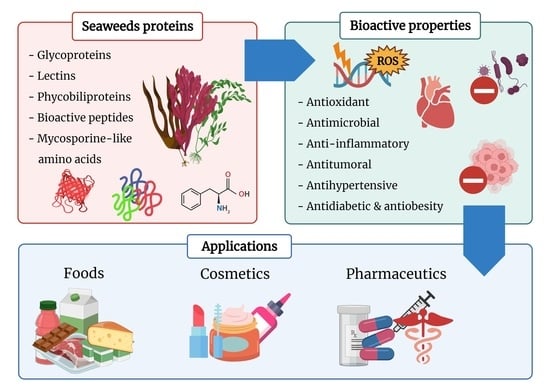
Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives
Abstract
1. Introduction
| Species | Protein (% dw) | AA Composition (g/100 g Protein) | Free AA (mg/g) | EAA (%TAA) | Ref. |
|---|---|---|---|---|---|
| Rhodophyceae | |||||
| Palmaria palmata | 12.5 | 4.7 Thr, 6.1 Val, 3.6 Iso, 5.9 Leu, 0.5 Tyr, 3.8 Phe, 4.6 His, 5.6 Lys, 2.7 Met, 4.1 Cya, 3 Tau, 10.2 Asp, 5 Ser, 15.5 Glu, 5.8 Gly, 6.3 Ala, 2.1 Cys, 6 Arg, 4.4 Pro. | 112.18 | 37.7 | [14] |
| 16.2 | 7.6 Ala, 6.8 Arg, 12.5 Asp, 12.3 Glu, 6.5 Gly, 1.6 His, 4 Ile, 7 Leu, 7.7 Lys, 2.2 Met, 5 Phe, 5.5 Pro, 6 Ser, 5.3 Thr, 3.4 Tyr, 6.6 Val | 124 | 24.8 | [15] | |
| Porphyra dioica | 28.7 | 3.3 Asp, 3.1 Glu, 3 Ala, 2.3 Arg, 1.8 Gly, 1.6 Ser, 1 Tyr, 0.9 Pro, 1.1 Phe, 0.6 His, 1.1 Ile, 2.2 Leu, 2.2 Lys, 0.5 Met, 1.2 Thr, 1.2 Val | 286.6 | 39.8 | [3] |
| Porphyra purpurea | 33.2 | 6.6 Asp, 4.6 Ser, 8.3 Glu, 7.5 Gly, 2.2 His, 9 Arg, 5 Thr, 8 Ala, 3.8 Pro, 1.3 Met, 0.4 Cys, 4.8 Val, 2.3 Lys, 3.4 Ile, 5.3 Leu, 7.8 Phe, 2.9 Tyr | n.a. | 41.0 | [16] |
| Pyropia columbina | 24.6 | 12.2 Asp, 10.5 Glu, 6.1 Ser, 1.2 His, 8.8 Gly, 5.9 Thr, 6.1 Arg, 12.5 Ala, 3.9 Pro, 2.5 Tyr, 3.7 Phe, 5.8 Val, 1.6 Met, 1.9 Cys, 2.7 Ile, 0.6 Trp, 7.3 Leu, 6 Lys | n.a. | 35.0 | [10] |
| Chondrus crispus | 35.2 | 5.5 Thr, 6.2 Val, 4.5 Ile, 6.9 Leu, 2.7 Tyr, 4.3 Phe, 2.1 His, 5.3 Lys, 3.3 Met, 2.9 Cya, 1.2 Tau, 12 Asp, 5.1 Ser, 12.1 Glu, 5.2 Gly, 7.5 Ala, 0.7 Cys, 6.5 Arg, 5.6 Pro | 226.2 | 40.9 | [14] |
| 19.5 | 3.6 Asp, 3.1 Glu, 4.6 Ser, 1.4 Thr, 1.8 His, 0.3 Gln, 0.3 Tau, 2.8 Arg, 0.4 Ala, 1.4 Tyr, 3.4 Lys, 2 Val, 0.2 Met, 1.5 Phe, 1.6 Ile, 2 Leu, 1.5 Hyp | 72.8 | 46.7 | [17] | |
| Osmundea pinnatifida | 24.3 | 4.8 Asp, 4.5 Glu, 3.9 Ser, 2.7 Thr, 3.7 His, 0.3 Gly, 0.1 Gln, 0.4 Tau, 2.4 Arg, 1.1 Ala, 1.7 Tyr, 3 Lys, 2.6 Val, 0.5 Met, 1.5 Phe, 2.1 Ile, 2.5 Leu, 1 Hyp | 66.9 | 47.9 | [17] |
| 20.7 | 13.6 Asp, 12.1 Glu, 2.7 Ser, 2.5 Gly, 0.9 His, 3.7 Arg, 5.7, Thr, 0.7 Ala, 15.8 Pro, 2 Tyr, 2.2 Val, 1.9 Met, 16.5 Ile, 2.2 Phe, 2.7 Lys | n.a. | 41.6 | [7] | |
| Gracilaria chilensis | 13.7 | 1.1 Asp, 1.5 Glu, 0.7 Ser, 1.1 His, 0.4 Gly, 0.6 Thr, 0.6 Arg, 0.6 Ala, 0.3 Tyr, 0.7 Val, 1.8 Met, 0.7 Cys, 0.8 Ile, 0.4 Leu, 1 Phe, 0.6 Lys | n.a. | 42.8 | [18] |
| Gracilaria gracilis | 18.7 | 1.4 Arg, 0.1 His, 1.3 Lys, 1 Thr, 0.9 Ile, 1.2 Leu, 1 Val, 0.3 Met, 0.9 Phe, 0.9 Pro, 1.2 Ala, 0.6 Tyr, 2.1 Asp, 2.5 Glu, 1.3 Gly, 1.2 Ser | n.a. | 45.6 | [19] |
| Gelidium corneum | 21 | 1.9 Ala, 0.8 Gly, 1.4 Val, 1.6 Leu, 0.9 Ile, 0.7 Thr, 0.8 Ser, 1.5 Pro, 2 Asp, 0.1 Met, 1.6 Glu, 1 Phe, 1.2 Lys, 0.3 His, 0.7 Tyr | n.a. | 44.1 | [20] |
| Phaeophyceae | |||||
| Sargasum maclurei | 8.4 | 3.6 Thr, 6.2 Leu, 3.7 Ile, 4 Phe, 4.1 Lys, 1.3 Met, 2.1 Tyr, 2.3 Trp, 8.2 Asp, 29.7 Glu, 3.5 Cys, 1.3 His, 4.2 Gly, 3.9 Pro, 7.9 Ala, 3.8 Arg | 74.9 | 27.8 | [21] |
| Fucus vesiculosus | 12.9 | 6.1 Thr, 5.8 Val, 2.1 Met, 5 Ile, 8.6 Leu, 5.4 Phe, 8 Lys, 1.9 His, 5.5 Arg, 3.2 Tyr, 16.7 Asn, 6.3 Ser, 19.7 Glu, 6.5 Gly, 9.8 Ala, 5.7 Pro, 2 Cys | 119 | 40.9 | [22] |
| Fucus spiralis | 11.8 | 5.2 Arg, 7.2 Glu, 5.5 Ser, 2.7 Thr, 1.6 His, 0.1 Gln, 0.7 Tau, 1.5 Arg, 0.7 Ala, 3.7 Lys, 2.2 Val, 0.2 Met, 0,1 Trp, 1.2 Phe, 1,9 Ile, 1.8 Hyp | 130 | 38.7 | [17] |
| 9.7 | 5.6 Asp, 12.1 Glu, 11.4 Ser, 7.4 Gly, 3.2 His, 11.7 Arg, 10.8 Thr, 4 Ala, 6.9 Pro, 7.7 Tyr, 11.4 Val, 6.3 Met, 15.4 Leu, 15.3 Ile, 9.8 Phe, 12.5 Lys | n.a. | 63.5 | [7] | |
| Ascophylum nodosum | 4.5 | 6.9 Ala, 4.4 Arg, 16 Asp, 16.3 Glu, 5.9 Gly, 1.4 His, 4.4 Ile, 7.5 Leu, 5.5 Lys, 2 Met, 5.3 Phe, 4.5 Pro, 5.4 Ser, 5.8 Thr, 2.9 Tyr, 6 Val | 35 | 29.2 | [15] |
| 9.4 | 4.1 Asp, 7.2 Glu, 3.9 Ser, 1.9 Thr, 1.1 His, 0.7 Tau, 1.7 Arg, 1.5 Ala, 0.9 Tyr, 3.3 Lys, 1.9 Val, 0.4 Met, 0.1 Trp, 1.2 Phe, 1.6 Ile, 2.3 Leu, 1.6 Hyp | 133 | 39.2 | [17] | |
| Saccharina latissima | 12 | 11 Ala, 4.8 Arg, 13.4 Asp, 13.8 Glu, 5.6 Gly, 1.6 His, 4.4 Ile, 7.9 Leu, 5.9 Lys, 2.4 Met, 5.2 Phe, 4.5 Pro, 5 Ser, 5.3 Thr, 3.1 Tyr, 6 Val | 98 | 32.7 | [15] |
| Bifurcaria bifurcata | 8.9 | 3.6 Thr, 3.7 Val, 1.7 Met, 2.9 Iso, 5.2 Leu, 3.3 Phe, 3.9 Lys, 1.3 His, 3.3 Arg, 1.7 Tyr, 8 Asn, 3.5 Ser, 15 Glu, 3.9 Gly, 8.4 Ala, 3.1 Pro | 73.2 | 39.9 | [22] |
| Undaria pinnatifida | 16.5 | 4.3 Asp, 7.6 Glu, 5.8 Ser, 2.4 Thr, 1.4 His, 0.2 Gly, 0.6 Tau, 2.7 Arg, 3.4 Ala, 1.5 Tyr, 2.8 Lys, 2.5 Val, 0.7 Met, 1.7 Phe, 2 Ile, 3 Leu, 0.9 Hyp | 64.7 | 37.2 | [17] |
| 16.8 | 7.5 Asp, 4.1 Ser, 12 Glu, 6.5 Gly, 1.7 His, 8.8 Arg, 2.9 Thr, 9.7 Ala, 4.4 Pro, 0.1 Met, 0.3 Cys, 5.8 Val, 3.9 Lys, 5 Ile, 8.6 Leu, 4.8 Phe, 2 Tyr | n.a. | 23.4 | [16] | |
| Chlorophyceae | |||||
| Ulva spp. | 23.3 | 6.1 Asp, 5.2 Glu, 7.8 Ser, 2 Thr, 3.3 His, 0.2 Gly, 0.2 Gln, 0.3 Tau, 3.7Arg, 1.1 Ala, 1.9 Tyr, 3.2 Lys, 3.5 Val, 0.6 Met, 0.1 Trp, 2.4 Phe, 2.6 Ile, 3.5 Leu, 1.4 Hyp | 116.2 | 43.6 | [17] |
| Ulva rigida | 17.4 | 3.1 Ile, 5.2 Leu, 3.7 Lys, 1.5 Met, 1.1 Cys, 3.3 Phe, 2.2 Tyr, 5 Thr, 5.6 Val, 1.4 His, 13 Asp, 9.4 Glu, 4.3 Pro, 6.1 Ser, 7.8 Gly, 12.3 Ala, 4.6 Arg | n.a. | 30.8 | [9] |
| 9.6 | 12.5 Asp, 9.4, 8.4 Ala, 6 Arg,6 Gly, 5.5 Ser, 3.2 Tyr, 4.4 Pro, 1 Hyp, 5.7 Phe, 2.9 His, 4.4 Ile, 7.8 Leu, 4.7 Lys, 1.3 Met, 4.8 Thr, o.4 Trp, 6.8 Val | 4.9 | 40.8 | [3] | |
| Ulva lactuca | 16.4 | 3.7 Ile, 6.7 Leu, 4.2 Lys, 1.6 Met, 0.4 Cys, 4 Phe, 2.1 Tyr, 4.7 Thr, 6.2 Val, 1.8 His, 12.3 Asp, 9 Glu, 5.3 Pro, 5.9 Ser, 10.7 Gly, 14.2 Ala, 3.6 Arg | n.a. | 33.6 | [9] |
| 15 | 8.4 Ala, 6.4 Arg, 12.1 Asp, 13.5 Glu, 6.4 Gly, 1.8, His, 4.2 Ile, 8 Leu, 5.5 Lys, 2.2 Met, 5.6 Phe, 4.7 Pro, 5.5 Ser, 5.5 Thr, 3.5 Tyr, 6.4 Val | 106.1 | 36.9 | [15] | |
| Ulva capensis | 17.3 | 3.5 Ile, 6-8 Leu, 3.7 Lys, 1.5 Met, 4 Phe, 2 Tyr, 5 Thr, 6.3 Val, 1.7 His, 17.2 Asp, 10.9 Glu, 3.6 Pro, 6.4 Ser, 8.8 Gly, 11.8 Ala, 3.3 Arg | n.a. | 32.7 | [9] |
| Codium fragile | 10.8 | 0.8 Asp, 1 Glu, 0.5 Ser, 0.09 His, 0.5 Gly, 0.5 Thr, 0.4 Arg, 0.6 Ala, 0.3 Tyr, 1.4 Val, 0.9 Met, 0.1 Cys, 0.4 Ile, 0.7 Leu, 0.4 Phe, 0.5 Lys | n.a. | 44.7 | [18] |
| Cladophora rupestris | 12 | 5.5 Ala, 6.5 Arg, 15.3 Asp, 15.3 Glu, 6.7 Gly, 1.4 His, 3.6 Ile, 7 Leu, 7.4 Lys, 1.8 Met, 4.5 Phe, 5.7 Pro, 4.3 Ser, 5.1 Thr, 4.3 Tyr, 5.8 Val | 95.9 | 38.1 | [15] |
2. Seaweed Proteins and Derived Peptides
2.1. Glycoproteins
2.2. Lectins
2.3. Phycobiliproteins
2.4. Mycosporine-Like Amino Acids
2.5. Protein-Derived Hydrolysates and Peptides
3. Bioactive Properties and Mechanisms of Action
3.1. Antioxidants
3.2. Antimicrobials and Antivirals
| Species | Protein(s) | Bioactivity | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Rhodophyceae | ||||
| Griffithsia sp. | Lectin (Griffithsin) | In vivo, antiviral | Mannose-binding lectin binds with viral envelope GPs, inhibiting viral infection of HIV-1, HSV, and HPV | [75] |
| In vitro, antiviral | Mannose-binding lectin binds with Spike GPs, inhibiting viral infection of SARS-CoV | [82] | ||
| In vitro, antiviral | Griffithsin and synthetic polymers showed high virucidal activity in cell-associated HIV-1 and in the presence of seminal and vaginal simulants. Blocking of CD4+ viral binding | [83] | ||
| Grateloupia chiangii | Lectin | In vitro, antiviral | Mannose-binding lectin binds with viral envelope GPs, inhibiting viral infection of Influenza H1N1 and HSV1/2 | [79] |
| Kappaphyrus alvarezii | Lectin | In vitro, antiviral | Mannose-binding lectin binds to Influenza hemagglutinin and HIV GP gp120, avoiding infection | [84] |
| Kappaphycus striatum | Lectin | In vitro, antimicrobial | Growth inhibition of Vibrio alginolyticus (25.4 μg/mL) and Enterococcus cloacae (101.6 μg/mL). Did not inhibit other Vibrio spp., Staphylococcus aureus, or Escherichia coli | [85] |
| Gracilaria fisheri | Lectin | In vivo, antimicrobial | There was >50% mortality reduction in 100 μg/mL treated shrimp, related with agglutination of Vibrio parahaemolyticus | [25] |
| Hydropuntia cornea | PBPs | In vitro, antifungal | Dosage of 0.3 mg/mL inhibited Botrytis cinerea growth (33%) and spore germination (80%) | [80] |
| Neopyropia yezoensis | GP | In vitro, anti-inflammatory | Inhibition of TLR4 and ERK1/2 activation, inhibition of NF-κB release | [86] |
| Amansia multifida | Lectin | In vivo, anti-inflammatory | Paw edema reduction against several pro-inflammatory agents. Reduction in TNF-α, IL-1β levels, and neutrophil migration. Increased glutathione levels | [87] |
| Eucheuma cottonii | Lectin-rich extract | In vivo, anti-inflammatory | Decreased mucin synthesis, leucocyte infiltration, and TNF-α, NF-κB, IL-4, MMP-9, EGFR levels in asthmatic rats | [88] |
| Briothamniun triquetum | Lectin | In vivo, anti-inflammatory | Reduced peritonitis and paw edema in treated mice. Reduction in TNF-α, IL-1β, MPO levels, and neutrophil migration. Better results than the positive control | [42] |
| Soliera filiformis | Lectin | In vitro, antitumor | Apoptosis induction. Bcl-2 downregulation, upregulation of caspases 3,8,9 | [89] |
| Gracilaria lemaneiformis | PE | In vitro, antitumor | Apoptosis induction. Increased caspase 3/9 and p53 expression | [90] |
| Portieria hornemannii | R-PE | In vitro, antitumor | Apoptosis induction. Cell cycle arrest at G2/M phase, membrane blebbing, and >80% cell deaths at 1 mg/mL | [91] |
| Eucheuma serra | Lectin | In vitro, antitumor | Apoptosis induction. Increased caspase-3 expression, binding to mannose in human sarcoma cells. Complete cancer cell death at 2 μg/mL, but not in normal cells | [92] |
| Bryothamnion seaforthii | Lectin | In vivo, wound healing | Induced greater inflammatory response, fibroblast proliferation, and collagen synthesis. Reduced wound size and faster closure in treated mice | [93] |
| Phaeophyceae | ||||
| Sargassum fusiforme | Lectin | In vitro, antioxidant | Dosage of 1.6 mg/mL inhibited 77.23% DPPH; 4 mg/mL inhibited 68.97% ABTS | [40] |
| Saccharina japonica | GP | In vitro, antioxidant | DPPH (IC50 = 0.12 mg/mL), ABTS (IC50 = 0.05 mg/mL), FRAP (IC50 = 0.3 mg/mL) and xanthine oxidase (70%, 0.2 mg/mL) inhibition | [94] |
| Undaria pinnatifida | GP | In vitro, antidiabetic | Rat intestinal (IC50 = 0.29 mg/mL) and yeast (IC50 = 0.11 mg/mL) α-glucosidase inhibition. Retained >60% of inhibitory activity after thermal treatments | [95] |
| Chlorophyceae | ||||
| Codium isthmocladum | 2 lectins | In vitro, antibiofilm | Binding to monosaccharide residues of the biofilms with preference to α-linkages | [71] |
| Halimeda renschii | Lectin | In vitro, antiviral | High mannose-binding lectin, bonded with Influenza H3N2 viral envelope hemagglutinin, preventing infection | [96] |
| Boodlea coacta | Lectin | In vitro, antiviral | High mannose-binding lectin binds to Influenza hemagglutinin and HIV-1 GP gp120, avoiding infection | [97] |
| Caulerpa cupressoides | Lectin | In vivo, anti-inflammatory and antinociceptive | Analgesic effect as measured by paw licking time. Reduced neutrophil infiltration (65.9%), better than the positive control | [98] |
| Lectin | In vivo, anti-inflammatory and antinociceptive | Reduced levels of TNF-α, IL-1β, MPO, and leukocyte infiltration | [99] | |
| Lectin | In vivo, anti-inflammatory | IL-1β, IL-6, TNF-α and COX-2. Lower neutrophil infiltration | [27] | |
| Codium decorticatum | GP | In vitro, antitumor | Apoptosis induction. Mitochondrial membrane alterations, caspase 3 and Bcl-2 genes upregulation | [35] |
3.3. Antihypertensive
| Species | Hydrolysis | Sequence/s | Bioactivity | Results | Ref. |
|---|---|---|---|---|---|
| Rhodophyceae | |||||
| Gracilariopsis lemaneiformis | Trypsin; E:S (1:25), 37 °C, pH 8, 8 h | QVEY | In vitro, antihypertensive | ACE inhibitory activity. IC50= 0.25 mg/mL | [52] |
| α-Chymotrypsin; E:S (1:25), 37 °C, pH 8, 2 h | ELWKTF | In vitro, antioxidant | DPPH radical scavenging. EC50 = 1.51 mg/mL | [58] | |
| Porphyra spp. | Pepsin; E:S (1:100), pH 2, 45 °C, 4 h | GGSK, ELS | In vitro, antidiabetic | α-Amylase inhibition. IC50(GGSK) = 0.8 mg/mL, IC50(ELS)= 0.9 mg/mL | [54] |
| Porphyra dioica | Alcalase® and Flavourzyme®; E:S (1:100), 50 °C, pH 7, 4 h | DYYLR, AGFY, YLVA, AFIT, MKTPITE, TYIA, LDLW | In vitro, antioxidant | Most antioxidant on ORAC assay: IC50(AFIT) = 0.4 μg/mL, IC50(MKTPITE) = 0.007 mg/mL | [60] |
| DYYLR, AGFY, YLVA, TYIA | In vitro, antihypertensive | Most inhibiting BAPs: IC50(TYIA) = 0.04 mg/mL, IC50(TYIA) = 0.07 mg/mL | [60] | ||
| YLVA | In vitro, antidiabetic | DPP-IV inhibition, IC50 = 0.2 mg/mL | [60] | ||
| Prolyve®; E:S (1:100), 50 °C, pH 8, 2 h | n.a., increased production of <1 kDa peptides | In vitro, antioxidant | ORAC (IC50 = 2.7 mmol TE/g), DPPH (IC50 = 0.2 mmol TE/g), FRAP (IC50 = 0.4 mmol TE/g) | [107] | |
| Neopyropia yezoensis | Chemical synthesis | IY, MKY, AKTSY, LRY | Clinical trial, antihypertensive | Blood pression reduction from 157/95 to 142/86 mmHg with 1.8 g/day for 35 days | [106] |
| Pepsin; E:S (1:40), pH 2, 45 °C, 2 h | NMEKGSSSVVSSRM | Ex vivo, anticoagulant | Blood clotting retardation; IC50 = 4.49 μg/mL | [56] | |
| Chemical synthesis | “PPY” peptide | In vitro, antitumor | Doses ≥125 ng/mL induced autophagy and apoptosis in MCF-7 cells via the mTOR pathway | [108] | |
| Chemical synthesis | “PPY” peptide | In vitro, anti-inflammatory | Doses ≥250 ng/mL inhibited expression of inflammatory cytokines in murine macrophages | [29] | |
| Pyropia columbina | Alkaline protease; E:S (1:40), 55 °C, pH 9.5, 2 h | n.a., ~2.4 kDa peptides | In vitro, antihypertensive | ACE inhibitory activity. IC50 = 1.2 mg/mL | [109] |
| In vitro, anticoagulant | Antiplatelet aggregation. 2.8 mg/mL achieved 18.7% inhibition | [109] | |||
| Trypsin; E:S (1:20), 50 °C, pH 8, 4 h | n.a., >400 Da peptides | In vitro, antioxidant and anti-inflammatory | DPPH (IC50 = 2.8 mg/mL), ABTS (IC50 = 2.4 mg/mL); upregulation of IL10 at 0.1 mg/mL | [50] | |
| Fungal protease; E:S (1:20), 55 °C, pH 4.3, 3 h and Flavourzyme®; E:S (1:50), 55 °C, pH 7, 4 h | n.a. | In vitro, anti-inflammatory | Upregulation of IL-10 in murine spenocytes, macrophages and lymphocytes at ≥0.01 mg/mL | [110] | |
| Palmaria palmata | Chymotrypsin; E:S (1:20), pH 8, 30 °C, 24 h | 33 BAPs < 10 kDa | In vitro, antioxidant and antihypertensive | A <10 kDa fraction was the most bioactive at ≥0.75 mg/mL | [55] |
| Thermolysin; E:S (1:100), 70 °C, pH 7, 3 h | LRY | In vitro, antihypertensive | ACE inhibitory IC50 = 0.01 mg/mL | [59] | |
| Papain; (20.7 U/ mg protein), 60 °C, pH 6, 24 h | IRLIIVLMPILMA | In vitro, antihypertensive | Renin inhibition. IC50 = 0.3 mg/mL | [103] | |
| Chemical synthesis | IRLIIVLMPILMA | In vivo, antihypertensive | Systolic blood pressure reduction from 187 to 155 mm Hg at 50 mg/kg bw | [104] | |
| Bangia fusco-purpurea | Pepsin; E:S (1:200), pH 7.5, 37 °C, 90 min | ALLAGDPSVLEDR, VVGGTGPVDEWGIAGAR | In vitro, antihypertensive | ACE inhibition. IC50(ALLAGDPSVLEDR) = 57.2 mg/mL, IC50(VVGGTGPVDEWGIAGAR) = 66.2 μg/mL | [102] |
| Phaeophyceae | |||||
| Saccharina longicruris | Trypsin; E:S (1:20), pH 7, 30 °C, 24 h | EAESSLTGGNGCAK, IGNGGELPR, ILVLQSNQIR, ISAILPSR, ISGLIYEETR, LPDAALNR, MALSSLPR, QVHPDTGISK, TITLDVEPSDTIDGVK | In vitro, antimicrobial | Mix of all peptides (2.50 mg/mL) inhibited 40% Staphylococcus aureus growth | [81] |
| Sargassum maclurei | Pepsin; (80 U/g protein), 37 °C, pH 2, 2 h and Papain; (60 U/g protein), 50 °C, pH 7, 3 h | RWDISQPY | In vivo and in vitro, antihypertensive | Systolic blood pressure reduction from 170 to 150 mm Hg at 100 mg/kg bw; 25% endothelin-1 inhibition at 1.5 mg/mL | [21] |
| Laminaria japonica | Alcalase®, papain, trypsin, and pepsin; 55 °C, pH 7.5. E:S and time not specified | KY, GKY, SKTY, AKY, AKY, AKYSY, KKFY, FY, KFKY | In vitro, antihypertensive | ACE inhibition. Hydrolysate of all combined proteases achieved IC50 = 0.6 mg/mL | [111] |
| Undaria pinnatifida | Hot water; 93 °C, 20 min | YH, KW, KY, KF, VW, VF, IY, IW, VY | In vivo, antihypertensive | Systolic blood pressure reduction (25–34 mm Hg) by 10 mg/kg bw for 1 week | [112] |
| Pepsin; E:S (100:1), 45 °C, pH 2, 18 h | YH, KW, KY, KF, VW, VF, IY, IW, VY | In vivo, antihypertensive | Systolic blood pressure reduction (14–21 mm Hg) by 1 mg/kg bw for 1 week | [105] | |
| Bromelain; (12 kU/g protein), 45 °C, pH 6, 4 h | KNFL | In vitro, antihypertensive | ACE inhibition. IC50 = 1.3 μg/mL | [113] | |
| Chlorophyceae | |||||
| Ulva intestinalis | Trypsin; E:S (1:25), 37 °C, pH 8, 5 h | FGMPLDR, MELVLR | In vitro, antihypertensive | ACE inhibition. IC50(FGMPLDR, MELVLR) = 0.18 mg/mL | [53] |
| Ulva rigida | Pepsin; E:S (1:100), pH 2.0, 37 °C 20 h | IP, AFL | In vitro, antihypertensive | ACE inhibition. IC50(IP, AFL) = 0.2 mg/mL | [57] |
| Ulva lactuca | Papain; E:S (1:100), 60 °C, pH 6, 24 h | 55 non-allergenic BAPs identified | In vitro, antihypertensive | ACE inhibition (93%) in >1 kDa hydrolysate fraction | [114] |
| Enteromorpha clathrata | Alcalase®; 2.9 kU/g protein, T not stated, pH 7.6, 90 min | PAFG | In vitro, antihypertensive | ACE inhibition. IC50 = 0.014 mg/mL | [115] |
| Caulerpa lentillifera | Thermolysin; E:S (1:50), 60 °C, pH 8.5, 16 h | FDGIP, AIDPVRA | In vitro, antihypertensive | ACE inhibition. IC50 (FDGIP)= 0.03 mg/mL, IC50 (AIDPVRA)= 0.04 mg/mL | [101] |
3.4. Anti-Inflammatory
3.5. Antitumoral
3.6. Anti-Diabetic and Anti-Obesity
4. Potential Applications
4.1. Seaweed Proteins
4.2. Bioactive Peptides
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SP | Seaweed proteins |
| AA | Amino acids |
| EAA | Essential amino acids |
| NEAA | Non-essential amino acids |
| BAPs | Bioactive peptides |
| GP | Glycoprotein |
| PBP | Phycobiliprotein |
| PE | Phycoerythrin |
| MAAs | Mycosporine-like amino acids |
| λmax | Absorption maxima |
| ORAC | Oxygen radical absorption capacity |
| FRAP | Ferric reducing antioxidant power |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| UV | Ultraviolet |
| ACE | Angiotensin-I converter enzyme |
| ROS | Reactive oxygen species |
| HIV | Human immunodeficiency virus |
| HSV | Herpes simplex virus |
| HPV | Human papilloma virus |
| SARS-CoV | Severe acute respiratory syndrome-coronavirus |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| COX-2 | Cyclooxygenase-2 |
| NO | Nitric oxide synthase |
| iNOS | Nitric oxide |
| TLR | Toll-like receptors |
| TGF-β | Transforming growth factor-β |
| NF-κB | Nuclear factor κb |
| MAPK | Mitogen-activated protein kinase |
| LPS | Lipopolysaccharides |
| MPO | Myeloperoxidase |
| HO-2 | Hemeoxygenase-2 |
| JNK | Jun N-terminal kinase |
| ERK | Extracellular signal-related kinase |
| mTOR | Mammalian target of rapamycin |
| IGF-IR | Insulin-like growth factor-I receptor |
| PI3K | Phosphatidylinositol 3-kinase |
| DPP-IV | Dipeptidyl peptidase-IV |
| GLP-1 | Glucagon-like peptide-1 |
| IRS-I | Insulin receptor substrate-I |
References
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino acid profile and protein quality assessment of macroalgae produced in an integrated multi-trophic aquaculture system. Foods 2020, 9, 1382. [Google Scholar] [CrossRef]
- Cermeño, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy, P.A.; FitzGerald, R.J. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef] [PubMed]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Cian, R.E.; Fajardo, M.A.; Alaiz, M.; Vioque, J.; González, R.J.; Drago, S.R. Chemical composition, nutritional and antioxidant properties of the red edible seaweed Porphyra columbina. Int. J. Food Sci. Nutr. 2014, 65, 299–305. [Google Scholar] [CrossRef]
- Poojary, M.M.; Orlien, V.; Olsen, K. Conventional and enzyme-assisted green extraction of umami free amino acids from Nordic seaweeds. J. Appl. Phycol. 2019, 31, 3925–3939. [Google Scholar] [CrossRef]
- Bocanegra, A.; Macho-González, A.; Garcimartín, A.; Benedí, J.; Sánchez-Muniz, F.J. Whole Alga, Algal Extracts, and Compounds as Ingredients of Functional Foods: Composition and Action Mechanism Relationships in the Prevention and Treatment of Type-2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3816. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive proteins, peptides, and amino acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- O’Connor, J.; Meaney, S.; Williams, G.A.; Hayes, M. Extraction of protein from four different seaweeds using three different physical pre-treatment strategies. Molecules 2020, 25, 2005. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino acid composition, protein content, and nitrogen-to-protein conversion factors of 21 seaweed species from Norwegian waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Taboada, C.; Millan, R.; Miguez, I. Evaluation of marine algae Undaria pinnatifida and Porphyra purpurea as a food supplement: Composition, nutritional value and effect of intake on intestinal, hepatic and renal enzyme activities in rats. J. Sci. Food Agric. 2013, 93, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef]
- Ferreira, M.; Teixeira, C.; Abreu, H.; Silva, J.; Costas, B.; Kiron, V.; Valente, L.M.P. Nutritional value, antimicrobial and antioxidant activities of micro- and macroalgae, single or blended, unravel their potential use for aquafeeds. J. Appl. Phycol. 2021, 33, 3507–3518. [Google Scholar] [CrossRef]
- Trigueros, E.; Sanz, M.T.; Alonso-Riaño, P.; Beltrán, S.; Ramos, C.; Melgosa, R. Recovery of the protein fraction with high antioxidant activity from red seaweed industrial solid residue after agar extraction by subcritical water treatment. J. Appl. Phycol. 2021, 33, 1181–1194. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; San, S. Efficacy of a novel ACE-inhibitory peptide from Sargassum maclurei in hypertension and reduction of intracellular endothelin-1. Nutrients 2020, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification and Applications. Mar. Drugs 2021, 19, 500. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Santos, J.P.; Chow, F.; Pena Ferreira, M.J.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Boonsri, N.; Rudtanatip, T.; Withyachumnarnkul, B.; Wongprasert, K. Protein extract from red seaweed Gracilaria fisheri prevents acute hepatopancreatic necrosis disease (AHPND) infection in shrimp. J. Appl. Phycol. 2017, 29, 1597–1608. [Google Scholar] [CrossRef]
- Seca, A.; Pinto, D. Overview on the Antihypertensive and Anti-Obesity Effects of Secondary Metabolites from Seaweeds. Mar. Drugs 2018, 16, 237. [Google Scholar] [CrossRef]
- de Queiroz, I.N.L.; Quinderé, A.L.G.; Rodrigues, J.A.G.; de Sousa Oliveira Vanderlei, E.; Ribeiro, N.A.; da Conceição Rivanor, R.L.; Ribeiro, K.A.; Coura, C.O.; Pereira, K.M.A.; Chaves, H.V.; et al. Dual effects of a lectin from the green seaweed Caulerpa cupressoides var. lycopodium on inflammatory mediators in classical models of inflammation. Inflamm. Res. 2015, 64, 971–982. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, I.H.; Nam, T.J. Bioactive peptide from Pyropia yezoensis and its anti-inflammatory activities. Int. J. Mol. Med. 2015, 36, 1701–1706. [Google Scholar] [CrossRef]
- Conde, E.; Balboa, E.M.; Parada, M.; Falqué, E. Algal proteins, peptides and amino acids. In Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Ed.; Woodhead Publishing Elsevier: Cambridge, UK, 2013; pp. 135–180. ISBN 9780857095121. [Google Scholar]
- Hayes, M.; Tiwari, B.K. Bioactive carbohydrates and peptides in foods: An overview of sources, downstream processing steps and associated bioactivities. Int. J. Mol. Sci. 2015, 16, 22485–22508. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Conlon, M.A.; Franco, C.M.M.; Zhang, W. The development of seaweed-derived bioactive compounds for use as prebiotics and nutraceuticals using enzyme technologies. Trends Food Sci. Technol. 2017, 70, 20–33. [Google Scholar] [CrossRef]
- Yoshiie, T.; Maeda, M.; Kimura, M.; Hama, Y.; Uchida, M.; Kimura, Y. Structural features of N-glycans of seaweed glycoproteins: Predominant occurrence of high-mannose type N-glycans in marine plants. Biosci. Biotechnol. Biochem. 2012, 76, 1996–1998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wijesekara, I.; Lang, M.; Marty, C.; Gemin, M.-P.; Boulho, R.; Douzenel, P.; Wickramasinghe, I.; Bedoux, G.; Bourgougnon, N. Different extraction procedures and analysis of protein from Ulva sp. in Brittany, France. J. Appl. Phycol. 2017, 29, 2503–2511. [Google Scholar] [CrossRef]
- Thangam, R.; Senthilkumar, D.; Suresh, V.; Sathuvan, M.; Sivasubramanian, S.; Pazhanichamy, K.; Gorlagunta, P.K.; Kannan, S.; Gunasekaran, P.; Rengasamy, R.; et al. Induction of ROS-Dependent Mitochondria-Mediated Intrinsic Apoptosis in MDA-MB-231 Cells by Glycoprotein from Codium decorticatum. J. Agric. Food Chem. 2014, 62, 3410–3421. [Google Scholar] [CrossRef]
- Barre, A.; Simplicien, M.; Benoist, H.; Van Damme, E.J.M.; Rougé, P. Mannose-Specific Lectins from Marine Algae: Diverse Structural Scaffolds Associated to Common Virucidal and Anti-Cancer Properties. Mar. Drugs 2019, 17, 440. [Google Scholar] [CrossRef]
- Frenkel, J.; Vyverman, W.; Pohnert, G. Pheromone signaling during sexual reproduction in algae. Plant J. 2014, 79, 632–644. [Google Scholar] [CrossRef]
- Damme, E.J.M.; Van Peumans, W.J.; Barre, A.; Rougé, P. Plant Lectins: A Composite of Several Distinct Families of Structurally and Evolutionary Related Proteins with Diverse Biological Roles. CRC Crit. Rev. Plant Sci. 1998, 17, 575–692. [Google Scholar] [CrossRef]
- Ambrosio, A.L.; Sanz, L.; Sánchez, E.I.; Wolfenstein-Todel, C.; Calvete, J.J. Isolation of two novel mannan- and L-fucose-binding lectins from the green alga Enteromorpha prolifera: Biochemical characterization of EPL-2. Arch. Biochem. Biophys. 2003, 415, 245–250. [Google Scholar] [CrossRef]
- Wu, M.; Tong, C.; Wu, Y.; Liu, S.; Li, W. A novel thyroglobulin-binding lectin from the brown alga Hizikia fusiformis and its antioxidant activities. Food Chem. 2016, 201, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Günaydın, G.; Edfeldt, G.; Garber, D.A.; Asghar, M.; Noȅl-Romas, L.; Burgener, A.; Wählby, C.; Wang, L.; Rohan, L.C.; Guenthner, P.; et al. Impact of Q-Griffithsin anti-HIV microbicide gel in non-human primates: In situ analyses of epithelial and immune cell markers in rectal mucosa. Sci. Rep. 2019, 9, 18120. [Google Scholar] [CrossRef]
- Fontenelle, T.P.C.; Lima, G.C.; Mesquita, J.X.; Lopes, J.L.; de Brito, T.V.; Vieira-Júnior, F.C.; Sales, A.B.; Aragão, K.S.; de Souza, M.H.L.P.; Barbosa, A.L.R.; et al. Lectin obtained from the red seaweed Bryothamnion triquetrum: Secondary structure and anti-inflammatory activity in mice. Int. J. Biol. Macromol. 2018, 112, 1122–1130. [Google Scholar] [CrossRef]
- Nair, D.; Krishna, J.G.; Panikkar, M.V.N.; Nair, B.G.; Pai, J.G.; Nair, S.S. Identification, purification, biochemical and mass spectrometric characterization of novel phycobiliproteins from a marine red alga, Centroceras clavulatum. Int. J. Biol. Macromol. 2018, 114, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Antioxidant activity of the phycoerythrobilin compound formed from a dried Korean purple laver (Porphyra sp.) during in vitro digestion. Food Sci. Technol. Res. 2010, 16, 347–351. [Google Scholar] [CrossRef]
- Chen, X.; Wu, M.; Yang, Q.; Wang, S. Preparation, characterization of food grade phycobiliproteins from Porphyra haitanensis and the application in liposome-meat system. LWT Food Sci. Technol. 2017, 77, 468–474. [Google Scholar] [CrossRef]
- Li, W.; Su, H.; Pu, Y.; Chen, J.; Liu, L.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Guan, X.; Wang, J.; Zhu, J.; Yao, C.; Liu, J.; Qin, S.; Jiang, P. Photosystem II Photochemistry and Phycobiliprotein of the Red Algae Kappaphycus alvarezii and Their Implications for Light Adaptation. BioMed Res. Int. 2013, 2013, 256549. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, Contents, and Types of Mycosporine-Like Amino Acids (MAAs) in Marine Macroalgae and a Database for MAAs Based on These Characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur. Food Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and identification of ACE inhibitory peptides from the marine macroalga Ulva intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Sirois, M.; Tamigneaux, É. Evaluation of the in vitro biological activity of protein hydrolysates of the edible red alga, Palmaria palmata (dulse) harvested from the Gaspe coast and cultivated in tanks. J. Appl. Phycol. 2016, 28, 3101–3115. [Google Scholar] [CrossRef]
- Indumathi, P.; Mehta, A. A novel anticoagulant peptide from the Nori hydrolysate. J. Funct. Foods 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.I.; Baptista, J. Isolation and characterization of angiotensin I-converting enzyme (ACE) inhibitory peptides from Ulva rigida C. Agardh protein hydrolysate. J. Funct. Foods 2016, 26, 65–76. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, D.; Sun, X.; Sun, S.; Xu, N. Preparation and identification of antioxidant peptides from protein hydrolysate of marine alga Gracilariopsis lemaneiformis. J. Appl. Phycol. 2019, 31, 2585–2596. [Google Scholar] [CrossRef]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide identification from a Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Purification and identification of dipeptidyl peptidase (DPP) IV inhibitory peptides from the macroalga Palmaria palmata. Food Chem. 2015, 172, 400–406. [Google Scholar] [CrossRef]
- Cian, R.E.; Hernández-Chirlaque, C.; Gámez-Belmonte, R.; Drago, S.R.; Sánchez de Medina, F.; Martínez-Augustin, O. Green alga Ulva spp. hydrolysates and their peptide fractions regulate cytokine production in splenic macrophages and lymphocytes involving the TLR4-NFκB/MAPK pathways. Mar. Drugs 2018, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yin, Y.; Zhao, W.; Wang, F.; Yu, Y.; Liu, B.; Liu, J.; Chen, F. Characterization of ACE-Inhibitory Peptide Associated with Antioxidant and Anticoagulation Properties. J. Food Sci. 2011, 76, C1149–C1155. [Google Scholar] [CrossRef]
- Senthilkumar, D.; Jayanthi, S. Antioxidant activities of purified glycoprotein extracted from Codium decorticatum. J. Appl. Pharm. Sci. 2015, 5, 101–104. [Google Scholar] [CrossRef]
- Suh, H.-J.; Lee, H.-W.; Jung, J. Mycosporine Glycine Protects Biological Systems Against Photodynamic Damage by Quenching Singlet Oxygen with a High Efficiency. Photochem. Photobiol. 2003, 78, 109. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef]
- Wen, L.; Tan, S.; Zeng, L.; Wang, Z.; Ke, X.; Zhang, Z.; Tang, H.; Guo, H.; Xia, E. Ultrasound-assisted extraction and in vitro simulated digestion of Porphyra haitanensis proteins exhibiting antioxidative and α-glucosidase inhibitory activity. J. Food Meas. Charact. 2020, 14, 3291–3298. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Cermeño, M.; Kleekayai, T.; Machado, S.; Rego, A.; Fernandes, E.; Alves, R.C.; Oliveira, M.B.P.P.; FitzGerald, R.J. Contribution of in vitro simulated gastrointestinal digestion to the antioxidant activity of Porphyra dioica conchocelis. Algal Res. 2020, 51, 102085. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Cermeño, M.; Kleekayai, T.; Harnedy, P.A.; Fernandes, E.; Alves, R.C.; Oliveira, M.B.P.P.; FitzGerald, R.J. Enzymatic Modification of Porphyra dioica-Derived Proteins to Improve their Antioxidant Potential. Molecules 2020, 25, 2838. [Google Scholar] [CrossRef]
- Carneiro, R.F.; Duarte, P.L.; Chaves, R.P.; da Silva, S.R.; Feitosa, R.R.; de Sousa, B.L.; Alves, A.W.S.; de Vasconcelos, M.A.; da Rocha, B.A.M.; Teixeira, E.H.; et al. New lectins from Codium isthmocladum Vickers show unique amino acid sequence and antibiofilm effect on pathogenic bacteria. J. Appl. Phycol. 2020, 32, 4263–4276. [Google Scholar] [CrossRef]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and characterization of Griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, P.; Albecka, A.; Belouzard, S.; Vercauteren, K.; Verhoye, L.; Wychowski, C.; Leroux-Roels, G.; Palmer, K.E.; Dubuisson, J. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob. Agents Chemother. 2011, 55, 5159–5167. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Bewley, C.A. Griffithsin: An antiviral lectin with outstanding therapeutic potential. Viruses 2016, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Derby, N.; Lal, M.; Aravantinou, M.; Kizima, L.; Barnable, P.; Rodriguez, A.; Lai, M.; Wesenberg, A.; Ugaonkar, S.; Levendosky, K.; et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Barre, A.; Damme, E.J.M.V.; Van Simplicien, M.; Benoist, H.; Rougé, P. Man-Specific, GalNAc/T/Tn-Specific and Neu5Ac-Specific Seaweed Lectins as Glycan Probes for the SARS-CoV-2 (COVID-19) Coronavirus. Mar. Drugs 2020, 18, 543. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.; Kouokam, J.C.; Hurst, H.; Palmer, K.E. Pharmacokinetics of the antiviral lectin griffithsin administered by different routes indicates multiple potential uses. Viruses 2016, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Micewicz, E.D.; Cole, A.L.; Jung, C.L.; Luong, H.; Phillips, M.L.; Pratikhya, P.; Sharma, S.; Waring, A.J.; Cole, A.M.; Ruchala, P. Grifonin-1: A small HIV-1 entry inhibitor derived from the algal lectin, griffithsin. PLoS ONE 2010, 5, e14360. [Google Scholar] [CrossRef]
- Hwang, H.-J.; Han, J.-W.; Jeon, H.; Cho, K.; Kim, J.; Lee, D.-S.; Han, J.W. Characterization of a Novel Mannose-Binding Lectin with Antiviral Activities from Red Alga, Grateloupia chiangii. Biomolecules 2020, 10, 333. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Di Foggia, M.; Quintana, A.M.; Roberti, R. Preliminary Study on the Activity of Phycobiliproteins against Botrytis cinerea. Mar. Drugs 2020, 18, 600. [Google Scholar] [CrossRef]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.E.E.; Turgeon, S.L.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.S.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-Spectrum In Vitro Activity and In Vivo Efficacy of the Antiviral Protein Griffithsin against Emerging Viruses of the Family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef]
- Alexandre, K.; Malatji, K.; Mulaudzi, T. Comparison of the antiviral activity of the microbicide candidate griffithsin and its tandemers derivatives against different modes of HIV-1 transmission. Virology 2020, 544, 12–20. [Google Scholar] [CrossRef]
- Hirayama, M.; Shibata, H.; Imamura, K.; Sakaguchi, T.; Hori, K. High-Mannose Specific Lectin and Its Recombinants from a Carrageenophyta Kappaphycus alvarezii Represent a Potent Anti-HIV Activity Through High-Affinity Binding to the Viral Envelope Glycoprotein gp120. Mar. Biotechnol. 2016, 18, 144–160. [Google Scholar] [CrossRef]
- Hung, L.D.; Hirayama, M.; Ly, B.M.; Hori, K. Biological activity, cDNA cloning and primary structure of lectin KSA-2 from the cultivated red alga Kappaphycus striatum (Schmitz) Doty ex Silva. Phytochem. Lett. 2015, 14, 99–105. [Google Scholar] [CrossRef]
- Shin, E.S.; Hwang, H.J.; Kim, I.H.; Nam, T.J. A glycoprotein from Porphyra yezoensis produces anti-inflammatory effects in liposaccharide-stimulated macrophages via the TLR4 signaling pathway. Int. J. Mol. Med. 2011, 28, 809–815. [Google Scholar] [CrossRef]
- Mesquita, J.X.; de Brito, T.V.; Fontenelle, T.P.C.; Damasceno, R.O.S.; de Souza, M.H.L.P.; Lopes, J.L.S.; Beltramini, L.M.; Barbosa, A.L.R.; Freitas, A.L.P. Lectin from red algae Amansia multifida Lamouroux: Extraction, characterization and anti-inflammatory activity. Int. J. Biol. Macromol. 2021, 170, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, N.; Anyanji, V.U.; Mustapha, N.M.; Lim, S.L.; Mohamed, S. Seaweed (Eucheuma cottonii) reduced inflammation, mucin synthesis, eosinophil infiltration and MMP-9 expressions in asthma-induced rats compared to Loratadine. J. Funct. Foods 2015, 19, 710–722. [Google Scholar] [CrossRef]
- Chaves, R.P.; da Silva, S.R.; do Nascimento-Neto, L.G.; Carneiro, R.F.; da Silva, A.L.C.; Sampaio, A.H.; de Sousa, B.L.; Cabral, M.G.; Videira, P.A.; Teixeira, E.H.; et al. Structural characterization of two isolectins from the marine red alga Solieria filiformis (Kützing) P.W. Gabrielson and their anticancer effect on MCF-7 breast cancer cells. Int. J. Biol. Macromol. 2018, 107, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ying, J.; Chang, Q.; Zhu, W.; Yang, G.; Xu, T.; Yi, H.; Pan, R.; Zhang, E.; Zeng, X.; et al. Effects of phycoerythrin from Gracilaria lemaneiformis in proliferation and apoptosis of SW480 cells. Oncol. Rep. 2016, 36, 3536–3544. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Kurinjimalar, C.; Thangam, R.; Suresh, V.; Kavitha, G.; Gunasekaran, P.; Rengasamy, R. Further studies and biological activities of macromolecular protein R-Phycoerythrin from Portieria hornemannii. Int. J. Biol. Macromol. 2013, 62, 107–116. [Google Scholar] [CrossRef]
- Hayashi, K.; Walde, P.; Miyazaki, T.; Sakayama, K.; Nakamura, A.; Kameda, K.; Masuda, S.; Umakoshi, H.; Kato, K. Active Targeting to Osteosarcoma Cells and Apoptotic Cell Death Induction by the Novel Lectin Eucheuma serra Agglutinin Isolated from a Marine Red Alga. J. Drug Deliv. 2012, 2012, 842785. [Google Scholar] [CrossRef]
- do Nascimento-Neto, L.G.; Carneiro, R.F.; da Silva, S.R.; da Silva, B.R.; Arruda, F.V.S.; Carneiro, V.A.; do Nascimento, K.S.; Saker-Sampaio, S.; da Silva, V.A.; Porto, A.L.F.; et al. Characterization of Isoforms of the Lectin Isolated from the Red Algae Bryothamnion seaforthii and Its Pro-Healing Effect. Mar. Drugs 2012, 10, 1936–1954. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Kim, Y.-R.; Nam, T.-J.; Kong, I.-S. Antioxidant and DNA protection activities of a glycoprotein isolated from a seaweed, Saccharina japonica. Int. J. Food Sci. Technol. 2012, 47, 1020–1027. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Min Lee, J.; Ahmed, R.; Lee, J.-H.; Kim, J.-M.; Kong, I.-S. Characterisation of the hypoglycaemic activity of glycoprotein purified from the edible brown seaweed, Undaria pinnatifida. Int. J. Food Sci. Technol. 2015, 50, 143–150. [Google Scholar] [CrossRef]
- Mu, J.; Hirayama, M.; Sato, Y.; Morimoto, K.; Hori, K. A novel high-mannose specific lectin from the green alga Halimeda renschii exhibits a potent anti-influenza virus activity through high-affinity binding to the viral hemagglutinin. Mar. Drugs 2017, 15, 255. [Google Scholar] [CrossRef]
- Sato, Y.; Hirayama, M.; Morimoto, K.; Yamamoto, N.; Okuyama, S.; Hori, K. High mannose-binding lectin with preference for the cluster of α1-2-mannose from the green alga Boodlea coacta is a potent entry inhibitor of HIV-1 and influenza viruses. J. Biol. Chem. 2011, 286, 19446–19458. [Google Scholar] [CrossRef]
- Vanderlei, E.S.O.; Patoilo, K.K.N.R.; Lima, N.A.; Lima, A.P.S.; Rodrigues, J.A.G.; Silva, L.M.C.M.; Lima, M.E.P.; Lima, V.; Benevides, N.M.B. Antinociceptive and anti-inflammatory activities of lectin from the marine green alga Caulerpa cupressoides. Int. Immunopharmacol. 2010, 10, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Rivanor, R.L.; Chaves, H.V.; Do Val, D.R.; De Freitas, A.R.; Lemos, J.C.; Rodrigues, J.A.G.; Pereira, K.M.A.; De Araújo, I.W.F.; Bezerra, M.M.; Benevides, N.M.B. A lectin from the green seaweed Caulerpa cupressoides reduces mechanical hyper-nociception and inflammation in the rat temporomandibular joint during zymosan-induced arthritis. Int. Immunopharmacol. 2014, 21, 34–43. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Joel, C.; Sutopo, C.; Prajitno, A.; Su, J.-H.; Hsu, J.-L. Screening of Angiotensin-I Converting Enzyme Inhibitory Peptides Derived from Caulerpa lentillifera. Molecules 2018, 23, 3005. [Google Scholar] [CrossRef]
- Wu, Q.; Cai, Q.-F.; Yoshida, A.; Sun, L.-C.; Liu, Y.-X.; Liu, G.-M.; Su, W.-J.; Cao, M.-J. Purification and characterization of two novel angiotensin I-converting enzyme inhibitory peptides derived from R-phycoerythrin of red algae (Bangia fusco-purpurea). Eur. Food Res. Technol. 2017, 243, 779–789. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and Characterization of Bioactive Pro-Peptides with in Vitro Renin Inhibitory Activities from the Macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Aluko, R.E.; Hossain, M.; Rai, D.K.; Hayes, M. Potential of a Renin Inhibitory Peptide from the Red Seaweed Palmaria palmata as a Functional Food Ingredient Following Confirmation and Characterization of a Hypotensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2014, 62, 8352–8356. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Wakame (Undaria pinnatifida) and Their Antihypertensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef]
- Saito, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K. Antihypertensive effect of Nori-peptides derived from red alga Porphyra yezoensis in hypertensive patients. Am. J. Hypertens. 2002, 15, A210. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Machado, M.; Cermeño, M.; Kleekayai, T.; Machado, S.; Rego, A.M.; Abreu, M.H.; Alves, R.C.; Oliveira, M.B.P.P.; FitzGerald, R.J. Enzyme-Assisted Release of Antioxidant Peptides from Porphyra dioica Conchocelis. Antioxidants 2021, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Ryu, J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Activation of the mTOR signaling pathway in breast cancer MCF-7 cells by a peptide derived from Porphyra yezoensis. Oncol. Rep. 2015, 33, 19–24. [Google Scholar] [CrossRef]
- Cian, R.E.; Garzón, A.G.; Ancona, D.B.; Guerrero, L.C.; Drago, S.R. Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT Food Sci. Technol. 2015, 64, 881–888. [Google Scholar] [CrossRef]
- Cian, R.E.; López-Posadas, R.; Drago, S.R.; Sánchez de Medina, F.; Martínez-Augustin, O. A Porphyra columbina hydrolysate upregulates IL-10 production in rat macrophages and lymphocytes through an NF-κB, and p38 and JNK dependent mechanism. Food Chem. 2012, 134, 1982–1990. [Google Scholar] [CrossRef]
- Chen, J.-C.C.; Wang, J.; Zheng, B.-D.D.; Pang, J.; Chen, L.-J.J.; Lin, H.T.; Guo, X. Simultaneous Determination of 8 Small Antihypertensive Peptides with Tyrosine at the C-Terminal in Laminaria japonica Hydrolysates by RP-HPLC Method. J. Food Process. Preserv. 2016, 40, 492–501. [Google Scholar] [CrossRef]
- Suetsuna, K.; Maekawa, K.; Chen, J.-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef]
- Feng, X.; Liao, D.; Sun, L.; Wu, S.; Lan, P.; Wang, Z.; Li, C.; Zhou, Q.; Lu, Y.; Lan, X. Affinity Purification of Angiotensin Converting Enzyme Inhibitory Peptides from Wakame (Undaria Pinnatifida) Using Immobilized ACE on Magnetic Metal Organic Frameworks. Mar. Drugs 2021, 19, 177. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Mora, L.; Hayes, M. In Vitro and In Silico Approaches to Generating and Identifying Angiotensin-Converting Enzyme I Inhibitory Peptides from Green Macroalga Ulva lactuca. Mar. Drugs 2019, 17, 204. [Google Scholar] [CrossRef]
- Pan, S.; Wang, S.; Jing, L.; Yao, D. Purification and characterisation of a novel angiotensin-I converting enzyme (ACE)-inhibitory peptide derived from the enzymatic hydrolysate of Enteromorpha clathrata protein. Food Chem. 2016, 211, 423–430. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Tornatore, L.; Thotakura, A.K.; Bennett, J.; Moretti, M.; Franzoso, G. The nuclear factor kappa B signaling pathway: Integrating metabolism with inflammation. Trends Cell Biol. 2012, 22, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y. Antiproliferative and Antioxidant Activities and Mycosporine-Like Amino Acid Profiles of Wild-Harvested and Cultivated Edible Canadian Marine Red Macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef]
- Park, S.J.; Ryu, J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Induction of apoptosis by a peptide from Porphyra yezoensis: Regulation of the insulin-like growth factor I receptor signaling pathway in MCF-7 cells. Int. J. Oncol. 2014, 45, 1011–1016. [Google Scholar] [CrossRef]
- Mennella, I.; Fogliano, V.; Vitaglione, P. Salivary lipase and α-amylase activities are higher in overweight than in normal weight subjects: Influences on dietary behavior. Food Res. Int. 2014, 66, 463–468. [Google Scholar] [CrossRef]
- Opinto, G.; Natalicchio, A.; Marchetti, P. Physiology of incretins and loss of incretin effect in type 2 diabetes and obesity. Arch. Physiol. Biochem. 2013, 119, 170–178. [Google Scholar] [CrossRef]
- Stack, J.; Tobin, P.R.; Gietl, A.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Seasonal variation in nitrogenous components and bioactivity of protein hydrolysates from Porphyra dioica. J. Appl. Phycol. 2017, 29, 2439–2450. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J. Appl. Phycol. 2013, 25, 1793–1803. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, I.H.; Choi, Y.H.; Choi, J.W.; Kim, Y.M.; Nam, T.J. The proliferative effects of Pyropia yezoensis peptide on IEC-6 cells are mediated through the epidermal growth factor receptor signaling pathway. Int. J. Mol. Med. 2015, 35, 909–914. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.M.; Sharkey, S.J.; Harnedy, P.A.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Twice daily oral administration of Palmaria palmata protein hydrolysate reduces food intake in streptozotocin induced diabetic mice, improving glycaemic control and lipid profiles. J. Funct. Foods 2020, 73, 104101. [Google Scholar] [CrossRef]
- McLaughlin, C.M.; Harnedy, P.A.; Lafferty, R.A.; Sharkey, S.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Macroalgal protein hydrolysates from Palmaria palmata influence the ‘incretin effect’ in vitro via DPP-4 inhibition and upregulation of insulin, GLP-1 and GIP secretion. Eur. J. Nutr. 2021, 60, 4439–4452. [Google Scholar] [CrossRef]
- Cian, R.E.; Salgado, P.R.; Drago, S.R.; González, R.J.; Mauri, A.N. Development of naturally activated edible films with antioxidant properties prepared from red seaweed Porphyra columbina biopolymers. Food Chem. 2014, 146, 6–14. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Ferraretto, A.; Arranz, E.; Stuknytė, M.; Bottani, M.; O’Connor, P.M.; Kelly, P.M.; De Noni, I.; Buckin, V.; Giblin, L. Bovine whey peptides transit the intestinal barrier to reduce oxidative stress in muscle cells. Food Chem. 2019, 288, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Sakamoto, Y.; Toh, M.; Ohara, N.; Hatanaka, Y.; Naka, A.; Kishimoto, Y.; Kondo, K.; Iida, K. Milk-derived peptide Val-Pro-Pro (VPP) inhibits obesity-induced adipose inflammation via an angiotensin-converting enzyme (ACE) dependent cascade. Mol. Nutr. Food Res. 2015, 59, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, I.H.; Choi, Y.H.; Nam, T.J. A peptide from Porphyra yezoensis stimulates the proliferation of IEC 6 cells by activating the Insulin-like growth factor I receptor signaling pathway. Int. J. Mol. Med. 2015, 35, 533–538. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echave, J.; Otero, P.; Garcia-Oliveira, P.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Simal-Gandara, J.; Prieto, M.A. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants 2022, 11, 176. https://doi.org/10.3390/antiox11010176
Echave J, Otero P, Garcia-Oliveira P, Munekata PES, Pateiro M, Lorenzo JM, Simal-Gandara J, Prieto MA. Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants. 2022; 11(1):176. https://doi.org/10.3390/antiox11010176
Chicago/Turabian StyleEchave, Javier, Paz Otero, Paula Garcia-Oliveira, Paulo E. S. Munekata, Mirian Pateiro, Jose M. Lorenzo, Jesus Simal-Gandara, and Miguel A. Prieto. 2022. "Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives" Antioxidants 11, no. 1: 176. https://doi.org/10.3390/antiox11010176
APA StyleEchave, J., Otero, P., Garcia-Oliveira, P., Munekata, P. E. S., Pateiro, M., Lorenzo, J. M., Simal-Gandara, J., & Prieto, M. A. (2022). Seaweed-Derived Proteins and Peptides: Promising Marine Bioactives. Antioxidants, 11(1), 176. https://doi.org/10.3390/antiox11010176