The Epidermis: Redox Governor of Health and Diseases
Abstract
1. Introduction and Overview
1.1. What Is Cornification for?
1.2. Revisiting Epidermal Sulfur Metabolism
2. Epidermal Differentiation and Skin Diseases
2.1. Psoriasis
2.1.1. EDC and Psoriasis
2.1.2. Antimicrobial Properties of the “Alternative” CE Precursors LCEs
2.2. Atopic Dermatitis
2.2.1. AD and the FLG Variant
2.2.2. AD and Corneocyte Adhesion
2.2.3. AD as an Antioxidative Response in the Epidermis
3. Redox Regulators of Skin Health and Diseases
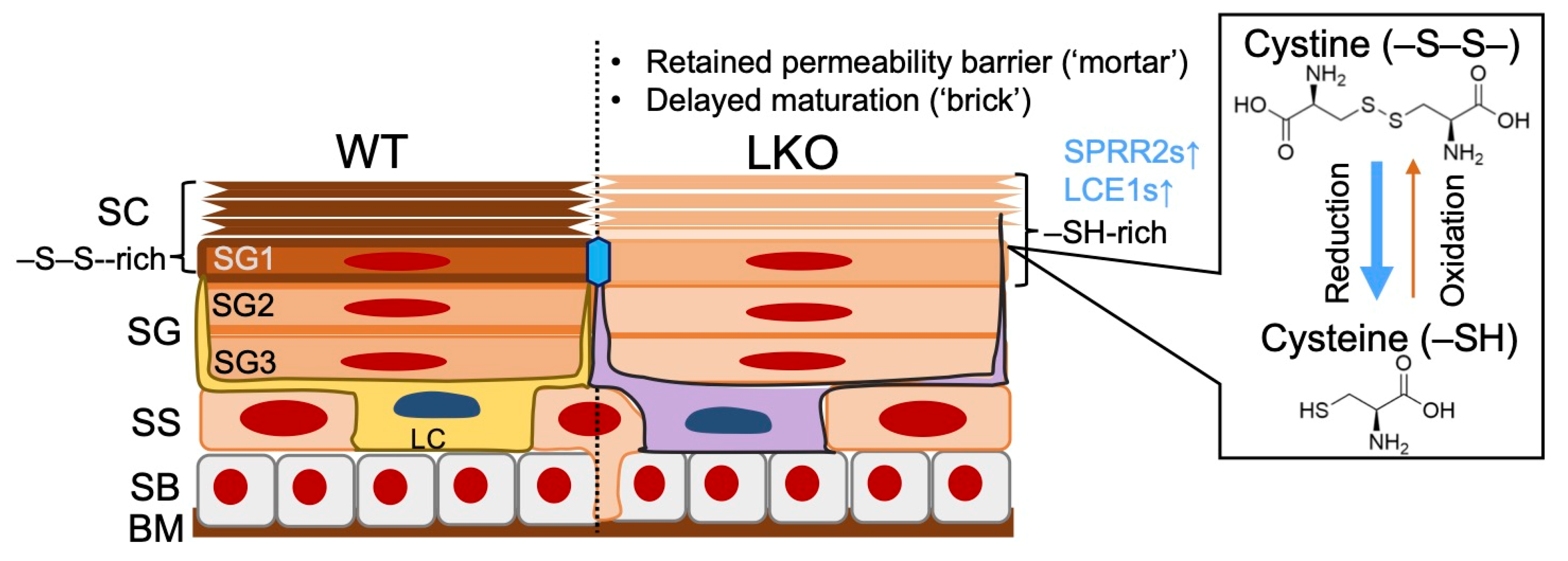
3.1. LOR, the “Finisher” of Cornification
3.2. “Structural Imprinting” of the Cutaneous Immune Effector Functions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD, ATOD | atopic dermatitis |
| CARD14 | caspase recruitment domain family member 14 |
| CDSN | corneosesmosin |
| CE | cornified cell envelope |
| CHS | contact hypersensitivity |
| CLDN1 | claudin-1 |
| DAMP | damage-associated molecular pattern |
| DC | dendritic cells |
| DMF | dimethyl fumarate |
| DSG | desmoglein |
| EDC | epidermal diffrentiation complex |
| FLG | filaggrin |
| FS | failure to thrive |
| HLA | human leukocyte antigen |
| IL36RN | interleukin-36 receptor antagonist protein |
| IMQ | imiquimod |
| IVL | involucrin |
| KEAP1 | Kelch-like erythroid cell-derived protein with the cap ’n’ collar homology-associated protein 1/NFE2-related factor 2 |
| KLK | kallikrein |
| LC | Langerhans cell |
| LCE | late cornified cell envelope protein |
| LEKTI | lymphoepithelial Kazal-type related inhibitor type 5 |
| LG | lamellar granule |
| LOF | loss-of-function |
| LOR | loricrin |
| MHC | major histocompatibility complex |
| MS | multiple sclerosis |
| NRF2 | NFE2-related factor 2 |
| NS | Netherton syndrome |
| OMIM | Online Mendelian Inheritance in Man |
| PSORS | psoriasis susceptibility |
| PSS | peeling skin syndrome |
| redox | reduction and oxidation |
| ROR-g | retinoic acid-receptor-related orphan receptor gamma |
| S. aureus | Staphylococcus aureus |
| SC | stratum corneum |
| SG | stratum granulosum |
| SPINK5 | serine protease inhibitor of Kasal-type 5 |
| SPRR | small prorine-rich protein |
| TGF-b1 | transforming growth factor-beta 1 |
| TH | T helper |
| TMEM79 | transmembrane protein 79 |
| TSLP | thymic stromal lymphopoietin |
| UV | ultraviolet |
References
- Eckhart, L.; Lippens, S.; Tschachler, E.; Declercq, W. Cell death by cornification. Biochim. Biophys. Acta 2013, 1833, 3471–3480. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Roop, D.R. Loricrin: Past, Present, and Future. Int. J. Mol. Sci. 2020, 21, 2271. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.H.; Green, H. The cornified envelope of terminally differentiated human epidermal keratinocytes consists of cross-linked protein. Cell 1977, 11, 417–422. [Google Scholar] [CrossRef]
- Doran, T.I.; Vidrich, A.; Sun, T.T. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell 1980, 22, 17–25. [Google Scholar] [CrossRef]
- Stark, H.J.; Baur, M.; Breitkreutz, D.; Mirancea, N.; Fusenig, N.E. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J. Invest. Dermatol. 1999, 112, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Mesa, K.R.; Kawaguchi, K.; Park, S.; Gonzalez, D.; Brown, S.; Boucher, J.; Klein, A.M.; Greco, V. Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 2016, 352, 1471–1474. [Google Scholar] [CrossRef]
- Menon, G.K.; Grayson, S.; Elias, P.M. Ionic calcium reservoirs in mammalian epidermis: Ultrastructural localization by ion-capture cytochemistry. J. Invest. Dermatol. 1985, 84, 508–512. [Google Scholar] [CrossRef]
- Xie, Z.; Komuves, L.; Yu, Q.C.; Elalieh, H.; Ng, D.C.; Leary, C.; Chang, S.; Crumrine, D.; Yoshizawa, T.; Kato, S.; et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J. Invest. Dermatol. 2002, 118, 11–16. [Google Scholar] [CrossRef]
- Saitou, M.; Sugai, S.; Tanaka, T.; Shimouchi, K.; Fuchs, E.; Narumiya, S.; Kakizuka, A. Inhibition of skin development by targeted expression of a dominant-negative retinoic acid receptor. Nature 1995, 374, 159–162. [Google Scholar] [CrossRef]
- Imakado, S.; Bickenbach, J.R.; Bundman, D.S.; Rothnagel, J.A.; Attar, P.S.; Wang, X.J.; Walczak, V.R.; Wisniewski, S.; Pote, J.; Gordon, J.S.; et al. Targeting expression of a dominant-negative retinoic acid receptor mutant in the epidermis of transgenic mice results in loss of barrier function. Genes Dev. 1995, 9, 317–329. [Google Scholar] [CrossRef]
- Matsui, T.; Kadono-Maekubo, N.; Suzuki, Y.; Furuichi, Y.; Shiraga, K.; Sasaki, H.; Ishida, A.; Takahashi, S.; Okada, T.; Toyooka, K.; et al. A unique mode of keratinocyte death requires intracellular acidification. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Matsui, T.; Amagai, M. Dissecting the formation, structure and barrier function of the stratum corneum. Int. Immunol. 2015, 27, 269–280. [Google Scholar] [CrossRef]
- Kalinin, A.; Marekov, L.N.; Steinert, P.M. Assembly of the epidermal cornified cell envelope. J. Cell Sci. 2001, 114, 3069–3070. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, Y.; Ogawa, T.; Nakamura, Y.; Kubota, N.; Fujisawa, Y.; Watanabe, R.; Okiyama, N.; Fujimoto, M.; Roop, D.R.; Ishida-Yamamoto, A. Loricrin and NRF2 Coordinate Cornification. JID Innov. 2022, 2, 100065. [Google Scholar] [CrossRef]
- Matsuki, M.; Yamashita, F.; Ishida-Yamamoto, A.; Yamada, K.; Kinoshita, C.; Fushiki, S.; Ueda, E.; Morishima, Y.; Tabata, K.; Yasuno, H.; et al. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase). Proc. Natl. Acad. Sci. USA 1998, 95, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, L.M.; Nachat, R.; Groot, K.R.; Klement, J.F.; Uitto, J.; Djian, P.; Maatta, A.; Watt, F.M. Mice deficient in involucrin, envoplakin, and periplakin have a defective epidermal barrier. J. Cell Biol. 2007, 179, 1599–1612. [Google Scholar] [CrossRef]
- Gallicano, G.I.; Kouklis, P.; Bauer, C.; Yin, M.; Vasioukhin, V.; Degenstein, L.; Fuchs, E. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J. Cell Biol. 1998, 143, 2009–2022. [Google Scholar] [CrossRef]
- Kugelmann, D.; Radeva, M.Y.; Spindler, V.; Waschke, J. Desmoglein 1 Deficiency Causes Lethal Skin Blistering. J. Invest. Dermatol. 2019, 139, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Godsel, L.M.; Roth-Carter, Q.R.; Koetsier, J.L.; Tsoi, L.C.; Huffine, A.L.; Broussard, J.A.; Fitz, G.N.; Lloyd, S.M.; Kweon, J.; Burks, H.E.; et al. Translational implications of Th17-skewed inflammation due to genetic deficiency of a cadherin stress sensor. J. Clin. Invest. 2021. [CrossRef] [PubMed]
- McAleer, M.A.; Pohler, E.; Smith, F.J.; Wilson, N.J.; Cole, C.; MacGowan, S.; Koetsier, J.L.; Godsel, L.M.; Harmon, R.M.; Gruber, R.; et al. Severe dermatitis, multiple allergies, and metabolic wasting syndrome caused by a novel mutation in the N-terminal plakin domain of desmoplakin. J. Allergy Clin. Immunol. 2015, 136, 1268–1276. [Google Scholar] [CrossRef]
- Samuelov, L.; Sarig, O.; Harmon, R.M.; Rapaport, D.; Ishida-Yamamoto, A.; Isakov, O.; Koetsier, J.L.; Gat, A.; Goldberg, I.; Bergman, R.; et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat. Genet. 2013, 45, 1244–1248. [Google Scholar] [CrossRef]
- Huber, M.; Rettler, I.; Bernasconi, K.; Frenk, E.; Lavrijsen, S.P.; Ponec, M.; Bon, A.; Lautenschlager, S.; Schorderet, D.F.; Hohl, D. Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science 1995, 267, 525–528. [Google Scholar] [CrossRef]
- Ishida-Yamamoto, A.; Igawa, S.; Kishibe, M. Molecular basis of the skin barrier structures revealed by electron microscopy. Exp. Dermatol. 2018, 27, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Skabytska, Y.; Wolbing, F.; Gunther, C.; Koberle, M.; Kaesler, S.; Chen, K.M.; Guenova, E.; Demircioglu, D.; Kempf, W.E.; Volz, T.; et al. Cutaneous innate immune sensing of Toll-like receptor 2–6 ligands suppresses T cell immunity by inducing myeloid-derived suppressor cells. Immunity 2014, 41, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Yoshino, M.; Yamazaki, H.; Naito, M.; Iyoda, T.; Omatsu, Y.; Shimoyama, S.; Letterio, J.J.; Nakabayashi, T.; Tagaya, H.; et al. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int. Immunol. 2001, 13, 695–704. [Google Scholar] [CrossRef]
- Kubo, A.; Nagao, K.; Yokouchi, M.; Sasaki, H.; Amagai, M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009, 206, 2937–2946. [Google Scholar] [CrossRef]
- Farache, J.; Koren, I.; Milo, I.; Gurevich, I.; Kim, K.W.; Zigmond, E.; Furtado, G.C.; Lira, S.A.; Shakhar, G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 2013, 38, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef]
- Smith, F.J.; Irvine, A.D.; Terron-Kwiatkowski, A.; Sandilands, A.; Campbell, L.E.; Zhao, Y.; Liao, H.; Evans, A.T.; Goudie, D.R.; Lewis-Jones, S.; et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat. Genet. 2006, 38, 337–342. [Google Scholar] [CrossRef]
- Kawasaki, H.; Nagao, K.; Kubo, A.; Hata, T.; Shimizu, A.; Mizuno, H.; Yamada, T.; Amagai, M. Altered stratum corneum barrier and enhanced percutaneous immune responses in filaggrin-null mice. J. Allergy Clin. Immunol. 2012, 129, 1538–1546.e6. [Google Scholar] [CrossRef] [PubMed]
- Moniaga, C.S.; Egawa, G.; Kawasaki, H.; Hara-Chikuma, M.; Honda, T.; Tanizaki, H.; Nakajima, S.; Otsuka, A.; Matsuoka, H.; Kubo, A.; et al. Flaky tail mouse denotes human atopic dermatitis in the steady state and by topical application with Dermatophagoides pteronyssinus extract. Am. J. Pathol. 2010, 176, 2385–2393. [Google Scholar] [CrossRef]
- Sasaki, T.; Furusyo, N.; Shiohama, A.; Takeuchi, S.; Nakahara, T.; Uchi, H.; Hirota, T.; Tamari, M.; Shimizu, N.; Ebihara, T. Filaggrin loss-of-function mutations are not a predisposing factor for atopic dermatitis in an Ishigaki Island under subtropical climate. J. Dermatol. Sci. 2014, 76, 10–15. [Google Scholar] [CrossRef]
- Spergel, J.M.; Mizoguchi, E.; Brewer, J.P.; Martin, T.R.; Bhan, A.K.; Geha, R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J. Clin. Invest. 1998, 101, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Has, C.; Jakob, T.; He, Y.; Kiritsi, D.; Hausser, I.; Bruckner-Tuderman, L. Loss of desmoglein 1 associated with palmoplantar keratoderma, dermatitis and multiple allergies. Br. J. Dermatol. 2015, 172, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Holstein, J.; Solimani, F.; Baum, C.; Meier, K.; Pollmann, R.; Didona, D.; Tekath, T.; Dugas, M.; Casadei, N.; Hudemann, C. Immunophenotyping in pemphigus reveals a TH17/TFH17 cell–dominated immune response promoting desmoglein1/3-specific autoantibody production. J. Allergy Clin. Immunol. 2021, 147, 2358–2369. [Google Scholar] [CrossRef]
- Ong, P.Y.; Leung, D.Y. Bacterial and Viral Infections in Atopic Dermatitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Amagai, M.; Matsuyoshi, N.; Wang, Z.; Andl, C.; Stanley, J. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat. Med. 2000, 6, 1275–1277. [Google Scholar] [CrossRef]
- Ouchi, T.; Kubo, A.; Yokouchi, M.; Adachi, T.; Kobayashi, T.; Kitashima, D.Y.; Fujii, H.; Clausen, B.E.; Koyasu, S.; Amagai, M.; et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 2011, 208, 2607–2613. [Google Scholar] [CrossRef]
- Nemes, Z.; Steinert, P.M. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Nagao, K.; Amagai, M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J. Clin. Investig. 2012, 122, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Van Scott, E.; Flesch, P. Sulfhydryl and disulfide in keratinization. Science 1954, 119, 70–71. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Roop, D.R. Loricrin Confers Photoprotective Function against UVB in Corneocytes. J. Invest. Dermatol. 2018, 138, 2684–2687. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Dutsch, S.; auf dem Keller, U.; Navid, F.; Schwarz, A.; Johnson, D.A.; Johnson, J.A.; Werner, S. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 2010, 24, 1045–1058. [Google Scholar] [CrossRef]
- Kawachi, Y.; Xu, X.; Taguchi, S.; Sakurai, H.; Nakamura, Y.; Ishii, Y.; Fujisawa, Y.; Furuta, J.; Takahashi, T.; Itoh, K.; et al. Attenuation of UVB-induced sunburn reaction and oxidative DNA damage with no alterations in UVB-induced skin carcinogenesis in Nrf2 gene-deficient mice. J. Invest. Dermatol. 2008, 128, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ishitsuka, Y.; Nakamura, Y.; Kubota, N.; Saito, A.; Fujisawa, Y.; Watanabe, R.; Okiyama, N.; Suga, Y.; Roop, D.R.; et al. NRF2 Augments Epidermal Antioxidant Defenses and Promotes Atopy. J Immunol 2020, 205, 907–914. [Google Scholar] [CrossRef]
- Kim, H.J.; Barajas, B.; Wang, M.; Nel, A.E. Nrf2 activation by sulforaphane restores the age-related decrease of T(H)1 immunity: Role of dendritic cells. J. Allergy Clin. Immunol. 2008, 121, 1255–1261.e7. [Google Scholar] [CrossRef]
- Ramos-Gomez, M.; Kwak, M.K.; Dolan, P.M.; Itoh, K.; Yamamoto, M.; Talalay, P.; Kensler, T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 3410–3415. [Google Scholar] [CrossRef]
- Bern, H.A.; Harkness, D.R.; Blair, S.M. Radioautographic Studies of Keratin Formation. Proc. Natl. Acad. Sci. USA 1955, 41, 55–60. [Google Scholar] [CrossRef]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.D.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005, 202, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.E.; Jaramillo, A.M.; Shenoy, S.K.; Raclawska, D.; Emezienna, N.A.; Richardson, V.L.; Hara, N.; Harder, A.Q.; NeeDell, J.C.; Hennessy, C.E.; et al. Disulfide disruption reverses mucus dysfunction in allergic airway disease. Nat. Commun. 2021, 12, 249. [Google Scholar] [CrossRef]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W.C. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Cao, Y.; Hur, J.; Mehta, R.S.; Sikavi, D.R.; Wang, Y.; Ma, W.; Wu, K.; Song, M.; Giovannucci, E.L.; et al. The Sulfur Microbial Diet Is Associated With Increased Risk of Early-Onset Colorectal Cancer Precursors. Gastroenterology 2021, 161, 1423–1432.e4. [Google Scholar] [CrossRef]
- Mehrel, T.; Hohl, D.; Rothnagel, J.A.; Longley, M.A.; Bundman, D.; Cheng, C.; Lichti, U.; Bisher, M.E.; Steven, A.C.; Steinert, P.M.; et al. Identification of a major keratinocyte cell envelope protein, loricrin. Cell 1990, 61, 1103–1112. [Google Scholar] [CrossRef]
- Huebner, A.J.; Dai, D.; Morasso, M.; Schmidt, E.E.; Schafer, M.; Werner, S.; Roop, D.R. Amniotic fluid activates the nrf2/keap1 pathway to repair an epidermal barrier defect in utero. Dev. Cell 2012, 23, 1238–1246. [Google Scholar] [CrossRef]
- Koch, P.J.; de Viragh, P.A.; Scharer, E.; Bundman, D.; Longley, M.A.; Bickenbach, J.; Kawachi, Y.; Suga, Y.; Zhou, Z.; Huber, M.; et al. Lessons from loricrin-deficient mice: Compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J. Cell Biol. 2000, 151, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol Rev 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Ogawa, T.; Roop, D. The KEAP1/NRF2 Signaling Pathway in Keratinization. Antioxidants 2020, 9, 751. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Huebner, A.J.; Rice, R.H.; Koch, P.J.; Speransky, V.V.; Steven, A.C.; Roop, D.R. Lce1 Family Members Are Nrf2-Target Genes that Are Induced to Compensate for the Loss of Loricrin. J. Invest. Dermatol. 2016, 136, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, Z.A.; de Guzman Strong, C. Recent Positive Selection in Genes of the Mammalian Epidermal Differentiation Complex Locus. Front. Genet. 2016, 7, 227. [Google Scholar] [CrossRef]
- Ogawa, T.; Ishitsuka, Y.; Inoue, S.; Nakamura, Y.; Saito, A.; Okiyama, N.; Fujisawa, Y.; Furuta, J.I.; Watanabe, R.; Fujimoto, M. Nuclear factor erythroid 2-related factor 2 (Nrf2) regulates epidermal keratinization under psoriatic skin inflammation. Am. J. Pathol. 2020. [Google Scholar] [CrossRef]
- Baurecht, H.; Hotze, M.; Brand, S.; Buning, C.; Cormican, P.; Corvin, A.; Ellinghaus, D.; Ellinghaus, E.; Esparza-Gordillo, J.; Folster-Holst, R.; et al. Genome-wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms. Am. J. Hum. Genet. 2015, 96, 104–120. [Google Scholar] [CrossRef]
- He, H.; Bissonnette, R.; Wu, J.; Diaz, A.; Proulx, E.S.-C.; Maari, C.; Jack, C.; Louis, M.; Estrada, Y.; Krueger, J.G. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J. Allergy Clin. Immunol. 2021, 147, 199–212. [Google Scholar] [CrossRef]
- Ishitsuka, Y.; Roop, D.R.; Ogawa, T. “Structural imprinting” of the cutaneous immune effector function. Tissue Barriers 2020. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, A.; Scott, A.F.; Amberger, J.S.; Bocchini, C.A.; McKusick, V.A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005, 33, D514–D517. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Balato, A.; Enerback, C.; Sabat, R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 2021, 397, 754–766. [Google Scholar] [CrossRef]
- McKenzie, B.S.; Kastelein, R.A.; Cua, D.J. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006, 27, 17–23. [Google Scholar] [CrossRef]
- Pinkus, H.; Mehregan, A.H. The primary histologic lesion of seborrheic dermatitis and psoriasis. J. Invest. Dermatol. 1966, 46, 109–116. [Google Scholar] [CrossRef]
- McGonagle, D.; McDermott, M.F. A proposed classification of the immunological diseases. PLoS Med. 2006, 3, e297. [Google Scholar] [CrossRef] [PubMed]
- Szekanecz, Z.; McInnes, I.B.; Schett, G.; Szamosi, S.; Benko, S.; Szucs, G. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat. Rev. Rheumatol. 2021, 17, 585–595. [Google Scholar] [CrossRef]
- Kurihara, K.; Fujiyama, T.; Phadungsaksawasdi, P.; Ito, T.; Tokura, Y. Significance of IL-17A-producing CD8+ CD103+ skin resident memory T cells in psoriasis lesion and their possible relationship to clinical course. J. Dermatol. Sci. 2019, 95, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Psoriasis and connective tissue diseases. Int. J. Mol. Sci. 2020, 21, 5803. [Google Scholar] [CrossRef]
- Hammer, R.E.; Maika, S.D.; Richardson, J.A.; Tang, J.P.; Taurog, J.D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta 2m: An animal model of HLA-B27-associated human disorders. Cell 1990, 63, 1099–1112. [Google Scholar] [CrossRef]
- Kunstfeld, R.; Hirakawa, S.; Hong, Y.K.; Schacht, V.; Lange-Asschenfeldt, B.; Velasco, P.; Lin, C.; Fiebiger, E.; Wei, X.; Wu, Y.; et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood 2004, 104, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Chan, K.S.; Carbajal, S.; Clifford, J.; Peavey, M.; Kiguchi, K.; Itami, S.; Nickoloff, B.J.; DiGiovanni, J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 2005, 11, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Tortola, L.; Rosenwald, E.; Abel, B.; Blumberg, H.; Schäfer, M.; Coyle, A.J.; Renauld, J.-C.; Werner, S.; Kisielow, J.; Kopf, M. Psoriasiform dermatitis is driven by IL-36–mediated DC-keratinocyte crosstalk. J. Clin. Invest. 2012, 122, 3965–3976. [Google Scholar] [CrossRef]
- Shepherd, J.; Little, M.C.; Nicklin, M.J. Psoriasis-like cutaneous inflammation in mice lacking interleukin-1 receptor antagonist. J. Invest. Dermatol. 2004, 122, 665–669. [Google Scholar] [CrossRef]
- Boyman, O.; Hefti, H.P.; Conrad, C.; Nickoloff, B.J.; Suter, M.; Nestle, F.O. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med. 2004, 199, 731–736. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Kemeny, L. The golden ages of inflammatory skin diseases: Skyrocketing developments in the therapy of psoriasis and atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 2239–2240. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, T.R.; Griesinger, F. Two cases of psoriasis responding to erlotinib: Time to revisiting inhibition of epidermal growth factor receptor in psoriasis therapy? Dermatology 2012, 225, 179–182. [Google Scholar] [CrossRef]
- Cheuk, S.; Schlums, H.; Gallais Serezal, I.; Martini, E.; Chiang, S.C.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Introini, A.; Forkel, M.; et al. CD49a Expression Defines Tissue-Resident CD8(+) T Cells Poised for Cytotoxic Function in Human Skin. Immunity 2017, 46, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Vo, S.; Watanabe, R.; Koguchi-Yoshioka, H.; Matsumura, Y.; Ishitsuka, Y.; Nakamura, Y.; Okiyama, N.; Fujisawa, Y.; Fujimoto, M. CD8 resident memory T cells with interleukin 17A-producing potential are accumulated in disease-naïve nonlesional sites of psoriasis possibly in correlation with disease duration. Br. J. Dermatol. 2019, 181, 410–412. [Google Scholar] [CrossRef]
- Morimoto, S.; Yoshikawa, K.; Kozuka, T.; Kitano, Y.; Imanaka, S.; Fukuo, K.; Koh, E.; Kumahara, Y. An open study of vitamin D3 treatment in psoriasis vulgaris. Br. J. Dermatol. 1986, 115, 421–429. [Google Scholar] [CrossRef]
- Fujiyama, T.; Ito, T.; Umayahara, T.; Ikeya, S.; Tatsuno, K.; Funakoshi, A.; Hashizume, H.; Tokura, Y. Topical application of a vitamin D3 analogue and corticosteroid to psoriasis plaques decreases skin infiltration of TH17 cells and their ex vivo expansion. J. Allergy Clin. Immunol. 2016, 138, 517–528. [Google Scholar] [CrossRef]
- Hashimoto, K.; Matsumoto, K.; Higashiyama, M.; Nishida, Y.; Yoshikawa, K. Growth-inhibitory effects of 1,25-dihydroxyvitamin D3 on normal and psoriatic keratinocytes. Br. J. Dermatol. 1990, 123, 93–98. [Google Scholar] [CrossRef]
- Takahashi, H.; Ibe, M.; Kinouchi, M.; Ishida-Yamamoto, A.; Hashimoto, Y.; Iizuka, H. Similarly potent action of 1,25-dihydroxyvitamin D3 and its analogues, tacalcitol, calcipotriol, and maxacalcitol on normal human keratinocyte proliferation and differentiation. J. Dermatol. Sci. 2003, 31, 21–28. [Google Scholar] [CrossRef]
- Mischke, D.; Korge, B.P.; Marenholz, I.; Volz, A.; Ziegler, A. Genes encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex (“epidermal differentiation complex”) on human chromosome 1q21. J. Invest. Dermatol. 1996, 106, 989–992. [Google Scholar] [CrossRef]
- Backendorf, C.; Hohl, D. A common origin for cornified envelope proteins? Nat. Genet. 1992, 2, 91. [Google Scholar] [CrossRef]
- de Cid, R.; Riveira-Munoz, E.; Zeeuwen, P.L.; Robarge, J.; Liao, W.; Dannhauser, E.N.; Giardina, E.; Stuart, P.E.; Nair, R.; Helms, C.; et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat. Genet. 2009, 41, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Giardina, E.; Capon, F.; De Rosa, M.C.; Mango, R.; Zambruno, G.; Orecchia, A.; Chimenti, S.; Giardina, B.; Novelli, G. Characterization of the loricrin (LOR) gene as a positional candidate for the PSORS4 psoriasis susceptibility locus. Ann. Hum. Genet. 2004, 68, 639–645. [Google Scholar] [CrossRef]
- Zhao, Y.; Terron-Kwiatkowski, A.; Liao, H.; Lee, S.P.; Allen, M.H.; Hull, P.R.; Campbell, L.E.; Trembath, R.C.; Capon, F.; Griffiths, C.E.; et al. Filaggrin null alleles are not associated with psoriasis. J. Invest. Dermatol. 2007, 127, 1878–1882. [Google Scholar] [CrossRef]
- Archer, N.K.; Dilolli, M.N.; Miller, L.S. Pushing the envelope in psoriasis: Late cornified envelope proteins possess antimicrobial activity. J. Invest. Dermatol. 2017, 137, 2257–2259. [Google Scholar] [CrossRef][Green Version]
- Jarnik, M.; de Viragh, P.A.; Scharer, E.; Bundman, D.; Simon, M.N.; Roop, D.R.; Steven, A.C. Quasi-normal cornified cell envelopes in loricrin knockout mice imply the existence of a loricrin backup system. J. Invest. Dermatol. 2002, 118, 102–109. [Google Scholar] [CrossRef]
- Bergboer, J.G.; Tjabringa, G.S.; Kamsteeg, M.; van Vlijmen-Willems, I.M.; Rodijk-Olthuis, D.; Jansen, P.A.; Thuret, J.Y.; Narita, M.; Ishida-Yamamoto, A.; Zeeuwen, P.L.; et al. Psoriasis risk genes of the late cornified envelope-3 group are distinctly expressed compared with genes of other LCE groups. Am. J. Pathol. 2011, 178, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Bergboer, J.G.; Oostveen, A.M.; de Jager, M.E.; Zeeuwen, P.L.; Joosten, I.; Seyger, M.M.; Schalkwijk, J. Koebner phenomenon in psoriasis is not associated with deletion of late cornified envelope genes LCE3B and LCE3C. J. Invest. Dermatol. 2012, 132, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Niehues, H.; Tsoi, L.C.; van der Krieken, D.A.; Jansen, P.A.M.; Oortveld, M.A.W.; Rodijk-Olthuis, D.; van Vlijmen, I.; Hendriks, W.; Helder, R.W.; Bouwstra, J.A.; et al. Psoriasis-Associated Late Cornified Envelope (LCE) Proteins Have Antibacterial Activity. J. Invest. Dermatol. 2017, 137, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzo, M.; Bandiera, A.; Gennaro, R.; Benincasa, M.; Pacor, S.; Antcheva, N.; Scocchi, M. Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 2007, 66, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Z.; Lone, A.G.; Artami, M.; Edwards, M.; Stein, M.; Harris-Tryon, T.A. Small proline-rich proteins 1 and 2 function as antimicrobial proteins in the skin. bioRxiv 2021. [Google Scholar] [CrossRef]
- McFadden, J.; Baker, B.; Powles, A.; Fry, L. Psoriasis and streptococci: The natural selection of psoriasis revisited. Br. J. Dermatol. 2009, 160, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Riveira-Munoz, E.; He, S.-M.; Escaramís, G.; Stuart, P.E.; Hüffmeier, U.; Lee, C.; Kirby, B.; Oka, A.; Giardina, E.; Liao, W. Meta-analysis confirms the LCE3C_LCE3B deletion as a risk factor for psoriasis in several ethnic groups and finds interaction with HLA-Cw6. J. Invest. Dermatol. 2011, 131, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Moyle, M.; Cevikbas, F.; Harden, J.L.; Guttman-Yassky, E. Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches. Exp. Dermatol. 2019, 28, 756–768. [Google Scholar] [CrossRef]
- Kobayashi, T.; Motomura, Y.; Moro, K. Discovery of group 2 innate lymphoid cells has changed the concept of type 2 immune diseases. Int. Immunol. 2021. [Google Scholar] [CrossRef]
- Gowthaman, U.; Chen, J.S.; Zhang, B.; Flynn, W.F.; Lu, Y.; Song, W.; Joseph, J.; Gertie, J.A.; Xu, L.; Collet, M.A.; et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science 2019, 365. [Google Scholar] [CrossRef]
- Kupper, T.S.; Ballard, D.W.; Chua, A.O.; McGuire, J.S.; Flood, P.M.; Horowitz, M.C.; Langdon, R.; Lightfoot, L.; Gubler, U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J. Exp. Med. 1986, 164, 2095–2100. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301–305. [Google Scholar] [CrossRef]
- Gahring, L.C.; Buckley, A.; Daynes, R.A. Presence of epidermal-derived thymocyte activating factor/interleukin 1 in normal human stratum corneum. J. Clin. Invest. 1985, 76, 1585–1591. [Google Scholar] [CrossRef]
- Kezic, S.; O’Regan, G.M.; Lutter, R.; Jakasa, I.; Koster, E.S.; Saunders, S.; Caspers, P.; Kemperman, P.M.; Puppels, G.J.; Sandilands, A.; et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J. Allergy Clin. Immunol. 2012, 129, 1031–1039.e1. [Google Scholar] [CrossRef]
- Harding, C.R.; Aho, S.; Bosko, C.A. Filaggrin—Revisited. Int. J. Cosmet. Sci. 2013, 35, 412–423. [Google Scholar] [CrossRef]
- Ishida-Yamamoto, A.; Igawa, S.; Kishibe, M. Order and disorder in corneocyte adhesion. J. Dermatol. 2011, 38, 645–654. [Google Scholar] [CrossRef]
- Jang, Y.H.; Choi, J.K.; Jin, M.; Choi, Y.-A.; Ryoo, Z.Y.; Lee, H.-S.; Park, P.-H.; Kim, S.-U.; Kwon, T.K.; Jang, M.H. House dust mite increases pro-Th2 cytokines IL-25 and IL-33 via the activation of TLR1/6 signaling. J. Invest. Dermatol. 2017, 137, 2354–2361. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Goswami, R.; Benede, S.; Grishina, G.; Dunkin, D.; Jarvinen, K.M.; Maleki, S.J.; Sampson, H.A.; Berin, M.C. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J. Clin. Invest. 2014, 124, 4965–4975. [Google Scholar] [CrossRef]
- Al Kindi, A.; Williams, H.; Matsuda, K.; Alkahtani, A.M.; Saville, C.; Bennett, H.; Alshammari, Y.; Tan, S.Y.; O’Neill, C.; Tanaka, A. Staphylococcus aureus second immunoglobulin-binding protein drives atopic dermatitis via IL-33. J. Allergy Clin. Immunol. 2021, 147, 1354–1368.e3. [Google Scholar] [CrossRef]
- Moriwaki, M.; Iwamoto, K.; Niitsu, Y.; Matsushima, A.; Yanase, Y.; Hisatsune, J.; Sugai, M.; Hide, M. Staphylococcus aureus from atopic dermatitis skin accumulates in the lysosomes of keratinocytes with induction of IL-1alpha secretion via TLR9. Allergy 2019, 74, 560–571. [Google Scholar] [CrossRef]
- Archer, N.K.; Jo, J.H.; Lee, S.K.; Kim, D.; Smith, B.; Ortines, R.V.; Wang, Y.; Marchitto, M.C.; Ravipati, A.; Cai, S.S.; et al. Injury, dysbiosis, and filaggrin deficiency drive skin inflammation through keratinocyte IL-1alpha release. J. Allergy Clin. Immunol. 2019, 143, 1426–1443.e6. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Briggs, W.H.; Cheng, G.C.; Rossiter, H.B.; Libby, P.; Kupper, T. Mechanical deformation promotes secretion of IL-1 alpha and IL-1 receptor antagonist. J. Immunol. 1997, 159, 5084–5088. [Google Scholar] [PubMed]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.R.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Invest. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Galand, C.; Kam, C.; Burton, O.; Gurish, M.; Musser, M.A.; Goldsmith, J.D.; Hait, E.; Nurko, S.; Brombacher, F.; et al. Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity 2019, 50, 1262–1275.e4. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Igyarto, B.Z.; Honda, T.; Egawa, G.; Otsuka, A.; Hara-Chikuma, M.; Watanabe, N.; Ziegler, S.F.; Tomura, M.; Inaba, K.; et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J. Allergy Clin. Immunol. 2012, 129, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; Larson, R.P.; Ziegler, S.F.; Geha, R.S. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2010, 126, 976–984, 984.e1-5. [Google Scholar] [CrossRef]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, 170ra116. [Google Scholar] [CrossRef]
- Wilson, S.R.; The, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kabata, H.; Kabashima, K.; Asano, K. Anti-TSLP antibodies: Targeting a master regulator of type 2 immune responses. Allergol. Int. 2020, 69, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suarez-Farinas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Cartron, A.M.; Nguyen, T.H.; Roh, Y.S.; Kwatra, M.M.; Kwatra, S.G. Janus kinase inhibitors for atopic dermatitis: A promising treatment modality. Clin. Exp. Dermatol. 2021, 46, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Ishizaki, I.; Kubo, A.; Kawasaki, H.; Nagao, K.; Ohashi, Y.; Amagai, M. The stratum corneum comprises three layers with distinct metal-ion barrier properties. Sci. Rep. 2013, 3, 1731. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Cantieri, J.S.; Teller, D.C.; Lonsdale-Eccles, J.D.; Dale, B.A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc. Natl. Acad. Sci. USA 1981, 78, 4097–4101. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Guttman-Yassky, E. Racial differences in atopic dermatitis. Ann. Allergy Asthma Immunol. 2019, 122, 449–455. [Google Scholar] [CrossRef]
- Margolis, D.J.; Gupta, J.; Apter, A.J.; Ganguly, T.; Hoffstad, O.; Papadopoulos, M.; Rebbeck, T.R.; Mitra, N. Filaggrin-2 variation is associated with more persistent atopic dermatitis in African American subjects. J. Allergy Clin. Immunol. 2014, 133, 784–789. [Google Scholar] [CrossRef]
- Muhandes, L.; Chapsa, M.; Pippel, M.; Behrendt, R.; Ge, Y.; Dahl, A.; Yi, B.; Dalpke, A.; Winkler, S.; Hiller, M.; et al. Low Threshold for Cutaneous Allergen Sensitization but No Spontaneous Dermatitis or Atopy in FLG-Deficient Mice. J. Invest. Dermatol. 2021, 141, 2611–2619. [Google Scholar] [CrossRef]
- Fallon, P.G.; Sasaki, T.; Sandilands, A.; Campbell, L.E.; Saunders, S.P.; Mangan, N.E.; Callanan, J.J.; Kawasaki, H.; Shiohama, A.; Kubo, A.; et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat. Genet. 2009, 41, 602–608. [Google Scholar] [CrossRef]
- Saunders, S.P.; Moran, T.; Floudas, A.; Wurlod, F.; Kaszlikowska, A.; Salimi, M.; Quinn, E.M.; Oliphant, C.J.; Nunez, G.; McManus, R.; et al. Spontaneous atopic dermatitis is mediated by innate immunity, with the secondary lung inflammation of the atopic march requiring adaptive immunity. J. Allergy Clin. Immunol. 2016, 137, 482–491. [Google Scholar] [CrossRef]
- Gruber, R.; Elias, P.M.; Crumrine, D.; Lin, T.K.; Brandner, J.M.; Hachem, J.P.; Presland, R.B.; Fleckman, P.; Janecke, A.R.; Sandilands, A.; et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am. J. Pathol. 2011, 178, 2252–2263. [Google Scholar] [CrossRef]
- Saunders, S.P.; Goh, C.S.; Brown, S.J.; Palmer, C.N.; Porter, R.M.; Cole, C.; Campbell, L.E.; Gierlinski, M.; Barton, G.J.; Schneider, G.; et al. Tmem79/Matt is the matted mouse gene and is a predisposing gene for atopic dermatitis in human subjects. J. Allergy Clin. Immunol. 2013, 132, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Shiohama, A.; Kubo, A.; Kawasaki, H.; Ishida-Yamamoto, A.; Yamada, T.; Hachiya, T.; Shimizu, A.; Okano, H.; Kudoh, J.; et al. A homozygous nonsense mutation in the gene for Tmem79, a component for the lamellar granule secretory system, produces spontaneous eczema in an experimental model of atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 1111–1120. [Google Scholar] [CrossRef]
- Emrick, J.J.; Mathur, A.; Wei, J.; Gracheva, E.O.; Gronert, K.; Rosenblum, M.D.; Julius, D. Tissue-specific contributions of Tmem79 to atopic dermatitis and mast cell-mediated histaminergic itch. Proc. Natl. Acad. Sci. USA 2018, 115, E12091–E12100. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Amado, N.; Tan, J.; Reis, A.; Ge, M.; Abreu, J.G.; He, X. TMEM79/MATTRIN defines a pathway for Frizzled regulation and is required for Xenopus embryogenesis. Elife 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Jonca, N.; Guerrin, M.; Haftek, M.; Bernard, D.; Caubet, C.; Egelrud, T.; Schmidt, R.; Serre, G. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J. Biol. Chem. 2001, 276, 20292–20299. [Google Scholar] [CrossRef] [PubMed]
- Hohl, D.; Mehrel, T.; Lichti, U.; Turner, M.L.; Roop, D.R.; Steinert, P.M. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J. Biol. Chem. 1991, 266, 6626–6636. [Google Scholar] [CrossRef]
- Serre, G.; Mils, V.; Haftek, M.; Vincent, C.; Croute, F.; Reano, A.; Ouhayoun, J.P.; Bettinger, S.; Soleilhavoup, J.P. Identification of late differentiation antigens of human cornified epithelia, expressed in re-organized desmosomes and bound to cross-linked envelope. J. Invest. Dermatol. 1991, 97, 1061–1072. [Google Scholar] [CrossRef]
- Samuelov, L.; Sprecher, E. Peeling off the genetics of atopic dermatitis-like congenital disorders. J. Allergy Clin. Immunol. 2014, 134, 808–815. [Google Scholar] [CrossRef]
- Descargues, P.; Deraison, C.; Bonnart, C.; Kreft, M.; Kishibe, M.; Ishida-Yamamoto, A.; Elias, P.; Barrandon, Y.; Zambruno, G.; Sonnenberg, A.; et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nat. Genet. 2005, 37, 56–65. [Google Scholar] [CrossRef]
- Furio, L.; de Veer, S.; Jaillet, M.; Briot, A.; Robin, A.; Deraison, C.; Hovnanian, A. Transgenic kallikrein 5 mice reproduce major cutaneous and systemic hallmarks of Netherton syndrome. J. Exp. Med. 2014, 211, 499–513. [Google Scholar] [CrossRef]
- Leclerc, E.A.; Huchenq, A.; Mattiuzzo, N.R.; Metzger, D.; Chambon, P.; Ghyselinck, N.B.; Serre, G.; Jonca, N.; Guerrin, M. Corneodesmosin gene ablation induces lethal skin-barrier disruption and hair-follicle degeneration related to desmosome dysfunction. J. Cell Sci. 2009, 122, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Zhou, Y.; Matsuo, S.; Nakanishi, H.; Hirose, K.; Oura, H.; Arase, S.; Ishida-Yamamoto, A.; Bando, Y.; Izumi, K.; et al. Targeted deletion of the murine corneodesmosin gene delineates its essential role in skin and hair physiology. Proc. Natl. Acad. Sci. USA 2008, 105, 6720–6724. [Google Scholar] [CrossRef] [PubMed]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans Cells—The Macrophage in Dendritic Cell Clothing. Trends Immunol 2017, 38, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Suarez-Farinas, M.; Ungar, B.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef]
- Esaki, H.; Brunner, P.M.; Renert-Yuval, Y.; Czarnowicki, T.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; Johnson, D.B.; et al. Early-onset pediatric atopic dermatitis is TH2 but also TH17 polarized in skin. J. Allergy Clin. Immunol. 2016, 138, 1639–1651. [Google Scholar] [CrossRef]
- Hoogendoorn, A.; Avery, T.D.; Li, J.; Bursill, C.; Abell, A.; Grace, P.M. Emerging Therapeutic Applications for Fumarates. Trends Pharmacol. Sci. 2021, 42, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, S.; Koch, M.; Hiebert, H.; Bapst, A.; Gravina, A.; Bloch, W.; Beer, H.D.; Werner, S.; Schafer, M. Genetic activation of Nrf2 reduces cutaneous symptoms in a murine model of Netherton syndrome. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef]
- Schafer, M.; Farwanah, H.; Willrodt, A.H.; Huebner, A.J.; Sandhoff, K.; Roop, D.; Hohl, D.; Bloch, W.; Werner, S. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol. Med. 2012, 4, 364–379. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003, 35, 238–245. [Google Scholar] [CrossRef]
- Schafer, M.; Willrodt, A.H.; Kurinna, S.; Link, A.S.; Farwanah, H.; Geusau, A.; Gruber, F.; Sorg, O.; Huebner, A.J.; Roop, D.R.; et al. Activation of Nrf2 in keratinocytes causes chloracne (MADISH)-like skin disease in mice. EMBO Mol. Med. 2014, 6, 442–457. [Google Scholar] [CrossRef]
- Pickard, C.; Louafi, F.; McGuire, C.; Lowings, K.; Kumar, P.; Cooper, H.; Dearman, R.J.; Cumberbatch, M.; Kimber, I.; Healy, E.; et al. The cutaneous biochemical redox barrier: A component of the innate immune defenses against sensitization by highly reactive environmental xenobiotics. J. Immunol. 2009, 183, 7576–7584. [Google Scholar] [CrossRef]
- Kitagaki, H.; Fujisawa, S.; Watanabe, K.; Hayakawa, K.; Shiohara, T. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J. Invest. Dermatol. 1995, 105, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, K.; Dykes, P.J.; Marks, R. Inflammation due to intra-cutaneous implantation of stratum corneum. Br. J. Exp. Pathol. 1984, 65, 107–115. [Google Scholar] [PubMed]
- Allen, A.; Siegfried, E.; Silverman, R.; Williams, M.L.; Elias, P.M.; Szabo, S.K.; Korman, N.J. Significant absorption of topical tacrolimus in 3 patients with Netherton syndrome. Arch. Dermatol. 2001, 137, 747–750. [Google Scholar] [PubMed]
- O’Shaughnessy, R.F.; Choudhary, I.; Harper, J.I. Interleukin-1 alpha blockade prevents hyperkeratosis in an in vitro model of lamellar ichthyosis. Hum. Mol. Genet. 2010, 19, 2594–2605. [Google Scholar] [CrossRef]
- Murphy, J.E.; Morales, R.E.; Scott, J.; Kupper, T.S. IL-1 alpha, innate immunity, and skin carcinogenesis: The effect of constitutive expression of IL-1 alpha in epidermis on chemical carcinogenesis. J. Immunol. 2003, 170, 5697–5703. [Google Scholar] [CrossRef]
- auf dem Keller, U.; Huber, M.; Beyer, T.A.; Kumin, A.; Siemes, C.; Braun, S.; Bugnon, P.; Mitropoulos, V.; Johnson, D.A.; Johnson, J.A.; et al. Nrf transcription factors in keratinocytes are essential for skin tumor prevention but not for wound healing. Mol. Cell Biol. 2006, 26, 3773–3784. [Google Scholar] [CrossRef]
- Xu, C.; Huang, M.T.; Shen, G.; Yuan, X.; Lin, W.; Khor, T.O.; Conney, A.H.; Kong, A.N. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006, 66, 8293–8296. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.R.; Holscher, C.; Mohrs, M.; Arendse, B.; Schwegmann, A.; Radwanska, M.; Leeto, M.; Kirsch, R.; Hall, P.; Mossmann, H.; et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 2004, 20, 623–635. [Google Scholar] [CrossRef]
- Dalessandri, T.; Crawford, G.; Hayes, M.; Castro Seoane, R.; Strid, J. IL-13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat. Commun. 2016, 7, 12080. [Google Scholar] [CrossRef]
- Strid, J.; Sobolev, O.; Zafirova, B.; Polic, B.; Hayday, A. The intraepithelial T cell response to NKG2D-ligands links lymphoid stress surveillance to atopy. Science 2011, 334, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Strid, J.; Roberts, S.J.; Filler, R.B.; Lewis, J.M.; Kwong, B.Y.; Schpero, W.; Kaplan, D.H.; Hayday, A.C.; Girardi, M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 2008, 9, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Magin, T.M. A keaper and a striker maintain epidermal homeostasis. Nat. Genet. 2003, 35, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Mlitz, V.; Hermann, M.; Rice, R.H.; Eigenheer, R.A.; Alibardi, L.; Tschachler, E.; Eckhart, L. Evolutionary origin and diversification of epidermal barrier proteins in amniotes. Mol. Biol. Evol. 2014, 31, 3194–3205. [Google Scholar] [CrossRef]
- Ehrlich, F.; Fischer, H.; Langbein, L.; Praetzel-Wunder, S.; Ebner, B.; Figlak, K.; Weissenbacher, A.; Sipos, W.; Tschachler, E.; Eckhart, L. Differential Evolution of the Epidermal Keratin Cytoskeleton in Terrestrial and Aquatic Mammals. Mol. Biol. Evol. 2019, 36, 328–340. [Google Scholar] [CrossRef]
- Simon, M.; Tazi-Ahnini, R.; Jonca, N.; Caubet, C.; Cork, M.J.; Serre, G. Alterations in the desquamation-related proteolytic cleavage of corneodesmosin and other corneodesmosomal proteins in psoriatic lesional epidermis. Br. J. Dermatol. 2008, 159, 77–85. [Google Scholar] [CrossRef]
- Allen, M.; Ishida-Yamamoto, A.; McGrath, J.; Davison, S.; Iizuka, H.; Simon, M.; Guerrin, M.; Hayday, A.; Vaughan, R.; Serre, G.; et al. Corneodesmosin expression in psoriasis vulgaris differs from normal skin and other inflammatory skin disorders. Lab Invest. 2001, 81, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.H.; Durbin-Johnson, B.P.; Ishitsuka, Y.; Salemi, M.; Phinney, B.S.; Rocke, D.M.; Roop, D.R. Proteomic Analysis of Loricrin Knockout Mouse Epidermis. J. Proteome Res. 2016, 15, 2560–2566. [Google Scholar] [CrossRef] [PubMed]
- Suga, Y.; Jarnik, M.; Attar, P.S.; Longley, M.A.; Bundman, D.; Steven, A.C.; Koch, P.J.; Roop, D.R. Transgenic mice expressing a mutant form of loricrin reveal the molecular basis of the skin diseases, Vohwinkel syndrome and progressive symmetric erythrokeratoderma. J. Cell Biol. 2000, 151, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Rabia, S.; Baala, L.; Vabres, P.; Hamel-Teillac, D.; Jacquemin, E.; Fabre, M.; Lyonnet, S.; De Prost, Y.; Munnich, A.; Hadchouel, M.; et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterology 2004, 127, 1386–1390. [Google Scholar] [CrossRef]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Tokumasu, R.; Yamaga, K.; Yamazaki, Y.; Murota, H.; Suzuki, K.; Tamura, A.; Bando, K.; Furuta, Y.; Katayama, I.; Tsukita, S. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proc. Natl. Acad. Sci. USA 2016, 113, E4061–E4068. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef]
- Mohammed, J.; Beura, L.K.; Bobr, A.; Astry, B.; Chicoine, B.; Kashem, S.W.; Welty, N.E.; Igyarto, B.Z.; Wijeyesinghe, S.; Thompson, E.A.; et al. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-beta. Nat. Immunol. 2016, 17, 414–421. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-beta structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef]
- Mulcahy, M.E.; Geoghegan, J.A.; Monk, I.R.; O’Keeffe, K.M.; Walsh, E.J.; Foster, T.J.; McLoughlin, R.M. Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin. PLoS Pathog. 2012, 8, e1003092. [Google Scholar] [CrossRef] [PubMed]
- Nishibu, A.; Ward, B.R.; Jester, J.V.; Ploegh, H.L.; Boes, M.; Takashima, A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J. Invest. Dermatol. 2006, 126, 787–796. [Google Scholar] [CrossRef]
- Bynoe, M.S.; Evans, J.T.; Viret, C.; Janeway, C.A., Jr. Epicutaneous immunization with autoantigenic peptides induces T suppressor cells that prevent experimental allergic encephalomyelitis. Immunity 2003, 19, 317–328. [Google Scholar] [CrossRef][Green Version]
- Walczak, A.; Siger, M.; Ciach, A.; Szczepanik, M.; Selmaj, K. Transdermal application of myelin peptides in multiple sclerosis treatment. JAMA Neurol. 2013, 70, 1105–1109. [Google Scholar] [CrossRef]
- Tordesillas, L.; Lozano-Ojalvo, D.; Dunkin, D.; Mondoulet, L.; Agudo, J.; Merad, M.; Sampson, H.A.; Berin, M.C. PDL2(+) CD11b(+) dermal dendritic cells capture topical antigen through hair follicles to prime LAP(+) Tregs. Nat. Commun. 2018, 9, 5238. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Mondoulet, L.; Blazquez, A.B.; Benhamou, P.H.; Sampson, H.A.; Berin, M.C. Epicutaneous immunotherapy induces gastrointestinal LAP(+) regulatory T cells and prevents food-induced anaphylaxis. J. Allergy Clin. Immunol. 2017, 139, 189–201.e4. [Google Scholar] [CrossRef] [PubMed]
- Irvine, A.D.; McLean, W.H. Breaking the (un)sound barrier: Filaggrin is a major gene for atopic dermatitis. J. Invest. Dermatol. 2006, 126, 1200–1202. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishitsuka, Y.; Roop, D.R. The Epidermis: Redox Governor of Health and Diseases. Antioxidants 2022, 11, 47. https://doi.org/10.3390/antiox11010047
Ishitsuka Y, Roop DR. The Epidermis: Redox Governor of Health and Diseases. Antioxidants. 2022; 11(1):47. https://doi.org/10.3390/antiox11010047
Chicago/Turabian StyleIshitsuka, Yosuke, and Dennis R. Roop. 2022. "The Epidermis: Redox Governor of Health and Diseases" Antioxidants 11, no. 1: 47. https://doi.org/10.3390/antiox11010047
APA StyleIshitsuka, Y., & Roop, D. R. (2022). The Epidermis: Redox Governor of Health and Diseases. Antioxidants, 11(1), 47. https://doi.org/10.3390/antiox11010047





