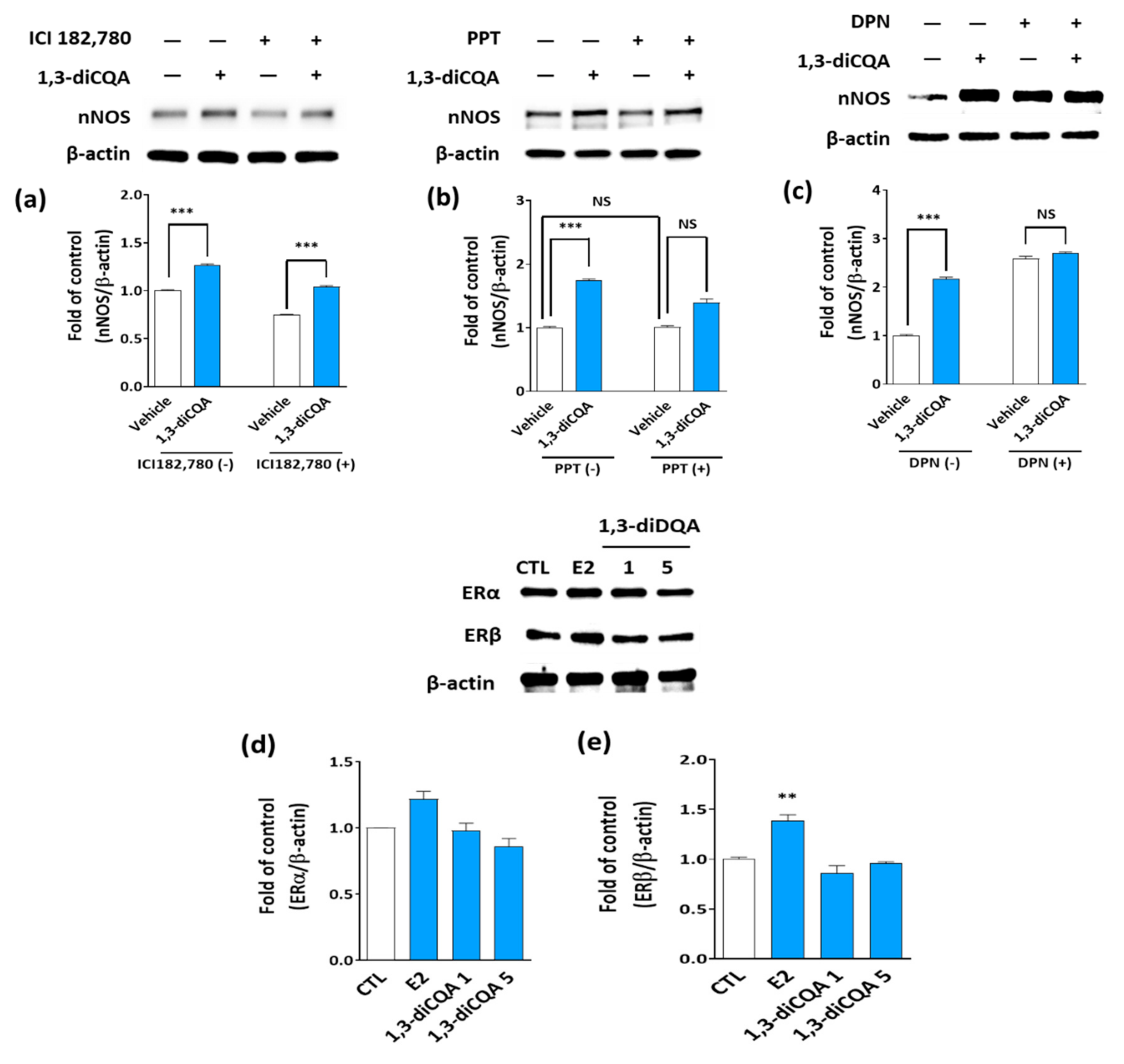
3.5. 1,3-diCQA Directly Regulates nNOS Expression without Affecting Estrogen Receptors
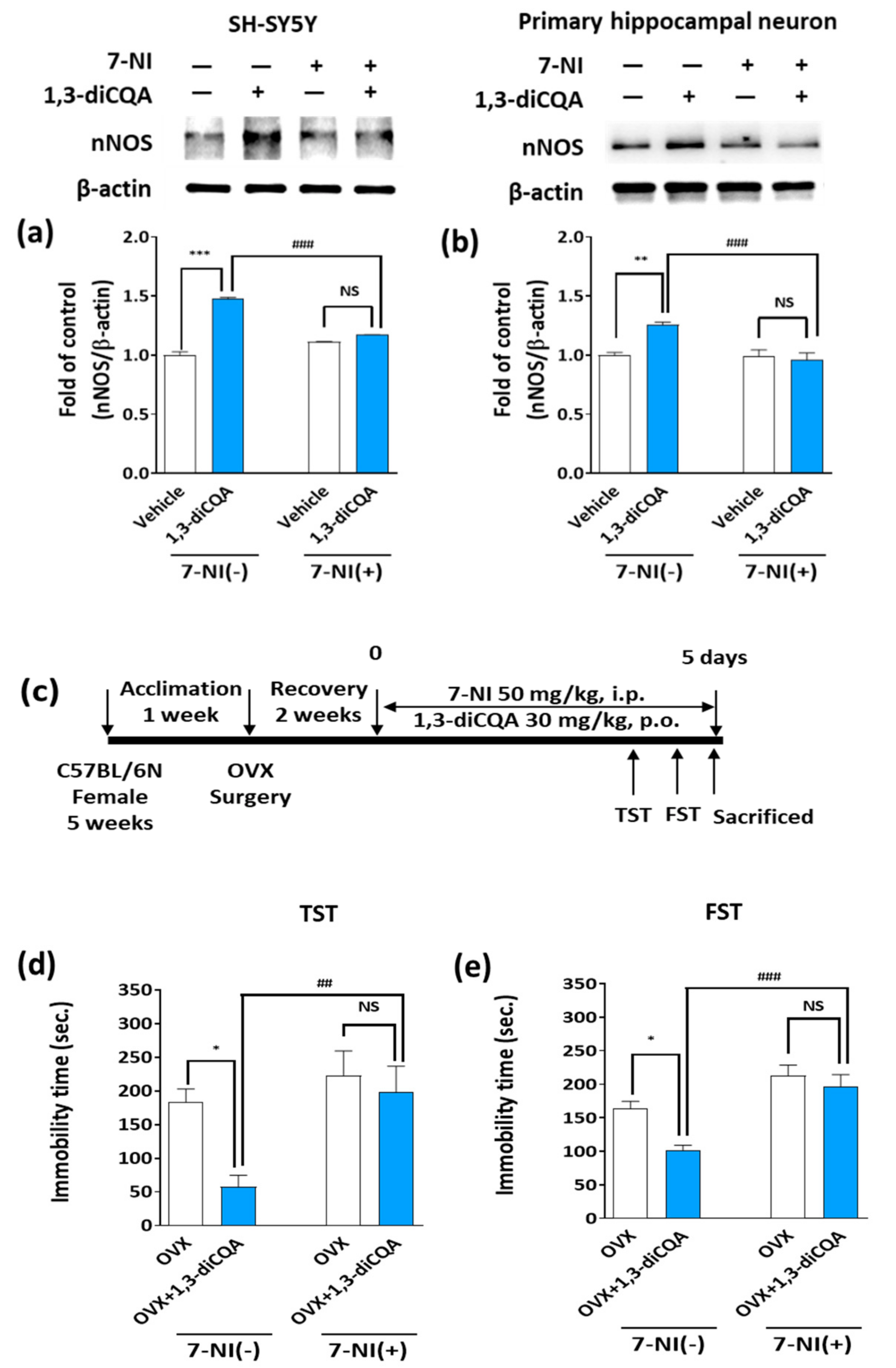
3.6. 7-NI Abolished the Behavioral Effects of 1,3-diCQA in OVX Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noble, R.E. Depression in women. Metabolism 2005, 54, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Steiner, M.; Dunn, E.; Born, L. Hormones and mood: From menarche to menopause and beyond. J. Affect. Disord. 2003, 74, 67–83. [Google Scholar] [CrossRef]
- Rasgon, N.L.; Altshuler, L.L.; Fairbanks, L.A.; Dunkin, J.J.; Davtyan, C.; Elman, S.; Rapkin, A.J. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J. Clin. Psychiatry 2002, 63, 45–48. [Google Scholar] [PubMed]
- Pickar, J.H.; Archer, D.F.; Kagan, R.; Pinkerton, J.V.; Taylor, H.S. Safety and benefit considerations for menopausal hormone therapy. Expert Opin. Drug Saf. 2017, 16, 941–954. [Google Scholar] [CrossRef]
- Toffol, E.; Heikinheimo, O.; Partonen, T. Hormone therapy and mood in perimenopausal and postmenopausal women: A narrative review. J. N. Am. Menopause Soc. 2015, 22, 564–578. [Google Scholar] [CrossRef]
- Knowles, R.G.; Palacios, M.; Palmer, R.M.; Moncada, S. Formation of nitric oxide from L-arginine in the central nervous system: A transduction mechanism for stimulation of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 1989, 86, 5159–5162. [Google Scholar] [CrossRef] [Green Version]
- Harkin, A.; Connor, T.J.; Burns, M.P.; Kelly, J.P. Nitric oxide synthase inhibitors augment the effects of serotonin re-uptake inhibitors in the forced swimming test. Eur. Neuropsychopharmacol. 2004, 14, 274–281. [Google Scholar] [CrossRef]
- Dhir, A.; Kulkarni, S.K. Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur. J. Pharmacol. 2007, 568, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kaster, M.P.; Ferreira, P.K.; Santos, A.R.S.; Rodrigues, A.L.S. Effects of potassium channel inhibitors in the forced swimming test: Possible involvement of L-arginine-nitric oxide-soluble guanylate cyclase pathway. Behav. Brain Res. 2005, 165, 204–209. [Google Scholar] [CrossRef]
- Karolewicz, B.; Szebeni, K.; Stockmeier, C.A.; Konick, L.; Overholser, J.C.; Jurjus, G.; Roth, B.L.; Ordway, G.A. Low nNOS protein in the locus coeruleus in major depression. J. Neurochem. 2004, 91, 1057–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicinelli, E.; Ignarro, L.J.; Matteo, M.G.; Galantino, P.; Schonauer, L.M.; Falco, N. Effects of estrogen replacement therapy on plasma levels of nitric oxide in postmenopausal women. Am. J. Obstet. Gynecol. 1999, 180, 334–339. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, D.L.; Luo, C.X.; Zhu, L.J.; Zhang, J.; Wu, H.Y.; Zhu, D.Y. Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 14224–14229. [Google Scholar] [CrossRef] [Green Version]
- Gingerich, S.; Krukoff, T.L. Estrogen modulates endothelial and neuronal nitric oxide synthase expression via an estrogen receptor beta-dependent mechanism in hypothalamic slice cultures. Endocrinology 2005, 146, 2933–2941. [Google Scholar] [CrossRef]
- Dhir, A.; Kulkarni, S.K. Nitric oxide and major depression. Nitric Oxide 2011, 24, 125–131. [Google Scholar] [CrossRef]
- Rodriguez, J.M.F.; de Souza, A.R.C.; Kruger, R.L.; Bombardelli, M.C.M.; Machado, C.S.; Corazza, M.L. Kinetics, composition and antioxidant activity of burdock (Arctium lappa) root extracts obtained with supercritical CO2 and co-solvent. J. Supercrit. Fluids 2018, 135, 25–33. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Yu, J.; Li, Y.B.; Wang, L.; Hu, L.; Zhang, L.; Zhou, Y.H. Extraction and antioxidant activities of polysaccharides from roots of Arctium lappa L. Int. J. Biol. Macromol. 2019, 123, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Skowronska, W.; Granica, S.; Dziedzic, M.; Kurkowiak, J.; Ziaja, M.; Bazylko, A. Arctium lappa and Arctium tomentosum, Sources of Arctii radix: Comparison of Anti-Lipoxygenase and Antioxidant Activity as well as the Chemical Composition of Extracts from Aerial Parts and from Roots. Plants 2021, 10, 78. [Google Scholar] [CrossRef]
- Liu, J.Y.; Cai, Y.Z.; Wong, R.N.S.; Lee, C.K.F.; Tang, S.C.W.; Sze, S.C.W.; Tong, Y.; Zhang, Y.B. Comparative Analysis of Caffeoylquinic Acids and Lignans in Roots and Seeds among Various Burdock (Arctium lappa) Genotypes with High Antioxidant Activity. J. Agric. Food Chem. 2012, 60, 4067–4075. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.; Alharbi, A.J.; Oraif, A. The Role of Progestogens in Threatened and Idiopathic Recurrent Miscarriage. Int. J. Womens Health 2020, 12, 253. [Google Scholar] [CrossRef] [Green Version]
- Gentil, M.; Pereira, J.V.; Sousa, Y.T.C.S.; Pietro, R.; Neto, M.D.S.; Vansan, L.P.; Franca, S.D. In vitro evaluation of the antibacterial activity of Arctium lappa as a phytotherapeutic agent used in intracanal dressings. Phytother. Res. 2006, 20, 184–186. [Google Scholar] [CrossRef]
- Lin, S.C.; Chung, T.C.; Lin, C.C.; Ueng, T.H.; Lin, Y.H.; Lin, S.Y.; Wang, L.Y. Hepatoprotective effects of Arctium lappa on carbon tetrachloride- and acetaminophen-induced liver damage. Am. J. Chin. Med. 2000, 28, 163–173. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Baggio, C.H.; Freitas, C.S.; Lepieszynski, J.; Mayer, B.; Twardowschy, A.; Missau, F.C.; dos Santos, E.P.; Pizzolatti, M.G.; Marques, M.C.A. Gastroprotective activity of the chloroform extract of the roots from Arctium lappa L. J. Pharm. Pharmacol. 2008, 60, 795–801. [Google Scholar] [CrossRef]
- Tian, X.; Sui, S.; Huang, J.; Bai, J.P.; Ren, T.S.; Zhao, Q.C. Neuroprotective effects of Arctium lappa L. roots against glutamate-induced oxidative stress by inhibiting phosphorylation of p38, JNK and ERK 1/2 MAPKs in PC12 cells. Environ. Toxicol. Pharmacol. 2014, 38, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Haghi, G.; Hatami, A.; Mehran, M. UPLC and HPLC of caffeoyl esters in wild and cultivated Arctium lappa L. Food Chem. 2013, 138, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules 2018, 23, 999. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.W.; Park, J.; Jung, J.; Kim, S.H.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Han, D.; Lee, C.; Lee, J. Dicaffeoylquinic acids alleviate memory loss via reduction of oxidative stress in stress-hormone-induced depressive mice. Pharmacol. Res. 2020, 161, 105252. [Google Scholar] [CrossRef]
- Edwards, S.E.; Rocha, I.; Williamson, E.M.; Heinrich, M. Phytopharmacy: An Evidence-Based Guide to Herbal Medical Products; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.W.; Park, J.; Han, D.; Lee, J.; Kim, Y.T.; Lee, C. Anti-Inflammatory Effects of Asian Fawn Lily (Erythronium japonicum) Extract on Lipopolysaccharide-Induced Depressive-Like Behavior in Mice. Nutrients 2020, 12, 3809. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, T.; Jung, J.; Song, Y.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Cho, S.; Kim, I.H.; Han, D.; et al. Chlorogenic Acid from Hawthorn Berry (Crataegus pinnatifida Fruit) Prevents Stress Hormone-Induced Depressive Behavior, through Monoamine Oxidase B-Reactive Oxygen Species Signaling in Hippocampal Astrocytes of Mice. Mol. Nutr. Food Res. 2018, 62, 1800029. [Google Scholar] [CrossRef]
- Mu, H.Y.; Wang, Z.F.; Zhang, X.J.; Qian, D.M.; Wang, Y.Y.; Jiang, S.S.; Liang, S.Z.; Wang, B. HCMV-encoded IE2 induces anxiety-depression and cognitive impairment in UL122 genetically-modified mice. Int. J. Clin. Exp. Pathol. 2019, 12, 4087–4095. [Google Scholar]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Vidaurre, O.G.; Adula, K.P.; Kezunovic, N.; Wentling, M.; Huntley, G.W.; Casaccia, P. Subcellular Distribution of HDAC1 in Neurotoxic Conditions Is Dependent on Serine Phosphorylation. J. Neurosci. 2017, 37, 7547–7559. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Hong, J.; Zhang, T.T.; Wu, J.; Chen, L. Activation of Sigma-1 Receptor Alleviates Postpartum Estrogen Withdrawal-Induced “Depression” through Restoring Hippocampal nNOS-NO-CREB Activities in Mice. Mol. Neurobiol. 2017, 54, 3017–3030. [Google Scholar] [CrossRef]
- MacQueen, G.; Frodl, T. The hippocampus in major depression: Evidence for the convergence of the bench and bedside in psychiatric research? Mol. Psychiatry 2011, 16, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.Y.; Zhang, Y.Q.; Qiao, M.Q. Mechanisms of extracellular signal-regulated kinase/cAMP response element-binding protein/brain-derived neurotrophic factor signal transduction pathway in depressive disorder. Neural Regen. Res. 2013, 8, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Birkhauser, M. Depression, menopause and estrogens: Is there a correlation? Maturitas 2002, 41, S3–S8. [Google Scholar] [CrossRef]
- Okada, M.; Hayashi, N.; Kometani, M.; Nakao, K.; Inukai, T. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn. J. Pharmacol. 1997, 73, 93–96. [Google Scholar] [CrossRef]
- Rocha, B.A.; Fleischer, R.; Schaeffer, J.M.; Rohrer, S.P.; Hickey, G.J. 17 beta-Estradiol-induced antidepressant-like effect in the Forced Swim Test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology 2005, 179, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Heydarpour, P.; Salehi-Sadaghiani, M.; Javadi-Paydar, M.; Rahimian, R.; Fakhfouri, G.; Khosravi, M.; Khoshkish, S.; Gharedaghi, M.H.; Ghasemi, M.; Dehpour, A.R. Estradiol reduces depressive-like behavior through inhibiting nitric oxide/cyclic GMP pathway in ovariectomized mice. Horm. Behav. 2013, 63, 361–369. [Google Scholar] [CrossRef]
- Joca, S.R.; Guimaraes, F.S. Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology 2006, 185, 298–305. [Google Scholar] [CrossRef]
- Lopez-Jaramillo, P.; Teran, E. Improvement in functions of the central nervous system by estrogen replacement therapy might be related with an increased nitric oxide production. J. Endothel. Cell Res. 1999, 6, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Gass, P.; Riva, M.A. CREB, neurogenesis and depression. Bioessays 2007, 29, 957–961. [Google Scholar] [CrossRef]
- Nibuya, M.; Nestler, E.J.; Duman, R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996, 16, 2365–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leem, Y.H.; Yoon, S.S.; Kim, Y.H.; Jo, S.A. Disrupted MEK/ERK signaling in the medial orbital cortex and dorsal endopiriform nuclei of the prefrontal cortex in a chronic restraint stress mouse model of depression. Neurosci. Lett. 2014, 580, 163–168. [Google Scholar] [CrossRef]
- Chen, M.J.; Russo-Neustadt, A.A. Running Exercise-Induced Up-Regulation of Hippocampal Brain-Derived Neurotrophic Factor Is CREB-Dependent. Hippocampus 2009, 19, 962–972. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Lin, W.; Li, J.; Li, H.; Wang, W.; Wang, D.; Sun, M. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol. Dis. 2008, 31, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Altar, C.A. Neurotrophins and depression. Trends Pharmacol. Sci. 1999, 20, 59–61. [Google Scholar] [CrossRef]
- Ding, Q.; Li, H.; Tian, X.; Shen, Z.; Wang, X.; Mo, F.; Huang, J.; Shen, H. Zinc and imipramine reverse the depression-like behavior in mice induced by chronic restraint stress. J. Affect. Disord. 2016, 197, 100–106. [Google Scholar] [CrossRef]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef]
- Xu, Y.J.; Sheng, H.; Bao, Q.Y.; Wang, Y.J.; Lu, J.Q.; Ni, X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav. Immun. 2016, 56, 175–186. [Google Scholar] [CrossRef]
- Spiteri, T.; Musatov, S.; Ogawa, S.; Ribeiro, A.; Pfaff, D.W.; Agmo, A. The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav. Brain Res. 2010, 210, 211–220. [Google Scholar] [CrossRef]
- Sasayama, D.; Sugiyama, N.; Yonekubo, S.; Pawlak, A.; Murasawa, H.; Nakamura, M.; Hayashi, M.; Ogawa, T.; Moro, M.; Washizuka, S.; et al. Novel oestrogen receptor beta-selective ligand reduces obesity and depressive-like behaviour in ovariectomized mice. Sci. Rep. 2017, 7, 4663. [Google Scholar] [CrossRef] [Green Version]
- Benmansour, S.; Privratsky, A.A.; Adeniji, O.S.; Frazer, A. Signaling mechanisms involved in the acute effects of estradiol on 5-HT clearance. Int. J. Neuropsychopharmacol. 2014, 17, 765–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, D.; Pesaresi, M.; Maschi, O.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Effect of Short-and Long-Term Gonadectomy on Neuroactive Steroid Levels in the Central and Peripheral Nervous System of Male and Female Rats. J. Neuroendocrinol. 2010, 22, 1137–1147. [Google Scholar] [CrossRef]
- Brinton, R.D. Minireview: Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology 2012, 153, 3571–3578. [Google Scholar] [CrossRef] [PubMed]
- Kermath, B.A.; Riha, P.D.; Woller, M.J.; Wolfe, A.; Gore, A.C. Hypothalamic Molecular Changes Underlying Natural Reproductive Senescence in the Female Rat. Endocrinology 2014, 155, 3597–3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyer, P.B.; Devine, P.J.; Hu, X.M.; Thompson, K.E.; Sipes, I.G. Ovarian toxicity of 4-vinylcyclohexene diepoxide: A mechanistic model. Toxicol. Pathol. 2001, 29, 91–99. [Google Scholar] [CrossRef]


| Antibody | Source | Identifier | Dilution |
|---|---|---|---|
| Mouse monoclonal anti-β-actin | Santa Cruz | Cat# sc-47778 | 1:1000 |
| Rabbit monoclonal anti-CREB | Cell signaling technology | Cat# 9197S | 1:1000 |
| Rabbit monoclonal anti-p-CREB | Cell signaling technology | Cat# 9198S | 1:1000 |
| Rabbit monoclonal anti-p44/42 MAPK (Erk1/2) | Cell signaling technology | Cat# 4695S | 1:1000 |
| Rabbit monoclonal anti-p-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | Cell signaling technology | Cat# 4377S | 1:1000 |
| Rabbit monoclonal anti-TrkB | Cell signaling technology | Cat# 4603S | 1:1000 |
| Rabbit polyclonal anti-p-TrkB (Tyr816) | Millipore | Cat# ABN1381 | 1:500 |
| Rabbit monoclonal anti-nNOS | Abcam | Cat# ab76067 | 1:1000 |
| Rabbit polyclonal anti-ERβ | Abcam | Cat# ab3567 | 1:1000 |
| Rabbit monoclonal anti-BDNF | Abcam | Cat# ab108319 | 1:1000 |
| Anti-Neurofilament heavy polypeptide antibody | Abcam | Cat# ab4680 | 1:1000 |
| Alexa 488-conjugated Goat anti-Rabbit IgG | Abcam | Cat# ab150077 | 1:200 |
| Alexa 555-conjugated Goat anti-Chicken IgG | Abcam | Cat# ab150170 | 1:200 |
| Goat Anti-Rabbit IgG Antibody (H+L), Peroxidase | Vector laboratories | Cat# PI-1000 | 1:5000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.W.; Kim, M.; Yoon, M.; Lee, J.; Lee, C.; Um, M.Y. 1,3-Dicaffeoylquinic Acid as an Active Compound of Arctium lappa Root Extract Ameliorates Depressive-Like Behavior by Regulating Hippocampal Nitric Oxide Synthesis in Ovariectomized Mice. Antioxidants 2021, 10, 1281. https://doi.org/10.3390/antiox10081281
Lim DW, Kim M, Yoon M, Lee J, Lee C, Um MY. 1,3-Dicaffeoylquinic Acid as an Active Compound of Arctium lappa Root Extract Ameliorates Depressive-Like Behavior by Regulating Hippocampal Nitric Oxide Synthesis in Ovariectomized Mice. Antioxidants. 2021; 10(8):1281. https://doi.org/10.3390/antiox10081281
Chicago/Turabian StyleLim, Dong Wook, Minji Kim, Minseok Yoon, Jaekwang Lee, Changho Lee, and Min Young Um. 2021. "1,3-Dicaffeoylquinic Acid as an Active Compound of Arctium lappa Root Extract Ameliorates Depressive-Like Behavior by Regulating Hippocampal Nitric Oxide Synthesis in Ovariectomized Mice" Antioxidants 10, no. 8: 1281. https://doi.org/10.3390/antiox10081281
APA StyleLim, D. W., Kim, M., Yoon, M., Lee, J., Lee, C., & Um, M. Y. (2021). 1,3-Dicaffeoylquinic Acid as an Active Compound of Arctium lappa Root Extract Ameliorates Depressive-Like Behavior by Regulating Hippocampal Nitric Oxide Synthesis in Ovariectomized Mice. Antioxidants, 10(8), 1281. https://doi.org/10.3390/antiox10081281







