N-Acetylcysteine Reverses Monocrotophos Exposure-Induced Hepatic Oxidative Damage via Mitigating Apoptosis, Inflammation and Structural Changes in Rats
Abstract
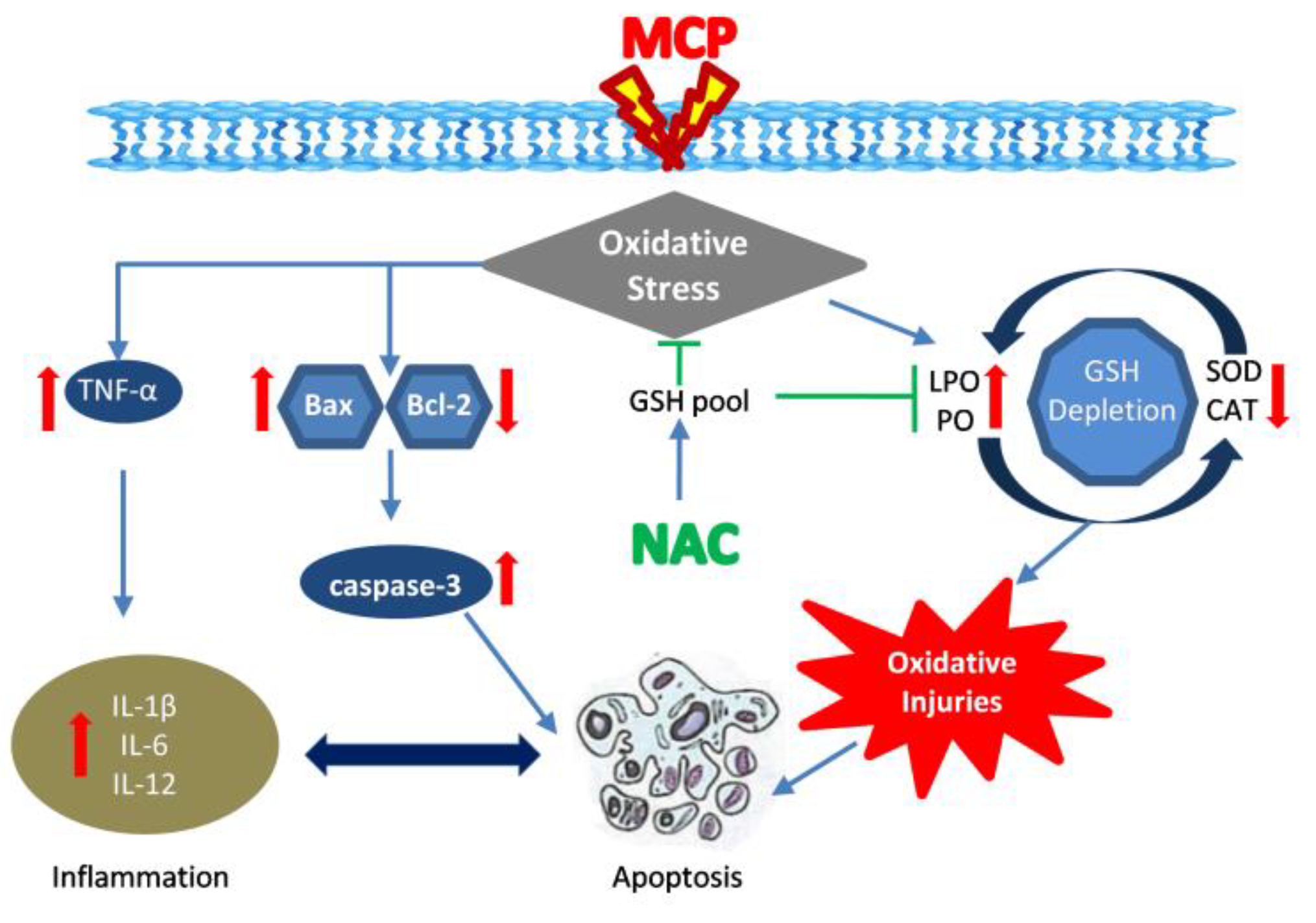
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Their Care
2.3. Experimental Design
- A total of 20 rats were randomly assigned to the following four groups containing 5 rats in each group: Control group (Cont): Rats received 1 mL of distilled water (vehicle), intragastrically, for 28 days.
- N-acetylcysteine Treated Group: Rats received 1 mL of NAC (200 mg/kg b.wt) dissolved in distilled water, intragastrically, for 28 days.
- Monocrotophos Exposure Group: Rats received MCP (0.9 mg/kg b.wt) dissolved in distilled water, intragastrically, for 28 days.
- N-acetylcysteine + MCP Coexposure Group: Rats received MCP and NAC dissolved in distilled water, intragastrically, for 28 days. In this group, NAC was given 2 h before MCP administration.
2.4. Tissue Homogenate Preparation
2.5. Acetylcholinesterase (AChE) Activity Assay
2.6. Estimation of Lipid Peroxidation (LPO)
2.7. Estimation of Protein Oxidation
2.8. Antioxidant Enzyme Assays
2.9. Glutathione Estimation (GSH)
2.10. Gene Expression Analysis of Inflammatory Cytokines
2.11. Western Blot Analysis
2.12. Fourier Transforms Infrared Analysis
2.13. Histolopathological Study
2.14. Electron Microscopy
2.15. Statistical Analysis
3. Results
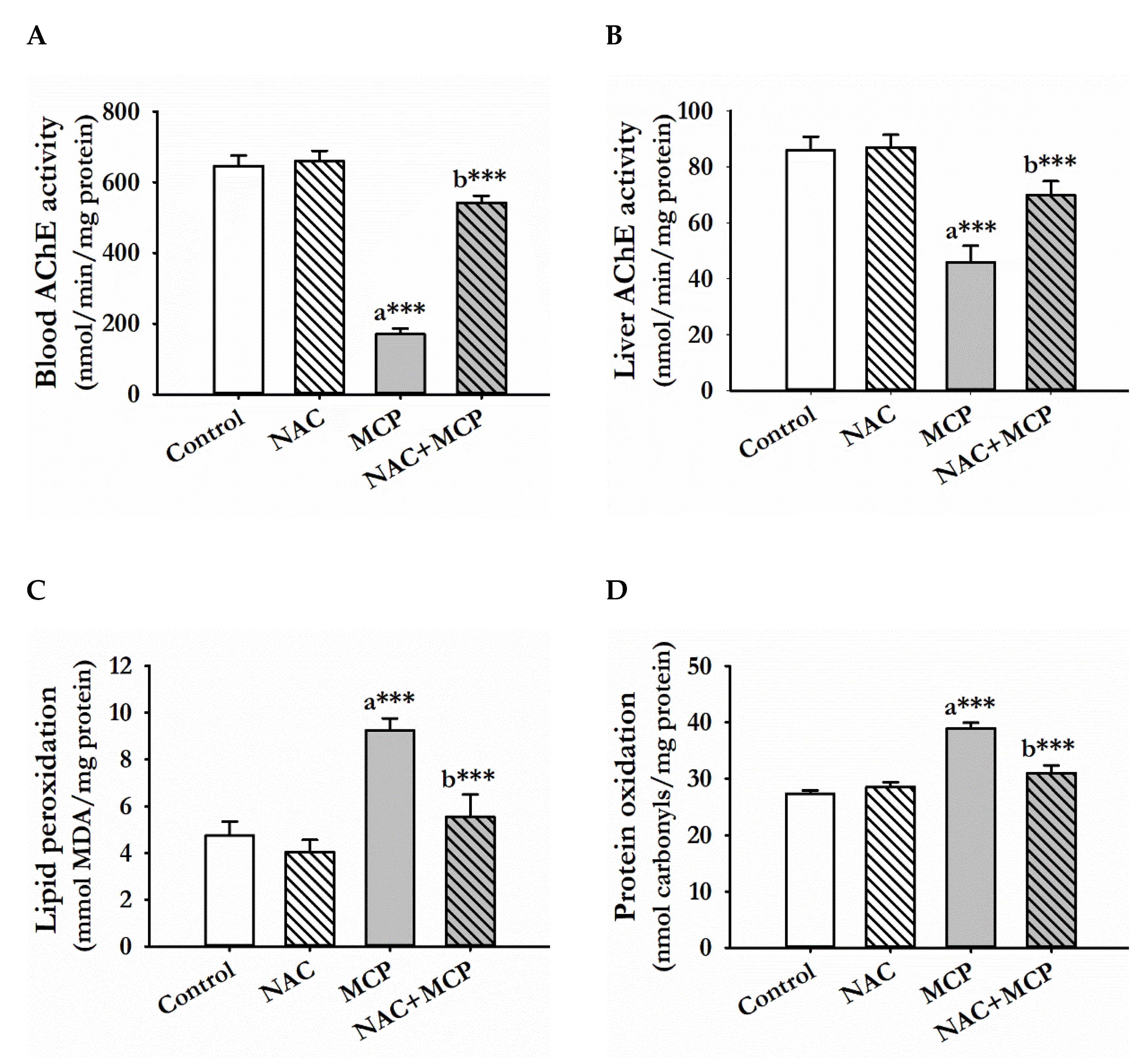
3.1. Acetylcholine Esterase Activity
3.2. Lipid Peroxidation
3.3. Protein Oxidation
3.4. Antioxidant Enzyme Activities
3.5. Glutathione Content
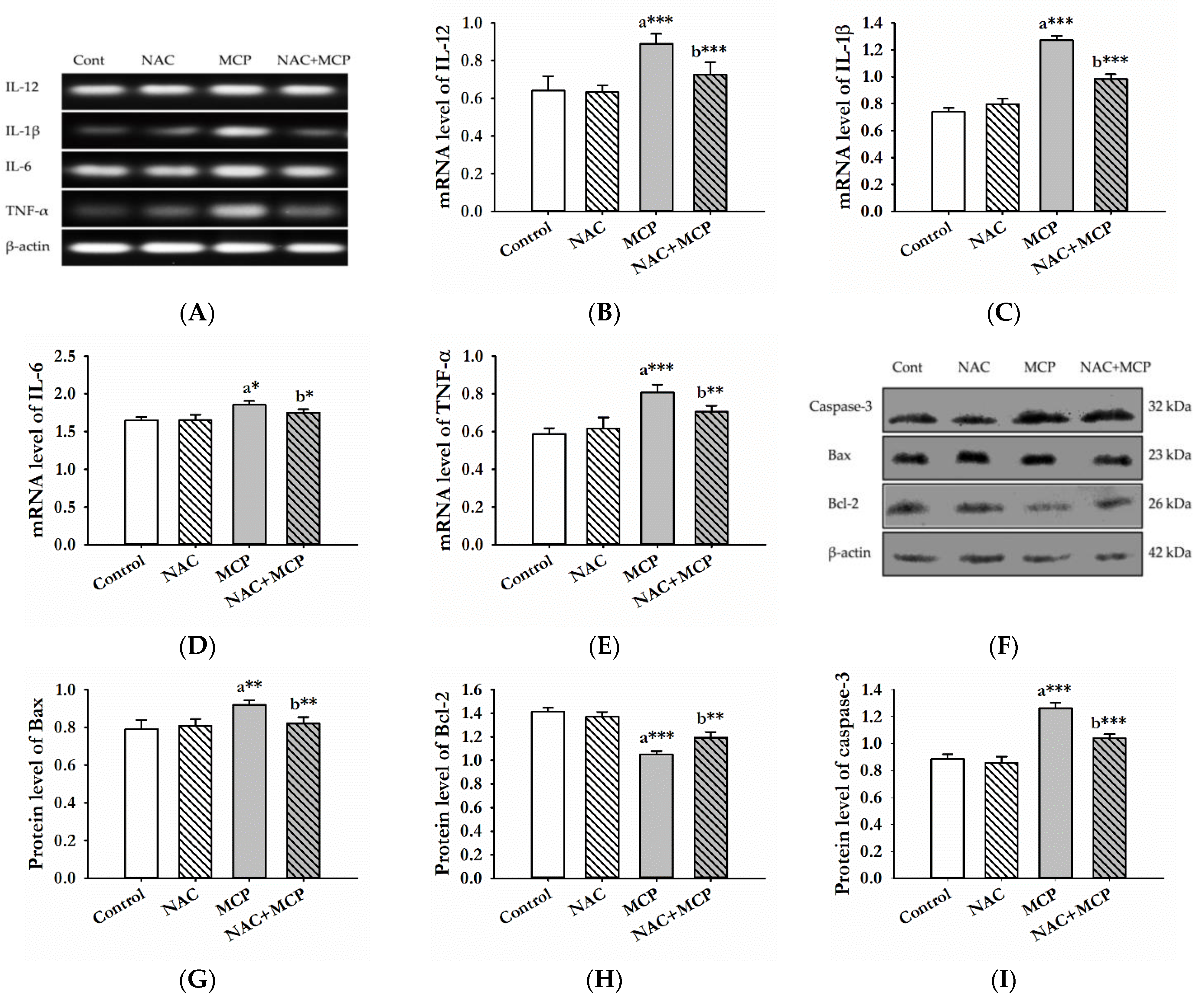
3.6. Evaluation of Pro-Inflammatory Cytokines
3.7. Western Blot Analysis
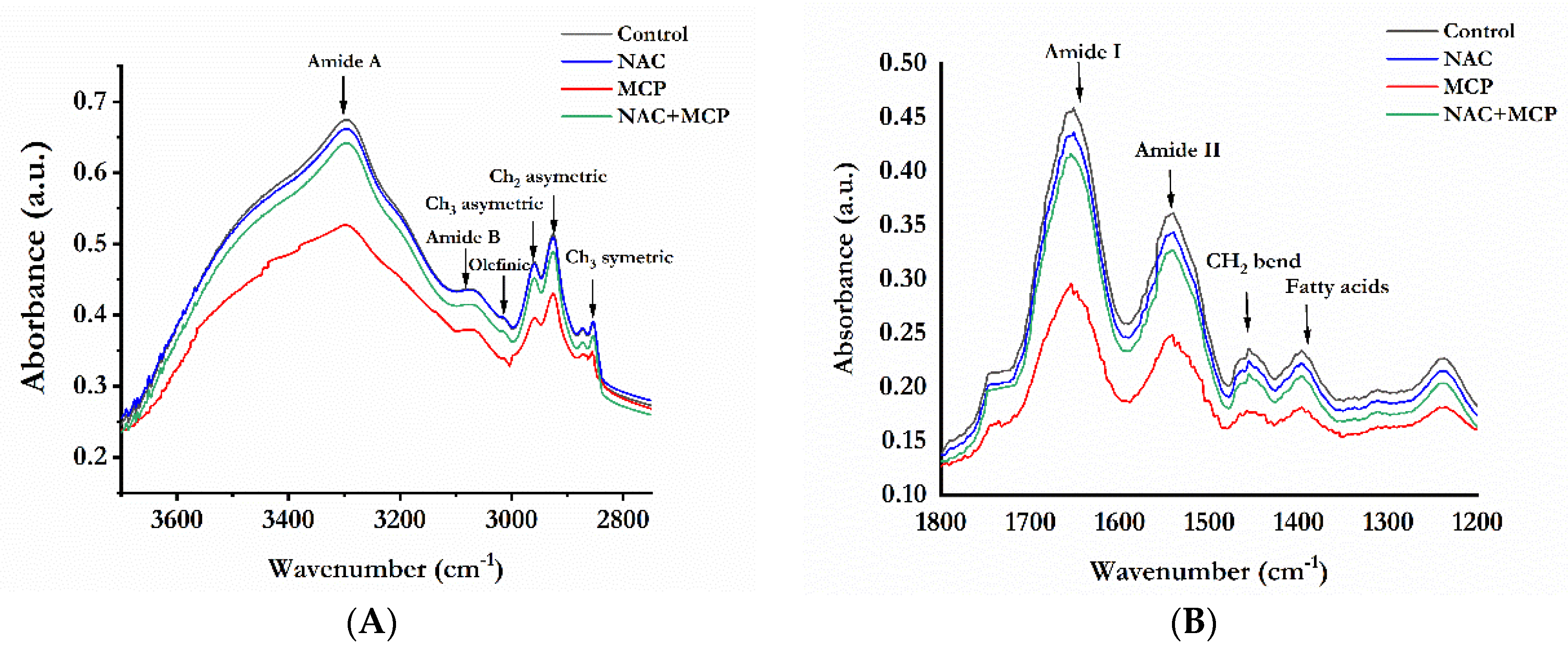
3.8. Fourier Transforms Infrared Analysis of Lipids and Proteins
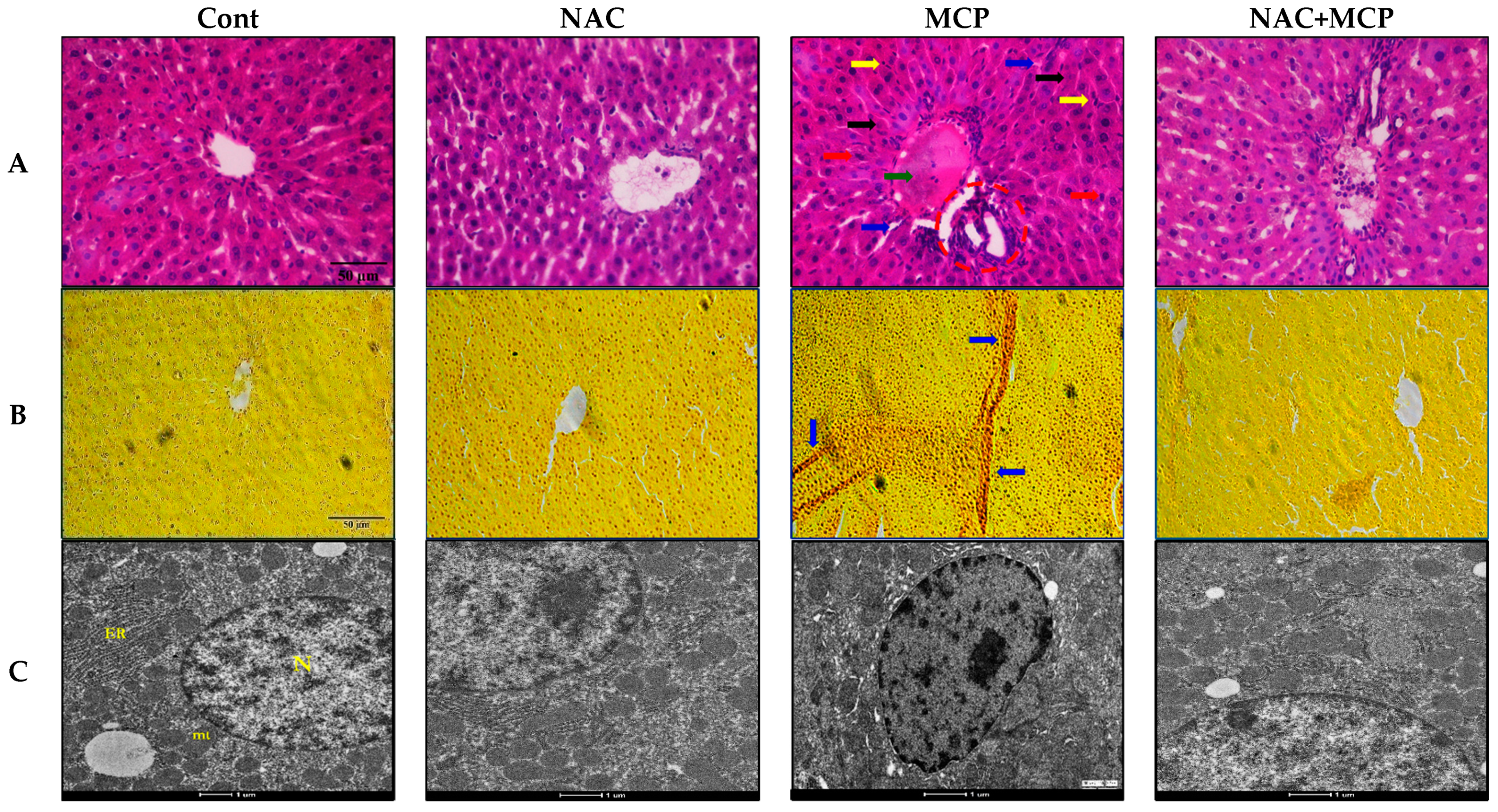
3.9. Histopathology Analysis
3.10. Transmission Electron Microscopy
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dash, D.M.; Osborne, J.W. Biodegradation of Monocrotophos by a Plant Growth Promoting Bacillus Aryabhattai (VITNNDJ5) Strain in Artificially Contaminated Soil. Int. J. Environ. Sci. Technol. 2020, 17, 1475–1490. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.; Singh, R.; Upadhyay, N.; Singh, J.; Pant, P.; Singh, R.; Srivastava, B.; Singh, A.; Subhose, V. Spectral, Structural and Energetic Study of Acephate, Glyphosate, Monocrotophos and Phorate: An Experimental and Computational Approach. J. Taibah Univ. Sci. 2018, 12, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Waite, D.T.; Sommerstad, H.; Grover, R.; Kerr, L.; Westcott, N.D. Pesticides in Ground Water, Surface Water and Spring Runoff in a Small Saskatchewan Watershed. Environ. Toxicol. Chem. Int. J. 1992, 11, 741–748. [Google Scholar] [CrossRef]
- Imran, A.; Hussain, T.; Nadeem, A. Chromatographic Determination of Residual Contents of Pesticides in Rice Samples from Different Geographical Regions of Punjab. FUUAST J. Biol. 2016, 6, 155–160. [Google Scholar]
- Huang, Y.; Shi, T.; Luo, X.; Xiong, H.; Min, F.; Chen, Y.; Nie, S.; Xie, M. Determination of Multi-Pesticide Residues in Green Tea with a Modified QuEChERS Protocol Coupled to HPLC-MS/MS. Food Chem. 2019, 275, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Lee, T.C.; Lin, M.F.; Lin, S.Y. Induction of Sister-Chromatid Exchanges by Pesticides in Primary Rat Tracheal Epithelial Cells and Chinese Hamster Ovary Cells. Mutat. Res./Genet. Toxicol. 1987, 188, 311–321. [Google Scholar] [CrossRef]
- Kumari, B.; Madan, V.K.; Singh, J.; Singh, S.; Kathpal, T.S. Monitoring of Pesticidal Contamination of Farmgate Vegetables from Hisar. Environ. Monit. Assess. 2004, 90, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Arora, K. Toxic Effects of Monocrotophos (an Organophosphate) on Histoarchitecture of Liver–Histopathological Studies. Int. J. Biotechnol. Biochem. 2009, 5, 445–450. [Google Scholar]
- Malik, V.; Singh, J.; Kumar, A.; Kumar, V. Protective Effect of Coenzyme Q10 Nanoparticles against Monocrotophos Induced Oxidative Stress in Kidney Tissues of Rats. Biologia 2021, 76, 1849–1857. [Google Scholar] [CrossRef]
- Sankhwar, M.L.; Yadav, R.S.; Shukla, R.K.; Singh, D.; Ansari, R.W.; Pant, A.B.; Parmar, D.; Khanna, V.K. Monocrotophos Induced Oxidative Stress and Alterations in Brain Dopamine and Serotonin Receptors in Young Rats. Toxicol. Ind. Health 2016, 32, 422–436. [Google Scholar] [CrossRef]
- Mahboob, M.; Rahman, M.F.; Danadevi, K.; Banu, B.S.; Grover, P. Detection of DNA Damage in Mouse Peripheral Blood Leukocytes by the Comet Assay after Oral Administration of Monocrotophos. Drug Chem. Toxicol. 2002, 25, 65–74. [Google Scholar] [CrossRef]
- Konradsen, F. Acute Pesticide Poisoning—A Global Public Health Problem. Dan. Med. Bull. 2007, 54, 58–59. [Google Scholar]
- Hirshhorn, N. Study of the Occupational Health of Indonesian Farmers Who Spray Pesticides. In The Indonesian National IPM Program; FAO: Jakarta, Indonesia, 1993; Volume 23, pp. 12–16. [Google Scholar]
- Loevinsohn, M. Insecticide Use and Increased Mortality in Rural Central Luzon, Philippines. Lancet 1987, 329, 1359–1362. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Kanwar, R.; Wani, A.B.; Gill, J.P.K.; Ramamurthy, P.C.; Singh, J.; Garg, V.K. Toxicity and Detoxification of Monocrotophos from Ecosystem Using Different Approaches: A Review. Chemosphere 2021, 275, 130051. [Google Scholar] [CrossRef]
- Kumar, V.; Upadhyay, N.; Kumar, V.; Kaur, S.; Singh, J.; Singh, S.; Datta, S. Environmental Exposure and Health Risks of the Insecticide Monocrotophos—A Review. J. Biodivers. Environ. Sci. 2014, 5, 111–120. [Google Scholar]
- Yaduvanshi, S.K.; Ojha, A.; Pant, S.C.; Lomash, V.; Srivastava, N. Monocrotophos Induced Lipid Peroxidation and Oxidative DNA Damage in Rat Tissues. Pestic. Biochem. Physiol. 2010, 97, 214–222. [Google Scholar] [CrossRef]
- Sunmonu, T.O.; Oloyede, O.B. Monocrotophos–Induced Enzymatic Changes as Toxicity Bio-Markers in Wistar Rat Liver. Agric. Biol. J. N. Am. 2012, 2151, 7525. [Google Scholar] [CrossRef]
- Begum, K.; Rajini, P.S. Monocrotophos Augments the Early Alterations in Lipid Profile and Organ Toxicity Associated with Experimental Diabetes in Rats. Pestic. Biochem. Physiol. 2011, 99, 33–38. [Google Scholar] [CrossRef]
- Karumuri, S.B.; Singh, H.; Naqvi, S.; Mishra, A.; Flora, S.J.S. Impact of Chronic Low Dose Exposure of Monocrotophos in Rat Brain: Oxidative/ Nitrosative Stress, Neuronal Changes and Cholinesterase Activity. Toxicol. Rep. 2019, 6, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, J.; Yu, X.; Tao, W.; Jiang, F.; Yin, Z.; Liu, C. Protective Effects of Chlorogenic Acid on Acute Hepatotoxicity Induced by Lipopolysaccharide in Mice. Inflamm. Res. 2010, 59, 871–877. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, Y.-H.; Wang, S.-T.; Ren, J.; Camer, D.; Hua, Y.-Z.; Zhang, Q.; Huang, J.; Xue, D.-L.; Zhang, X.-F.; et al. Chlorogenic Acid Protects D-Galactose-Induced Liver and Kidney Injury via Antioxidation and Anti-Inflammation Effects in Mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canbay, A.; Friedman, S.; Gores, G.J. Apoptosis: The Nexus of Liver Injury and Fibrosis. Hepatology 2004, 39, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-X.; Liu, S.-W.; Wang, L.-W.; Wu, S.-H. Physiopathology of Multiple Organ Dysfunctions in Severely Monocrotophos-Poisoned Rabbits. Chem.-Biol. Interact. 2017, 278, 9–14. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Tawil, O.S.; Bungǎu, S.G.; Abdel-Daim, M.M.; Atanasov, A.G. Antioxidants: Scientific Literature Landscape Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 8278454. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, S.; Wang, Y.; Li, J.; Shan, L.; Liu, Q.; Liu, Y.; Song, Q.; Yu, F.; Yu, H. N-Acetyl Cysteine Depletes Reactive Oxygen Species and Prevents Dental Monomer-Induced Intrinsic Mitochondrial Apoptosis in Vitro in Human Dental Pulp Cells. PLoS ONE 2016, 11, e0147858. [Google Scholar] [CrossRef]
- Malik, F.; Kumar, A.; Bhushan, S.; Khan, S.; Bhatia, A.; Suri, K.A.; Qazi, G.N.; Singh, J. Reactive Oxygen Species Generation and Mitochondrial Dysfunction in the Apoptotic Cell Death of Human Myeloid Leukemia HL-60 Cells by a Dietary Compound Withaferin A with Concomitant Protection by N-Acetyl Cysteine. Apoptosis 2007, 12, 2115–2133. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11. [Google Scholar]
- Rakshit, S.; Bagchi, J.; Mandal, L.; Paul, K.; Ganguly, D.; Bhattacharjee, S.; Ghosh, M.; Biswas, N.; Chaudhuri, U.; Bandyopadhyay, S. N-Acetyl Cysteine Enhances Imatinib-Induced Apoptosis of Bcr-Abl+ Cells by Endothelial Nitric Oxide Synthase-Mediated Production of Nitric Oxide. Apoptosis 2009, 14, 298–308. [Google Scholar] [CrossRef]
- Wang, B.; Navath, R.S.; Romero, R.; Kannan, S.; Kannan, R. Anti-Inflammatory and Anti-Oxidant Activity of Anionic Dendrimer–N-Acetyl Cysteine Conjugates in Activated Microglial Cells. Int. J. Pharm. 2009, 377, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-Acetylcysteine (NAC) in Neurological Disorders: Mechanisms of Action and Therapeutic Opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef] [Green Version]
- Minarini, A.; Ferrari, S.; Galletti, M.; Giambalvo, N.; Perrone, D.; Rioli, G.; Galeazzi, G.M. N-Acetylcysteine in the Treatment of Psychiatric Disorders: Current Status and Future Prospects. Expert Opin. Drug Metab. Toxicol. 2017, 13, 279–292. [Google Scholar] [CrossRef]
- Ooi, S.L.; Green, R.; Pak, S.C. N-Acetylcysteine for the Treatment of Psychiatric Disorders: A Review of Current Evidence. BioMed Res. Int. 2018, 2018, 2469486. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.S.; Rani, G.P.; Sainath, S.B.; Meena, R.; Supriya, C. Protective Effects of N-Acetylcysteine against Arsenic-Induced Oxidative Stress and Reprotoxicity in Male Mice. J. Trace Elem. Med. Biol. 2011, 25, 247–253. [Google Scholar] [CrossRef]
- Luczak, M.W.; Zhitkovich, A. Role of Direct Reactivity with Metals in Chemoprotection by N-Acetylcysteine against Chromium (VI), Cadmium (II), and Cobalt (II). Free Radic. Biol. Med. 2013, 65, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Rossignol, D.A. The Use of N-Acetylcysteine as a Chelator for Metal Toxicity. In The Therapeutic Use of N-Acetylcysteine (NAC) in Medicine; Springer: Singapore, 2019; pp. 169–179. [Google Scholar]
- Abdel-Daim, M.M.; Dessouki, A.A.; Abdel-Rahman, H.G.; Eltaysh, R.; Alkahtani, S. Hepatorenal Protective Effects of Taurine and N-Acetylcysteine against Fipronil-Induced Injuries: The Antioxidant Status and Apoptotic Markers Expression in Rats. Sci. Total Environ. 2019, 650, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, R.; Birdane, Y.O.; Demirel, H.H.; Yavuz, H.; Kabu, M.; Ince, S. Antioxidant and Cytoprotective Effects of N-Acetylcysteine against Subchronic Oral Glyphosate-Based Herbicide-Induced Oxidative Stress in Rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 11427–11437. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Akomolafe, A.P.; Imosemi, I.O.; Odunola, O.A.; Oyelere, A.K. N-Acetyl Cysteine Co-Treatment Abates Perfluorooctanoic Acid-Induced Reproductive Toxicity in Male Rats. Andrologia 2021, 53, e14037. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.C.; Zaretsky, M.D.; Dubs, J.G.; Roederer, M.; Anderson, M.; Green, A.; Mitra, D.; Watanabe, N.; Nakamura, H.; Tjioe, I. N-Acetylcysteine Replenishes Glutathione in HIV Infection. Eur. J. Clin. Investig. 2000, 30, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-Acetyl-Cysteine Supplementation on Sperm Quality, Chromatin Integrity and Level of Oxidative Stress in Infertile Men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devi, N.; Boya, C.; Chhabra, M.; Bansal, D. N-Acetyl-Cysteine as Adjuvant Therapy in Female Infertility: A Systematic Review and Meta-Analysis. J. Basic Clin. Physiol. Pharmacol. 2020, 32. [Google Scholar] [CrossRef]
- Ghafarizadeh, A.; Malmir, M.; Naderi Noreini, S.; Faraji, T. Antioxidant Effects of N-Acetylcysteine on the Male Reproductive System: A Systematic Review. Andrologia 2021, 53, e13898. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukla, S.; Kumar, A.; Singh, B.K.; Kumar, V.; Chauhan, A.K.; Singh, D.; Pandey, H.P.; Singh, C. Biochemical and Molecular Mechanisms of N-Acetyl Cysteine and Silymarin-Mediated Protection against Maneb- and Paraquat-Induced Hepatotoxicity in Rats. Chem.-Biol. Interact. 2013, 201, 9–18. [Google Scholar] [CrossRef]
- Galal, A.A.A.; Ramadan, R.A.; Metwally, M.M.M.; El-Sheikh, S.M.A. Protective Effect of N-Acetylcysteine on Fenitrothion-Induced Toxicity: The Antioxidant Status and Metabolizing Enzymes Expression in Rats. Ecotoxicol. Environ. Saf. 2019, 171, 502–510. [Google Scholar] [CrossRef]
- Nagaraju, R.; Joshi, A.K.R.; Vamadeva, S.G.; Rajini, P.S. Deregulation of Hepatic Lipid Metabolism Associated with Insulin Resistance in Rats Subjected to Chronic Monocrotophos Exposure. J. Biochem. Mol. Toxicol. 2020, 34, e22506. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Estimation by Lowry’s Method. J. Biol. Chem. 1951, 193, 265. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Wills, E.D. Mechanisms of Lipid Peroxide Formation in Animal Tissues. Biochem. J. 1966, 99, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Meth. Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.H. A Spectrophotometric Method for Determination of Catalase Activity in Small Tissue Samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A Simple Method for Clinical Assay of Superoxide Dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Prakash, C.; Kumar, V. Chronic Arsenic Exposure-Induced Oxidative Stress Is Mediated by Decreased Mitochondrial Biogenesis in Rat Liver. Biol. Trace Elem. Res. 2016, 173, 87–95. [Google Scholar] [CrossRef]
- Akkas, S.B.; Severcan, M.; Yilmaz, O.; Severcan, F. Effects of Lipoic Acid Supplementation on Rat Brain Tissue: An FTIR Spectroscopic and Neural Network Study. Food Chem. 2007, 105, 1281–1288. [Google Scholar] [CrossRef]
- Chakroun, S.; Ezzi, L.; Grissa, I.; Kerkeni, E.; Neffati, F.; Bhouri, R.; Sallem, A.; Najjar, M.F.; Hassine, M.; Mehdi, M.; et al. Hematological, Biochemical, and Toxicopathic Effects of Subchronic Acetamiprid Toxicity in Wistar Rats. Environ. Sci. Pollut. Res. 2016, 23, 25191–25199. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Jaiswal, P.; Mishra, A. Curcumin Loaded Nanoparticles Reversed Monocrotophos Induced Motor Impairment and Memory Deficit: Role of Oxidative Stress and Intracellular Calcium Level. J. Drug Deliv. Sci. Technol. 2020, 56, 101559. [Google Scholar] [CrossRef]
- Velmurugan, G.; Venkatesh Babu, D.D.; Ramasamy, S. Prolonged Monocrotophos Intake Induces Cardiac Oxidative Stress and Myocardial Damage in Rats. Toxicology 2013, 307, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Rencuzogullari, E.; Canli, M. Investigations on the Effects of Etoxazole in the Liver and Kidney of Wistar Rats. Environ. Sci. Pollut. Res. 2017, 24, 19635–19639. [Google Scholar] [CrossRef]
- Milošević, M.D.; Paunović, M.G.; Matić, M.M.; Ognjanović, B.I.; Saičić, Z.S. Role of Selenium and Vitamin C in Mitigating Oxidative Stress Induced by Fenitrothion in Rat Liver. Biomed. Pharmacother. 2018, 106, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Refaie, A.A.; Ramadan, A.; Sabry, N.M.; Khalil, W.K.B.; Mossa, A.-T.H. Over-Gene Expression in the Apoptotic, Oxidative Damage and Liver Injure in Female Rats Exposed to Butralin. Environ. Sci. Pollut. Res. 2020, 27, 31383–31393. [Google Scholar] [CrossRef]
- Firouzian, F.; Pourshoja, P.; Nili-Ahmadabadi, A.; Ranjbar, A. Hepatoprotective Effect of N-Acetylcystein Loaded Niosomes on Liver Function in Paraquat-Induced Acute Poisoning. Pestic. Biochem. Physiol. 2019, 160, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; Castellano, V.; Martínez, M.A.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A. Cytotoxicity Induced by Deltamethrin and Its Metabolites in SH-SY5Y Cells Can Be Differentially Prevented by Selected Antioxidants. Toxicol. Vitr. 2012, 26, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.K.R.; Rajini, P.S. Hyperglycemic and Stressogenic Effects of Monocrotophos in Rats: Evidence for the Involvement of Acetylcholinesterase Inhibition. Exp. Toxicol. Pathol. 2012, 64, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Bhutia, Y.D.; Kumar, V.; Yadav, P.; Kushwaha, P.; Swarnkar, H.; Flora, S.J.S. Effects of Combined Exposure to Dichlorvos and Monocrotophos on Blood and Brain Biochemical Variables in Rats. Hum. Exp. Toxicol. 2010, 29, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Shadnia, S.; Dasgar, M.; Taghikhani, S.; Mohammadirad, A.; Khorasani, R.; Abdollahi, M. Protective Effects of Alpha-Tocopherol and N-Acetyl-Cysteine on Diazinon-Induced Oxidative Stress and Acetylcholinesterase Inhibition in Rats. Toxicol. Mech. Methods 2007, 17, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.S.; Chopra, K.; Sandhir, R. Neuroprotective Effect of N-Acetylcysteine in the Development of Diabetic Encephalopathy in Streptozotocin-Induced Diabetes. Metab. Brain Dis. 2008, 23, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Oldham, K.M.; Bowen, P.E. Oxidative Stress in Critical Care: Is Antioxidant Supplementation Beneficial? J. Am. Diet. Assoc. 1998, 98, 1001–1008. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Kaur, M.; Sandhir, R. Comparative Effects of Acute and Chronic Carbofuran Exposure on Oxidative Stress and Drug-Metabolizing Enzymes in Liver. Drug Chem. Toxicol. 2006, 29, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lasram, M.M.; Lamine, A.J.; Dhouib, I.B.; Bouzid, K.; Annabi, A.; Belhadjhmida, N.; Ahmed, M.B.; El Fazaa, S.; Abdelmoula, J.; Gharbi, N. Antioxidant and Anti-Inflammatory Effects of N-Acetylcysteine against Malathion-Induced Liver Damages and Immunotoxicity in Rats. Life Sci. 2014, 107, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E. Role of Antioxidants in Paraquat Toxicity. Toxicology 2002, 180, 65–77. [Google Scholar] [CrossRef]
- Tarantino, G.; Di Minno, M.N.D.; Capone, D. Drug-Induced Liver Injury: Is It Somehow Foreseeable? World J. Gastroenterol. WJG 2009, 15, 2817. [Google Scholar] [CrossRef]
- Kartheek, R.M.; David, M. Assessment of Fipronil Toxicity on Wistar Rats: A Hepatotoxic Perspective. Toxicol. Rep. 2018, 5, 448–456. [Google Scholar] [CrossRef]
- Shivanoor, S.M.; David, M. Reversal of Deltamethrin-Induced Oxidative Damage in Rat Neural Tissues by Turmeric-Diet: Fourier Transform-Infrared and Biochemical Investigation. J. Basic Appl. Zool. 2016, 77, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, R.; Sil, P.C. The Protein Fraction of Phyllanthus Niruri Plays a Protective Role against Acetaminophen Induced Hepatic Disorder via Its Antioxidant Properties. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 595–601. [Google Scholar]
- Rao, J.V. Biochemical Alterations in Euryhaline Fish, Oreochromis Mossambicus Exposed to Sub-Lethal Concentrations of an Organophosphorus Insecticide, Monocrotophos. Chemosphere 2006, 65, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Custódio, J.B.; Cardoso, C.M.; Almeida, L.M. Thiol Protecting Agents and Antioxidants Inhibit the Mitochondrial Permeability Transition Promoted by Etoposide: Implications in the Prevention of Etoposide-Induced Apoptosis. Chem.-Biol. Interact. 2002, 140, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Sadegh Soltan-Sharifi, M.; Mojtahedzadeh, M.; Najafi, A.; Reza Khajavi, M.; Reza Rouini, M.; Moradi, M.; Mohammadirad, A.; Abdollahi, M. Improvement by N-Acetylcysteine of Acute Respiratory Distress Syndrome through Increasing Intracellular Glutathione, and Extracellular Thiol Molecules and Anti-Oxidant Power: Evidence for Underlying Toxicological Mechanisms. Hum. Exp. Toxicol. 2007, 26, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Eraslan, G.; Kanbur, M.; Silici, S. Effect of Carbaryl on Some Biochemical Changes in Rats: The Ameliorative Effect of Bee Pollen. Food Chem. Toxicol. 2009, 47, 86–91. [Google Scholar] [CrossRef]
- Yurumez, Y.; Cemek, M.; Yavuz, Y.; Birdane, Y.O.; Buyukokuroglu, M.E. Beneficial Effect of N-Acetylcysteine against Organophosphate Toxicity in Mice. Biol. Pharm. Bull. 2007, 30, 490–494. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.K.J.; Mahboob, M.; Mustafa, M. Hepatic and Extra Hepatic Glutathione Depletion and Glutathione-S-Transferase Inhibition by Monocrotophos and Its Two Thiol Analogues. Toxicology 1990, 64, 271–279. [Google Scholar] [CrossRef]
- Sadowska, A.M.; Manuel-Y-Keenoy, B.; De Backer, W.A. Antioxidant and Anti-Inflammatory Efficacy of NAC in the Treatment of COPD: Discordant in Vitro and in Vivo Dose-Effects: A Review. Pulm. Pharmacol. Ther. 2007, 20, 9–22. [Google Scholar] [CrossRef]
- El-Bini Dhouib, I.; Annabi, A.; Jrad, A.; El-Golli, N.; Gharbi, N.; Lasram, M.M.; El-Fazaa, S. Carbosulfan-Induced Oxidative Damage Following Subchronic Exposure and the Protective Effects of N-Acetylcysteine in Rats. Gen. Physiol. Biophys. 2015, 34, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Mostafalou, S.; Abdollahi, M.; Eghbal, M.A.; Saeedi Kouzehkonani, N. Protective Effect of NAC against Malathion-Induced Oxidative Stress in Freshly Isolated Rat Hepatocytes. Adv. Pharm. Bull. 2012, 2, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Zhou, B. Antioxidative, Anti-Inflammatory and Anti-Apoptotic Effects of Ellagic Acid in Liver and Brain of Rats Treated by D-Galactose. Sci. Rep. 2018, 8, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, J.; Gong, X.; Zhang, M.; Kang, S.; Shu, B.; Wei, Z.; Huang, Z.-S.; Li, D. Upregulation of BCL-2 by Acridone Derivative through Gene Promoter i-Motif for Alleviating Liver Damage of NAFLD/NASH. Nucleic Acids Res. 2020, 48, 8255–8268. [Google Scholar] [CrossRef] [PubMed]
- Karami-Mohajeri, S.; Ahmadipour, A.; Rahimi, H.-R.; Abdollahi, M. Adverse Effects of Organophosphorus Pesticides on the Liver: A Brief Summary of Four Decades of Research. Arh. Hig. Rada Toksikol. 2017, 68, 261–275. [Google Scholar] [CrossRef] [Green Version]
| Gene | Accession # | Direction | Sequence (5′ to 3′) |
|---|---|---|---|
| β-actin | V01217.1 | Forward | TTGCCCTAGACTTCGAGAAA |
| Reverse | AGACTTACAGTGTGGCCTCC | ||
| IL-1β | NM_031512.2 | Forward | GGGATGATGACGACCTGCTA |
| Reverse | TGTCGTTGCTTGTCTCTCCT | ||
| IL-6 | NM_012589.2 | Forward | AGCCAGAGTCATTCAGAGCA |
| Reverse | GGTCTTGGTCCTTAGCCACT | ||
| IL-12 | NM_022611.1 | Forward | GATGCTGGCCAATACACCTG |
| Reverse | CAAGTCCGTGTTTCTGTGCA | ||
| TNF-α | NM_012675.3 | Forward | CATGAGCACGGAAAGCATGA |
| Reverse | TAGACAGAAGAGCGTGGTGG |
| Group | SOD (U/mg Protein) | Catalase (μmol H2 O2 Decomposed /min/mg Protein) | GSH (nmol/mg Protein) |
|---|---|---|---|
| Cont | 8.73 ± 0.35 | 50.80 ± 4.82 | 41.66 ± 4.08 |
| NAC | 8.41 ± 0.60 | 52.17 ± 4.60 | 43.73 ± 2.77 |
| MCP | 5.44 ± 0.51 a *** | 28.20 ± 3.56 a *** | 25.61 ± 2.03 a *** |
| NAC + MCP | 7.03 ± 0.37 b *** | 40.20 ± 5.07 b *** | 36.94 ± 2.01 b *** |
| Wavenumber (cm−1) | Peak Assignment | |||
|---|---|---|---|---|
| Control | NAC | MCP | NAC + MCP | |
| 3297 | 3297 | 3294 | 3296 | N-H stretch of proteins: mainly amide A |
| 3080 | 3081 | 3077 | 3080 | N-H stretch of proteins: mainly amide B |
| 3014 | 3014 | 3012 | 3014 | Olefinic = C-H stretch: unsaturated lipids |
| 2959 | 2961 | 2952 | 2957 | CH3asymmetric stretching: mainly lipids |
| 2925 | 2926 | 2921 | 2923 | CH2 asymmetrical stretching: mainly lipids |
| 2854 | 2854 | 2852 | 2854 | CH2symmetric stretch: lipids |
| 1745 | 1744 | 1738 | 1742 | Ester C = O stretch: lipids |
| 1652 | 1653 | 1649 | 1652 | C = O stretch of proteins: amide I |
| 1541 | 1541 | 1537 | 1540 | N-H bend, C-N stretch of proteins: amide II |
| 1456 | 1458 | 1451 | 1456 | CH2 bend: lipid and protein |
| 1397 | 1398 | 1393 | 1396 | COO- symmetric stretch: fatty acids |
| Peak Position | Experiment Groups | |||
|---|---|---|---|---|
| Cont | NAC | MCP | NAC + MCP | |
| 3297 cm−1 | 170.33 ± 3.42 | 169.95 ± 2.96 | 108.00 ± 4.07 a *** | 163.05 ± 3.17 b *** |
| 3080 cm−1 | 17.97 ± 1.35 | 18.44 ± 1.73 | 12.46 ± 0.93 a *** | 16.73 ± 1.12 b *** |
| 3014 cm−1 | 2.53 ± 0.35 | 2.69 ± 0.41 | 1.77 ± 0.14 a *** | 2.44 ± 0.27 b *** |
| 2959 cm−1 | 9.49 ± 0.86 | 9.53 ± 0.94 | 07.70 ± 1.01 a * | 08.72 ± 0.78 b * |
| 2925 cm−1 | 14.08 ± 0.61 | 14.14 ± 0.53 | 11.25 ± 0.76 a ** | 12.58 ± 0.62 b ** |
| 2854 cm−1 | 20.70 ± 0.77 | 21.07 ± 0.87 | 14.67 ± 1.12 a *** | 18.88 ± 0.68 b *** |
| 1745 cm−1 | 01.53 ± 0.15 | 01.62 ± 0.12 | 0.93 ± 0.12 a *** | 01.37 ± 0.08 b *** |
| 1652 cm−1 | 29.96 ± 1.73 | 29.12 ± 1.23 | 14.68 ± 1.52 a *** | 25.28 ± 1.28 b *** |
| 1540 cm−1 | 17.41 ± 1.27 | 16.77 ± 1.02 | 09.01 ± 1.36 a *** | 14.89 ± 1.11 b *** |
| 1456 cm−1 | 04.39 ± 0.38 | 04.58 ± 0.59 | 02.38 ± 0.37 a *** | 03.49 ± 0.45 b *** |
| 1397 cm−1 | 05.69 ± 0.72 | 05.87 ± 0.44 | 03.23 ± 0.60 a *** | 04.48 ± 0.19 b *** |
| Parameters | Control | NAC | MCP | NAC + MCP |
|---|---|---|---|---|
| H and E Stain | ||||
| Congestion of central vein | - | - | ++ | + |
| Sinusoidal space | - | - | +++ | + |
| Inflammation and infiltrated cells | - | - | +++ | + |
| Apoptotic hepatocytes | - | - | ++ | + |
| Pyknotic nuclei | - | - | +++ | - |
| Activated kuffer cells | - | - | ++ | - |
| Van Gieson’s Stain | ||||
| Focal necrosis plaques | - | - | ++ | + |
| Replication of collagen fibers | - | - | +++ | + |
| Fibrotic generation | - | - | ++ | - |
| Transmission Electron Microscopy | ||||
| Ovulated nucleus | - | - | +++ | + |
| Breaks in nuclear envelope | - | - | +++ | - |
| Hyper chromatic chromatids | - | - | +++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.; Phogat, A.; Prakash, C.; Chhikara, S.K.; Singh, S.; Malik, V.; Kumar, V. N-Acetylcysteine Reverses Monocrotophos Exposure-Induced Hepatic Oxidative Damage via Mitigating Apoptosis, Inflammation and Structural Changes in Rats. Antioxidants 2022, 11, 90. https://doi.org/10.3390/antiox11010090
Singh J, Phogat A, Prakash C, Chhikara SK, Singh S, Malik V, Kumar V. N-Acetylcysteine Reverses Monocrotophos Exposure-Induced Hepatic Oxidative Damage via Mitigating Apoptosis, Inflammation and Structural Changes in Rats. Antioxidants. 2022; 11(1):90. https://doi.org/10.3390/antiox11010090
Chicago/Turabian StyleSingh, Jagjeet, Annu Phogat, Chandra Prakash, Sunil Kumar Chhikara, Sandeep Singh, Vinay Malik, and Vijay Kumar. 2022. "N-Acetylcysteine Reverses Monocrotophos Exposure-Induced Hepatic Oxidative Damage via Mitigating Apoptosis, Inflammation and Structural Changes in Rats" Antioxidants 11, no. 1: 90. https://doi.org/10.3390/antiox11010090
APA StyleSingh, J., Phogat, A., Prakash, C., Chhikara, S. K., Singh, S., Malik, V., & Kumar, V. (2022). N-Acetylcysteine Reverses Monocrotophos Exposure-Induced Hepatic Oxidative Damage via Mitigating Apoptosis, Inflammation and Structural Changes in Rats. Antioxidants, 11(1), 90. https://doi.org/10.3390/antiox11010090






