Ultrasound-Assisted Alcoholic Extraction of Lesser Mealworm Larvae Oil: Process Optimization, Physicochemical Characteristics, and Energy Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Reagents
2.2. Conventional Solvent Extraction
2.3. Ultrasound-Assisted Extraction Process
2.4. Extraction Efficiency
2.5. DPPH⋅ Scavenging Capacity Assay
2.6. Reducing Power Assay
2.7. Single-Factor Exploratory Tests
2.8. Experimental Design and Response Surface Optimization
2.9. Evaluation of Total Phenolic Content
2.10. Determination of Total Carotenoid Content
2.11. Analysis of Fatty Acids Profile
2.12. Assessment of Physicochemical Properties
2.13. Estimation of Diffusion Coefficients
2.14. Calculation of Electric Energy Consumption
2.15. Scanning Electron Microscopy
2.16. Statistical Analysis
3. Results and Discussion
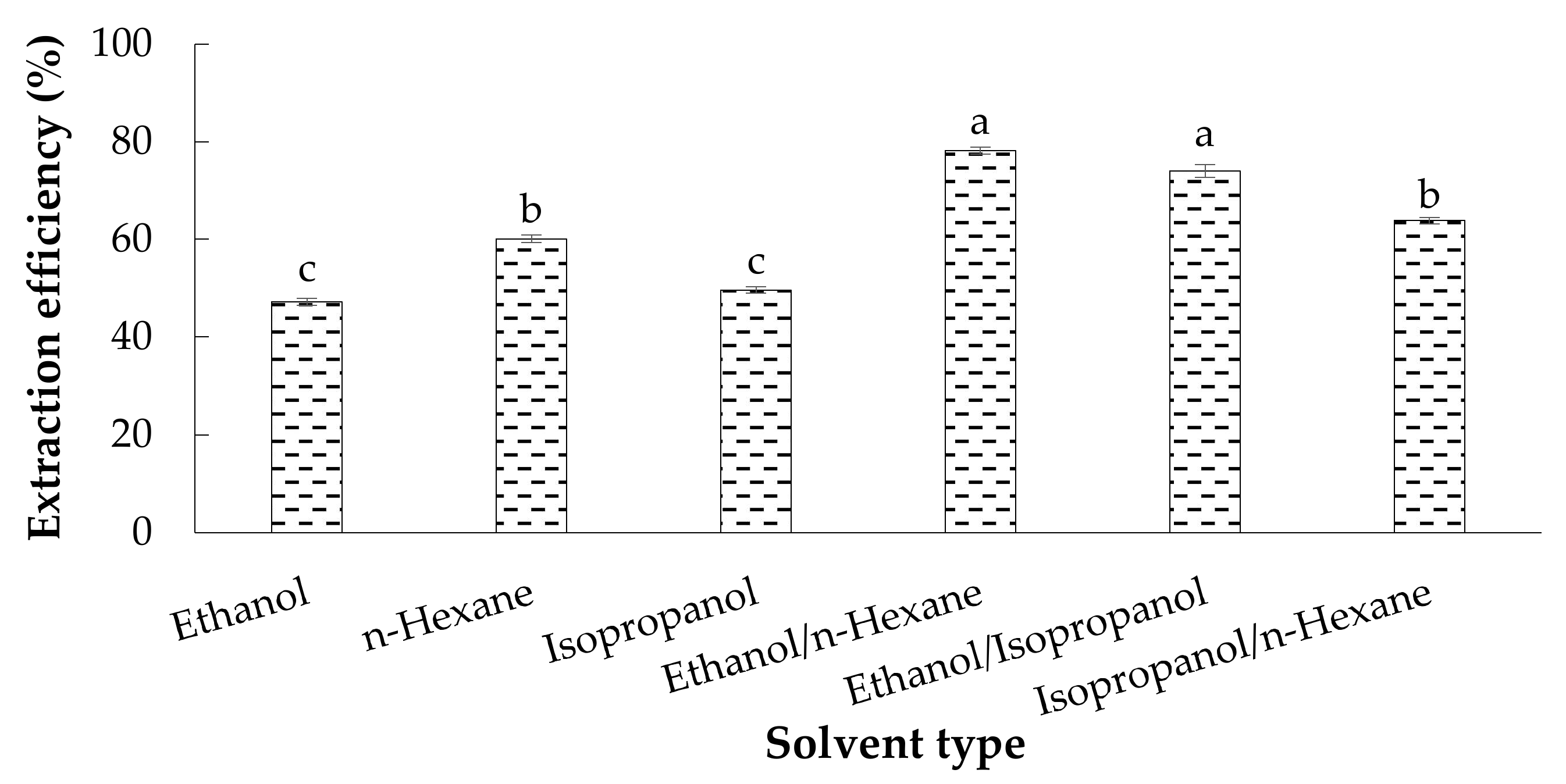
3.1. Selection of the Organic Solvent
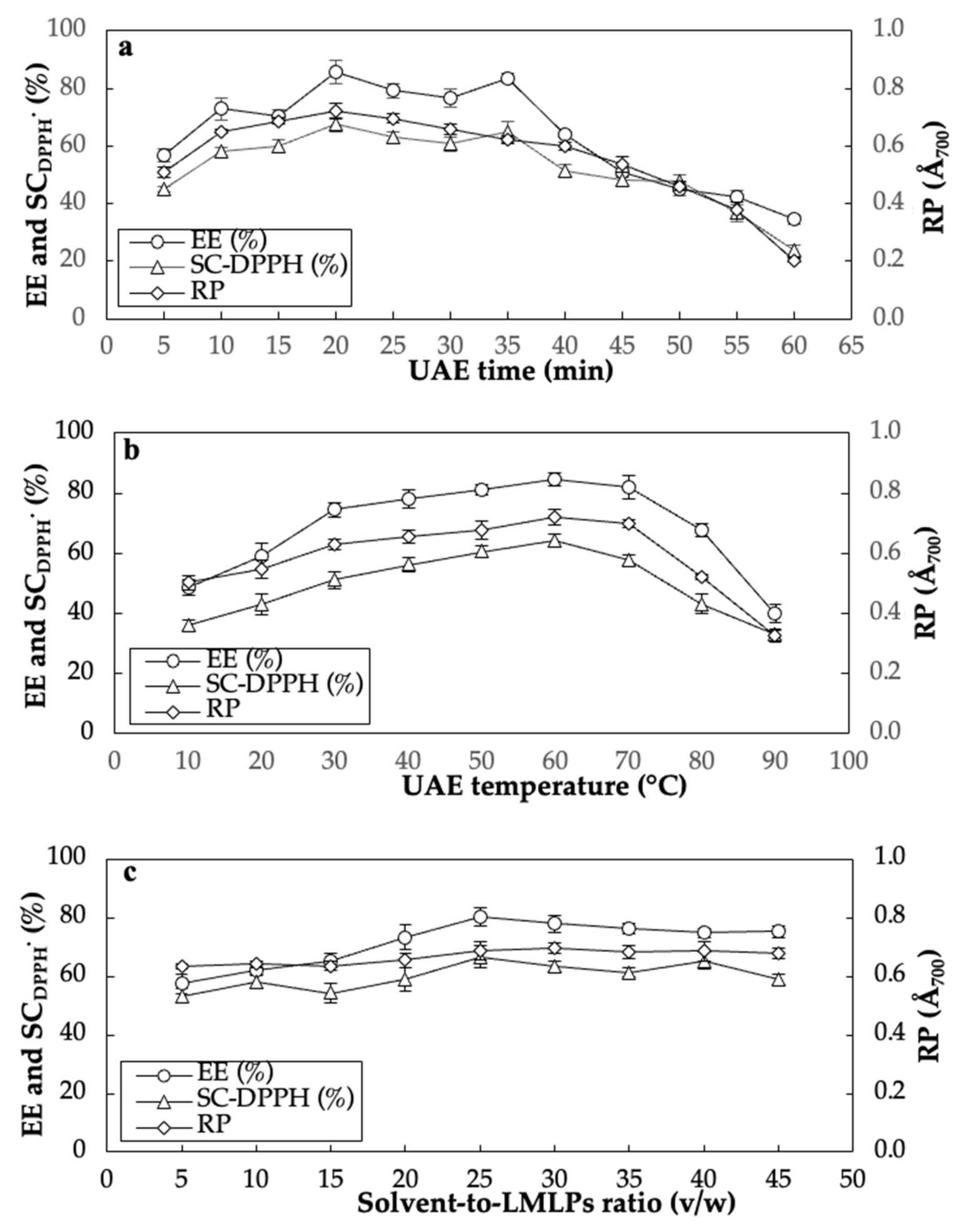
3.2. Single-Factor Experiments
3.3. Fitting the Mathematical Models
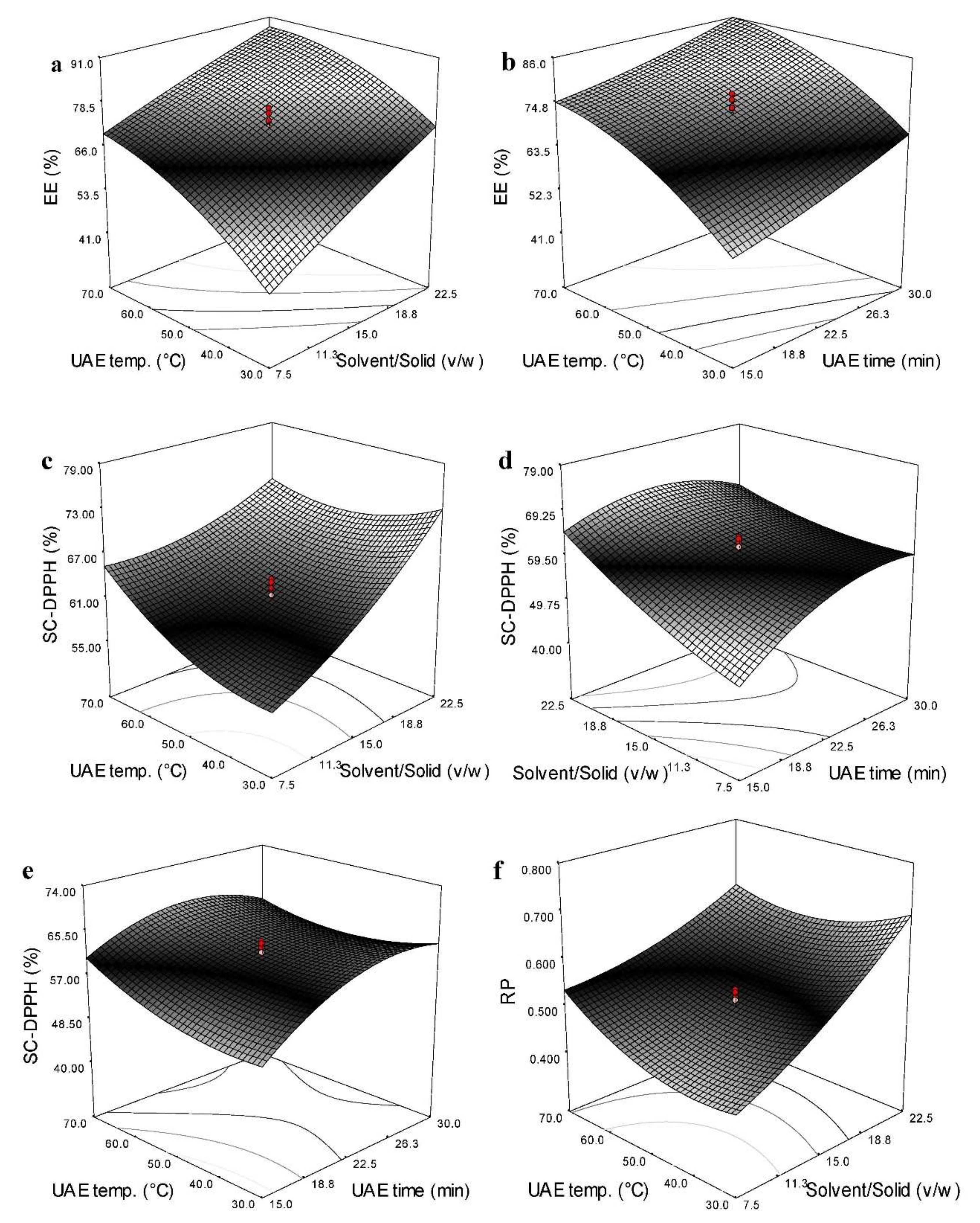
3.4. Effects of Independent Variables on the EE
3.5. Effects of Independent Variables on the Antioxidant Activity
3.6. Optimal Conditions and Verification of the Models
3.7. Comparison of Ultrasound and n-Hexane Extraction Methods
3.7.1. Process Efficiency
3.7.2. Bioactive Compounds and Antioxidant Activity
3.7.3. Fatty Acid Composition and Physicochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gharibzahedi, S.M.T.; Ansarifard, I.; Hasanabadi, Y.S.; Ghahderijani, M.; Yousefi, R. Physicochemical Properties of Moringa Peregrina Seed and Its Oil. Qual. Assur. Saf. Crops Food 2013, 5, 303–309. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; Van Valenberg, H.J.F.; Lakemond, C.M.M. Four Insect Oils as Food Ingredient: Physical and Chemical Characterisation of Insect Oils Obtained by an Aqueous Oil Extraction. J. Insects Food Feed 2019, 5, 279–292. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Hamedi, M.; Rezaei, K.; Khodaiyan, F. Evaluation of Physicochemical Properties and Antioxidant Activities of Persian Walnut Oil Obtained by Several Extraction Methods. Ind. Crops Prod. 2013, 45, 133–140. [Google Scholar] [CrossRef]
- Perrier, A.; Delsart, C.; Boussetta, N.; Grimi, N.; Citeau, M.; Vorobiev, E. Effect of Ultrasound and Green Solvents Addition on the Oil Extraction Efficiency from Rapeseed Flakes. Ultrason. Sonochem. 2017, 39, 58–65. [Google Scholar] [CrossRef]
- Ferreira, I.J.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M. Green Emerging Extraction Technologies to Obtain High-Quality Vegetable Oils from Nuts: A Review. Innov. Food Sci. Emerg. Technol. 2022, 76, 102931. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Rawski, M.; Kołodziejski, P.; Bryszak, M.; Józefiak, D. Insect Oil as an Alternative to Palm Oil and Poultry Fat in Broiler Chicken Nutrition. Animals 2019, 9, 116. [Google Scholar] [CrossRef]
- Fawole, F.J.; Labh, S.N.; Hossain, M.S.; Overturf, K.; Small, B.C.; Welker, T.L.; Hardy, R.W.; Kumar, V. Insect (Black Soldier Fly Larvae) Oil as a Potential Substitute for Fish or Soy Oil in the Fish Meal-Based Diet of Juvenile Rainbow Trout (Oncorhynchus mykiss). Anim. Nutr. 2021, 7, 1360–1370. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Dewettinck, K.; Gellynck, X.; Schouteten, J.J. Replacing Vegetable Oil by Insect Oil in Food Products: Effect of Deodorization on the Sensory Evaluation. Food Res. Int. 2021, 141, 110140. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M.; et al. Lesser Mealworm (Alphitobius diaperinus) Powder as a Novel Baking Ingredient for Manufacturing High-Protein, Mineral-Dense Snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef]
- Senrayan, J.; Venkatachalam, S. A Short Extraction Time of Vegetable Oil from Carica Papaya L. Seeds Using Continuous Ultrasound Acoustic Cavitation: Analysis of Fatty Acid Profile and Thermal Behavior. J. Food Process Eng. 2019, 42, e12950. [Google Scholar] [CrossRef] [Green Version]
- Chutia, H.; Mahanta, C.L. Green Ultrasound and Microwave Extraction of Carotenoids from Passion Fruit Peel Using Vegetable Oils as a Solvent: Optimization, Comparison, Kinetics, and Thermodynamic Studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. Fabrication of nanoemulsions by ultrasonication. In Nanoemulsions; Jafari, S.M., McClements, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 233–285. [Google Scholar]
- Kahraman, O.; Malvandi, A.; Vargas, L.; Feng, H. Drying Characteristics and Quality Attributes of Apple Slices Dried by a Non-Thermal Ultrasonic Contact Drying Method. Ultrason. Sonochem. 2021, 73, 105510. [Google Scholar] [CrossRef] [PubMed]
- Hashemifesharaki, R.; Xanthakis, E.; Altintas, Z.; Guo, Y.; Gharibzahedi, S.M.T. Microwave-assisted Extraction of Polysaccharides from the Marshmallow Roots: Optimization, Purification, Structure, and Bioactivity. Carbohydr. Polym. 2020, 240, 116301. [Google Scholar] [CrossRef] [PubMed]
- Gharibzahedi, S.M.T. Ultrasound-mediated Nettle Oil Nanoemulsions Stabilized by Purified Jujube Polysaccharide: Process Optimization, Microbial Evaluation and Physicochemical Storage Stability. J. Mol. Liq. 2017, 234, 240–248. [Google Scholar] [CrossRef]
- Kenari, R.E.; Dehghan, B. Optimization of Ultrasound-assisted Solvent Extraction of Hemp (Cannabis sativa L.) Seed Oil Using RSM: Evaluation of Oxidative Stability and Physicochemical Properties of Oil. Food Sci. Nutr. 2020, 8, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Daghaghele, S.; Kiasat, A.R.; Ardebili, S.M.S.; Mirzajani, R. Intensification of Extraction of Antioxidant Compounds from Moringa oleifera Leaves Using Ultrasound-assisted Approach: BBD-RSM Design. Int. J. Fruit Sci. 2021, 21, 693–705. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of Ultrasound-assisted Extraction of Moringa Peregrina Oil with Response Surface Methodology and Comparison with Soxhlet Method. Ind. Crops Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Pang, X.; Hua, P.; Gao, X.; Li, Q.; Li, Z. Simultaneous Optimization of Ultrasound-assisted Extraction for Flavonoids and Antioxidant Activity of Angelica keiskei Using Response Surface Methodology (RSM). Molecules 2019, 24, 3461. [Google Scholar] [CrossRef]
- Abreu, D.J.M.; Carvalho, E.E.N.; Boas, E.V.; Asquieri, E.R.; Damiani, C. Ultrasound-assisted Extraction of Red Mombin Seed Oil (Spondias purpurea L.): Phenolic Profile, Fatty Acid Profile and Chemical Characterization of the Cake, Residue from the Oil Extraction. Grasas Aceites 2022, 73, e451. [Google Scholar] [CrossRef]
- Susanti, E.P.; Rohman, A.; Setyaningsih, W. Dual Response Optimization of Ultrasound-assisted Oil Extraction from Red Fruit (Pandanus conoideus): Recovery and Total Phenolic Compounds. Agronomy 2022, 12, 523. [Google Scholar] [CrossRef]
- Alenyorege, E.A.; Hussein, Y.A.; Adongo, T.A. Extraction Yield, Efficiency and Loss of the Traditional Hot Water Floatation (HWF) Method of Oil Extraction from the Seeds of Allanblackia floribunda. Int. J. Sci. Technol. Res. 2015, 4, 92–95. [Google Scholar]
- Gharibzahedi, S.M.T.; Mousavi, S.M.; Hamedi, M.; Khodaiyan, F. Determination and Characterization of Kernel Biochemical Composition and Functional Compounds of Persian Walnut Oil. J. Food Sci. Technol. 2014, 51, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ogbunugafor, H.A.; Eneh, F.U.; Ozumba, A.N.; Igwo-Ezikpe, M.N.; Okpuzor, J.; Igwilo, I.O.; Adenekan, S.O.; Onyekwelu, O.A. Physico-Chemical and Antioxidant Properties of Moringa oleifera Seed Oil. Pak. J. Nutr. 2011, 10, 409–414. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, S.M. Developing an Emulsion Model System Containing Canthaxanthin Biosynthesized by Dietzia natronolimnaea HS-1. Int. J. Biol. Macromol. 2012, 51, 618–626. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Naebi, M.; Torbati, M.; Azadmard-Damirchi, S.; Siabi, S.; Savage, G.P. Changes in Physicochemical Properties of Cold Press Extracted Oil from Balangu (Lallemantia peltata) Seeds During Storage. J. Food Compost. Anal. 2022, 107, 104358. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Channip, A.A.; Hwa, P.C.H.; Ja’afar, F.; Yasin, H.M.; Usman, A. Physicochemical Properties, Antioxidant Capacities, and Metal Contents of Virgin Coconut Oil Produced by Wet and Dry Processes. Food Sci. Nutr. 2018, 6, 1298–1306. [Google Scholar] [CrossRef]
- Moigradean, D.; Poiana, M.A.; Alda, L.M.; Gogoasa, I. Quantitative Identification of Fatty Acids from Walnut and Coconut Oils Using GC-MS Method. J. Agroaliment. Processes Technol. 2013, 19, 459–463. [Google Scholar]
- Jeon, Y.H.; Son, Y.J.; Kim, S.H.; Yun, E.Y.; Kang, H.J.; Hwang, I.K. Physicochemical Properties and Oxidative Stabilities of Mealworm (Tenebrio molitor) Oils Under Different Roasting Conditions. Food Sci. Biotechnol. 2016, 25, 105–110. [Google Scholar] [CrossRef]
- Mariod, A.A.; Abdelwahab, S.I.; Gedi, M.A.; Solati, Z. Supercritical Carbon Dioxide Extraction of Sorghum Bug (Agonoscelis Pubescens) Oil Using Response Surface Methodology. J. Am. Oil Chem. Soc. 2010, 87, 849–856. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists Society, 5th ed.; AOCS Press: Champaign, IL, USA, 1998. [Google Scholar]
- ISO 3656; Animal and Vegetable Fats and Oils. Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. International Organisation for Standardisation: Geneva, Switzerland, 2011; pp. 1–8. Available online: https://www.iso.org/standard/51008.html (accessed on 3 May 2022).
- Almeida, D.T.D.; Viana, T.V.; Costa, M.M.; Silva, C.D.S.; Feitosa, S. Effects of Different Storage Conditions on the Oxidative Stability of Crude and Refined Palm Oil, Olein and Stearin (Elaeis guineensis). Food Sci. Technol. 2018, 39, 211–217. [Google Scholar] [CrossRef]
- Wang, D.; Fan, W.; Guan, Y.; Huang, H.; Yi, T.; Ji, J. Oxidative Stability of Sunflower Oil Flavored by Essential Oil from Coriandrum Sativum L. During Accelerated Storage. LWT 2018, 98, 268–275. [Google Scholar] [CrossRef]
- Ochoa-Martínez, C.I.; Ramaswamy, H.S.; Ayala-Aponte, A.A. Suitability of Crank’s Solutions to Fick’s Second Law for Water Diffusivity Calculation and Moisture Loss Prediction in Osmotic Dehydration of Fruits. J. Food Process Eng. 2009, 32, 933–943. [Google Scholar] [CrossRef]
- Chen, G.; Sun, F.; Wang, S.; Wang, W.; Dong, J.; Gao, F. Enhanced Extraction of Essential Oil from Cinnamomum Cassia Bark by Ultrasound Assisted Hydrodistillation. Chin. J. Chem. Eng. 2021, 36, 38–46. [Google Scholar] [CrossRef]
- Baümler, E.R.; Carrín, M.E.; Carelli, A.A. Extraction of Sunflower Oil Using Ethanol as Solvent. J. Food Eng. 2016, 178, 190–197. [Google Scholar] [CrossRef]
- Milani, N.N.; Ghiasi, R.; Forghaniha, A. The Impact of Solvent Polarity on the Stability, Electronic Properties, and 1H NMR Chemical Shift of the Conformers of 2-Chloro-3-Methylcyclohexan-1-One Oxime: A Conceptual DFT Approach. J. Appl. Spectrosc. 2020, 86, 1123–1131. [Google Scholar] [CrossRef]
- Espinosa-Pardo, F.A.; Savoire, R.; Subra-Paternault, P.; Harscoat-Schiavo, C. Oil and Protein Recovery from Corn Germ: Extraction Yield, Composition and Protein Functionality. Food Bioprod. Process. 2020, 120, 131–142. [Google Scholar] [CrossRef]
- Wang, J.L.; Yu, Z.L.; Yin, F.W.; Li, D.Y.; Liu, H.L.; Song, L.; Zhou, D.Y. Comparison of Different Solvents for Extraction of Oils from By-Products of Shrimps Penaeus vannamei and Procambarus clarkia. J. Food Process. Preserv. 2021, 45, e15754. [Google Scholar] [CrossRef]
- Xu, G.; Liang, C.; Huang, P.; Liu, Q.; Xu, Y.; Ding, C.; Li, T. Optimization of Rice Lipid Production from Ultrasound-assisted Extraction by Response Surface Methodology. J. Cereal Sci. 2016, 70, 23–28. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Ultrasound-microwave Assisted Extraction of Pectin from Fig (Ficus carica L.) Skin: Optimization, Characterization and Bioactivity. Carbohydr. Polym. 2019, 222, 114992. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, Z.; Zheng, B.; Lo, Y.M. Optimization of Ultrasonic-assisted Extraction of Pomegranate (Punica granatum L.) Seed Oil. Ultrason. Sonochem. 2013, 20, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.A.; Zhang, Z.Q.; Yue, X.F.; Fan, X.H.; Li, T.; Chen, S.F. Response Surface Optimization of Ultrasound-assisted Oil Extraction from Autoclaved Almond Powder. Food Chem. 2009, 116, 513–518. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, L.J.; Li, D.; Jiao, S.S.; Chen, X.D.; Mao, Z.H. Ultrasound-assisted Extraction of Oil from Flaxseed. Sep. Purif. Technol. 2008, 62, 192–198. [Google Scholar] [CrossRef]
- Khosravi, A.; Safari, M.; Khodaiyan, F.; Gharibzahedi, S.M.T. Bioconversion Enhancement of Conjugated Linoleic Acid by Lactobacillus Plantarum Using the Culture Media Manipulation and Numerical Optimization. J. Food Sci. Technol. 2015, 52, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in Ultrasound Assisted Extraction of Bioactive Compounds from Cash Crops—A Review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Fei, S.; Gu, H.; Yang, L. Optimization of Ultrasonic Circulating Extraction of Samara Oil from Acer Saccharum Using Combination of Plackett–Burman Design and Box–Behnken Design. Ultrason. Sonochem. 2017, 35, 161–175. [Google Scholar] [CrossRef]
- Rostami, H.; Gharibzahedi, S.M.T. Mathematical Modeling of Mucilage Extraction Kinetic from the Waste Hydrolysates of Fruiting Bodies of Zizyphus Jujuba Mill. J. Food Process. Preserv. 2017, 41, e13064. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; George, S.; Greiner, R.; Estevinho, B.N.; Frutos Fernández, M.J.; McClements, D.J.; Roohinejad, S. New Trends in the Microencapsulation of Functional Fatty Acid-Rich Oils Using Transglutaminase Catalyzed Crosslinking. Compr. Rev. Food Sci. Food Saf. 2018, 17, 274–289. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, S.M. Comparison of Antioxidant and Free Radical Scavenging Activities of Biocolorant Synthesized by Dietzia natronolimnaea HS-1 Cells Grown in Batch, Fed-Batch and Continuous Cultures. Ind. Crops Prod. 2013, 49, 10–16. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of Ultrasound-assisted Extraction of Oil from Papaya Seed by Response Surface Methodology: Oil Recovery, Radical Scavenging Antioxidant Activity, and Oxidation Stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Optimization of Ultrasound-assisted Extraction of Oil from Olive Pomace Using Response Surface Technology: Oil Recovery, Unsaponifiable Matter, Total Phenol Content and Antioxidant Activity. LWT-Food Sci. Technol. 2017, 79, 178–189. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of Ultrasound-assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Grape (Vitis vinifera) Seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of Ultrasound-assisted Extraction with Conventional Extraction Methods of Oil and Polyphenols from Grape (Vitis vinifera L.) Seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Ideris, F.; Nomanbhay, S.; Kusumo, F.; Silitonga, A.S.; Ong, M.Y.; Ong, H.C.; Mahlia, T.M.I. Optimization of Ultrasound-assisted Oil Extraction from Canarium odontophyllum Kernel as a Novel Biodiesel Feedstock. J. Clean. Prod. 2021, 288, 125563. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Razavi, S.H.; Mousavi, M. Optimal Development of a New Stable Nutraceutical Nanoemulsion Based on the Inclusion Complex of 2-Hydroxypropyl-Β-Cyclodextrin with Canthaxanthin Accumulated by Dietzia Natronolimnaea HS-1 Using Ultrasound-assisted Emulsification. J. Disper. Sci. Technol. 2015, 36, 614–625. [Google Scholar] [CrossRef]
- Senrayan, J.; Venkatachalam, S. Ultrasonic Acoustic-Cavitation as a Novel and Emerging Energy Efficient Technique for Oil Extraction from Kapok Seeds. Innov. Food Sci. Emerg. Technol. 2020, 62, 102347. [Google Scholar] [CrossRef]
- Yap, J.W.-L.; Lee, Y.-Y.; Tang, T.-K.; Chong, L.-C.; Kuan, C.-H.; Lai, O.-M.; Phuah, E.-T. Fatty Acid Profile, Minor Bioactive Constituents and Physicochemical Properties of Insect-Based Oils: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef]
- Nino, M.C.; Reddivari, L.; Ferruzzi, M.G.; Liceaga, A.M. Targeted Phenolic Characterization and Antioxidant Bioactivity of Extracts from Edible Acheta Domesticus. Foods 2021, 10, 2295. [Google Scholar] [CrossRef]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.; van Boekel, M.A.; Lakemond, C.M. Insect Lipid Profile: Aqueous Versus Organic Solvent-Based Extraction Methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Jalili, F.; Jafari, S.M.; Emam-Djomeh, Z.; Malekjani, N.; Farzaneh, V. Optimization of Ultrasound-assisted Extraction of Oil from Canola Seeds with the Use of Response Surface Methodology. Food Anal. Method 2018, 11, 598–612. [Google Scholar] [CrossRef]
- Shi, L.K.; Zheng, L.; Liu, R.J.; Chang, M.; Jin, Q.Z.; Wang, X.G. Chemical Characterization, Oxidative Stability, and In Vitro Antioxidant Capacity of Sesame Oils Extracted by Supercritical and Subcritical Techniques and Conventional Methods: A Comparative Study Using Chemometrics. Eur. J. Lipid Sci. Technol. 2018, 120, 1700326. [Google Scholar] [CrossRef]
- Dong, W.; Chen, Q.; Wei, C.; Hu, R.; Long, Y.; Zong, Y.; Chu, Z. Comparison of the Effect of Extraction Methods on the Quality of Green Coffee oil from Arabica Coffee Beans: Lipid Yield, Fatty Acid Composition, Bioactive Components, and Antioxidant Activity. Ultrason. Sonochem. 2021, 74, 105578. [Google Scholar] [CrossRef] [PubMed]

| Trial | Independent Variables | Response Variables | ||||
|---|---|---|---|---|---|---|
| UAE Time (min, X1) | Solvent/Solid (v/w, X2) | UAE Temperature (°C, X3) | EE (%, Y1) | SCDPPH⋅ (%, Y2) | RP (Y3) | |
| 1 | 15 (−1, Factorial) | 7.5 (−1, Factorial) | 30 (−1, Factorial) | 37.6 ± 1.2 | 45.0 ± 0.6 | 0.326 ± 0.010 |
| 2 | 30 (+1, Factorial) | 7.5 (−1, Factorial) | 30 (−1, Factorial) | 56.0 ± 2.4 | 58.1 ± 0.4 | 0.417 ± 0.014 |
| 3 | 5 (−1, Factorial) | 22.5 (+1, Factorial) | 30 (−1, Factorial) | 68.9 ± 1.8 | 66.6 ± 0.3 | 0.532 ± 0.009 |
| 4 | 15 (−1), Factorial | 22.5 (+1, Factorial) | 30 (−1, Factorial) | 79.5 ± 1.0 | 69.3 ± 1.0 | 0.666 ± 0.001 |
| 5 | 30 (+1, Factorial) | 7.5 (−1, Factorial) | 70 (+1, Factorial) | 63.0 ± 1.0 | 56.6 ± 0.3 | 0.432 ± 0.004 |
| 6 | 5 (−1, Factorial) | 7.5 (−1, Factorial) | 70 (+1, Factorial) | 78.1 ± 2.0 | 64.5 ± 1.1 | 0.509 ± 0.012 |
| 7 | 15 (−1, Factorial) | 22.5 (+1, Factorial) | 70 (+1, Factorial) | 84.7 ± 2.2 | 69.3 ± 0.7 | 0.575 ± 0.007 |
| 8 | 30 (+1, Factorial) | 22.5 (+1, Factorial) | 70 (+1, Factorial) | 92.3 ± 1.3 | 60.4 ± 0.5 | 0.532 ± 0.007 |
| 9 | 9.89 (−α, Axial) | 15 (0, Center) | 50 (0, Center) | 65.5 ± 0.6 | 40.0 ± 0.8 | 0.274 ± 0.005 |
| 10 | 35.11 (+α, Axial) | 15 (0, Center) | 50 (0, Center) | 85.4 ± 0.7 | 58.1 ± 1.7 | 0.434 ± 0.006 |
| 11 | 22.5 (0, Center) | 2.39 (−α, Axial) | 50 (0, Center) | 51.6 ± 1.0 | 56.4 ± 1.5 | 0.402 ± 0.013 |
| 12 | 22.5 (0, Center) | 27.61 (+α, Axial) | 50 (0, Center) | 90.4 ± 1.2 | 78.4 ± 1.1 | 0.796 ± 0.001 |
| 13 | 22.5 (0, Center) | 15 (0, Center) | 16.36 (−α, Axial) | 41.0 ± 1.5 | 65.3 ± 0.8 | 0.632 ± 0.003 |
| 14 | 22.5 (0, Center) | 15 (0, Center) | 83.64 (+α, Axial) | 80.2 ± 1.2 | 73.9 ± 0.8 | 0.686 ± 0.013 |
| 15 | 22.5 (0, Center) | 15 (0, Center) | 50 (0, Center) | 73.3 ± 0.7 | 59.5 ± 1.2 | 0.479 ± 0.014 |
| 16 | 22.5 (0, Center) | 15 (0, Center) | 50 (0, Center) | 76.9 ± 0.4 | 60.3 ± 0.6 | 0.488 ± 0.009 |
| 17 | 22.5 (0, Center) | 15 (0, Center) | 50 (0, Center) | 71.5 ± 0.9 | 63.5 ± 0.4 | 0.545 ± 0.005 |
| 18 | 22.5 (0, Center) | 15 (0, Center) | 50 (0, Center) | 75.4 ± 1.3 | 62.3 ± 0.5 | 0.492 ± 0.004 |
| 19 | 22.5 (0, Center) | 15 (0, Center) | 50 (0, Center) | 73.4 ± 1.9 | 63.1 ± 0.3 | 0.535 ± 0.004 |
| 20 | 22.5 (0, Center) | 15 (0, Center) | 50 (0, Center) | 69.9 ± 1.0 | 61.3 ± 0.8 | 0.529 ± 0.002 |
| Source | DF | EE (%, Y1) 1 | SCDPPH⋅ (%, Y2) 1 | RP (Y3) 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | SS | p-Value | C | SS | p-Value | C | SS | p-Value | ||
| Model 2 | 9 | 73.37 | 4152.90 | <0.0001 | 61.72 | 1357.42 | <0.0001 | 0.51 | 0.26 | <0.0001 |
| X1 | 1 | 6.28 | 539.28 | <0.0001 | 3.31 | 149.35 | 0.0010 | 0.039 | 0.02 | 0.0076 |
| X2 | 1 | 11.40 | 1773.81 | <0.0001 | 5.74 | 449.44 | <0.0001 | 0.094 | 0.12 | <0.0001 |
| X3 | 1 | 10.37 | 1469.62 | <0.0001 | 1.93 | 50.78 | 0.0230 | - | 0.01 | 0.2409 ns |
| X1 2 | 1 | - | 14.29 | 0.1377 ns | −4.63 | 308.65 | <0.0001 | −0.064 | 0.06 | 0.0002 |
| X2 2 | 1 | - | 4.63 | 0.3800 ns | 1.84 | 49.00 | 0.0249 | - | 0.007 | 0.0748 ns |
| X3 2 | 1 | −4.25 | 260.41 | <0.0001 | 2.63 | 99.72 | 0.0037 | 0.044 | 0.028 | 0.0031 |
| X1X2 | 1 | −1.97 | 31.17 | 0.0385 | −3.39 | 91.89 | 0.0048 | - | 0.002 | 0.5403 ns |
| X1X3 | 1 | - | 5.53 | 0.3393 ns | −2.09 | 35.07 | 0.0499 | - | 0.0007 | 0.1469 ns |
| X2X3 | 1 | −2.31 | 42.74 | 0.0191 | −3.02 | 73.14 | 0.0092 | −0.036 | 0.004 | 0.0386 |
| Residual | 10 | 54.91 | 70.55 | 0.018 | ||||||
| LoF 1 | 5 | 22.88 | 0.6395 ns | 58.13 | 0.0578 ns | 0.014 | 0.0911 ns | |||
| Pure error | 5 | 32.03 | 12.42 | 0.004 | ||||||
| Total | 19 | 4207.81 | 1427.97 | 0.28 | ||||||
| R2 | 0.9870 | 0.9506 | 0.9348 | |||||||
| R2adj | 0.9752 | 0.9061 | 0.8760 | |||||||
| CV | 3.31 | 4.31 | 8.35 | |||||||
| AP | 33.86 | 18.36 | 15.41 | |||||||
| Property | Extraction Method 1,2 | |
|---|---|---|
| CSE with n-Hexane | UAE with Ethanol/Isopropanol | |
| Process efficiency, energy, and diffusion coefficient | ||
| Extraction efficiency (%) | 60.09 ± 1.32 b | 89.41 ± 1.87 a |
| Diffusion coefficient (D, ×10−9 m2/s) | 5.07 × 10−11 b | 0.97 ± 0.05 × 10−9 a |
| Electric energy consumption (EEC, kW.h/g) | 0.647 ± 0.009 a | 0.035 ± 0.003 b |
| Bioactive compounds and antioxidant activity | ||
| Total carotenoid content (TCC, mg/g) | 0.645 ± 0.044 b | 0.778 ± 0.032 a |
| Total phenolic content (TPC, mg GAE/g) | 3.652 ± 0.015 b | 4.306 ± 0.029 a |
| DPPH scavenging capacity (SCDPPH⋅, %) | 60.10 ± 2.32 b | 71.31 ± 0.84 a |
| Reducing power (RP) | 0.517 ± 0.012 b | 0.663 ± 0.016 a |
| Physicochemical properties | ||
| Browning index (BI) | 0.316 ± 0.009 a | 0.299 ± 0.05 a |
| Photometric color index (PCI) | 14.98 ± 0.09 a | 15.22 ± 0.11 a |
| Specific gravity | 0.9005 ± 0.0003 a | 0.9003 ± 0.0003 a |
| Refractive index | 1.452 ± 0.003 a | 1.450 ± 0.002 a |
| Apparent visocisty (cP) | 300.78 ± 5.62 a | 300.29 ± 7.01 a |
| Acid value (AV, mg KOH/g) | 1.78 ± 0.09 a | 1.67 ± 0.10 b |
| Saponification value (SV, mg KOH/g) | 221.05 ± 1.35 a | 220.35 ± 0.19 a |
| Peroxide value (PV, meq O2/kg) | 0.303 ± 0.009 a | 0.269 ± 0.011 b |
| p-Anisidine value (AnV) | 0.201 ± 0.04 a | 0.193 ± 0.02 b |
| Totox value (TxV) | 0.807 ± 0.005 a | 0.731 ± 0.005 b |
| Iodine value (IV, g iodine/100 g) | 88.70 ± 0.71 a | 83.52 ± 0.34 b |
| Conjugated diene (K232) | 1.64 ± 0.07 a | 1.55 ± 0.03 b |
| Conjugated triene (K270) | 0.126 ± 0.005 a | 0.109 ± 0.004 b |
| Induction time (IT, h) | 17.22 ± 0.61 b | 19.56 ± 0.40 a |
| Free fatty acid (FFA, mg/kg) | 0.34 ± 0.04 a | 0.24 ± 0.03 b |
| Fatty acids profile | ||
| Caproic acid (C6:0) | 0.15 ± 0.02 a | 0.15 ± 0.01 a |
| Lauric acid (C12:0) | 0.32 ± 0.02 a | 0.29 ± 0.01 a |
| Myristic acid (C14:0) | 0.91 ± 0.03 a | 0.93 ± 0.07 a |
| Palmitic acid (C16:0) | 28.65 ± 0.26 a | 26.32 ± 0.18 a |
| Palmitoleic acid (C16:1) | 0.30 ± 0.02 a | 0.28 ± 0.00 a |
| Stearic acid (C18:0) | 7.68 ± 0.06 a | 7.56 ± 0.38 a |
| Oleic acid (C18:1Δ9c) | 28.01 ± 0.76 a | 29.54 ± 0.25 a |
| Linoleic acid (C18:2n-6) | 30.18 ± 0.44 a | 31.52 ± 0.63 a |
| α-Linolenic acid (C18:3n-3) | 2.05 ± 0.11 a | 2.01 ± 0.05 a |
| Arachidic acid (C20:0) | 0.31 ± 0.05 a | 0.30 ± 0.02 a |
| Gondoic acid (C20:1Δ11) | 0.38 ± 0.03 a | 0.34 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharibzahedi, S.M.T.; Altintas, Z. Ultrasound-Assisted Alcoholic Extraction of Lesser Mealworm Larvae Oil: Process Optimization, Physicochemical Characteristics, and Energy Consumption. Antioxidants 2022, 11, 1943. https://doi.org/10.3390/antiox11101943
Gharibzahedi SMT, Altintas Z. Ultrasound-Assisted Alcoholic Extraction of Lesser Mealworm Larvae Oil: Process Optimization, Physicochemical Characteristics, and Energy Consumption. Antioxidants. 2022; 11(10):1943. https://doi.org/10.3390/antiox11101943
Chicago/Turabian StyleGharibzahedi, Seyed Mohammad Taghi, and Zeynep Altintas. 2022. "Ultrasound-Assisted Alcoholic Extraction of Lesser Mealworm Larvae Oil: Process Optimization, Physicochemical Characteristics, and Energy Consumption" Antioxidants 11, no. 10: 1943. https://doi.org/10.3390/antiox11101943
APA StyleGharibzahedi, S. M. T., & Altintas, Z. (2022). Ultrasound-Assisted Alcoholic Extraction of Lesser Mealworm Larvae Oil: Process Optimization, Physicochemical Characteristics, and Energy Consumption. Antioxidants, 11(10), 1943. https://doi.org/10.3390/antiox11101943






