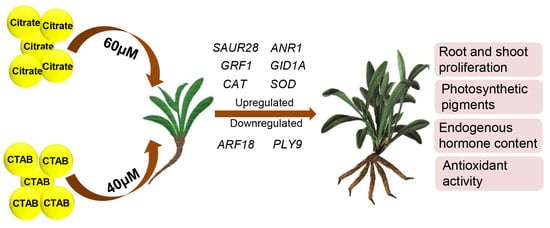
Charged Gold Nanoparticles Promote In Vitro Proliferation in Nardostachys jatamansi by Differentially Regulating Chlorophyll Content, Hormone Concentration, and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Citrate-AuNPs
2.3. Synthesis of CTAB-AuNPs
2.4. Characterization of Synthesized AuNPs
2.5. Plant Material and Treatment Conditions
2.6. Morphological Analysis
2.7. Chlorophyll Estimation
2.8. Total Soluble Sugar Content
2.9. Estimation of Non-Enzymatic Antioxidants
2.10. DPPH Radical Scavenging Activity
2.11. Superoxide Dismutase (SOD) Enzyme Activity
2.12. Endogenous Hormone Content Measurement
2.13. RNA Isolation and qRT-PCR Analysis
2.14. Statistical Analyses
3. Results
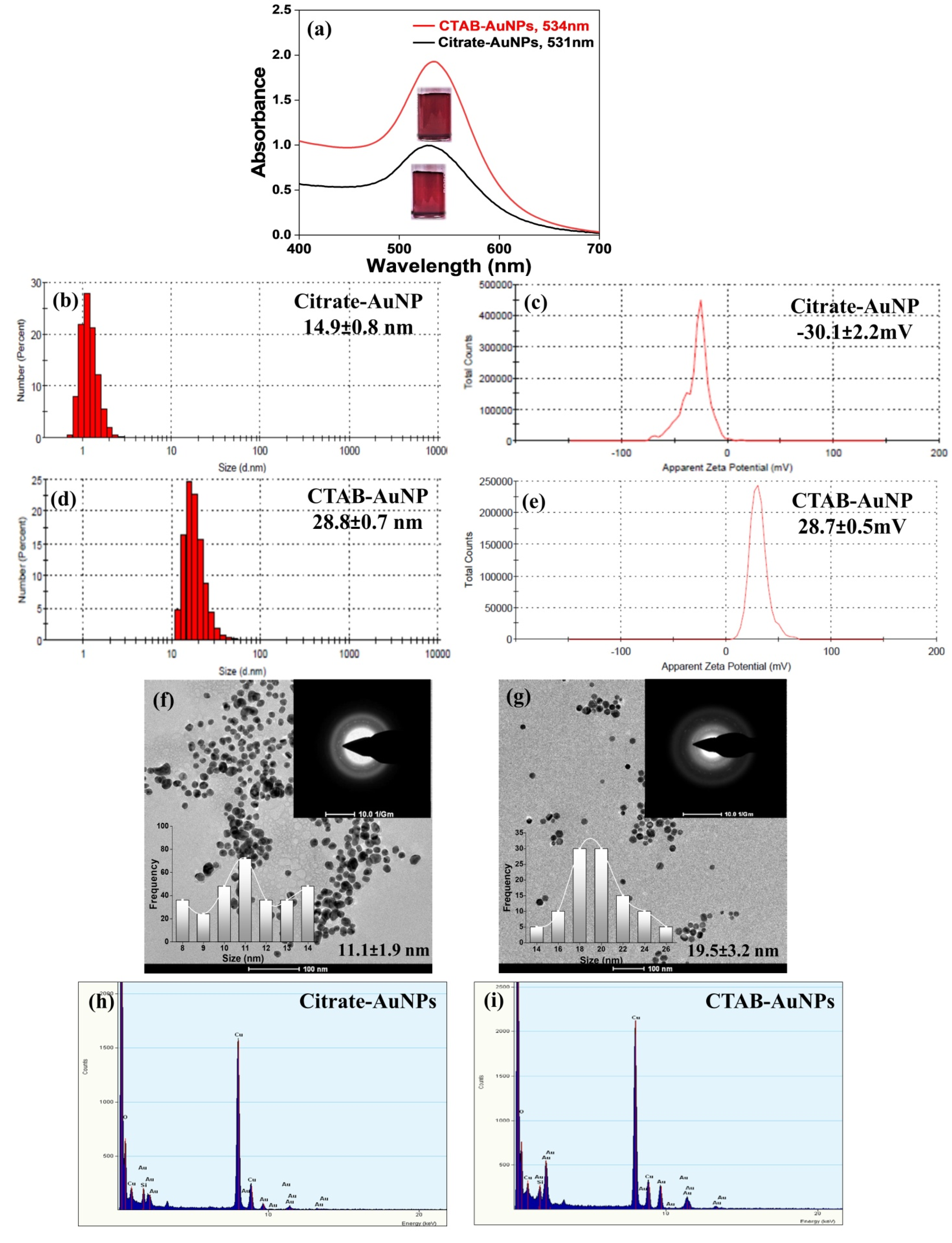
3.1. Characterization of AuNPs
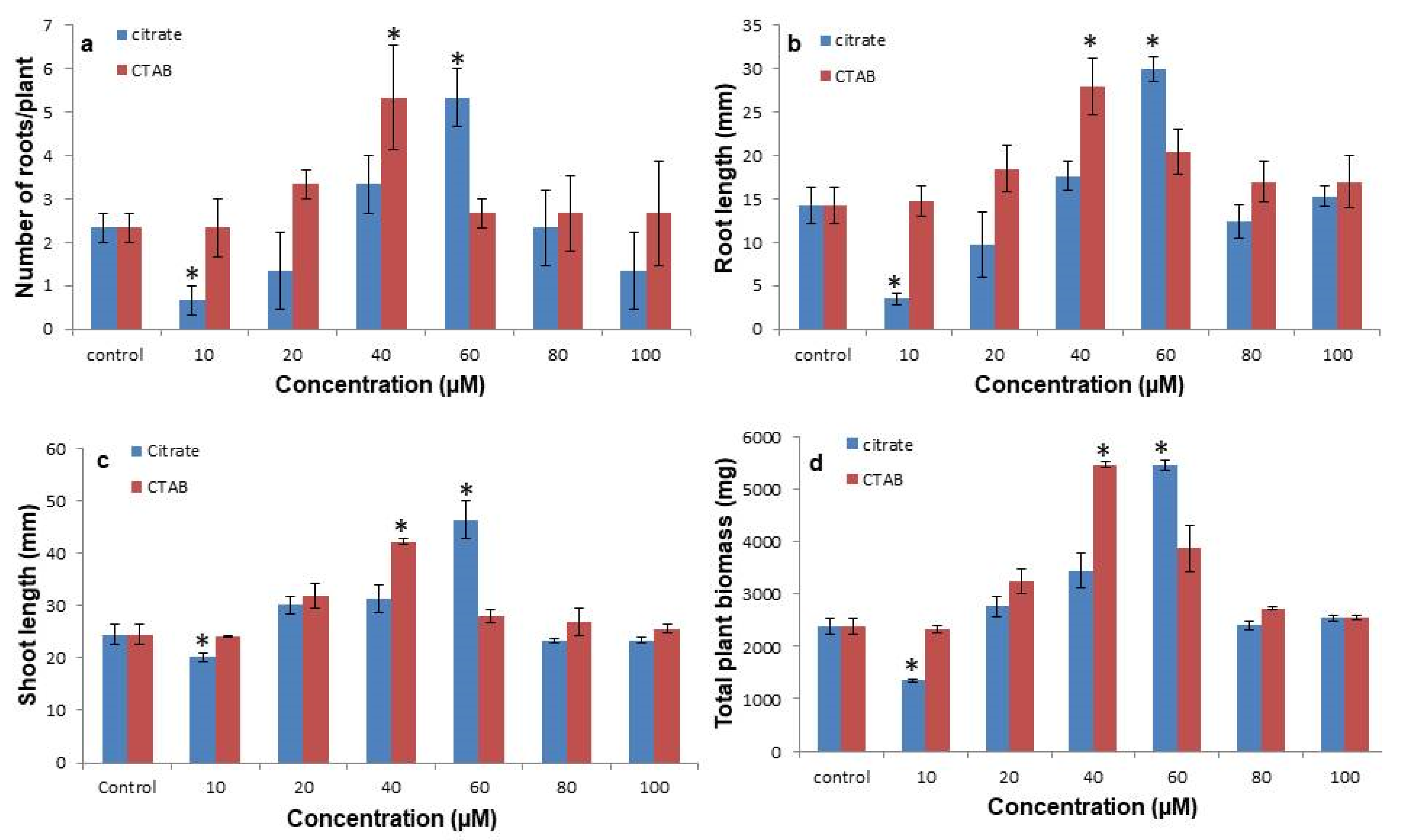
3.2. Citrate-AuNPs and CTAB-AuNPs Improves the Shoot and Root Proliferation and Biomass
3.3. Citrate-AuNPs and CTAB-AuNPs Treatment Improves Photosynthetic Pigment and Total Soluble Sugar Content
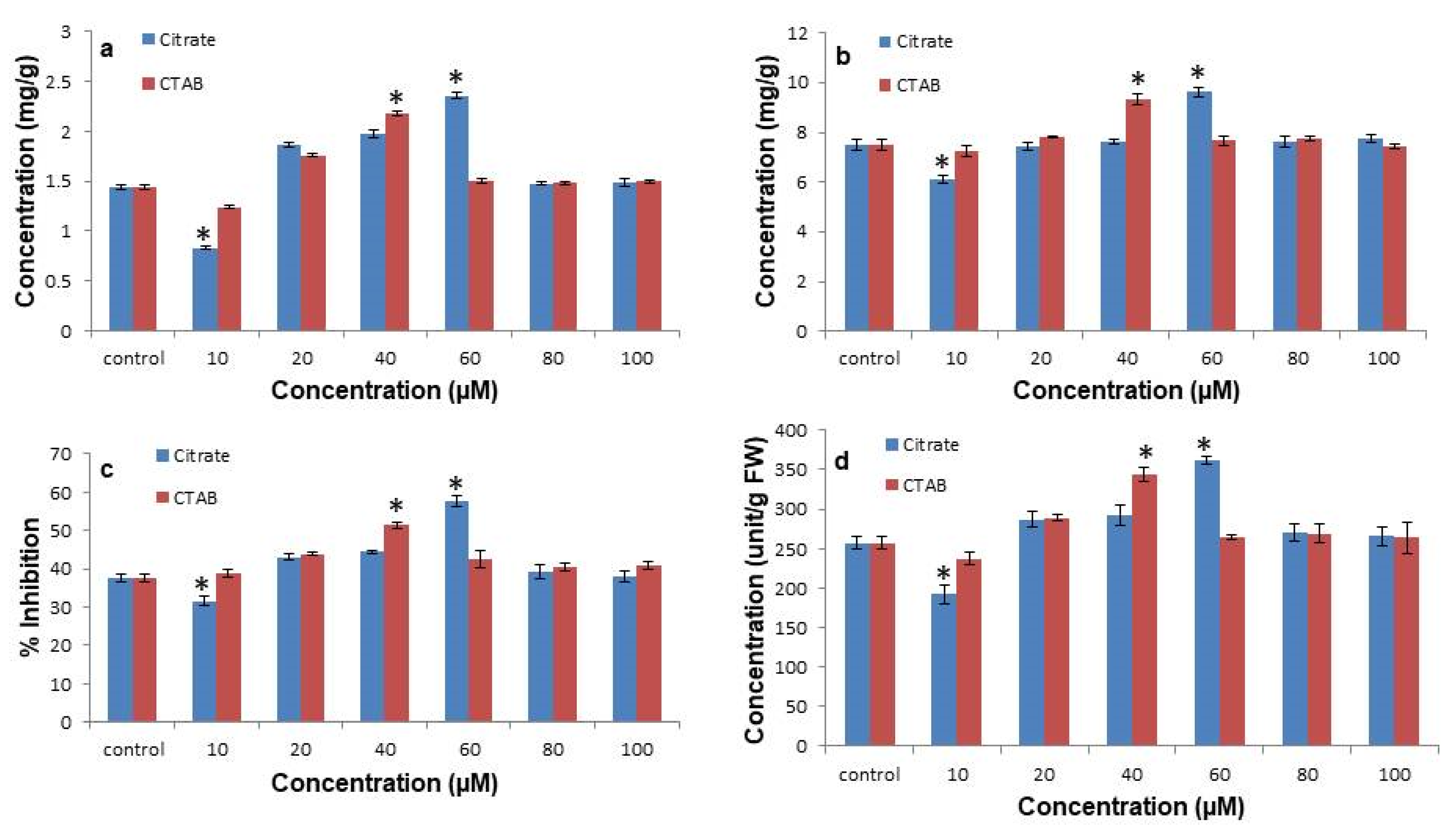
3.4. Citrate-AuNPs and CTAB-AuNPs Enhances Total Phenolics, Flavonoids, DPPH Radical Scavenging, and Superoxide Dismutase Enzymatic Activity
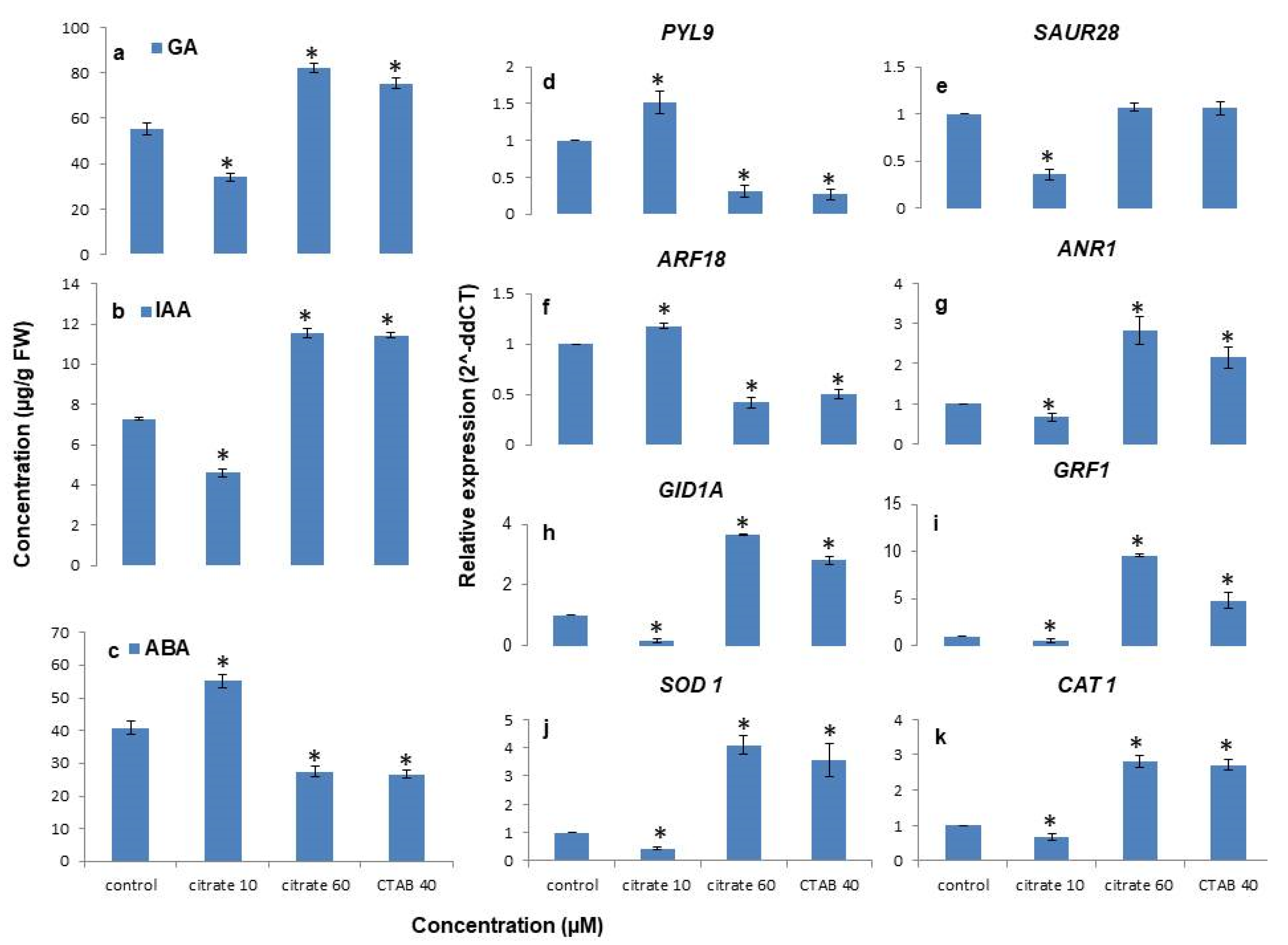
3.5. Citrate-AuNPs and CTAB-AuNPs Treatment Improves Endogenous Hormones Levels
3.6. Citrate-AuNPs and CTAB-AuNPs Modulates Genes Regulating Hormone Content and Antioxidant Enzyme Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, K.H.; Oli, S.; Bisht, K.A.; Meredith, C.; Leaman, D. Review of the biology, uses and conservation of the critically endangered endemic Himalayan species Nardostachys jatamansi (Caprifoliaceae). Biodivers. Conserv. 2021, 30, 3315–3333. [Google Scholar] [CrossRef]
- Bose, B.; Kumaria, S.; Tandon, P. Physiological insights into the role of temperature and light conditions on in vitro growth, membrane thermostability and antioxidative activity of Nardostachys jatamansi, an IUCN Red-listed critically endangered therapeutic plant. S. Afr. J. Bot. 2022, 146, 365–374. [Google Scholar] [CrossRef]
- Dhiman, N.; Bhattacharya, A. Nardostachys jatamansi (D. Don) DC.-Challenges and opportunities of harnessing the untapped medicinal plant from the Himalayas. J. Ethnopharmacol. 2020, 246, 112211. [Google Scholar] [CrossRef] [PubMed]
- Bose, B.; Tripathy, D.; Chatterjee, A.; Tandon, P.; Kumaria, S. Secondary metabolite profiling, cytotoxicity, anti-inflammatory potential and in vitro inhibitory activities of Nardostachys jatamansi on key enzymes linked to hyperglycemia, hypertension and cognitive disorders. Phytomedicine 2019, 55, 58–69. [Google Scholar] [CrossRef]
- Dhiman, N.; Devi, K.; Bhattacharya, A. Development of low cost micropropagation protocol for Nardostachys jatamansi: A critically endangered medicinal herb of Himalayas. S. Afr. J. Bot. 2021, 140, 468–477. [Google Scholar] [CrossRef]
- Foyer, C.H.; Hanke, G. ROS production and signalling in chloroplasts: Cornerstones and evolving concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Suskova, S.; Mandzhieva, S.; Tsitsuashvili, V.; Chapligin, V.; Fedorenko, A. Effects of copper nanoparticles (CuO NPs) on crop plants: A mini review. BioNanoScience 2018, 8, 36–42. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold nanoparticles (GNPs) in biomedical and clinical applications: A review. Nano Sel. 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Venzhik, Y.V.; Moshkov, I.E.; Dykman, L.A. Gold Nanoparticles in Plant Physiology: Principal Effects and Prospects of Application. Russ. J. Plant Physl. 2021, 68, 401–412. [Google Scholar] [CrossRef]
- Milewska-Hendel, A.; Gepfert, W.; Zubko, M.; Kurczyńska, E. Morphological, Histological and Ultrastructural Changes in Hordeum vulgare (L.) Roots That Have Been Exposed to Negatively Charged Gold Nanoparticles. Appl. Sci. 2022, 12, 3265. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, L.; Rose, A.; Grassian, V.H. Impact of surface adsorbed biologically and environmentally relevant coatings on TiO 2 nanoparticle reactivity. Environ. Sci. Nano 2020, 7, 3783–3793. [Google Scholar] [CrossRef]
- Dar, A.I.; Walia, S.; Acharya, A. Citric acid-coated gold nanoparticles for visual colorimetric recognition of pesticide dimethoate. J. Nanopart. Res. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Dar, A.I.; Abidi, S.M.; Randhawa, S.; Joshi, R.; Kumar, R.; Acharya, A. Protein-Cloaked Nanoparticles for Enhanced Cellular Association and Controlled Pathophysiology via Immunosurveillance Escape. ACS Appl. Mater. Interfaces 2022, 14, 337–349. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Joshi, R.; Ramanarao, M.V.; Baisakh, N. Arabidopsis plants constitutively overexpressing a myo-inositol 1-phosphate synthase gene (SaINO1) from the halophyte smooth cordgrass exhibits enhanced level of tolerance to salt stress. Plant Physiol. Biochem. 2013, 65, 61–66. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl. Biochem. Biotechnol. 2016, 180, 1076–1092. [Google Scholar] [CrossRef]
- Ahmed, M.; Ji, M.; Qin, P.; Gu, Z.; Liu, Y.; Sikandar, A.; Iqbal, M.F.; Javeed, A. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019, 17, 6961–6979. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Ghawana, S.; Paul, A.; Kumar, H.; Kumar, A.; Singh, H.; Bhardwaj, P.K.; Rani, A.; Singh, R.S.; Raizada, J.; Singh, K.; et al. An RNA isolation system for plant tissues rich in secondary metabolites. BMC Res. Notes 2011, 4, 85. [Google Scholar] [CrossRef]
- Dhiman, N.; Kumar, A.; Kumar, D.; Bhattacharya, A. De novo transcriptome analysis of the critically endangered alpine Himalayan herb Nardostachys jatamansi reveals the biosynthesis pathway genes of tissue-specific secondary metabolites. Sci. Rep. 2020, 10, 17186. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984; Volume 630, pp. 83–556. [Google Scholar]
- Joshi, R.; Bhattacharya, P.; Sairam, R.K.; Sathee, L.; Chinnusamy, V. Identification and characterization of NADH kinase-3 from a stress-tolerant wild mung bean species (Vigna luteola (Jacq.) Benth.) with a possible role in waterlogging tolerance. Plant Mol. Biol. Rep. 2020, 38, 137–150. [Google Scholar] [CrossRef]
- Asl, K.R.; Hosseini, B.; Sharafi, A.; Palazon, J. Influence of nano-zinc oxide on tropane alkaloid production, h6h gene transcription and antioxidant enzyme activity in Hyoscyamus reticulatus L. hairy roots. Eng. Life Sci. 2019, 19, 73–89. [Google Scholar] [CrossRef]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Minkina, T.; Singh, R.K. Zinc Oxide Nanoparticles Improve Salt Tolerance in Rice Seedlings by Improving Physiological and Biochemical Indices. Agriculture 2022, 12, 1014. [Google Scholar] [CrossRef]
- Tiede, K.; Hassellöv, M.; Breitbarth, E.; Chaudhry, Q.; Boxall, A.B. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J. Chromatogr. 2009, 1216, 503–509. [Google Scholar] [CrossRef]
- Siegel, J.; Záruba, K.; Švorčík, V.; Kroumanová, K.; Burketová, L.; Martinec, J. Round-shape gold nanoparticles: Effect of particle size and concentration on Arabidopsis thaliana root growth. Nanoscale Res. Lett. 2018, 13, 95. [Google Scholar] [CrossRef]
- Zhu, Z.J.; Wang, H.; Yan, B.; Zheng, H.; Jiang, Y.; Miranda, O.R.; Rotello, V.M.; Xing, B.; Vachet, R.W. Effect of surface charge on the uptake and distribution of gold nanoparticles in four plant species. Envir. Sci. Technol. 2012, 46, 12391–12398. [Google Scholar] [CrossRef]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef]
- Anwar, N.; Wahid, J.; Uddin, J.; Khan, A.; Shah, M.; Shah, S.A.; Subhan, F.; Khan, M.A.; Ali, K.; Rauf, M.; et al. Phytosynthesis of poly (ethylene glycol) methacrylate-hybridized gold nanoparticles from C. tuberculata: Their structural characterization and potential for in vitro growth in banana. Vitr. Cell. Dev. Biol. 2021, 57, 248–260. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A.; Jedrzejczyk, I.; Winiecki, J. Gold nanoparticles and electromagnetic irradiation in tissue culture systems of bleeding heart: Biochemical, physiological, and (cyto) genetic effects. Plant Cell Tissue Organ Cult. 2022, 149, 715–734. [Google Scholar] [CrossRef]
- Ferrari, E.; Barbero, F.; Busquets-Fité, M.; Franz-Wachtel, M.; Köhler, H.R.; Puntes, V.; Kemmerling, B. Growth-Promoting Gold Nanoparticles Decrease Stress Responses in Arabidopsis Seedlings. Nanomaterials 2021, 11, 3161. [Google Scholar] [CrossRef] [PubMed]
- Stortenbeker, N.; Bemer, M. The SAUR gene family: The plant’s toolbox for adaptation of growth and development. J. Exp. Bot. 2019, 70, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Lor, V.S.; Ren, H.; Olszewski, N.E.; Miller, N.D.; Wu, G.; Spalding, E.P.; Gray, W.M. Constitutive expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in tomato confers auxin-independent hypocotyl elongation. Plant Physiol. 2017, 173, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Du, G.; Xiang, J.; Hu, C.; Li, X.; Wang, W.; Sui, J. Genome-wide identification of auxin response factor (ARF) gene family and the miR160-ARF18-mediated response to salt stress in peanut (Arachis hypogaea L.). Genomics 2022, 114, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Zhao, X.; Wu, B.; Wang, C.; Wu, C.; Yang, S.; Xue, Z. Auxin response factors are ubiquitous in plant growth and development, and involved in crosstalk between plant hormones: A review. Appl. Sci. 2022, 12, 1360. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Gaj, M.D. Expression profiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep. 2017, 36, 843–858. [Google Scholar] [CrossRef]
- Quintana-Escobar, A.O.; Nic-Can, G.I.; Avalos, R.M.G.; Loyola-Vargas, V.M.; Gongora-Castillo, E. Transcriptome analysis of the induction of somatic embryogenesis in Coffea canephora and the participation of ARF and Aux/IAA genes. Peer J. 2019, 7, 7752. [Google Scholar] [CrossRef]
- Liu, J.; Hua, W.; Hu, Z.; Yang, H.; Zhang, L.; Li, R.; Deng, L.; Sun, X.; Wang, X.; Wang, H. Natural variation in ARF18 gene simultaneously affects seed weight and silique length in polyploid rapeseed. Proc. Natl. Acad. Sci. USA 2015, 112, 5123–5132. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.H.; Yu, J.Q.; Wen, L.Z.; Guo, Y.H.; Sun, X.; Hao, Y.J.; Hu, D.G.; Zheng, C.S. Chrysanthemum MADS-box transcription factor CmANR1 modulates lateral root development via homo-/heterodimerization to influence auxin accumulation in Arabidopsis. Plant Sci. 2018, 266, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.H.; Wang, J.H.; Gu, K.D.; Zhang, P.; Zhang, X.Y.; Zheng, C.S.; Hu, D.G.; Ma, F. New insights into the role of MADS-box transcription factor gene CmANR1 on root and shoot development in chrysanthemum (Chrysanthemum morifolium). BMC Plant Biol. 2021, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Montejo, S.D.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Shoja, A.A.; Çirak, C.; Ganjeali, A.; Cheniany, M. Stimulation of phenolic compounds accumulation and antioxidant activity in in vitro culture of Salvia tebesana Bunge in response to nano-TiO2 and methyl jasmonate elicitors. Plant Cll Tissue Organ Cult. 2022, 149, 423–440. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Singh, A.; Dwivedi, P. Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: A review. J. Pharmacogn. Phytochem. 2018, 7, 750–757. [Google Scholar]
- Kokina, I.; Gerbreders, V.; Sledevskis, E.; Bulanovs, A. Penetration of nanoparticles in flax (Linum usitatissimum L.) calli and regenerants. J. Biotechnol. 2013, 165, 27–132. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Med. J. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ferreira, G.K.; Cardoso, E.; Vuolo, F.S.; Michels, M.; Zanoni, E.T.; Carvalho-Silva, M.; Gomes, L.M.; Dal-Pizzol, F.; Rezin, G.T.; Streck, E.L.; et al. Gold nanoparticles alter parameters of oxidative stress and energy metabolism in organs of adult rats. Biochem. Cell Biol. 2015, 93, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Khai, H.D.; Mai, N.T.N.; Tung, H.T.; Luan, V.Q.; Cuong, D.M.; Ngan, H.T.M.; Chau, N.H.; Buu, N.Q.; Vinh, N.Q.; Dung, D.M.; et al. Selenium nanoparticles as in vitro rooting agent, regulates stomata closure and antioxidant activity of gerbera to tolerate acclimatization stress. Plant Cell Tissue Organ Cult. 2022, 150, 113–128. [Google Scholar] [CrossRef]
- Lu, Y.; Meng, Y.; Zeng, J.; Luo, Y.; Feng, Z.; Bian, L.; Gao, S. Coordination between GROWTH-REGULATING FACTOR1 and GRF-INTERACTING FACTOR1 plays a key role in regulating leaf growth in rice. BMC Plant Biol. 2020, 20, 200–212. [Google Scholar] [CrossRef]
- Lantzouni, O.; Alkofer, A.; Falter-Braun, P.; Schwechheimer, C. GROWTH-REGULATING FACTORS interact with DELLAs and regulate growth in cold stress. Plant Cell 2020, 32, 1018–1034. [Google Scholar] [CrossRef] [PubMed]
- Piya, S.; Liu, J.; Burch-Smith, T.; Baum, T.J.; Hewezi, T. A role for Arabidopsis growth-regulating factors 1 and 3 in growth–stress antagonism. J. Exp. Bot. 2020, 71, 1402–1417. [Google Scholar] [CrossRef]
- Hauvermale, A.L.; Ariizumi, T.; Steber, C.M. The roles of the GA receptors GID1a, GID1b, and GID1c in sly1-independent GA signaling. Plant Signal. Behav. 2014, 9, 2125–2139. [Google Scholar] [CrossRef]
- Willige, B.C.; Ghosh, S.; Nill, C.; Zourelidou, M.; Dohmann, E.M.; Maier, A.; Schwechheimer, C. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. The Plant Cell 2007, 19, 1209–1220. [Google Scholar] [CrossRef]
- Syu, Y.Y.; Hung, J.H.; Chen, J.C.; Chuang, H.W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Xing, L.; Zhao, Y.; Gao, J.; Xiang, C.; Zhu, J.K. The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Sci. Rep. 2016, 6, 27177. [Google Scholar] [CrossRef]


| Primer Name | Sequence | Product Length (bp) |
|---|---|---|
| PYL9_F | CAGGAACGATGGTGATTGAATC | 61 |
| PYL9_R | TCCTCTTTAGTGTTTCCATCAGG | |
| ARF18_F | AGAGAAGGTTCACTGGCACT | 150 |
| ARF18_R | TGTAAGGTTCGATTTCCCATGG | |
| SAUR28_F | AGCTGTTTACGTTGGAGAGAA | 150 |
| SAUR28_R | CTGCAAGGAATTGTGAGACCA | |
| ANR1_F | CAGCCAGACAAGTGACATTTTC | 100 |
| ANR1_R | AAAATGATGAGAGCAACTTCAGC | |
| GID1A_F | GATCTCTATCGTCGCCTTCC | 50 |
| GID1A_R | TAGAGATTGGTATTGGCGAGCA | |
| GRF1_F | CACAGGCTTTCTTGAACGGT | 178 |
| GRF1_R | TCAACATGTGGGATGGAATTGT | |
| SOD_F | CAGCAGGATTGTAATGGGGTC | 100 |
| SOD_R | CTTTCTGGCCTTGCACCTG | |
| CAT_F | CTCCTCATCCCTGTGCATGA | 60 |
| CAT_R | TGTGCCCATCATAATAATCACCA | |
| Actin_F | TCAAATCACGACCGGCCATA | 100 |
| Actin_R | TTCCGGTGATGGAGTCACTCA |
| S. No. | Sample | Hydrodynamic Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|---|
| 1 | Citrate-AuNPs | 14.9 ± 0.8 | −30.1 ± 2.2 | 0.25 ± 0.03 |
| 2 | CTAB-AuNPs | 28.8 ± 0.7 | 28.7 ± 0.5 | 0.21 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, S.; Dar, A.I.; Acharya, A.; Joshi, R. Charged Gold Nanoparticles Promote In Vitro Proliferation in Nardostachys jatamansi by Differentially Regulating Chlorophyll Content, Hormone Concentration, and Antioxidant Activity. Antioxidants 2022, 11, 1962. https://doi.org/10.3390/antiox11101962
Joshi S, Dar AI, Acharya A, Joshi R. Charged Gold Nanoparticles Promote In Vitro Proliferation in Nardostachys jatamansi by Differentially Regulating Chlorophyll Content, Hormone Concentration, and Antioxidant Activity. Antioxidants. 2022; 11(10):1962. https://doi.org/10.3390/antiox11101962
Chicago/Turabian StyleJoshi, Shubham, Aqib I. Dar, Amitabha Acharya, and Rohit Joshi. 2022. "Charged Gold Nanoparticles Promote In Vitro Proliferation in Nardostachys jatamansi by Differentially Regulating Chlorophyll Content, Hormone Concentration, and Antioxidant Activity" Antioxidants 11, no. 10: 1962. https://doi.org/10.3390/antiox11101962
APA StyleJoshi, S., Dar, A. I., Acharya, A., & Joshi, R. (2022). Charged Gold Nanoparticles Promote In Vitro Proliferation in Nardostachys jatamansi by Differentially Regulating Chlorophyll Content, Hormone Concentration, and Antioxidant Activity. Antioxidants, 11(10), 1962. https://doi.org/10.3390/antiox11101962