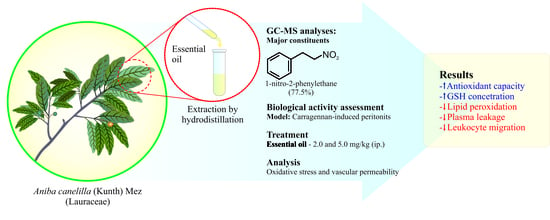
Aniba canelilla (Kunth) Mez (Lauraceae) Essential Oil: Effects on Oxidative Stress and Vascular Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals
2.3. Plant Material, Extraction, and Analysis of Essential Oil
2.4. Drugs and Solutions
2.5. Carrageenan-Induced Peritonitis
2.6. Oxidative Biochemistry Assays
2.6.1. Total Antioxidant Capacity
2.6.2. Reduced Glutathione Level (GSH)
2.6.3. Lipid Peroxidation
2.7. Vascular Permeability Assays
2.7.1. Protein Concentration
2.7.2. Cell Migration Assessment
2.8. Statistical Analysis
3. Results
3.1. Essential Oil Composition
3.2. AcEO Antioxidant Activity
3.2.1. AcEO Improves Total Antioxidant Capacity
3.2.2. AcEO Prevents GSH Decrease
3.2.3. AcEO Prevents Lipid Peroxidation
3.3. AcEO Activity on Vascular Permeability
3.3.1. AcEO Inhibits Plasma Leakage into Peritoneal Cavity
3.3.2. AcEO Reduces Leukocyte Migration into the Peritoneal Cavity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008, 295, C849–C868. [Google Scholar] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [PubMed]
- Feitosa, C.M.; da Silva Oliveira, G.L.; do Nascimento Cavalcante, A.; Morais Chaves, S.K.; Rai, M. Determination of parameters of oxidative stress in vitro models of neurodegenerative diseases—A review. Curr. Clin. Pharmacol. 2018, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Gudkov, S.V.; Lankin, V.Z.; Novoselov, V.I. Role of Glutathione Peroxidases and Peroxiredoxins in Free Radical-Induced Pathologies. Biochemistry 2021, 86, 1418–1433. [Google Scholar]
- Filomeni, G.; de Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar]
- Baiano, A.; del Nobile, M.A. Antioxidant compounds from vegetable matrices: Biosynthesis, occurrence, and extraction systems. Crit. Rev. Food Sci. Nutr. 2016, 56, 2053–2068. [Google Scholar]
- Li, A.-N.; Li, S.; Li, H.-B.; Xu, D.-P.; Xu, X.-R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.-J.; Xu, D.-P.; Zhou, T.; Zhou, Y.; Li, S.; Li, H.-B. Bioactivities and health benefits of wild fruits. Int. J. Mol. Sci. 2016, 17, 1258. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [PubMed]
- Balmus, I.M.; Ciobica, A.; Trifan, A.; Stanciu, C. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2016, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Jenab, M.; Riboli, E.; Ferrari, P.; Sabate, J.; Slimani, N.; Norat, T.; Friesen, M.; Tjønneland, A.; Olsen, A.; Overvad, K. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006, 27, 2250–2257. [Google Scholar] [CrossRef]
- Pinto, A.C. O Brasil dos viajantes e dos exploradores e a química de produtos naturais brasileira. Química Nova 1995, 18, 608–615. [Google Scholar]
- Tropicos.org. Missouri Botanical Garden. Available online: https://www.tropicos.org/name/17800123 (accessed on 13 July 2022).
- Maia, J.G.S.; Zoghbi, M.; das, G.B.; de Aguilar Andrade, E.H. Plantas Aromáticas na Amazônia e Seus Óleos Essenciais; Museu Paraense Emílio Goeldi: Belem, PA, Brazil, 2001. [Google Scholar]
- Almeida, F.F. Estudo de Revisão da Literatura Sobre a Importância do Óleo Essencial de Aniba Canelilla e Estudo Experimental da Biotransformação do 1-Nitro-2-Feniletano; UNIFESSPA: Marabá, Brazil, 2022. [Google Scholar]
- Sousa, P.J.C.; Araujo, J.S.; Pereira, L.L.S.; Modro, M.N.R.; Maia, J.G.S.; Araujo, M.T.F.; Carvalho, J.C.T.; Perazzo, F.F. Phytochemical and Toxicological Evaluations of the Essential Oil From the Bark of Aniba canellila (HBK) Mez. J. Essent. Oil Res. 2009, 21, 381–384. [Google Scholar] [CrossRef]
- de Lima, A.B.; Santana, M.B.; Cardoso, A.S.; da Silva, J.K.R.; Maia, J.G.S.; Carvalho, J.C.T.; Sousa, P.J.C. Antinociceptive activity of 1-nitro-2-phenylethane, the main component of Aniba canelilla essential oil. Phytomedicine 2009, 16, 555–559. [Google Scholar] [CrossRef]
- Oyemitan, I.A.; Elusiyan, C.A.; Akanmu, M.A.; Olugbade, T.A. Hypnotic, anticonvulsant and anxiolytic effects of 1-nitro-2-phenylethane isolated from the essential oil of Dennettia tripetala in mice. Phytomedicine 2013, 20, 1315–1322. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Sousa, P.J.C.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant capacity and cytotoxicity of essential oil and methanol extract of Aniba canelilla (HBK) Mez. J. Agric. Food Chem. 2007, 55, 9422–9426. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.F. Estudo do Potencial Biotecnológico de Aniba Canelilla (HBK) Mez Para Obtenção de Cosméticos; Universidade do Estado do Amazonas: Manaus, Brazil, 2012. [Google Scholar]
- Mesquita, T.J.B.; da Silva, G.F.; Albuquerque, P.M.; Duvoisin, S., Jr. Análise fitoquímica e determinação da capacidade antioxidante em extratos de Aniba canelilla (HBK) Mez. In Anais Do XX Congresso Brasileiro de Engenharia Química; Blucher Chemical Engineering Proceedings: Florianópolis, SC, Brazil, 2014; Volume 8. [Google Scholar]
- Martins, F.J.; Caneschi, C.A.; Vieira, J.L.F.; Barbosa, W.; Raposo, N.R.B. Antioxidant activity and potential photoprotective from amazon native flora extracts. J. Photochem. Photobiol. B Biol. 2016, 161, 34–39. [Google Scholar] [CrossRef]
- Vale, J.K.L.; Lima, A.B.; Pinheiro, B.G.; Cardoso, A.S.; Silva, J.K.R.; Maia, J.G.S.; de Sousa, G.E.P.; da Silva, A.B.F.; Sousa, P.J.C.; Borges, R.S. Evaluation and theoretical study on the anti-inflammatory mechanism of 1-nitro-2-phenylethane. Planta Med. 2013, 79, 628–633. [Google Scholar] [CrossRef]
- Lahlou, S.; Magalhães, P.J.C.; de Siqueira, R.J.B.; Figueiredo, A.F.; Interaminense, L.F.L.; Maia, J.G.S.; da Cunha Sousa, P.J. Cardiovascular effects of the essential oil of Aniba canelilla bark in normotensive rats. J. Cardiovasc. Pharmacol. 2005, 46, 412–421. [Google Scholar] [CrossRef] [PubMed]
- De Siqueira, R.J.B.; Macedo, F.I.B.; de Fátima LealInteraminense, L.; Duarte, G.P.; Magalhães, P.J.C.; Brito, T.S.; da Silva, J.K.R.; Maia, J.G.S.; da CunhaSousa, P.J.; Leal-Cardoso, J.H.; et al. 1-Nitro-2-phenylethane, the main constituent of the essential oil of Aniba canelilla, elicits a vago-vagal bradycardiac and depressor reflex in normotensive rats. Eur. J. Pharmacol. 2010, 638, 90–98. [Google Scholar] [CrossRef]
- de Fátima Leal Interaminense, L.; de Siqueira, R.J.B.; Xavier, F.E.; Duarte, G.P.; Magalhães, P.J.C.; da Silva, J.K.; Maia, J.G.S.; da Cunha Sousa, P.J.; Leal-Cardoso, J.H.; Lahlou, S. Cardiovascular effects of 1-nitro-2-phenylethane, the main constituent of the essential oil of Aniba canelilla, in spontaneously hypertensive rats. Fundam. Clin. Pharmacol. 2011, 25, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cross, C.E. Oxygen-derived species: Their relation to human disease and environmental stress. Environ. Health Perspect. 1994, 102 (Suppl. S10), 5–12. [Google Scholar]
- Souza-Junior, F.J.C.; Luz-Moraes, D.; Pereira, F.S.; Barros, M.A.; Fernandes, L.M.P.; Queiroz, L.Y.; Maia, C.F.; Maia, J.G.S.; Fontes-Junior, E.A. Aniba canelilla (Kunth) mez (Lauraceae): A review of ethnobotany, phytochemical, antioxidant, anti-inflammatory, cardiovascular, and neurological properties. Front. Pharmacol. 2020, 11, 699. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Mondello, L. FFNSC Library—Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; Shimadzu Corporation: Kyoto, Japan, 2011. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A Generalization of The Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- De Souza, G.E.P.; Ferreira, S.H. Blockade by antimacrophage serum of the migration of PMN neutrophils into the inflamed peritoneal cavity. Agents Actions 1985, 17, 97–103. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- George, E.L. Tissue sulfhydryl groups. Arch. Biochem Biophys 1959, 82, 70–77. [Google Scholar]
- Kohn, H.I.; Liversedge, M. On a new aerobic metabolite whose production by brain is inhibited by apomorphine, emetine, ergotamine, epinephrine, and menadione. J. Pharmacol. Exp. Ther. 1994, 82, 292–300. [Google Scholar]
- Percario, S.; Vital, A.C.C.; Jablonka, F. Dosagem do malondialdeido. Newslab 1994, 2, 46–50. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the Biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Manhães, A.P.; da Veiga-Júnior, V.F.; Wiedemann, L.S.M.; Fernandes, K.S.; de Tarso Barbosa Sampaio, P. Biomass production and essential oil yield from leaves, fine stems and resprouts using pruning the crown of Aniba canelilla (HBK) (Lauraceae) in the Central Amazon. Acta Amaz. 2012, 42, 355–362. [Google Scholar] [CrossRef]
- Taveira, F.S.N.; de Lima, W.N.; Andrade, E.H.A.; Maia, J.G.S. Seasonal essential oil variation of Aniba canelilla. Biochem. Syst. Ecol. 2003, 31, 69–75. [Google Scholar] [CrossRef]
- De Moraes, B.C.; da Costa, J.M.N.; da Costa, A.C.L.; Costa, M.H. Variação espacial e temporal da precipitação no estado do Pará. Acta Amaz. 2005, 35, 207–214. [Google Scholar] [CrossRef]
- Markov, A.V.; Sen’kova, A.V.; Babich, V.O.; Odarenko, K.V.; Talyshev, V.A.; Salomatina, O.V.; Salakhutdinov, N.F.; Zenkova, M.A.; Logashenko, E.B. Dual effect of soloxolone methyl on LPS-induced inflammation in vitro and in vivo. Int. J. Mol. Sci. 2020, 21, 7876. [Google Scholar] [CrossRef]
- Falcão, T.R.; de Araújo, A.A.; Soares, L.A.L.; de Farias, I.B.; da Silva, W.A.V.; Ferreira, M.R.A.; de Araújo, R.F., Jr.; de Medeiros, J.S.; de Sousa Lopes, M.L.D.; Guerra, G.C.B. Libidibia ferrea fruit crude extract and fractions show anti-inflammatory, antioxidant, and antinociceptive effects in vivo and increase cell viability in vitro. Evid. Based Complementary Altern. Med. 2019, 2019, 6064805. [Google Scholar]
- Amorim, V.R.; do Nascimento Rodrigues, D.C.; do Nascimento Silva, J.; Ramos, C.L.S.; Almeida, L.M.N.; Almeida, A.A.C.; Pinheiro-Neto, F.R.; Almeida, F.R.C.; Rizzo, M.S.; Pereira-Freire, J.A. Anti-inflammatory mechanisms of fruits and by-products from Mauritia flexuosa, an exotic plant with functional benefits. J. Toxicol. Environ. Health 2021, 84, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, D.R.; Malkey, R.; Florian, T.L.; Filip, A.; Clichici, S.; Bidian, C.; Moldovan, R.; Hoteiuc, O.A.; Toader, A.M.; Baldea, I. Daily oral administration of chlorogenic acid prevents the experimental carrageenan-induced oxidative stress. J. Physiol Pharm. 2020, 71, 55–65. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Zalachoras, I.; Hollis, F.; Ramos-Fernández, E.; Trovo, L.; Sonnay, S.; Geiser, E.; Preitner, N.; Steiner, P.; Sandi, C.; Morató, L. Therapeutic potential of glutathione-enhancers in stress-related psychopathologies. Neurosci. Biobehav. Rev. 2020, 114, 134–155. [Google Scholar] [CrossRef]
- Bjørklund, G.; Peana, M.; Maes, M.; Dadar, M.; Severin, B. The glutathione system in Parkinson’s disease and its progression. Neurosci. Biobehav. Rev. 2021, 120, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Coté, H.; Boucher, M.-A.; Pichette, A.; Legault, J. Anti-inflammatory, antioxidant, antibiotic, and cytotoxic activities of Tanacetum vulgare L. essential oil and its constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef]
- Mehri, S.; Meshki, M.A.; Hosseinzadeh, H. Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug Chem. Toxicol. 2015, 38, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Et Biophys. Acta Gen. Subj. 2014, 1840, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Feng, X.-L.; Xu, X.-Y.; Cao, S.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, H.-B. Comparison of antioxidant activities of different grape varieties. Molecules 2018, 23, 2432. [Google Scholar] [CrossRef]
- Gomes Junior, A.L.; Islam, M.T.; Nicolau, L.A.D.; de Souza, L.K.M.; de Souza Lopes Araújo, T.; de Oliveira, G.A.L.; de Melo Nogueira, K.; da Silva Lopes, L.; Medeiros, J.-V.R.; Mubarak, M.S. Anti-inflammatory, antinociceptive, and antioxidant properties of anacardic acid in experimental models. ACS Omega 2020, 5, 19506–19515. [Google Scholar] [CrossRef] [PubMed]







| RI(C) | RI(L) | Compounds | % * |
|---|---|---|---|
| 850 | 850 a | 3Z-hexenol | tr |
| 933 | 932 a | α-pinene | 0.4 ± 0.3 |
| 958 | 952 a | benzaldehyde | 0.6 ± 0.4 |
| 977 | 974 a | β-pinene | 0.4 ± 0.3 |
| 1024 | 1020 a | p-cymene | 0.1 ± 0.0 |
| 1028 | 1024 a | limonene | 0.2 ± 0.2 |
| 1031 | 1026 a | 1,8-cineole | 0.1 ± 0.1 |
| 1041 | 1036 a | benzene acetaldehyde | 1.1 ± 0.1 |
| 1100 | 1095 a | linalool | 2.8 ± 0.7 |
| 1137 | 1134 a | benzeneacetonitrile | 0.2 ± 0.1 |
| 1190 | 1186 a | α-terpineol | 0.4 ± 0.1 |
| 1195 | 1195 a | myrtenal | 0.1 ± 0.0 |
| 1308 | 1294 a | 1-nitro-2-phenylethane | 77.5 ± 4.8 |
| 1357 | 1356 a | eugenol | 0.3 ± 0.1 |
| 1377 | 1374 a | α-copaene | 0.8 ± 0.4 |
| 1408 | 1417 a | β-longipinene | 1.6 ± 0.9 |
| 1420 | 1417 a | E-caryophyllene | 3.6 ± 2.7 |
| 1454 | 1452 a | α-humulene | 0.4 ± 0.3 |
| 1496 | 1498 a | α-selinene | 0.1 ± 0.0 |
| 1509 | 1505 a | β-bisabolene | 0.1 ± 0.0 |
| 1525 | 1522 a | δ-cadinene | 0.1 ± 0.1 |
| 1584 | 1582 a | caryophyllene oxide | 4.4 ± 0.9 |
| 1610 | 1608 a | humulene epoxide II | 0.3 ± 0.1 |
| 1634 | 1639 a | caryophyll-4(12),8(13)-dien-5-α-ol | 0.2 ± 0.0 |
| 1637 | 1639 a | caryophyll-4(12),8(13)-dien-5-β-ol | 0.7 ± 0.1 |
| 1656 | 1658 a | selin-11-en-4-α-ol | 0.8 ± 0.0 |
| 1672 | 1668 a | 14-hydroxy-9-epi-E-caryophyllene | 0.2 ± 0.2 |
| monoterpene hydrocarbons | 1.0 ± 0.9 | ||
| oxygenated monoterpenes | 3.5 ± 1.0 | ||
| sesquiterpene hydrocarbons | 6.9 ± 4.7 | ||
| oxygenated sesquiterpenes | 6.9 ± 1.6 | ||
| benzenoids | 80.0 ± 5.8 | ||
| Total identified | 98.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, E.K.S.; Kubota, K.; Luz, D.A.; Mendes, P.F.S.; Figueiredo, P.L.B.; Lima, R.R.; Maia, C.S.F.; Fontes-Júnior, E.A. Aniba canelilla (Kunth) Mez (Lauraceae) Essential Oil: Effects on Oxidative Stress and Vascular Permeability. Antioxidants 2022, 11, 1903. https://doi.org/10.3390/antiox11101903
Cardoso EKS, Kubota K, Luz DA, Mendes PFS, Figueiredo PLB, Lima RR, Maia CSF, Fontes-Júnior EA. Aniba canelilla (Kunth) Mez (Lauraceae) Essential Oil: Effects on Oxidative Stress and Vascular Permeability. Antioxidants. 2022; 11(10):1903. https://doi.org/10.3390/antiox11101903
Chicago/Turabian StyleCardoso, Eloise K. Serrão, Karen Kubota, Diandra Araújo Luz, Paulo Fernando S. Mendes, Pablo Luis B. Figueiredo, Rafael Rodrigues Lima, Cristiane S. Ferraz Maia, and Enéas Andrade Fontes-Júnior. 2022. "Aniba canelilla (Kunth) Mez (Lauraceae) Essential Oil: Effects on Oxidative Stress and Vascular Permeability" Antioxidants 11, no. 10: 1903. https://doi.org/10.3390/antiox11101903
APA StyleCardoso, E. K. S., Kubota, K., Luz, D. A., Mendes, P. F. S., Figueiredo, P. L. B., Lima, R. R., Maia, C. S. F., & Fontes-Júnior, E. A. (2022). Aniba canelilla (Kunth) Mez (Lauraceae) Essential Oil: Effects on Oxidative Stress and Vascular Permeability. Antioxidants, 11(10), 1903. https://doi.org/10.3390/antiox11101903