GPX4 Alleviates Diabetes Mellitus-Induced Erectile Dysfunction by Inhibiting Ferroptosis
Abstract
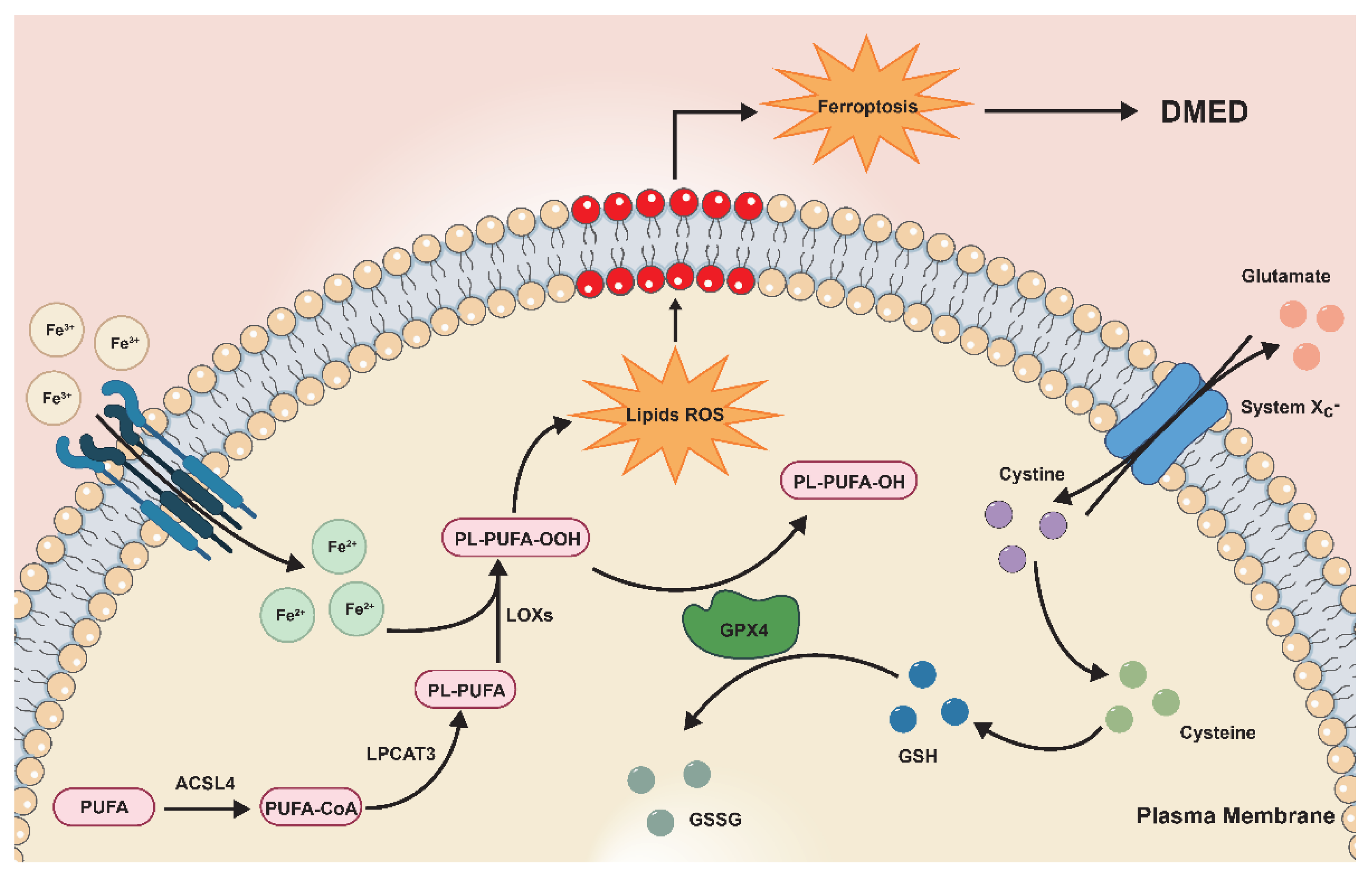
:1. Introduction
2. Materials and Methods
2.1. Lentivirus Construction, Package, and Examination
2.2. Animal and Experimental Designs
2.3. Erectile Function Evaluation
2.4. Histologic Assessment
2.4.1. Fluorescence Staining
2.4.2. Immunohistochemistry and Masson’s Trichrome Staining
2.4.3. Prussian Blue Staining
2.4.4. Transmission Electron Microscopy
2.5. Western Blotting
2.6. Oxidative Stress Levels and Iron Contents Detection
2.7. Nitric Oxide (NO) and Cyclic Guanosine Monophosphate (cGMP) Levels
2.8. Statistical Analysis
3. Results
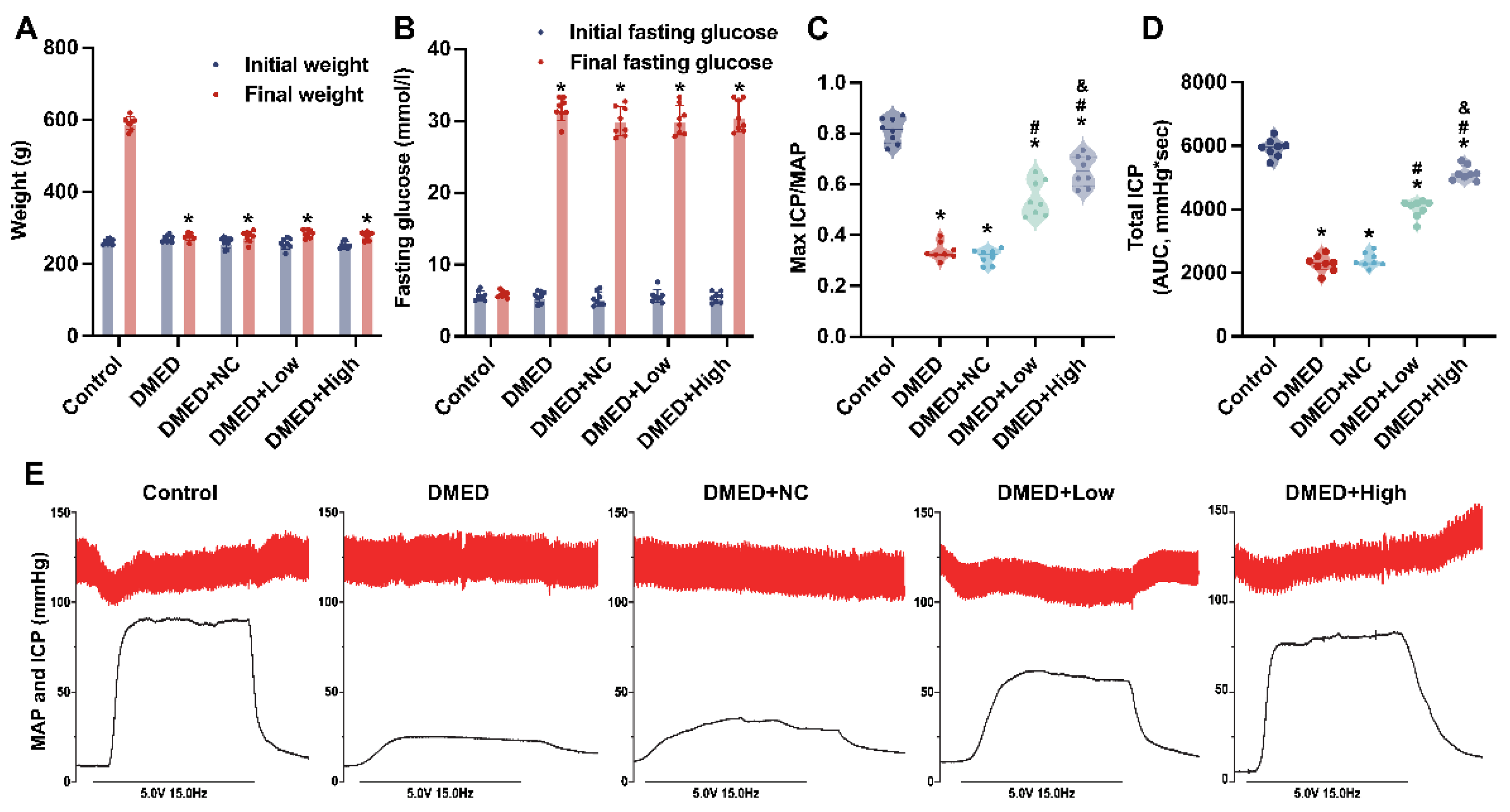
3.1. GPX4-LV Injection Had No Effect on Metabolic Indexes
3.2. GPX4-LV Injection Improved Erectile Function in a Dose-Dependent Manner
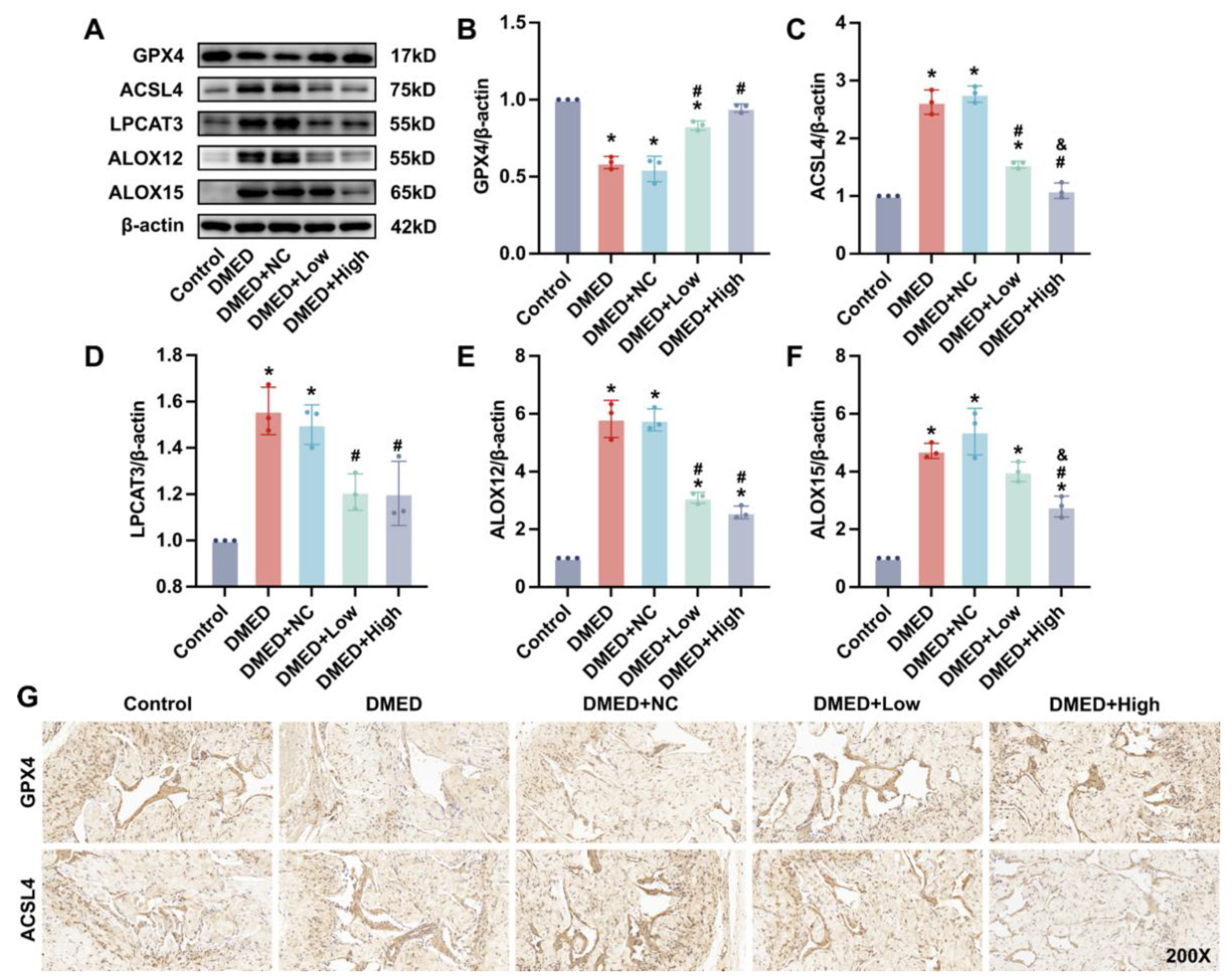
3.3. GPX4-LV Injection Alleviated Ferroptosis in the Corpus Cavernosum
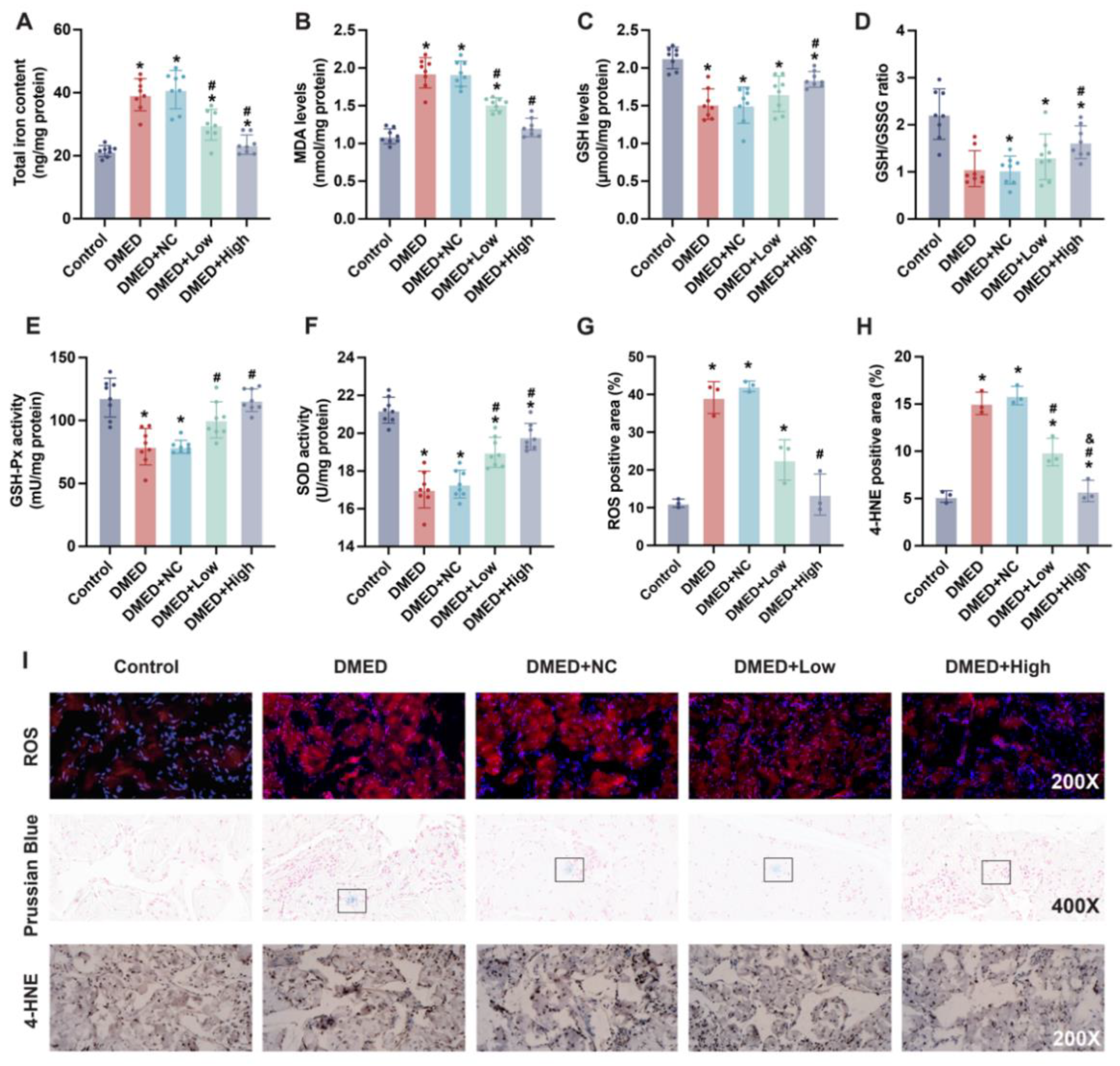
3.4. GPX4-LV Injection Ameliorated Iron Overload and Oxidative Stress
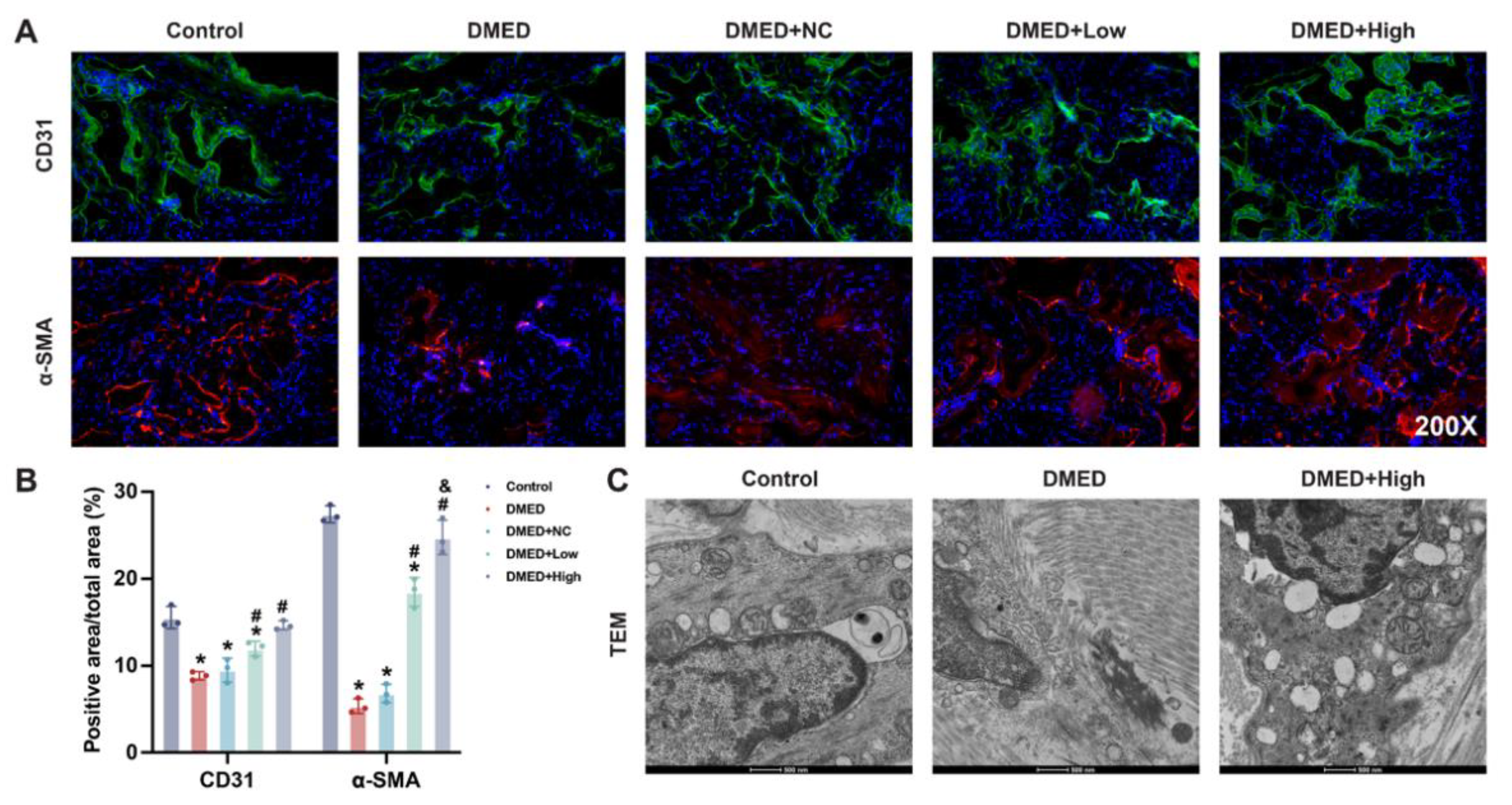
3.5. GPX4-LV Injection Restored Endothelial and Smooth Muscle Cell Numbers in the Corpus Cavernosum
3.6. GPX4-LV Injection Improved the NO-cGMP and RhoA-ROCK1/ROCK2 Signaling Pathway
3.7. GPX4-LV Injection Inhibited Fibrosis in the Corpus Cavernosum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thorve, V.S.; Kshirsagar, A.D.; Vyawahare, N.S.; Joshi, V.S.; Ingale, K.G.; Mohite, R.J. Diabetes-induced erectile dysfunction: Epidemiology, pathophysiology and management. J. Diabetes Complicat. 2011, 25, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-F.; Jiang, H.; Hong, K.; Zhao, L.-M.; Tang, W.-H.; Ma, L.-L. Influence of erectile dysfunction course on its progress and efficacy of treatment with phosphodiesterase type 5 inhibitors. Chin. Med. J. 2010, 123, 3258–3261. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.H. Antimicrobial and Wound Healing Properties of FeO Fabricated Chitosan/PVA Nanocomposite Sponge. Antibiotics 2021, 10, 524. [Google Scholar] [CrossRef]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2022. [Google Scholar] [CrossRef]
- Fang, X.; Cai, Z.; Wang, H.; Han, D.; Cheng, Q.; Zhang, P.; Gao, F.; Yu, Y.; Song, Z.; Wu, Q.; et al. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ. Res. 2020, 127, 486–501. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Manco, M. Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol. 2014, 2, 513–526. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Hadian, K.; Stockwell, B.R. SnapShot: Ferroptosis. Cell 2020, 181, 1188.e1. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Choi, S.E.; Kim, T.H.; Lee, H.J.; Lee, S.J.; Kang, Y.; Jeon, J.Y.; Kim, H.J.; Lee, K.W. Iron overload by transferrin receptor protein 1 regulation plays an important role in palmitate-induced insulin resistance in human skeletal muscle cells. FASEB J. 2019, 33, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, S.; Naito, Y.; Okuno, K.; Sawada, H.; Asakura, M.; Masuyama, T.; Ishihara, M. Effects of Heterozygous TfR1 (Transferrin Receptor 1) Deletion in Pathogenesis of Renal Fibrosis in Mice. Hypertension 2020, 75, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q.; et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm. Sin. B 2021, 12, 708–722. [Google Scholar] [CrossRef]
- Zhu, K.; Zhu, X.; Liu, S.; Yu, J.; Wu, S.; Hei, M.; Vasconcelos, S.M.M. Glycyrrhizin Attenuates Hypoxic-Ischemic Brain Damage by Inhibiting Ferroptosis and Neuroinflammation in Neonatal Rats via the HMGB1/GPX4 Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 8438528. [Google Scholar] [CrossRef]
- Lin, J.H.; Yang, K.T.; Lee, W.S.; Ting, P.C.; Luo, Y.P.; Lin, D.J.; Wang, Y.S.; Chang, J.C. Xanthohumol Protects the Rat Myocardium against Ischemia/Reperfusion Injury-Induced Ferroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 9523491. [Google Scholar] [CrossRef]
- Luo, E.F.; Li, H.X.; Qin, Y.H.; Qiao, Y.; Yan, G.L.; Yao, Y.Y.; Li, L.Q.; Hou, J.T.; Tang, C.C.; Wang, D. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J. Diabetes 2021, 12, 124–137. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Zhang, W.; Li, H.; Zhao, W.; Sun, J.; Yang, M. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxidative Med. Cell. Longev. 2020, 2020, 9067610. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef] [Green Version]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Baart, G.J.; Zomer, B.; de Haan, A.; van der Pol, L.A.; Beuvery, E.C.; Tramper, J.; Martens, D.E. Modeling Neisseria meningitidis metabolism: From genome to metabolic fluxes. Genome Biol. 2007, 8, R136. [Google Scholar] [CrossRef] [PubMed]
- Stoyanovsky, D.A.; Tyurina, Y.Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.A.; Protchenko, O.; Jadhav, S.; Bolevich, S.B.; Kozlov, A.V.; Vladimirov, Y.A.; et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic. Biol. Med. 2019, 133, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Guo, C.; Deng, H.J.; Dong, J.Q.; Lei, S.T.; Li, G.X. Construction of lentivirus-based inhibitor of hsa-microRNA-338-3p with specific secondary structure. Acta Pharmacol. Sin. 2013, 34, 167–175. [Google Scholar] [CrossRef]
- Sun, T.; Xu, W.; Wang, J.; Song, J.; Wang, T.; Wang, S.; Liu, K.; Liu, J. Paeonol ameliorates Paeonol ameliorates diabetic erectile dysfunction by inhibiting HMGB1/RAGE/NF-kB pathway. Andrology 2022. [Google Scholar] [CrossRef]
- Heaton, J.P.; Varrin, S.J.; Morales, A. The characterization of a bio-assay of erectile function in a rat model. J. Urol. 1991, 145, 1099–1102. [Google Scholar] [CrossRef]
- Elabbady, A.; Hassouna, M.M.; Elhilali, M. Apomorphine versus mating behavior in testing erectile capabilities of diabetic rats. Urology 1995, 45, 715–719. [Google Scholar] [CrossRef]
- Liu, G.; Sun, X.; Bian, J.; Wu, R.; Guan, X.; Ouyang, B.; Huang, Y.; Xiao, H.; Luo, D.; Atala, A.; et al. Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS ONE 2013, 8, e72790. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Liu, X.; Wang, T.; Wang, S.; Liu, J.; Jiang, H. JAK2 deficiency improves erectile function in diabetic mice through attenuation of oxidative stress, apoptosis, and fibrosis. Andrology 2021, 9, 1662–1671. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Z.; Yang, X.; Zhao, M.; Hou, Y.; Wang, D.; Tang, S.; Li, J.; Yu, J. Propofol Protects Myocardium From Ischemia/Reperfusion Injury by Inhibiting Ferroptosis Through the AKT/p53 Signaling Pathway. Front. Pharmacol. 2022, 13, 841410. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Wu, J.; Xu, Y.; Ding, C.C.; Mestre, A.A.; Lin, C.C.; Yang, W.H.; Chi, J.T. Zinc transporter ZIP7 is a novel determinant of ferroptosis. Cell Death Dis. 2021, 12, 198. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Wang, Y.; Leng, Y.; Xia, Z. Inhibition of DNMT-1 alleviates ferroptosis through NCOA4 mediated ferritinophagy during diabetes myocardial ischemia/reperfusion injury. Cell Death Discov. 2021, 7, 267. [Google Scholar] [CrossRef]
- Duarte, T.L.; Talbot, N.P.; Drakesmith, H. NRF2 and Hypoxia-Inducible Factors: Key Players in the Redox Control of Systemic Iron Homeostasis. Antioxidants Redox Signal. 2020, 35, 433–452. [Google Scholar] [CrossRef]
- Yue, X.F.; Shen, C.X.; Wang, J.W.; Dai, L.Y.; Fang, Q.; Long, L.; Zhi, Y.; Li, X.R.; Wang, Y.W.; Shen, G.F.; et al. The near-infrared dye IR-61 restores erectile function in a streptozotocin-induced diabetes model via mitochondrial protection. Asian J. Androl. 2021, 23, 249–258. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Ruan, Z.; Sun, D.; Zhang, H.; Lin, G.; Hu, L.; Zhao, S.; Fu, Q. Probucol enhances the therapeutic efficiency of mesenchymal stem cells in the treatment of erectile dysfunction in diabetic rats by prolonging their survival time via Nrf2 pathway. Stem Cell Res. Ther. 2020, 11, 302. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S.; Sun, Z.; Dong, H.; Yu, H.; Huang, M.; Gao, X. Ferroptosis Enhanced Diabetic Renal Tubular Injury via HIF-1alpha/HO-1 Pathway in db/db Mice. Front. Endocrinol. 2021, 12, 626390. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Yang, H.Z.; Wang, Y.J.; Chen, Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci. Rep. 2021, 41, BSR20202924. [Google Scholar] [CrossRef]
- Li, S.; Zheng, L.; Zhang, J.; Liu, X.; Wu, Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic. Biol. Med. 2020, 162, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, Q.; Wang, H.; Chen, C.; Luo, D. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp. Cell Res. 2021, 407, 112800. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Sun, T.; Wang, J.; Wang, T.; Wang, S.; Liu, J.; Li, H. GPX4 Alleviates Diabetes Mellitus-Induced Erectile Dysfunction by Inhibiting Ferroptosis. Antioxidants 2022, 11, 1896. https://doi.org/10.3390/antiox11101896
Xu W, Sun T, Wang J, Wang T, Wang S, Liu J, Li H. GPX4 Alleviates Diabetes Mellitus-Induced Erectile Dysfunction by Inhibiting Ferroptosis. Antioxidants. 2022; 11(10):1896. https://doi.org/10.3390/antiox11101896
Chicago/Turabian StyleXu, Wenchao, Taotao Sun, Jiaxin Wang, Tao Wang, Shaogang Wang, Jihong Liu, and Hao Li. 2022. "GPX4 Alleviates Diabetes Mellitus-Induced Erectile Dysfunction by Inhibiting Ferroptosis" Antioxidants 11, no. 10: 1896. https://doi.org/10.3390/antiox11101896




