Rosehip (Rosa canina L.) Meal as a Natural Antioxidant on Lipid and Protein Quality and Shelf-Life of Polyunsaturated Fatty Acids Enriched Eggs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Procedure
2.2. Experimental Materials
2.3. Birds’ Husbandry
2.4. Experimental Diets
2.5. Laying Performance and Egg Production
2.6. Egg Sampling
2.7. Primary Chemical Analysis
2.8. Amino Acids Analyses
2.8.1. Amino Acid Content (AA)
2.8.2. Estimations of Egg Yolk Protein Quality Indices
2.9. Egg Yolk Fatty Acids Analyses
2.9.1. Fatty Acids Content
2.9.2. Estimation of Lipid Health-Related Quality Indices
2.10. Total Polyphenol Concentration and Antioxidant Capacity Analyses
2.11. Shelf-Life of the Eggs
2.12. Statistical Analysis
3. Results
3.1. Chemical Composition of the Rosehip and Flaxseed Meal
3.2. Effect of Rosehip and Flaxseed Meals on Laying Hens’ Performances
3.3. Effect of Rosehip and Flaxseed Meal on Amino Acid Profile in Egg Yolk
3.4. Effect of Rosehip and Flaxseed Meal on Fatty Acid Content in Egg
3.5. Effect of Rosehip Meal on TAC and TPC Determined in Eggs
3.6. Effect of Rosehip and Flaxseed Meal on Shelf-Life of Eggs
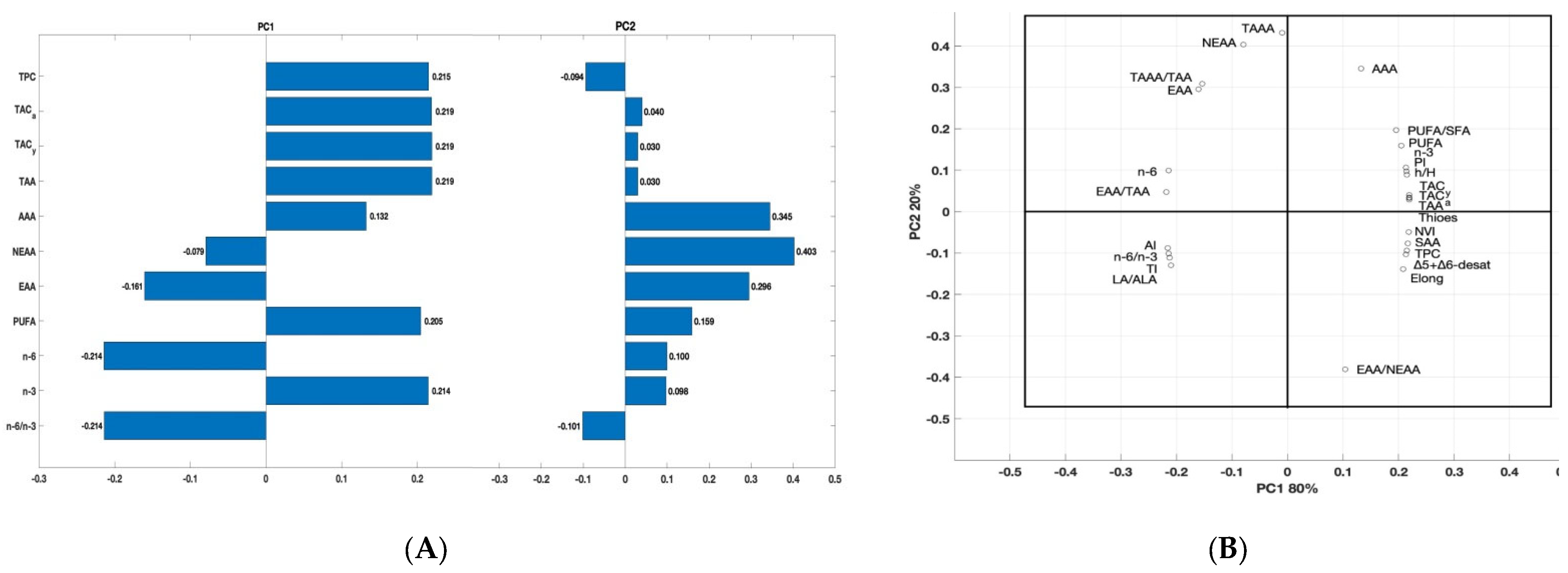
3.7. Principal Component Analysis (PCA)
4. Discussion
4.1. Chemical Composition of the Rosehip and Flaxseed Meals
4.2. Effect of Rosehip and Flaxseed Meals on Laying Hens’ Performances
4.3. Effect of Rosehip and Flaxseed Meal on Yolk Amino Acids and Protein Quality Indices
4.4. Effect of Rosehip and Flaxseed Meal on Yolk Fatty Acids and Lipid Quality Indices
4.5. Effect of Rosehip and Flaxseed Meal on Antioxidant Activity
4.6. Effect of Rosehip and Flaxseed Meal on Shelf-Life of Eggs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenzo, J.M.; Munekata, P.E.; Pateiro, M.; Fierro, E.M.; Brnčić, S.R.; Brnčić, M.; Barba, F.J. Functional foods. In Nutraceuticals and Dietary Supplements; Apple Academic Press: Candor, NY, USA, 2020; pp. 3–20. [Google Scholar]
- Birch, C.S.; Bonwick, G.A. Ensuring the future of functional foods. Int. J. Food Sci. Technol. 2019, 54, 1467–1485. [Google Scholar] [CrossRef]
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional Food—Consumer Motivations and Expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Li, L.; Wen, Z.G.; Yan, H.J.; Yang, P.L.; Tang, J.; Xie, M.; Hou, S.S. Dual functions of eicosapentaenoic acid-rich microalgae: Enrichment of yolk with n-3 polyunsaturated fatty acids and partial replacement for soybean meal in diet of laying hens. Poult. Sci. 2019, 98, 350–357. [Google Scholar] [CrossRef]
- Oomah, B.D. Flaxseed By-products. In Food Wastes and By-Products: Nutraceutical and Health Potential; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 267–289. [Google Scholar]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching laying hens eggs by feeding diets with different fatty acid composition and antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef]
- Shayan, M.; Kamalian, S.; Sahebkar, A.; Tayarani-Najaran, Z. Flaxseed for Health and Disease: Review of Clinical Trials. CCHTS 2020, 23, 699–722. [Google Scholar] [CrossRef]
- Igual, M.; Chiş, M.S.; Păucean, A.; Vodnar, D.C.; Muste, S.; Man, S.; Martínez-Monzó, J.; García-Segovia, P. Valorization of Rose Hip (Rosa canina) Puree Co-Product in Enriched Corn Extrudates. Foods 2021, 10, 2787. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Levickienė, D.; Hallmann, E. The Effect of Ripening Stages on the Accumulation of Carotenoids, Polyphenols and Vitamin C in Rosehip Species/Cultivars. Appl. Sci. 2021, 11, 6761. [Google Scholar] [CrossRef]
- Kubczak, M.; Khassenova, A.B.; Skalski, B.; Michlewska, S.; Wielanek, M.; Aralbayeva, A.N.; Murzakhmetova, M.K.; Zamaraeva, M.; Skłodowska, M.; Bryszewska, M.; et al. Bioactive Compounds and Antiradical Activity of the Rosa canina L. Leaf and Twig Extracts. Agronomy 2020, 10, 1897. [Google Scholar] [CrossRef]
- Patel, S. Rose hip as an underutilized functional food: Evidence-based review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Gjorgovska, N.; Grigorova, S.; Levkov, V. Application of Rose Hip Fruits as Feed Supplement in Animal Nutrition. J. Agric. Food Dev. 2021, 7, 12–15. [Google Scholar] [CrossRef]
- Konca, Y.; Kaliber, M.; Uzkulekci, H.H.; Cimen, B.; Yalcin, H. The effect of rosehip (Rosa canina L.) supplementation to diet on the performance, egg and meat quality, antioxidant activity in laying quail. Sains Malays. 2021, 50, 3617–3629. [Google Scholar] [CrossRef]
- Olteanu, M.; Panaite, T.D.; Turcu, R.P.; Ropota, M.; Vlaicu, P.A.; Mitoi, M. Using grapeseed meal as natural antioxidant in slow-growing Hubbard broiler diets enriched in polyunsaturated fatty acids. Rev. Mex. Cienc. Pecu. 2022, 13, 43–63. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.E.; Martura, T.; Olteanu, M.; Panaite, T.D.; Schitea, M.; Van, I. Development and validation of an RP-HPLC method for methionine, cystine and lysine separation and determination in corn samples. Rev. Chem. 2013, 64, 673–679. [Google Scholar]
- Food and Agriculture Organization (FAO); World Health Organization (WHO); United Nations University (UNU). Protein and Amino Acid Requirements in Human Nutrition; Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series No. 935; World Health Organization (WHO): Geneva, Switzerland, 2007. [Google Scholar]
- Turcu, R.P.; Olteanu, M.; Criste, R.D.; Panaite, T.D.; Ropotă, M.; Vlaicu, P.A.; Drăgotoiu, D. Grapeseed meal used as natural antioxidant in high fatty acid diets for Hubbard broilers. Braz. J. Poult. Sci. 2019, 21, 001–012. [Google Scholar] [CrossRef]
- Untea, A.E.; Varzaru, I.; Panaite, T.D.; Gavris, T.; Lupu, A.; Ropota, M. The Effects of dietary inclusion of bilberry and walnut leaves in laying hens’ diets on the antioxidant properties of eggs. Animals 2020, 10, 191. [Google Scholar] [CrossRef]
- FAO/WHO (Food and Agricultural Organization of the United Nations and World Health Organization). Fats and Fatty Acids in Human Nutrition; Report of an Extract Consultation; FAO Food Nutrition Papers; FAO: Rome, Italy, 2010; Volume 91. [Google Scholar]
- Olteanu, M.; Criste, R.D.; Panaite, T.D.; Ropotă, M.; Mitoi, M.; Vlaicu, P.A.; Șoica, C. Quality of the eggs obtained from hens fed diet formulations rich in polyunsaturated fatty acids and with grape seeds meal as antioxidant. Arch. Zootech. 2017, 20, 37–49. [Google Scholar]
- Vlaicu, P.A.; Panaite, T.D. Effect of dietary pumpkin (Cucurbita moschata) seed meal on layer performance and egg quality characteristics. Anim. Biosci. 2022, 35, 236–246. [Google Scholar] [CrossRef]
- Zimniewska, M.; Rozańska, W.; Gryszczynska, A.; Romanowska, B.; Kicinska-Jakubowska, A. Antioxidant Potential of Hemp and Flax Fibers Depending on Their Chemical Composition. Molecules 2018, 23, 1993. [Google Scholar] [CrossRef]
- Lemes, L.F.R.; Tarley, C.R.T. Combination of supramolecular solvent-based microextraction and ultrasound-assisted extraction for cadmium determination in flaxseed flour by thermospray flame furnace atomic absorption spectrometry. Food Chem. 2021, 357, 129695. [Google Scholar] [CrossRef]
- Moldovan, C.; Babotă, M.; Mocan, A.; Menghini, L.; Cesa, S.; Gavan, A.; Sisea, C.; Vodnar, D.C.; Dias, M.I.; Pereira, C.; et al. Optimization of the drying process of autumn fruits rich in antioxidants: A study focusing on rosehip (Rosa canina L.) and sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) and their bioactive properties. Food Funct. 2021, 12, 3939–3953. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, S.; Gjorgovska, N.; Levkov, V. Effects of rosehip feed supplementation on egg quality parameters, yolk lipid oxidation, and blood parameters of laying hens. Iran. J. Appl. Anim. Sci. 2021, 11, 827–833. [Google Scholar]
- Sepehr, A.; Kashani, R.B.; Esmaeili, M.; Safari, O.; Rombenso, A. Effects of extruded, milled, and whole flaxseed (Linum usitatissimum) on egg performance, lipid components, and fatty acids concentrations in yolk and blood, and antioxidant system of commercial laying hens. Anim. Feed. Sci. Technol. 2021, 276, 114877. [Google Scholar] [CrossRef]
- Huang, S.; Baurhoo, B.; Mustafa, A. Effects of extruded flaxseed on layer performance, nutrient retention and yolk fatty acid composition. Br. Poult. Sci. 2018, 59, 463–469. [Google Scholar] [CrossRef]
- Bean, L.D.; Leeson, S. Long-term effects of feeding flaxseed on performance and egg fatty acid composition of brown and white hens. Poult. Sci. 2003, 82, 388–394. [Google Scholar] [CrossRef]
- Imran, M.; Anjum, F.M.; Nadeem, M.; Ahmad, N.; Khan, M.K.; Mushtaq, Z.; Hussain, S. Production of Bio-omega-3 eggs through the supplementation of extruded flaxseed meal in hen diet. Lipids Health Dis. 2015, 14, 126. [Google Scholar] [CrossRef]
- Hatice, K.; Adem, K.; Esenbuğa, N.; Macit, M. The effect of rosehip seed supplementation into laying hens diets on performance, egg quality traits, yolk lipid profile and serum parameters. Alinteri J. Agric. Sci. 2019, 34, 84–87. [Google Scholar]
- Bortoluzzi, C.; Rochell, S.J.; Applegate, T.J. Threonine, arginine, and glutamine: Influences on intestinal physiology, immunology, and microbiology in broilers. Poult. Sci. 2018, 97, 937–945. [Google Scholar] [CrossRef]
- Debnath, B.C.; Biswas, P.; Roy, B. The effects of supplemental threonine on performance, carcass characteristics, immune response and gut health of broilers in subtropics during pre-starter and starter period. J. Anim. Phy. Anim. Nutr. 2019, 103, 29–40. [Google Scholar] [CrossRef]
- Ravindran, V. Poultry feed availability and nutrition in developing countries. Poult. Dev. Rev. 2013, 2, 60–63. [Google Scholar]
- Heger, J.; Frydrych, Z. Efficiency of utilization of amino acids. In Absorption and Utilization of Amino Acids; CRC Press: Boca Raton, FL, USA, 2019; pp. 31–56. [Google Scholar]
- Attia, Y.A.; Al-Harthi, M.A.; Korish, M.A.; Shiboob, M.H. Protein and Amino Acid Content in Four Brands of Commercial Table Eggs in Retail Markets in Relation to Human Requirements. Animals 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Ijiri, D.; Shimamoto, S.; Takaya, M.; Ohtsuka, A.; Goto, T. Genetic effect on free amino acid contents of egg yolk and albumen using five different chicken genotypes under floor rearing system. PLoS ONE 2021, 16, e0258506. [Google Scholar] [CrossRef] [PubMed]
- Tas, N.G.; Gokmen, V. Profiling of the contents of amino acids, water-soluble vitamins, minerals, sugars and organic acids in Turkish hazelnut varieties. Pol. J. Food Nutr. Sci. 2018, 68, 223–234. [Google Scholar] [CrossRef]
- Jensen, C.L.; Maude, M.; Anderson, R.E.; Heird, W.C. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am. J. Clin. Nutr. 2000, 71, 292s–299s. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Saracila, M.; Panaite, T.D.; Cornescu, G.M. Nutritional composition and bioactive compounds of basil, thyme and sage plant additives and their functionality on broiler thigh meat quality. Foods 2022, 11, 1105. [Google Scholar] [CrossRef]
- Fredriksson, S.; Elwinger, K.; Pickova, J. Fatty acid and carotenoid composition of egg yolk as an effect of microalgae addition to feed formula for laying hens. Food Chem. 2000, 99, 530–537. [Google Scholar] [CrossRef]
- Fraeye, I.; Bruneel, C.; Lemahieu, C.; Buyse, J.; Muylaert, K.; Foubert, I. Dietary enrichment of eggs with omega-3 fatty acids: A review. Food Res. Int. 2012, 48, 961–969. [Google Scholar] [CrossRef]
- Gładkowski, W.; Kiełbowicz, G.; Chojnacka, A.; Bobak, Ł.; Spychaj, R.; Dobrzański, Z.; Trziszka, T.; Wawrzeńczyk, C. The effect of feed supplementation with dietary sources of n-3 polyunsaturated fatty acids, flaxseed and algae Schizochytrium sp., on their incorporation into lipid fractions of J apanese quail eggs. Int. J. Food Sci. Technol. 2014, 49, 1876–1885. [Google Scholar] [CrossRef]
- Lemahieu, C.; Bruneel, C.; Termote-Verhalle, R.; Muylaert, K.; Buyse, J.; Foubert, I. Effect of different microalgal n− 3 PUFA supplementation doses on yolk color and n− 3 LC-PUFA enrichment in the egg. Algal Res. 2014, 6, 119–123. [Google Scholar] [CrossRef]
- Pottel, L.; Lycke, M.; Boterberg, T.; Foubert, I.; Pottel, H.; Duprez, F.; Goethals, L.; Debruyne, P.R. Omega-3 fatty acids: Physiology, biological sources and potential applications in supportive cancer care. Phytochem. Rev. 2014, 13, 223–244. [Google Scholar] [CrossRef]
- Gogus, U.; Smith, C. n-3 Omega fatty acids: A review of current knowledge. Int. J. Food Sci. Technol. 2010, 45, 417–436. [Google Scholar] [CrossRef]
- Kang, M.J.; Shin, M.S.; Park, J.N.; Lee, S.S. The effects of polyunsaturated: Saturated fatty acids ratios and peroxidisability index values of dietary fats on serum lipid profiles and hepatic enzyme activities in rats. Br. J. Nutr. 2005, 94, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Galobart, J.; Barroeta, A.C.; Baucells, M.D.; Codony, R.; Ternes, W. Effect of dietary supplementation with rosemary extract and α-tocopheryl acetate on lipid oxidation in eggs enriched with ω3-fatty acids. Poult. Sci. 2001, 80, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Harlina, P.W.; Ma, M.; Shahzad, R.; Khalifa, I. Effect of Rosemary Extract on Lipid Oxidation, Fatty Acid Composition, Antioxidant Capacity, and Volatile Compounds of Salted Duck Eggs. Food Sci. Anim. Resour. 2022, 42, 689. [Google Scholar] [CrossRef] [PubMed]
- Multescu, M.; Marinas, I.C.; Susman, I.E.; Belc, N. Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods 2022, 11, 253. [Google Scholar] [CrossRef]
- Hayat, Z.; Cherian, G.; Pasha, T.N.; Khattak, F.M.; Jabbar, M.A. Effect of feeding flax and two types of antioxidants on egg production, egg quality, and lipid composition of eggs. J. Appl. Poult. Res. 2009, 18, 541–551. [Google Scholar] [CrossRef]
- Hayat, Z.; Cherian, G.; Pasha, T.N.; Khattak, F.M.; Jabbar, M.A. Oxidative stability and lipid components of eggs from flax-fed hens: Effect of dietary antioxidants and storage. Poult. Sci. 2010, 89, 1285–1292. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Chiang, Y.-F.; Chen, H.-Y.; Ali, M.; Shieh, T.-M.; Huang, Y.-J.; Wang, K.-L.; Chang, H.-Y.; Huang, T.-C.; Hong, Y.-H.; Hsia, S.-M. The Role of Cell Proliferation and Extracellular Matrix Accumulation Induced by Food Additive Butylated Hydroxytoluene in Uterine Leiomyoma. Nutrients 2021, 13, 3074. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Curpan, A.S.; Impellitteri, F.; Plavan, G.; Ciobica, A.; Faggio, C. Mytilus galloprovincialis: An essential, low-cost model organism for the impact of xenobiotics on oxidative stress and public health. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109302. [Google Scholar] [CrossRef] [PubMed]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef] [PubMed]
- Daels-Rakotoarison, D.A.; Gressier, B.; Trotin, F.; Brunet, C.; Luyckx, M.; Dine, T.; Bailleul, F.; Cazin, M.; Cazin, J.C. Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2002, 16, 157–161. [Google Scholar] [CrossRef]
- Yuceer, M.; Caner, C. Antimicrobial lysozyme–chitosan coatings affect functional properties and shelf life of chicken eggs during storage. J. Sci. Food Agric. 2014, 94, 153–162. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Panaite, T.D.; Cornescu, G.M. Shelf life of eggs from hens fed diets rich in polyunsaturated fatty acids and antioxidants under the effect of different storage time and temperatures. Sci. Pap. Ser. D Anim. Sci. 2021, 64, 487–495. [Google Scholar]
- Feddern, V.; de Prá, M.C.; Mores, R.; Nicoloso, R.d.S.; Coldebella, A.; Abreu, P.G.d. Egg quality assessment at different storage conditions, seasons and laying hen strains. Ciênc. Agrotecnol. 2017, 41, 322–333. [Google Scholar] [CrossRef] [Green Version]
- Elwan, H.A.; Elnesr, S.S.; Mohany, M.; Al-Rejaie, S.S. The effects of dietary tomato powder (Solanum lycopersicum L.) supplementation on the haematological, immunological, serum biochemical and antioxidant parameters of growing rabbits. J. Anim. Physiol. Anim. Nutr. 2019, 103, 534–546. [Google Scholar] [CrossRef]
- Pires, P.G.D.S.; Leuven, A.F.R.; Franceschi, C.H.; Machado, G.S.; Pires, P.D.D.S.; Moraes, P.D.O.; Kindlein, L.; Andretta, I. Effects of rice protein coating enriched with essential oils on internal quality and shelf life of eggs during room temperature storage. Poult. Sci. 2020, 99, 604–611. [Google Scholar] [CrossRef]
- Eke, M.O.; Olaitan, N.I.; Ochefu, J.H. Effect of storage conditions on the quality attributes of shell (table) eggs. Niger. Food J. 2013, 31, 18–24. [Google Scholar] [CrossRef]
- Sati, N.M.; Oshibanjo, D.O.; Emennaa, P.E.; Mbuka, J.J.; Haliru, H.; Ponfa, S.B.; Abimiku, O.R.; Nwamo, A.C. Egg Quality Assessment within Day 0 to 10 as Affected by Storage Temperature. Asian J. Res. Anim. Vet. Sci. 2020, 6, 15–25. [Google Scholar]
- Adamski, M.; Kuzniacka, J.; Czarnecki, R.; Kucharska-Gaca, J.; Kowalska, E. Variation in egg quality traits depending on storage conditions. Pol. J. Natl. Sci. 2017, 32, 39–47. [Google Scholar]
- Marzec, A.; Damaziak, K.; Kowalska, H.; Riedel, J.; Michalczuk, M.; Koczywąs, E.; Cisneros, F.; Lenart, A.; Niemiec, J. Effect of Hens Age and Storage Time on Functional and Physiochemical Properties of Eggs. J. Appl. Poult. Res. 2019, 28, 290–300. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Schieber, A.; Wu, J. Effects of storage and cooking on the antioxidant capacity of laying hen eggs. Food Chem. 2016, 194, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.R.; Ward, G.E.; Regmi, P.; Karcher, D.M. Impact of egg handling and conditions during extended storage on egg quality. Poult. Sci. 2018, 97, 716–723. [Google Scholar] [CrossRef]
- De Araújo Soares, R.; Borges, S.V.; Dias, M.V.; Piccoli, R.H.; Fassani, E.J.; da Silva, E.M.C. Impact of whey protein isolate/sodium montmorillonite/sodium metabisulfite coating on the shelf life of fresh eggs during storage. LWT 2021, 139, 110611. [Google Scholar] [CrossRef]
- Heng, N.; Gao, S.; Guo, Y.; Chen, Y.; Wang, L.; Sheng, X.; Wang, X.; Xing, K.; Xiao, L.; Ni, H. Effects of supplementing natural astaxanthin from Haematococcus pluvialis to laying hens on egg quality during storage at 4 °C and 25 °C. Poult. Sci. 2020, 99, 6877–6883. [Google Scholar] [CrossRef]
- Tabib, I.; Onbaşilar, E.E.; Yalçin, S. The effects of cage type, oviposition time and egg storage period on the egg quality characteristics of laying hens. Ankara Univ. Vet. Fak. Derg. 2021, 68, 329–336. [Google Scholar]
- Lee, J.; Seo, H.G.; Lee, C.-H. Effects of lotus (Nelumbo nucifera) leaf hot water extracts on the quality and stability of eggs using ultrasonication treatment during storage. Food Sci. Anim. Res. 2020, 40, 1044–1054. [Google Scholar] [CrossRef]
- Yuan, N.; Wang, J.P.; Ding, X.M.; Bai, S.P.; Zeng, Q.F.; Su, Z.W.; Xuan, Y.; Peng, H.W.; Fraley, G.S.; Zhang, K.Y. Effects of supplementation with different rapeseed oil sources and levels on production performance, egg quality, and serum parameters in laying hens. Poult. Sci. 2019, 98, 1697–1705. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Liang, W.; Geng, Z.; Chen, X. Green tea powder supplementation increased viscosity and decreased lysozyme activity of egg white during storage of eggs frjmom Huainan partridge chicken. Ital. J. Anim. Sci. 2020, 19, 586–592. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; Gebert, R.R.; De Oliveira, R.C.; Boiago, M.M.; Souza, C.F.; Baldissera, M.D.; Vendruscolo, R.G.; Kempka, A.P.; Paiano, D.; Wagner, R.; et al. Impacts of the supplementation of acai lump flour in the diet of laying hens on productive performance, and fatty acid profiles and antioxidant capacity in the fresh and stocked eggs. J. Food Biochem. 2019, 43, e13022. [Google Scholar] [CrossRef] [PubMed]


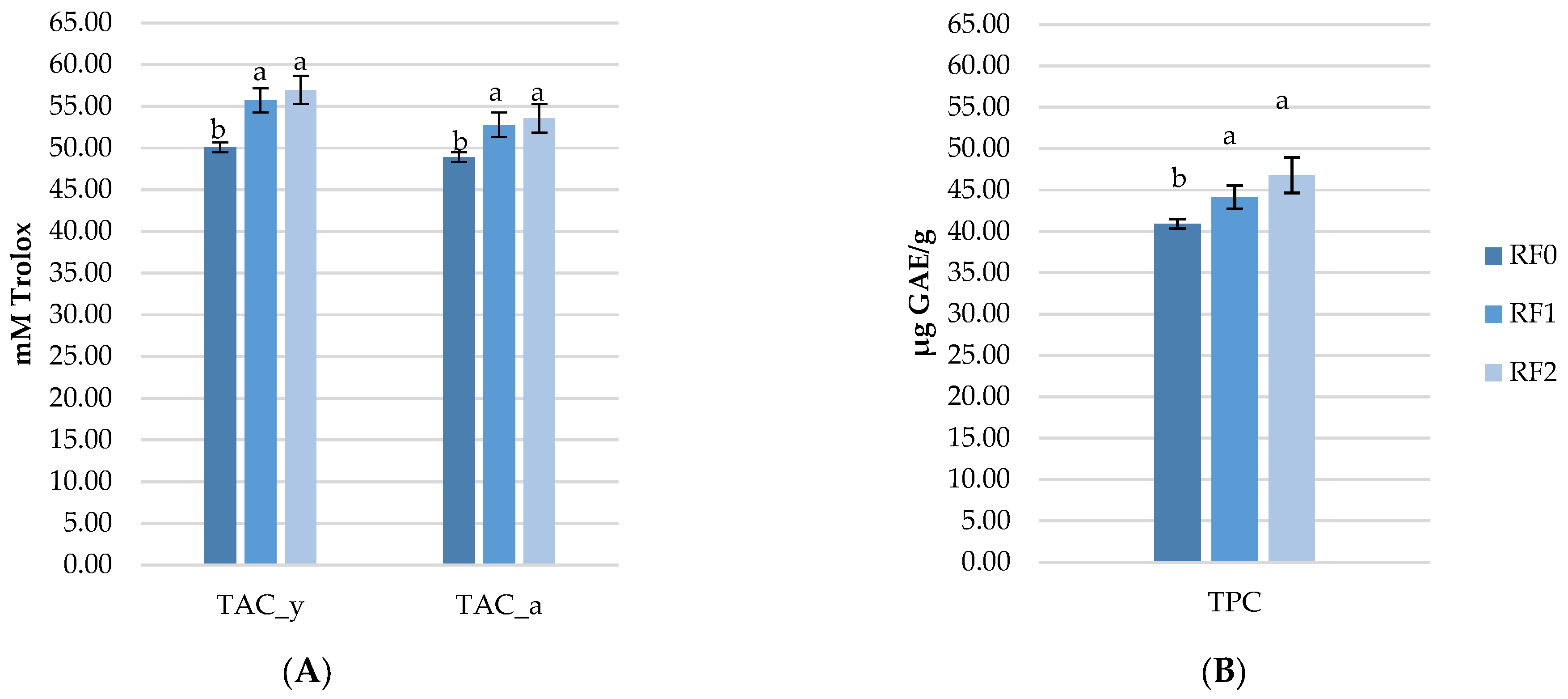
| Ingredients, % as Feed Basis | RF0 | RF1 | RF2 |
|---|---|---|---|
| Corn | 20.00 | 20.00 | 20.00 |
| Wheat | 28.25 | 24.47 | 24.18 |
| Rice bran | 10.00 | 10.00 | 10.00 |
| Soybean meal | 18.72 | 13.36 | 15.32 |
| Rapeseed meal | 8.00 | 8.00 | 4.68 |
| Vegetable sunflower oil | 3.53 | 3.84 | 3.9 |
| Flaxseed meal | 0.00 | 7.00 | 7.00 |
| Rosehip meal | 0.00 | 1.50 | 3.00 |
| DL-Methionine | 0.08 | 0.16 | 0.18 |
| L-Lysine HCl | 0.16 | 0.16 | 0.16 |
| Calcium Carbonate | 8.72 | 8.74 | 8.78 |
| Monocalcium phosphate | 1.27 | 1.33 | 1.36 |
| Chloride | 0.38 | 0.39 | 0.39 |
| Choline | 0.05 | 0.05 | 0.05 |
| Premix vitamin–mineral | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 |
| Nutritional composition—Analyzed, % | |||
| Dry matter | 90.16 | 90.73 | 90.68 |
| Crude protein | 17.50 | 17.50 | 17.50 |
| Crude fat | 5.07 | 7.29 | 7.42 |
| Crude fiber | 3.99 | 5.64 | 5.97 |
| Main fatty acid classes | |||
| Saturated fatty acids (SFA) | 18.45 | 15.99 | 15.24 |
| Monounsaturated fatty acids (MUFA) | 37.13 | 33.51 | 33.50 |
| Polyunsaturated fatty acids (PUFA) | 44.42 | 50.51 | 51.26 |
| Unsaturated fatty acids (UFA) | 81.55 | 84.04 | 84.76 |
| n-3 PUFA | 2.13 | 6.59 | 6.68 |
| n-6 PUFA | 42.29 | 43.92 | 44.58 |
| n-6/n-3 ratio | 19.88 | 6.67 | 6.68 |
| Antioxidant capacity, mM Trolox | 5.54 | 7.00 | 7.64 |
| Items | Rosehip Meal | Flaxseed Meal |
|---|---|---|
| Nutrients, % | ||
| Dry matter | 92.37 | 92.12 |
| Crude protein | 10.53 | 34.34 |
| Crude fat | 4.84 | 13.22 |
| Crude fibre | 49.35 | 12.51 |
| Antioxidant compounds | ||
| Total polyphenols, mg/g | 60.23 | nd |
| Antioxidant capacity, mM Trolox | 23.87 | nd |
| Flavonoids, mg Equiv. rutin/g | 12.18 | nd |
| Main fatty acids, (g/100 g FA) | ||
| Palmitic C16:0 | 5.55 | 6.80 |
| Stearic C18:0 | 2.98 | 3.20 |
| Oleic cis C18:1 | 22.46 | 18.48 |
| Linoleic cis C18:2n6 | 52.81 | 15.04 |
| Linolenic α C18:3n3 | 14.28 | 55.36 |
| PUFA | 67.65 | 70.63 |
| MUFA | 22.80 | 18.75 |
| SFA | 9.55 | 10.43 |
| n-3 PUFA | 14.28 | 55.36 |
| n-6 PUFA | 53.07 | 15.27 |
| n-6 to n-3 ratio | 3.72 | 0.28 |
| Amino acids, (g/100 g) | ||
| Arginine | 1.096 | 4.318 |
| Isoleucine | 0.401 | 1.681 |
| Leucine | 0.737 | 2.517 |
| Lysine | 0.242 | 1.497 |
| Methionine | 0.142 | 0.509 |
| Phenylalanine | 0.454 | 2.120 |
| Threonine | 0.419 | 1.849 |
| Valine | 0.444 | 1.970 |
| EAA | 3.935 | 15.953 |
| Alanine | 0.476 | 2.080 |
| Aspartic acid | 1.207 | 3.234 |
| Glutamic acid | 3.010 | 7.452 |
| Serine | 0.620 | 2.205 |
| Glycine | 0.671 | 2.174 |
| Tyrosine | 0.189 | 1.047 |
| Cystine | 0.204 | 0.649 |
| NEAA | 6.377 | 18.192 |
| TAA | 10.312 | 34.145 |
| Item | RF0 | RF1 | RF2 | SEM | p |
|---|---|---|---|---|---|
| Daily feed intake, g feed/day | 118.82 a | 116.41 b | 115.61 b | 0.129 | 0.0054 |
| Feed conversion ratio, kg feed/kg egg | 2.02 | 2.02 | 2.03 | 0.354 | 0.3214 |
| Laying percentage, % | 95.55 b | 96.67 ab | 98.38 a | 0.061 | 0.0013 |
| Egg mass, g | 64.60 b | 65.09 ab | 66.32 a | 0.021 | 0.0302 |
| Item (g/100 g) | RF0 | RF1 | RF2 | SEM | p |
|---|---|---|---|---|---|
| Crude protein | 31.44 b | 31.75 a | 31.80 a | 1.554 | 0.0450 |
| Arginine | 2.858 b | 2.986 a | 3.008 a | 0.927 | 0.0340 |
| Isoleucine | 1.691 b | 1.787 a | 1.796 a | 1.542 | 0.0315 |
| Leucine | 2.478 b | 2.689 a | 2.774 a | 0.024 | 0.0063 |
| Lysine | 2.384 b | 2.543 a | 2.549 a | 0.029 | 0.0075 |
| Methionine | 0.656 b | 0.722 a | 0.735 a | 0.845 | 0.0078 |
| Phenylalanine | 1.601 b | 1.905 a | 1.862 a | 1.224 | 0.0128 |
| Threonine | 2.348 b | 2.533 a | 2.521 a | 3.017 | 0.1440 |
| Valine | 1.505 b | 1.541 a | 1.557 a | 1.287 | 0.0030 |
| EAA | 15.521 b | 16.706 a | 16.802 a | 1.040 | 0.0432 |
| Alanine | 0.241 a | 0.191 b | 0.197 b | 0.010 | 0.0152 |
| Aspartic acid | 3.996 a | 3.614 b | 3.772 ab | 0.023 | 0.0081 |
| Glutamic acid | 4.602 | 4.347 | 4.352 | 1.108 | 0.1087 |
| Serine | 3.346 a | 3.192 a | 3.107 b | 0.209 | 0.0015 |
| Glycine | 0.831 | 0.847 | 0.851 | 0.715 | 0.0577 |
| Tyrosine | 1.712 | 1.621 | 1.653 | 0.334 | 0.2200 |
| Cystine | 0.835 b | 0.893 a | 0.911 a | 1.196 | 0.0319 |
| NEAA | 15.563 a | 14.705 b | 14.843 b | 2.557 | 0.0035 |
| TAA | 31.084 b | 31.411 a | 31.645 a | 0.222 | 0.0027 |
| Item, % | RF0 | RF1 | RF2 | SEM | p |
|---|---|---|---|---|---|
| TAAA | 3.313 b | 3.526 a | 3.515 a | 0.073 | 0.0020 |
| AAA | 10.590 b | 10.596 ab | 10.768 a | 1.609 | 0.0065 |
| SAA | 1.491 b | 1.615 a | 1.646 a | 1.079 | 0.0146 |
| TAAA/TAA | 0.107 b | 0.112 a | 0.111 a | 0.089 | 0.0205 |
| EAA/TAA | 0.499 b | 0.532 a | 0.531 a | 0.015 | 0.0026 |
| EAA/NEAA | 0.997 b | 1.136 a | 1.132 a | 0.004 | 0.0268 |
| Cystine/SAA | 0.560 a | 0.553 b | 0.554 b | 0.055 | 0.0071 |
| Item (g/100 g) | RF0 | RF1 | RF2 | SEM | p |
|---|---|---|---|---|---|
| Yolk fat | 27.66 | 28.01 | 28.34 | 1.457 | 0.2295 |
| Myristic C14:0 | 0.328 a | 0.263 b | 0.252 b | 0.022 | 0.0003 |
| Pentadecanoic C15:0 | 0.072 | 0.077 | 0.075 | 0.008 | 0.4451 |
| Palmitic C16:0 | 23.472 a | 21.968 b | 21.675 b | 0.882 | 0.0484 |
| Heptadecanoic C17:0 | 0.157 | 0.117 | 0.117 | 0.064 | 0.3766 |
| Stearic C18:0 | 11.583 | 11.415 | 12.297 | 1.846 | 0.7971 |
| Total SFA | 35.610 | 33.835 | 34.417 | 2.731 | 0.0825 |
| Myristioleic C14:1 | 0.040 a | 0.025 b | 0.030 ab | 0.008 | 0.0224 |
| Pentadecenoic C15:1 | 0.132 | 0.077 | 0.075 | 0.005 | 0.2569 |
| Palmitoleic C16:1 | 23.472 a | 21.968 b | 21.675 b | 0.512 | 0.0404 |
| Heptadecenoic C17:1 | 0.092 | 0.152 | 0.133 | 0.064 | 0.2049 |
| Oleic C18:1 | 31.790 a | 30.645 b | 30.465 b | 0.512 | 0.0153 |
| Erucic C22:1n9 | 0.083 | 0.078 | 0.070 | 0.041 | 0.6826 |
| Nervonic C24:1n9 | 0.400 a | 0.230 b | 0.245 b | 0.021 | <0.0001 |
| Total MUFA | 33.842 | 33.057 | 32.827 | 0.524 | 0.0591 |
| Linoleic C18:2n6 | 22.810 b | 23.801 a | 23.182 ab | 1.120 | 0.0011 |
| Linolenic γ C18:3n6 | 0.112 | 0.107 | 0.102 | 0.023 | 0.8217 |
| Eicosadienoic C20:2n6 | 0.172 | 0.170 | 0.151 | 0.018 | 0.7489 |
| Eicosatrienoic C20:3n6 | 0.332 | 0.298 | 0.292 | 0.045 | 0.2050 |
| Arachidonic C20:4n6 | 4.457 a | 3.363 b | 3.887 b | 1.219 | 0.0088 |
| Docosatetraenoic C22:4n6 | 1.790 a | 0.390 b | 0.355 b | 0.534 | <0.0001 |
| Total n-6 | 29.501 a | 28.192 ab | 27.969 b | 2.731 | 0.0312 |
| Linolenic α C18:3n3 | 0.310 b | 1.382 a | 1.350 a | 0.103 | <0.0001 |
| Eicosatrienoic C20:3n3 | 0.219 b | 0.235 a | 0.281 a | 0.205 | 0.0298 |
| Docosapentaenoic C22:5n3 | 0.084 b | 0.255 a | 0.288 a | 0.130 | <0.0001 |
| Docosahexaenoic C22:6n3 | 0.931 b | 2.695 a | 2.842 a | 0.076 | <0.0001 |
| Total n-3 | 1.544 b | 4.567 a | 4.761 a | 0.045 | <0.0001 |
| Total PUFA | 31.045 b | 32.648 a | 32.730 a | 0.454 | 0.0033 |
| Lipid Indices, % | RF0 | RF1 | RF2 | SEM | p |
|---|---|---|---|---|---|
| Qualitative | |||||
| PUFA/SFA | 0.862 b | 0.979 a | 0.952 a | 0.015 | 0.0009 |
| n-6/n-3 FA | 19.10 b | 6.17 b | 5.87 b | 1.565 | <0.0001 |
| Linoleic/α-Linolenic acids | 70.85 a | 17.38 b | 18.01 b | 6.295 | <0.0001 |
| Peroxidability Index | 65.36 b | 71.96 a | 71.98 a | 0.835 | <0.0001 |
| Nutritional | |||||
| Nutritional Value Index | 1.81 b | 1.92 a | 1.97 a | 0.021 | 0.0012 |
| Index of Atherogenicity | 0.39 a | 0.35 b | 0.35 b | 0.006 | 0.0056 |
| Index of Thrombogenicity | 0.99 a | 0.76 b | 0.77 b | 0.030 | <0.0001 |
| Hypocholesterolemic/Hypercholesterolemic | 2.59 b | 2.87 a | 2.88 a | 0.043 | 0.0017 |
| Health-Promoting Index | 2.60 | 2.87 | 2.89 | 0.043 | 0.0026 |
| Desirable Fatty Acids | 75.97 b | 77.58 a | 77.88 a | 0.263 | 0.0075 |
| Metabolic | |||||
| Elongase Index | 0.49 a | 0.52 ab | 0.56 b | 0.166 | 0.0130 |
| Thioesterase Index | 71.92 b | 84.16 a | 87.82 a | 5.140 | 0.0037 |
| Δ9-Desaturase (18) | 72.73 | 72.86 | 71.24 | 2.751 | 0.5558 |
| Δ5/Δ6-Desaturase | 21.15 | 21.14 | 22.49 | 0.075 | 0.2033 |
| Item | External Quality Parameters | Internal Quality Parameters | Shell Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Egg, g | Albumen, g | Yolk, g | Shell, g | Yolk pH | Albumen pH | HU | Thick Nesses | Breaking Strength | ||
| 0 d | RF0 | 64.23 | 39.67 | 16.54 | 8.02 | 5.72 | 8.11 | 82.70 | 0.36 | 3.99 |
| RF1 | 64.48 | 39.80 | 15.96 | 7.72 | 5.71 | 8.09 | 94.13 | 0.31 | 4.14 | |
| RF2 | 64.37 | 40.31 | 16.57 | 7.48 | 5.73 | 8.09 | 93.83 | 0.34 | 4.84 | |
| 14 d | 5 °C | |||||||||
| RF0 | 63.33 | 39.05 | 15.65 | 7.97 | 6.08 | 8.92 | 88.18 | 0.34 | 3.90 | |
| RF1 | 63.94 | 39.26 | 15.85 | 7.49 | 6.09 | 8.88 | 91.50 | 0.37 | 4.15 | |
| RF2 | 64.08 | 40.68 | 16.32 | 7.86 | 6.08 | 8.81 | 91.13 | 0.37 | 4.64 | |
| 21 °C | ||||||||||
| RF0 | 62.67 | 38.14 | 16.76 | 7.80 | 6.26 | 9.09 | 79.94 | 0.35 | 3.91 | |
| RF1 | 62.88 | 37.25 | 16.94 | 7.49 | 6.23 | 8.83 | 81.45 | 0.36 | 3.90 | |
| RF2 | 63.04 | 37.76 | 17.20 | 7.44 | 6.20 | 8.74 | 81.69 | 0.37 | 4.23 | |
| 28 d | 5 °C | |||||||||
| RF0 | 61.13 | 38.08 | 16.48 | 7.61 | 6.28 | 9.01 | 86.32 | 0.35 | 4.25 | |
| RF1 | 62.96 | 37.89 | 16.89 | 7.70 | 6.22 | 8.76 | 89.01 | 0.36 | 4.11 | |
| RF2 | 63.64 | 38.09 | 16.89 | 7.66 | 6.15 | 8.61 | 90.17 | 0.34 | 4.15 | |
| 21 °C | ||||||||||
| RF0 | 59.58 | 33.79 | 18.12 | 7.68 | 6.44 | 9.66 | 72.95 | 0.34 | 3.58 | |
| RF1 | 61.95 | 33.33 | 17.39 | 7.51 | 6.35 | 9.04 | 78.45 | 0.34 | 4.20 | |
| RF2 | 62.02 | 35.35 | 17.04 | 7.63 | 6.30 | 9.01 | 79.71 | 0.34 | 4.18 | |
| SEM | 0.203 | 0.193 | 0.122 | 0.047 | 0.007 | 0.007 | 0.485 | 0.003 | 0.061 | |
| Main effect | ||||||||||
| Time | ||||||||||
| 0 d | 64.36a | 39.93 a | 16.36 b | 7.74 | 5.72 c | 8.09 c | 90.22 a | 0.34 b | 4.32 | |
| 14 d | 63.01b | 38.69 a | 16.45 b | 7.68 | 6.16 b | 8.98 a | 85.65 b | 0.36 a | 4.12 | |
| 28 d | 60.88c | 36.09 a | 17.14 a | 7.63 | 6.29 a | 8.90 b | 82.27 c | 0.34 b | 4.09 | |
| Temperature | ||||||||||
| 5 °C | 63.12a | 38.84 a | 16.35 b | 7.71 | 6.15 b | 8.85 b | 89.22 a | 0.36 | 4.12 | |
| 21 °C | 60.77b | 35.94 b | 17.24 a | 7.59 | 6.30 a | 9.03 a | 78.70 b | 0.35 | 4.00 | |
| Diet | ||||||||||
| RF0 | 62.43 | 37.74 | 16.84 | 7.58 b | 6.16 a | 8.82 a | 81.02 b | 0.35 | 3.92b | |
| RF1 | 62.18 | 37.79 | 16.61 | 7.61 a | 6.12 ab | 8.76 b | 85.51 b | 0.35 | 4.10b | |
| RF2 | 62.67 | 38.15 | 16.67 | 7.82 a | 6.09 b | 8.73 b | 87.11 a | 0.35 | 4.41a | |
| p | ||||||||||
| time | <0.001 | <0.001 | 0.014 | 0.685 | <0.001 | <0.001 | 0.003 | 0.023 | 0.763 | |
| temp | <0.001 | <0.001 | 0.002 | 0.251 | <0.001 | <0.001 | <0.001 | 0.528 | 0.149 | |
| diet | 0.713 | 0.625 | 0.668 | 0.066 | 0.013 | <0.001 | <0.001 | 0.662 | 0.003 | |
| time × temp | 0.067 | 0.029 | 0.625 | 0.480 | 0.960 | 0.302 | 0.243 | 0.567 | 0.902 | |
| time × diet | 0.830 | 0.341 | 0.936 | 0.370 | 0.031 | 0.074 | 0.637 | 0.336 | 0.479 | |
| temp × diet | 0.322 | 0.187 | 0.929 | 0.763 | 0.553 | <0.001 | 0.982 | 0.510 | 0.753 | |
| time × temp × diet | 0.894 | 0.489 | 0.145 | 0.499 | 0.650 | 0.704 | 0.677 | 0.823 | 0.185 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Panaite, T.D.; Saracila, M. Rosehip (Rosa canina L.) Meal as a Natural Antioxidant on Lipid and Protein Quality and Shelf-Life of Polyunsaturated Fatty Acids Enriched Eggs. Antioxidants 2022, 11, 1948. https://doi.org/10.3390/antiox11101948
Vlaicu PA, Untea AE, Turcu RP, Panaite TD, Saracila M. Rosehip (Rosa canina L.) Meal as a Natural Antioxidant on Lipid and Protein Quality and Shelf-Life of Polyunsaturated Fatty Acids Enriched Eggs. Antioxidants. 2022; 11(10):1948. https://doi.org/10.3390/antiox11101948
Chicago/Turabian StyleVlaicu, Petru Alexandru, Arabela Elena Untea, Raluca Paula Turcu, Tatiana Dumitra Panaite, and Mihaela Saracila. 2022. "Rosehip (Rosa canina L.) Meal as a Natural Antioxidant on Lipid and Protein Quality and Shelf-Life of Polyunsaturated Fatty Acids Enriched Eggs" Antioxidants 11, no. 10: 1948. https://doi.org/10.3390/antiox11101948
APA StyleVlaicu, P. A., Untea, A. E., Turcu, R. P., Panaite, T. D., & Saracila, M. (2022). Rosehip (Rosa canina L.) Meal as a Natural Antioxidant on Lipid and Protein Quality and Shelf-Life of Polyunsaturated Fatty Acids Enriched Eggs. Antioxidants, 11(10), 1948. https://doi.org/10.3390/antiox11101948










