SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Animals
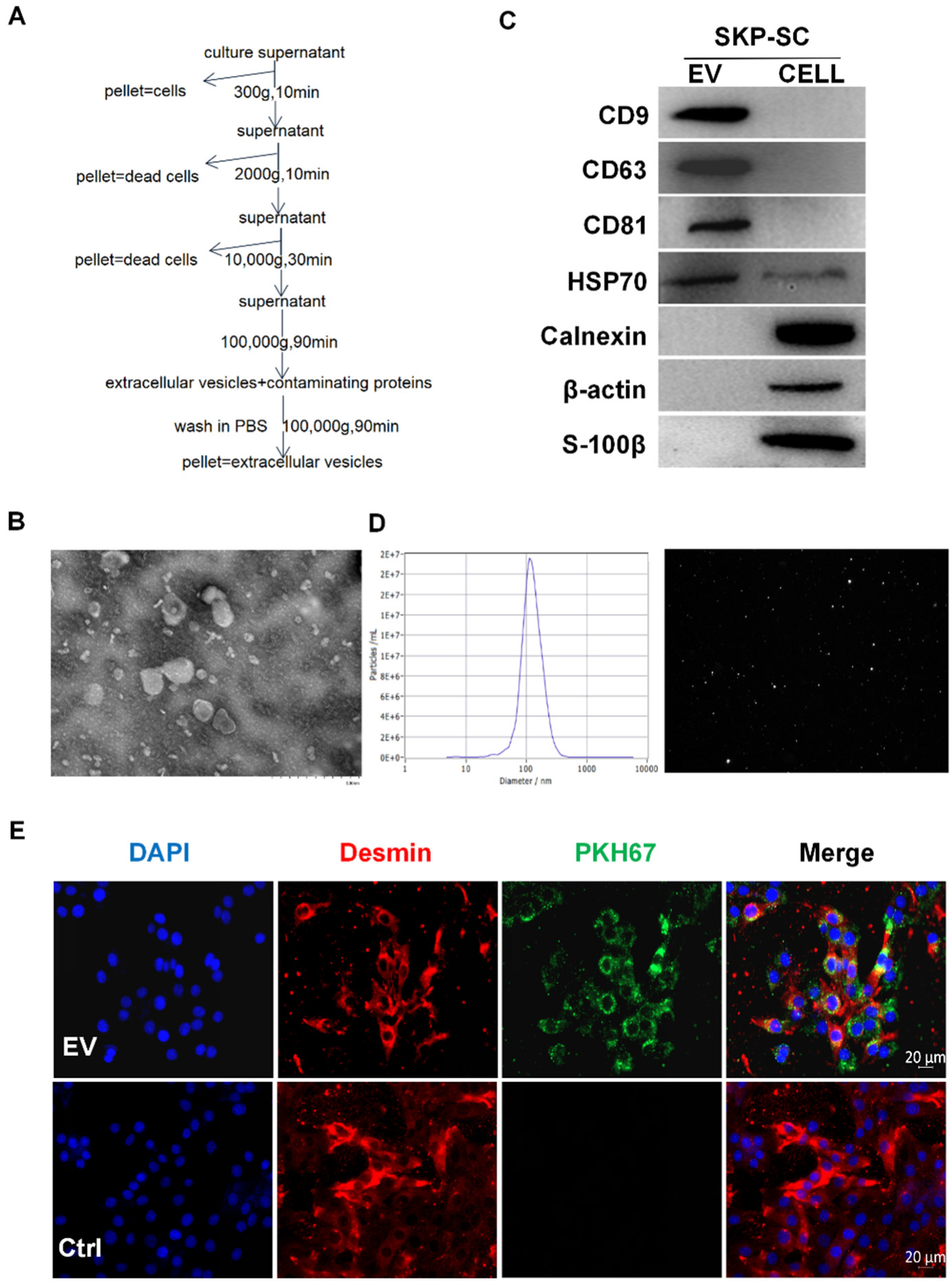
2.3. Isolation and Identification of Vesicles
2.4. Internalization of PKH67-Labelled SKP-SC-EVs
2.5. Immunofluorescence Staining
2.6. Immunohistochemical Analysis
2.7. Transmission Electron Microscopy Analysis
2.8. ROS Detection
2.9. Cell Proliferation Analysis
2.10. Quantitative Reverse Transcriptase-Polymerase Chain Reaction
2.11. Western Blot Analysis
2.12. Ultrasound Imaging Analysis
2.13. Statistical Analysis
3. Results
3.1. Identification of SKP-SCs
3.2. Isolation and Identification of SKP-SC-EVs
3.3. SKP-SC-EVs Promote C2C12 Proliferation and Differentiation
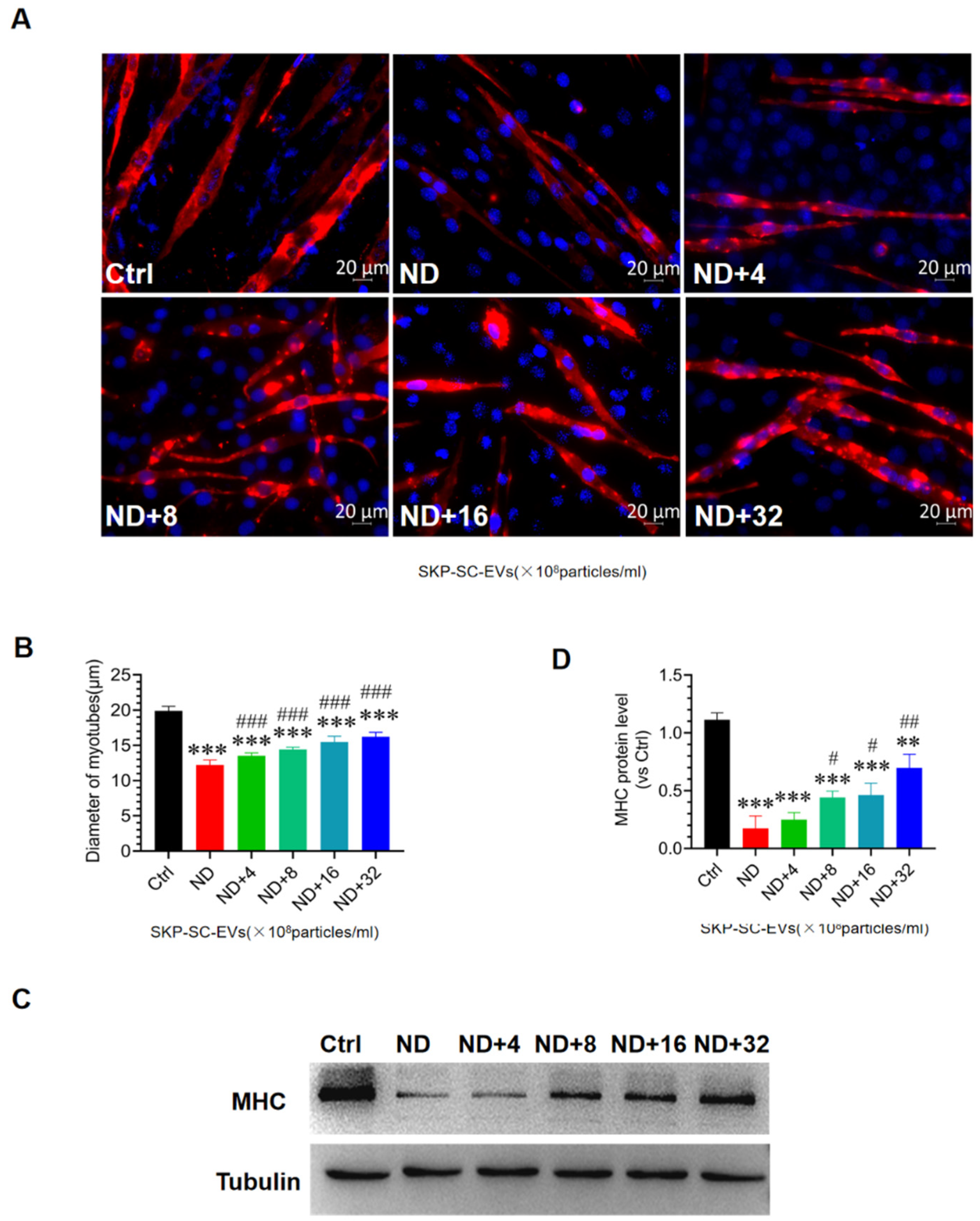
3.4. SKP-SC-EVs Mitigate C2C12 Myotube Atrophy Caused by Nutrient Deprivation
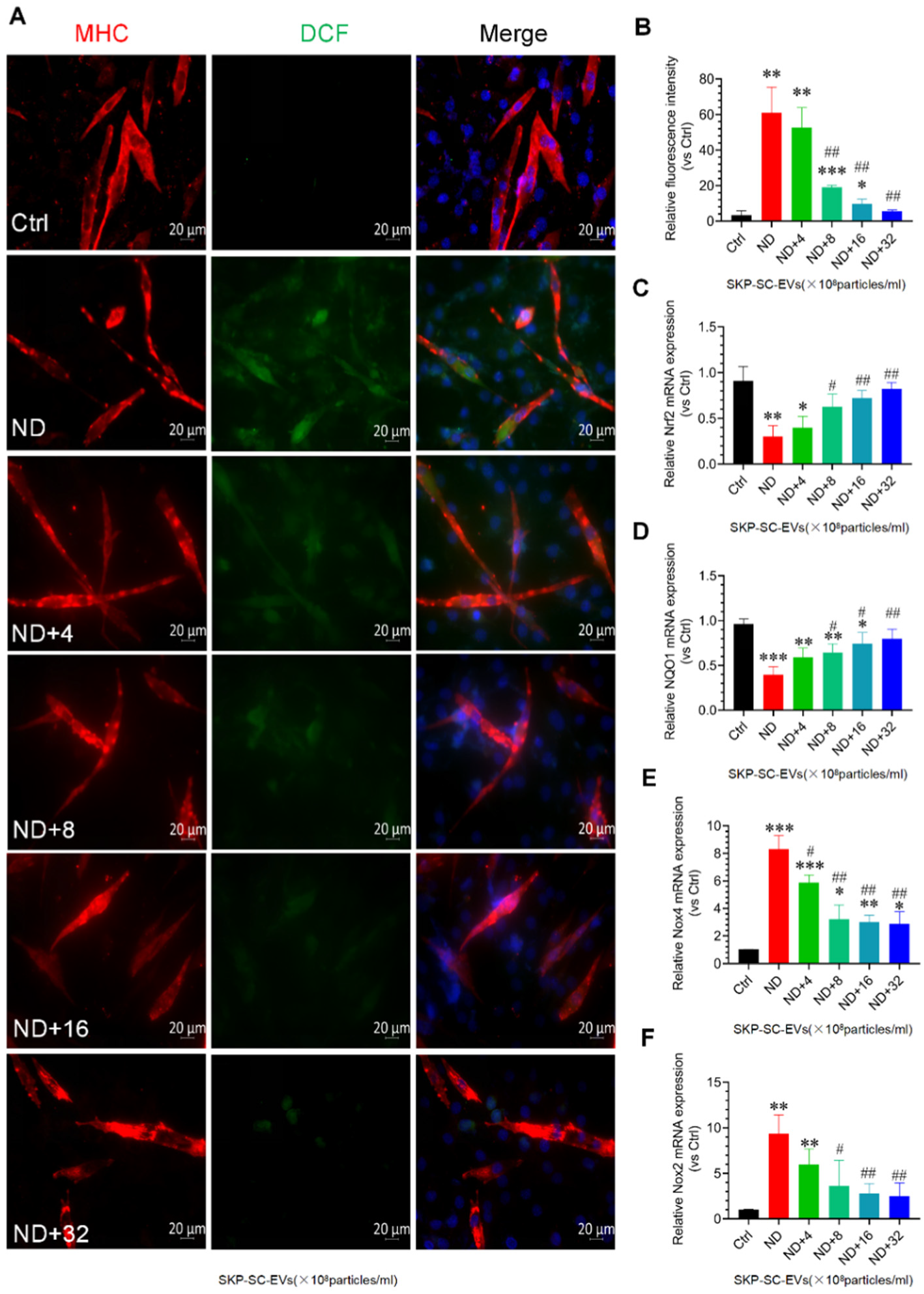
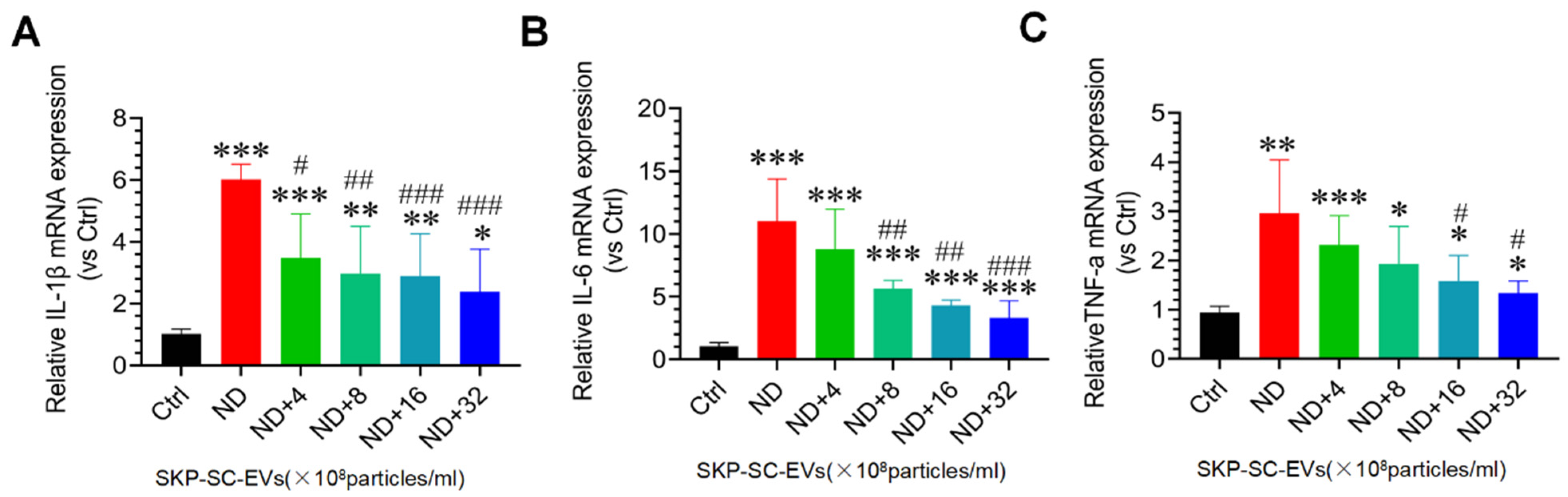
3.5. SKP-SC-EVs Inhibit Nutrient Deprivation-Induced Oxidative Stress and Inflammation in C2C12 Myotubes
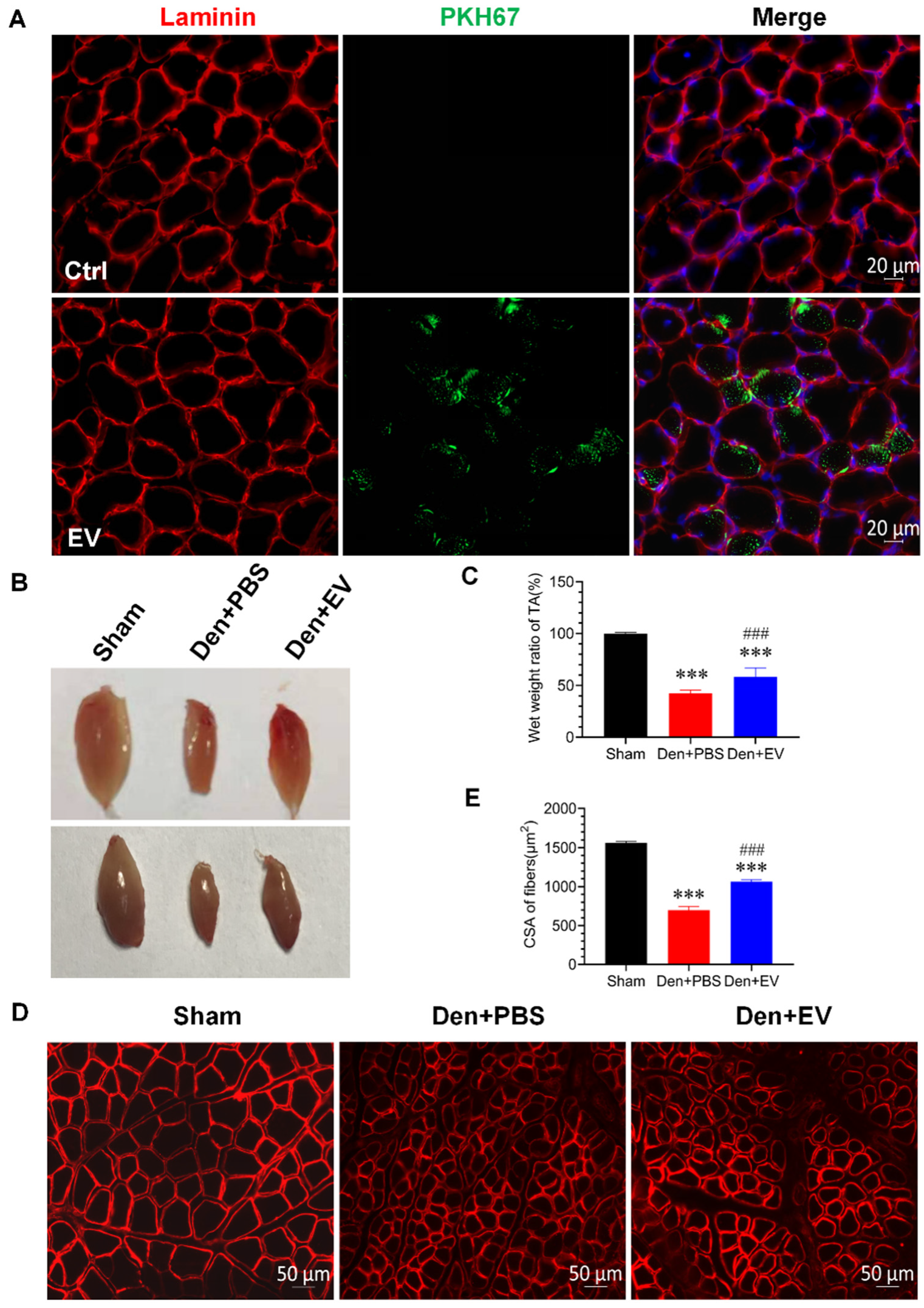
3.6. SKP-SC-EVs Relieve Skeletal Muscle Atrophy Caused by Denervation
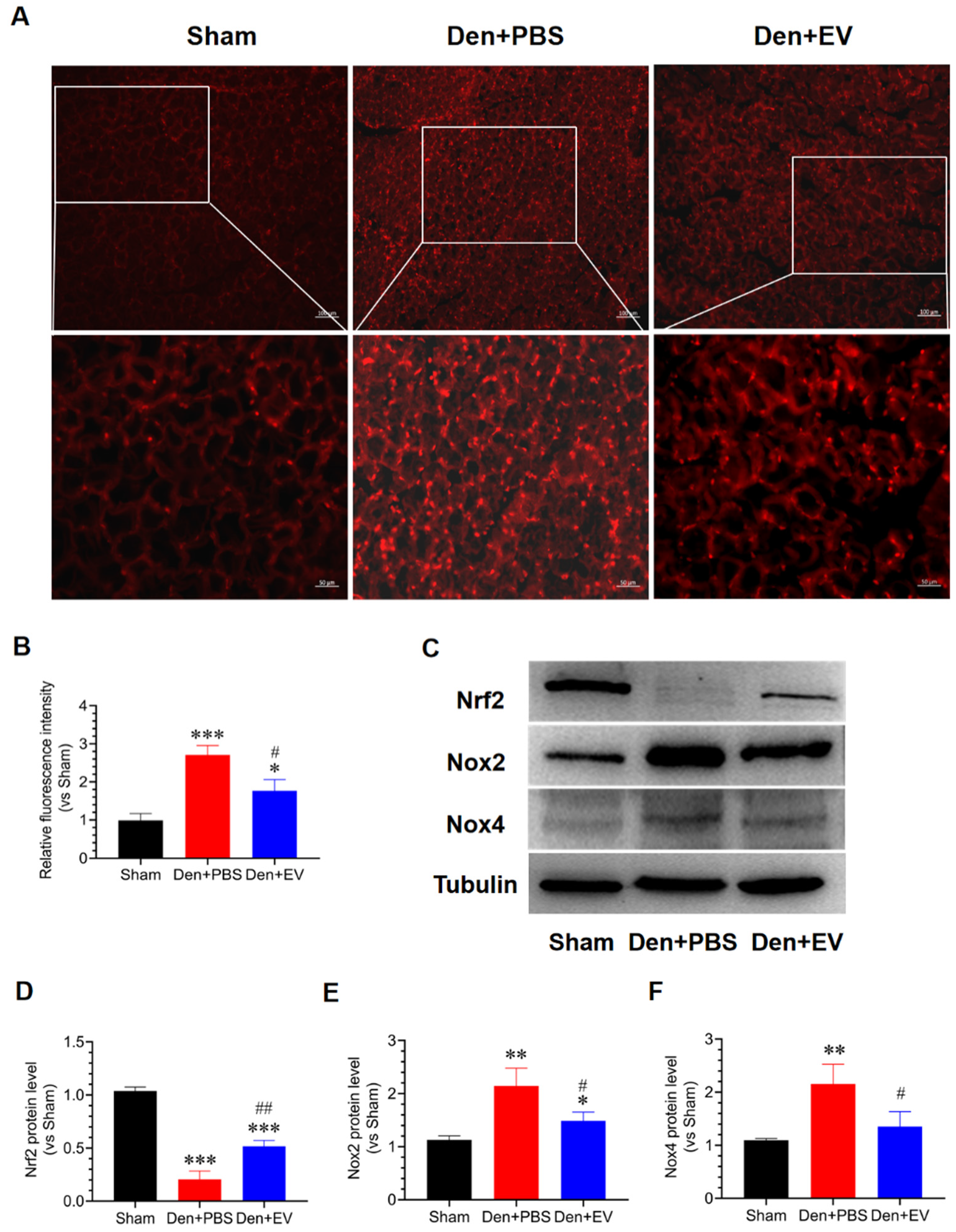
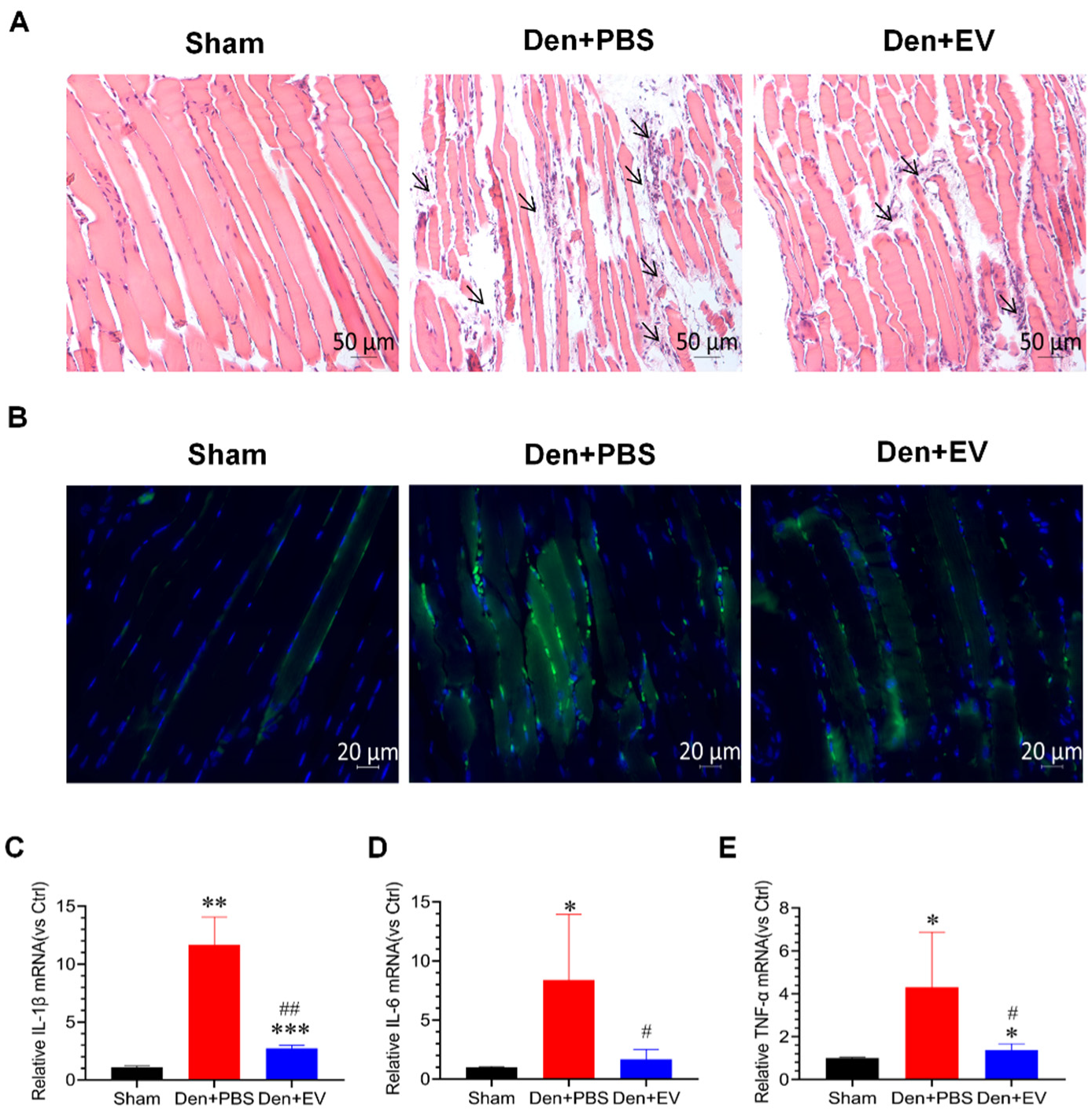
3.7. SKP-SC-EVs Inhibit Oxidative Stress and Inflammation during Denervated Muscle Atrophy
3.8. SKP-SC-EVs Inhibit the UPS Pathway during Denervated Muscle Atrophy
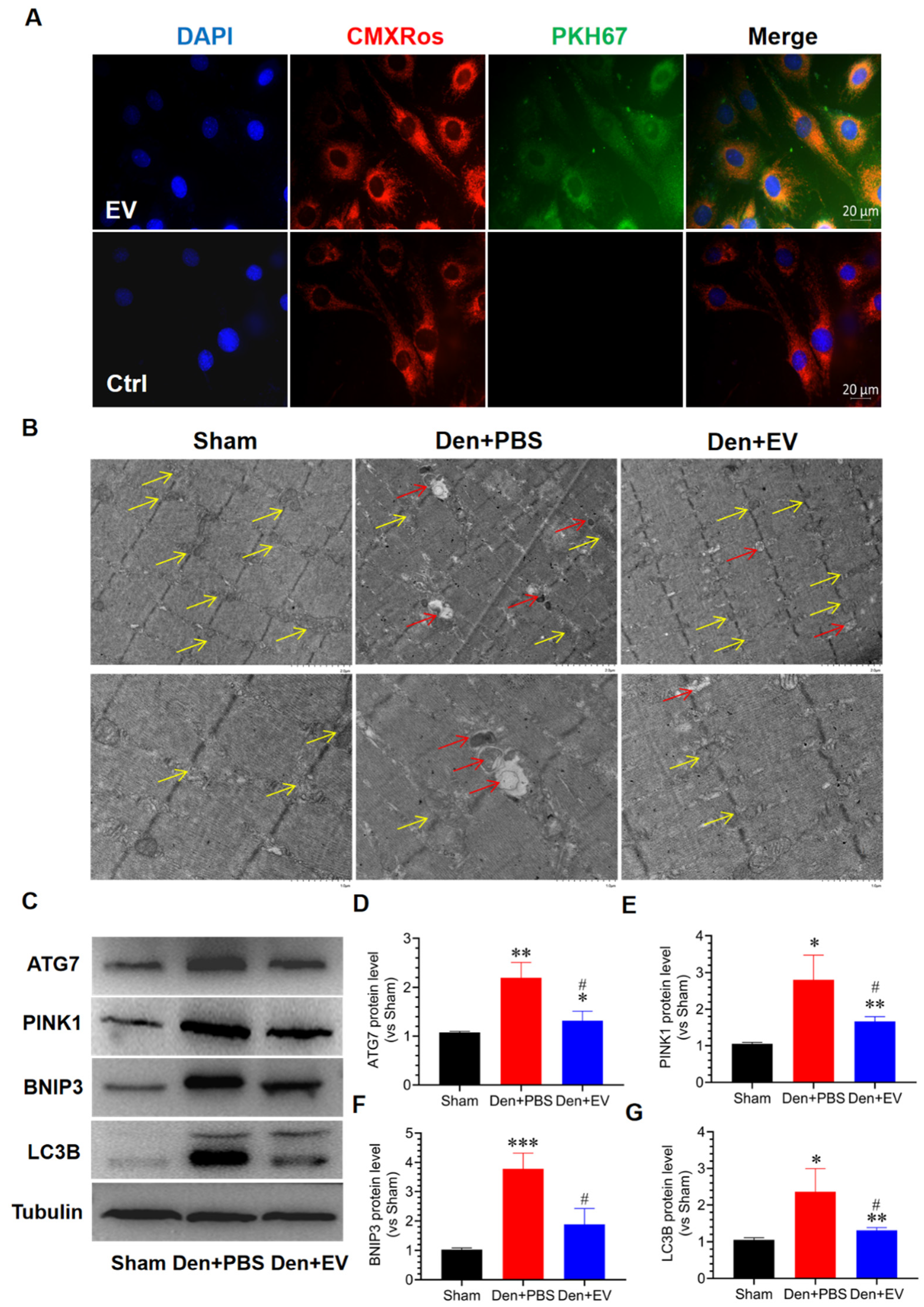
3.9. SKP-SC-EVs Inhibit Mitochondrial Autophagy during Denervated Muscle Atrophy
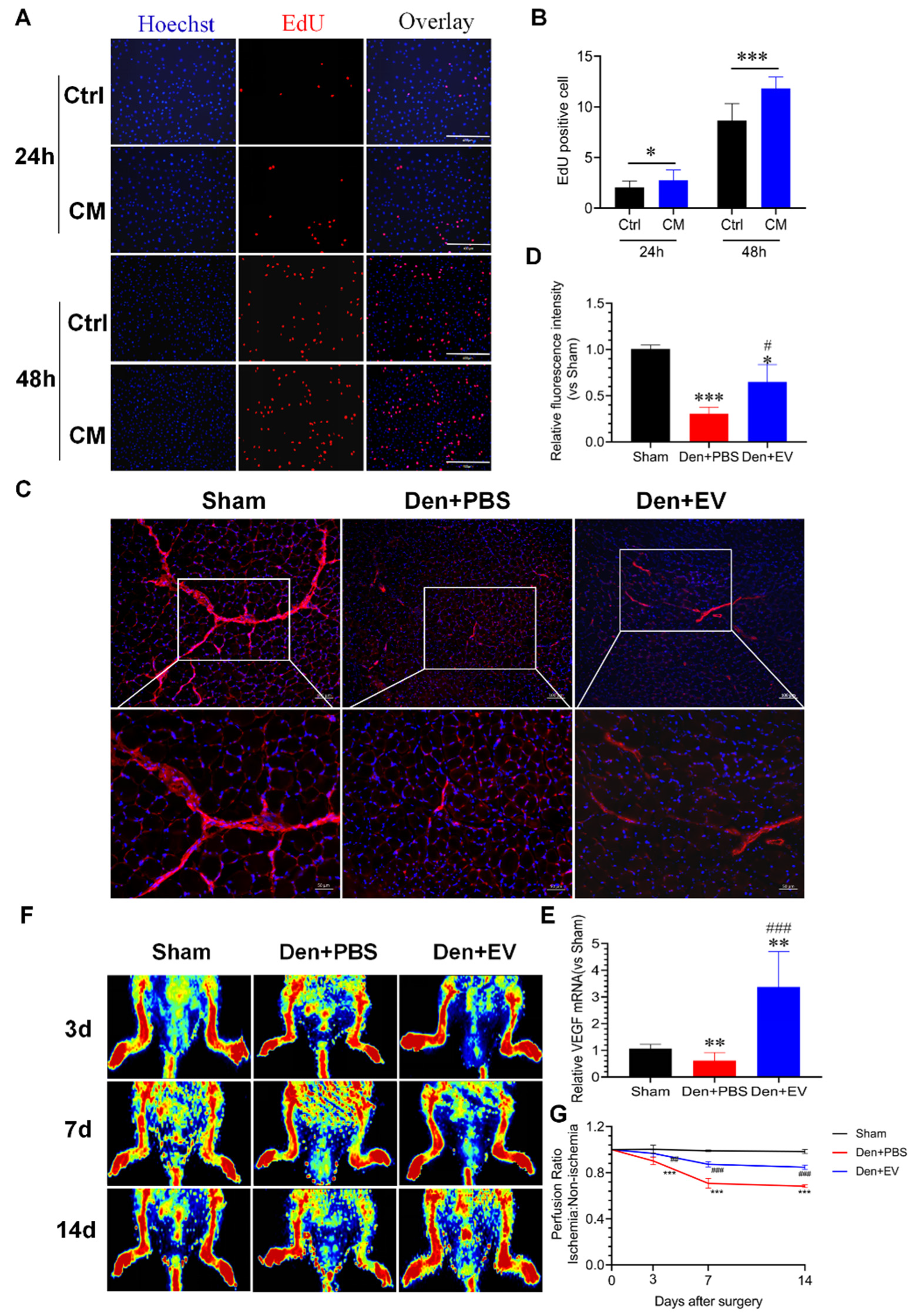
3.10. SKP-SC-EVs Improve Microvascular Blood Flow in the Denervated Skeletal Muscle
4. Discussion
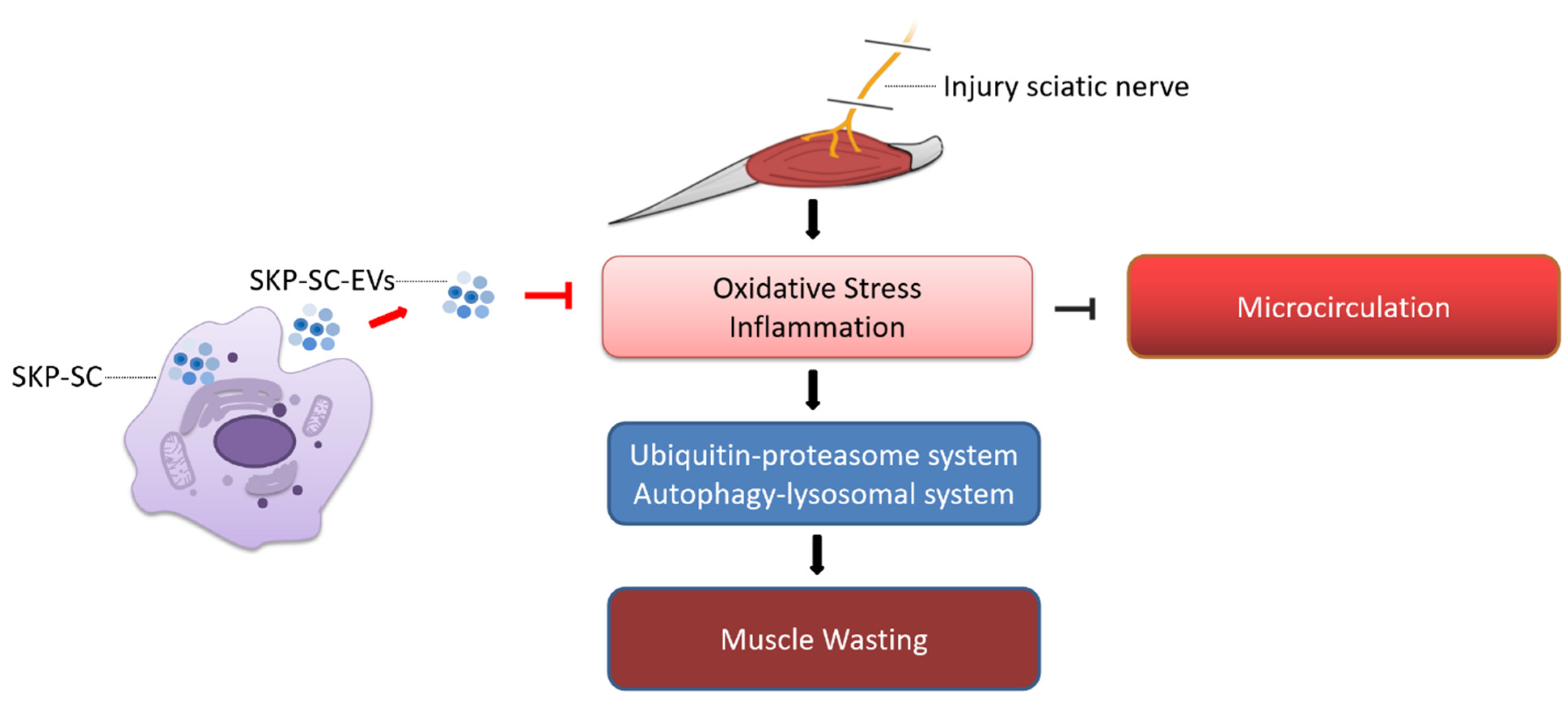
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagai, K.; Misonou, Y.; Fujisaki, Y.; Fuyuki, R.; Horii, Y. Topical application of l-carnosine to skeletal muscle excites the sympathetic nerve innervating the contralateral skeletal muscle in rats. Amino Acids 2019, 51, 39–48. [Google Scholar] [CrossRef]
- Carlson, B.M. The biology of long-term denervated skeletal muscle. Eur. J. Transl. Myol. 2014, 24, 3293. [Google Scholar] [CrossRef]
- Qiu, J.; Fang, Q.; Xu, T.; Wu, C.; Xu, L.; Wang, L.; Yang, X.; Yu, S.; Zhang, Q.; Ding, F.; et al. Mechanistic role of reactive oxygen species and therapeutic potential of antioxidants in denervation- or fasting-induced skeletal muscle atrophy. Front. Physiol. 2018, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Zhang, R.; Xu, L.; Wan, Q.; Zhu, J.; Gu, J.; Huang, Z.; Ma, W.; Shen, M.; Ding, F.; et al. Microarray analysis of gene expression provides new insights into denervation-induced skeletal muscle atrophy. Front. Physiol. 2019, 10, 1298. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex differences in muscle wasting. Ad. Exp. Med. Biol. 2017, 1043, 153–197. [Google Scholar]
- Matsui, Y. Pathological state or cause of sarcopenia. Clin. Calcium 2017, 27, 45–52. [Google Scholar]
- Powers, S.K.; Smuder, A.J.; Judge, A.R. Oxidative stress and disuse muscle atrophy: Cause or consequence? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Costamagna, D.; Costelli, P.; Sampaolesi, M.; Penna, F. Role of inflammation in muscle homeostasis and myogenesis. Mediat. Inflamm. 2015, 2015, 805172. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Sun, J.; Li, M.; Qian, L.; Zhang, L.; Huang, Z.; Shen, Y.; Law, B.Y.; Liu, L.; Gu, X. Transcriptome analysis of immune receptor activation and energy metabolism reduction as the underlying mechanisms in interleukin-6-induced skeletal muscle atrophy. Front. Immunol. 2021, 12, 730070. [Google Scholar] [CrossRef]
- Cai, X.; Yuan, Y.; Liao, Z.; Xing, K.; Zhu, C.; Xu, Y.; Yu, L.; Wang, L.; Wang, S.; Zhu, X.; et al. A-ketoglutarate prevents skeletal muscle protein degradation and muscle atrophy through phd3/adrb2 pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Gong, Y.; Qiu, J.; Chen, Y.; Ding, F.; Zhao, Q. Traf6 inhibition rescues dexamethasone-induced muscle atrophy. Int. J. Mol. Sci. 2014, 15, 11126–11141. [Google Scholar] [CrossRef]
- Khalil, R. Ubiquitin-proteasome pathway and muscle atrophy. Adv. Exp. Med. Biol. 2018, 1088, 235–248. [Google Scholar]
- Ma, W.; Cai, Y.; Shen, Y.; Chen, X.; Zhang, L.; Ji, Y.; Chen, Z.; Zhu, J.; Yang, X.; Sun, H. Hdac4 knockdown alleviates denervation-induced muscle atrophy by inhibiting myogenin-dependent atrogene activation. Front. Cell Neurosci. 2021, 15, 663384. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, L.; Zhu, J.; Xu, H.; Ma, W.; Zhang, L.; Shen, Y.; Law, B.Y.; Ding, F.; Gu, X.; et al. Inhibition of il-6/jak/stat3 pathway rescues denervation-induced skeletal muscle atrophy. Ann. Transl. Med. 2020, 8, 1681. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, L.; Wang, Y.; Zhang, Q.; Ma, W.; Fang, Q.; Sun, H.; Ding, F. Microrna351 targeting traf6 alleviates dexamethasone-induced myotube atrophy. J. Thorac. Dis. 2018, 10, 6238–6246. [Google Scholar] [CrossRef]
- Li, J.; Chan, M.C.; Yu, Y.; Bei, Y.; Chen, P.; Zhou, Q.; Cheng, L.; Chen, L.; Ziegler, O.; Rowe, G.C.; et al. Mir-29b contributes to multiple types of muscle atrophy. Nat. Commun. 2017, 8, 15201. [Google Scholar] [CrossRef] [Green Version]
- Ninfali, C.; Siles, L.; Darling, D.S.; Postigo, A. Regulation of muscle atrophy-related genes by the opposing transcriptional activities of zeb1/ctbp and foxo3. Nucleic Acids Res. 2018, 46, 10697–10708. [Google Scholar] [CrossRef] [Green Version]
- Baracos, V.E. Management of muscle wasting in cancer-associated cachexia: Understanding gained from experimental studies. Cancer 2001, 92, 1669–1677. [Google Scholar] [CrossRef]
- von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Kern, H.; Carraro, U. Home-based functional electrical stimulation for long-term denervated human muscle: History, basics, results and perspectives of the vienna rehabilitation strategy. Eur. J. Transl. Myol. 2014, 24, 3296. [Google Scholar] [CrossRef]
- Lomo, T. The response of denervated muscle to long-term stimulation (1985, revisited here in 2014). Eur. J. Transl. Myol. 2014, 24, 3294. [Google Scholar] [CrossRef]
- Shojaei, S.; Hashemi, S.M.; Ghanbarian, H.; Salehi, M.; Mohammadi-Yeganeh, S. Effect of mesenchymal stem cells-derived exosomes on tumor microenvironment: Tumor progression versus tumor suppression. J. Cell. Physiol. 2019, 234, 3394–3409. [Google Scholar] [CrossRef]
- Albertin, G.; Kern, H.; Hofer, C.; Guidolin, D.; Porzionato, A.; Rambaldo, A.; De Caro, R.; Piccione, F.; Marcante, A.; Zampieri, S. Two years of functional electrical stimulation by large surface electrodes for denervated muscles improve skin epidermis in sci. Eur. J. Transl. Myol. 2018, 28, 7373. [Google Scholar] [CrossRef] [Green Version]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Nooshabadi, V.T.; Mardpour, S.; Yousefi-Ahmadipour, A.; Allahverdi, A.; Izadpanah, M.; Daneshimehr, F.; Ai, J.; Banafshe, H.R.; Ebrahimi-Barough, S. The extracellular vesicles-derived from mesenchymal stromal cells: A new therapeutic option in regenerative medicine. J. Cell. Biochem. 2018, 119, 8048–8073. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [Green Version]
- Upadhya, R.; Madhu, L.N.; Attaluri, S.; Gitaí, D.L.G.; Pinson, M.R.; Kodali, M.; Shetty, G.; Zanirati, G.; Kumar, S.; Shuai, B.; et al. Extracellular vesicles from human ipsc-derived neural stem cells: Mirna and protein signatures, and anti-inflammatory and neurogenic properties. J. Extracell. Vesicles 2020, 9, 1809064. [Google Scholar] [CrossRef]
- Lerner, N.; Chen, I.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular vesicles mediate anti-oxidative response-in vitro study in the ocular drainage system. Int. J. Mol. Sci. 2020, 21, 6105. [Google Scholar] [CrossRef]
- Yadid, M.; Lind, J.U.; Ardoña, H.A.M.; Sheehy, S.P.; Dickinson, L.E.; Eweje, F.; Bastings, M.M.C.; Pope, B.; O’Connor, B.B.; Straubhaar, J.R.; et al. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci. Transl. Med. 2020, 12, 565. [Google Scholar] [CrossRef]
- Gu, X. Biodegradable materials and the tissue engineering of nerves. Engineering 2021. [Google Scholar] [CrossRef]
- Bataille, A.; Leschiera, R.; L’Herondelle, K.; Pennec, J.P.; Le Goux, N.; Mignen, O.; Sakka, M.; Plee-Gautier, E.; Brun, C.; Oddos, T.; et al. In vitro differentiation of human skin-derived cells into functional sensory neurons-like. Cells 2020, 9, 1000. [Google Scholar] [CrossRef] [Green Version]
- Krause, M.P.; Dworski, S.; Feinberg, K.; Jones, K.; Johnston, A.P.; Paul, S.; Paris, M.; Peles, E.; Bagli, D.; Forrest, C.R.; et al. Direct genesis of functional rodent and human schwann cells from skin mesenchymal precursors. Stem Cell Rep. 2014, 3, 85–100. [Google Scholar] [CrossRef]
- Mehrotra, P.; Tseropoulos, G.; Bronner, M.E.; Andreadis, S.T. Adult tissue-derived neural crest-like stem cells: Sources, regulatory networks, and translational potential. Stem Cells Transl. Med. 2020, 9, 328–341. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Zhang, W.; Wang, W.; Shen, J.; Cai, K.; Liu, M.; Cao, M. Skp-scs transplantation alleviates 6-ohda-induced dopaminergic neuronal injury by modulating autophagy. Cell Death Dis. 2021, 12, 674. [Google Scholar] [CrossRef]
- Khuong, H.T.; Kumar, R.; Senjaya, F.; Grochmal, J.; Ivanovic, A.; Shakhbazau, A.; Forden, J.; Webb, A.; Biernaskie, J.; Midha, R. Skin derived precursor schwann cells improve behavioral recovery for acute and delayed nerve repair. Exp. Neurol. 2014, 254, 168–179. [Google Scholar] [CrossRef]
- Jing, H.; He, X.; Zheng, J. Exosomes and regenerative medicine: State of the art and perspectives. Transl. Res. J. Lab. Clin. Med. 2018, 196, 1–16. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Cong, M.; Shen, M.; He, Q.; Ding, F.; Shi, H. Extracellular vesicles from skin precursor-derived schwann cells promote axonal outgrowth and regeneration of motoneurons via akt/mtor/p70s6k pathway. Ann. Transl. Med. 2020, 8, 1640. [Google Scholar] [CrossRef]
- Yu, M.; Gu, G.; Cong, M.; Du, M.; Wang, W.; Shen, M.; Zhang, Q.; Shi, H.; Gu, X.; Ding, F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor schwann cells. Acta Biomater 2021, 134, 190–203. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Y.; Wang, W.; Li, X. Mir-30a-5p ameliorates spinal cord injury-induced inflammatory responses and oxidative stress by targeting neurod 1 through mapk/erk signalling. Clin. Exp. Pharmacol. Physiol. 2018, 45, 68–74. [Google Scholar] [CrossRef]
- Tang, Q.; Len, Q.; Liu, Z.; Wang, W. Overexpression of mir-22 attenuates oxidative stress injury in diabetic cardiomyopathy via sirt 1. Cardiovasc. Ther. 2018, 36, e12318. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Li, M.; Wang, B.; Klein, J.D.; Price, S.R.; Wang, X.H. Mirna-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J. Cachexia Sarcopenia Ad Muscle 2018, 9, 755–770. [Google Scholar] [CrossRef]
- Hou, L.; Xu, J.; Jiao, Y.; Li, H.; Pan, Z.; Duan, J.; Gu, T.; Hu, C.; Wang, C. Mir-27b promotes muscle development by inhibiting mdfi expression. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 2271–2283. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Verrilli, M.A.; Picou, F.; Court, F.A. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 2013, 61, 1795–1806. [Google Scholar] [CrossRef]
- Abrigo, J.; Rivera, J.C.; Simon, F.; Cabrera, D.; Cabello-Verrugio, C. Transforming growth factor type beta (tgf-β) requires reactive oxygen species to induce skeletal muscle atrophy. Cell. Signal. 2016, 28, 366–376. [Google Scholar] [CrossRef]
- Wang, D.; Sun, H.; Song, G.; Yang, Y.; Zou, X.; Han, P.; Li, S. Resveratrol improves muscle atrophy by modulating mitochondrial quality control in stz-induced diabetic mice. Mol. Nutr. Food Res. 2018, 62, e1700941. [Google Scholar] [CrossRef] [Green Version]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta ct) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin-proteasome system by the foxo transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef] [Green Version]
- Guigni, B.A.; Callahan, D.M.; Tourville, T.W.; Miller, M.S.; Fiske, B.; Voigt, T.; Korwin-Mihavics, B.; Anathy, V.; Dittus, K.; Toth, M.J. Skeletal muscle atrophy and dysfunction in breast cancer patients: Role for chemotherapy-derived oxidant stress. Am. J. Physiol. Cell Physiol. 2018, 315, C744–C756. [Google Scholar] [CrossRef]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. Msc-derived exosomes-based therapy for peripheral nerve injury: A novel therapeutic strategy. BioMed Res. Int. 2019, 2019, 6458237. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Brennan, M.; Lötvall, J.; Breakefield, X.O.; El Andaloussi, S. Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med. 2019, 11, 492. [Google Scholar] [CrossRef]
- Kim, S.Y.; Khanal, D.; Tharkar, P.; Kalionis, B.; Chrzanowski, W. None of us is the same as all of us: Resolving the heterogeneity of extracellular vesicles using single-vesicle, nanoscale characterization with resonance enhanced atomic force microscope infrared spectroscopy (afm-ir). Nanoscale Horiz. 2018, 3, 430–438. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, J.; Xue, C.; Wang, Y.; Wang, S.; Bao, S.; Chen, R.; Li, Y.; Gu, Y. Skin derived precursor schwann cell-generated acellular matrix modified chitosan/silk scaffolds for bridging rat sciatic nerve gap. Neurosci. Res. 2018, 135, 21–31. [Google Scholar] [CrossRef]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef]
- Duan, L.; Huang, H.; Zhao, X.; Zhou, M.; Chen, S.; Wang, C.; Han, Z.; Han, Z.C.; Guo, Z.; Li, Z.; et al. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int. J. Mol. Med. 2020, 46, 1551–1561. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 1695–1710. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal stem cell-derived extracellular vesicles attenuate mitochondrial damage and inflammation by stabilizing mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of extracellular vesicle therapeutics: Challenges, considerations, and opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, H.; Wang, Y.; Zhang, L.; Wang, X. Roles of extracellular vesicles in the aging microenvironment and age-related diseases. J. Extracell. Vesicles 2021, 10, e12154. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular vesicles in angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Lopatina, T.; Favaro, E.; Grange, C.; Cedrino, M.; Ranghino, A.; Occhipinti, S.; Fallo, S.; Buffolo, F.; Gaykalova, D.A.; Zanone, M.M.; et al. Pdgf enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia. Sci. Rep. 2018, 8, 17458. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Extracellular vesicles from mesenchymal stem cells activates vegf receptors and accelerates recovery of hindlimb ischemia. J. Control. Release Off. J. Control. Release Soc. 2017, 264, 112–126. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Kuang, Y.; Zheng, X.; Zhang, L.; Ai, X.; Venkataramani, V.; Kilic, E.; Hermann, D.M.; Majid, A.; Bähr, M.; Doeppner, T.R. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of mir-25. J. Extracell. Vesicles 2020, 10, e12024. [Google Scholar] [CrossRef]
- Fujita, Y.; Araya, J.; Ito, S.; Kobayashi, K.; Kosaka, N.; Yoshioka, Y.; Kadota, T.; Hara, H.; Kuwano, K.; Ochiya, T. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in copd pathogenesis. J. Extracell. Vesicles 2015, 4, 28388. [Google Scholar] [CrossRef]


| Gene (Accession Number) | Primer Sequences (5′→3′) | |
|---|---|---|
| Nrf2 (18024) | Forward | TAGATGACCATGAGTCGCTTGC |
| Reverse | GCCAAACTTGCTCCATGTCC | |
| Nox2 (13058) | Forward | TGAATGCCAGAGTCGGGATTT |
| Reverse | CGAGTCACGGCCACATACA | |
| Nox4 (50490) | Forward | TGCCTGCTCATTTGGCTGT |
| Reverse | CCGGCACATAGGTAAAAGGATG | |
| NQO1 (18104) | Forward | AGGATGGGAGGTACTCGAATC |
| Reverse | TGCTAGAGATGACTCGGAAGG | |
| IL-6 (16193) | Forward | CTGCAAGAGACTTCCATCCAG |
| Reverse | AGTGGTATAGACAGGTCTGTTGG | |
| IL-1β (16176) | Forward | GAAATGCCACCTTTTGACAGTG |
| Reverse | TGGATGCTCTCATCAGGACAG | |
| TNF-α (21926) | Forward | CTGAACTTCGGGGTGATCGG |
| Reverse | GGCTTGTCACTCGAATTTTGAGA | |
| VEGF (22339) | Forward | GCTGCTGTAACGATGAAG |
| Reverse | ATCTGCTGTGCTGTAGGA | |
| 18s (19791) | Forward | CAGCCACCCGAGATTGAGCA |
| Reverse | TAGTAGCGACGGGCGGTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Shen, D.; Zhang, L.; Ji, Y.; Xu, L.; Chen, Z.; Shen, Y.; Gong, L.; Zhang, Q.; Shen, M.; et al. SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation. Antioxidants 2022, 11, 66. https://doi.org/10.3390/antiox11010066
Wang W, Shen D, Zhang L, Ji Y, Xu L, Chen Z, Shen Y, Gong L, Zhang Q, Shen M, et al. SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation. Antioxidants. 2022; 11(1):66. https://doi.org/10.3390/antiox11010066
Chicago/Turabian StyleWang, Wei, Dingding Shen, Lilei Zhang, Yanan Ji, Lai Xu, Zehao Chen, Yuntian Shen, Leilei Gong, Qi Zhang, Mi Shen, and et al. 2022. "SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation" Antioxidants 11, no. 1: 66. https://doi.org/10.3390/antiox11010066
APA StyleWang, W., Shen, D., Zhang, L., Ji, Y., Xu, L., Chen, Z., Shen, Y., Gong, L., Zhang, Q., Shen, M., Gu, X., & Sun, H. (2022). SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation. Antioxidants, 11(1), 66. https://doi.org/10.3390/antiox11010066






