The Impact of Oxidative Stress on Pediatrics Syndromes
Abstract
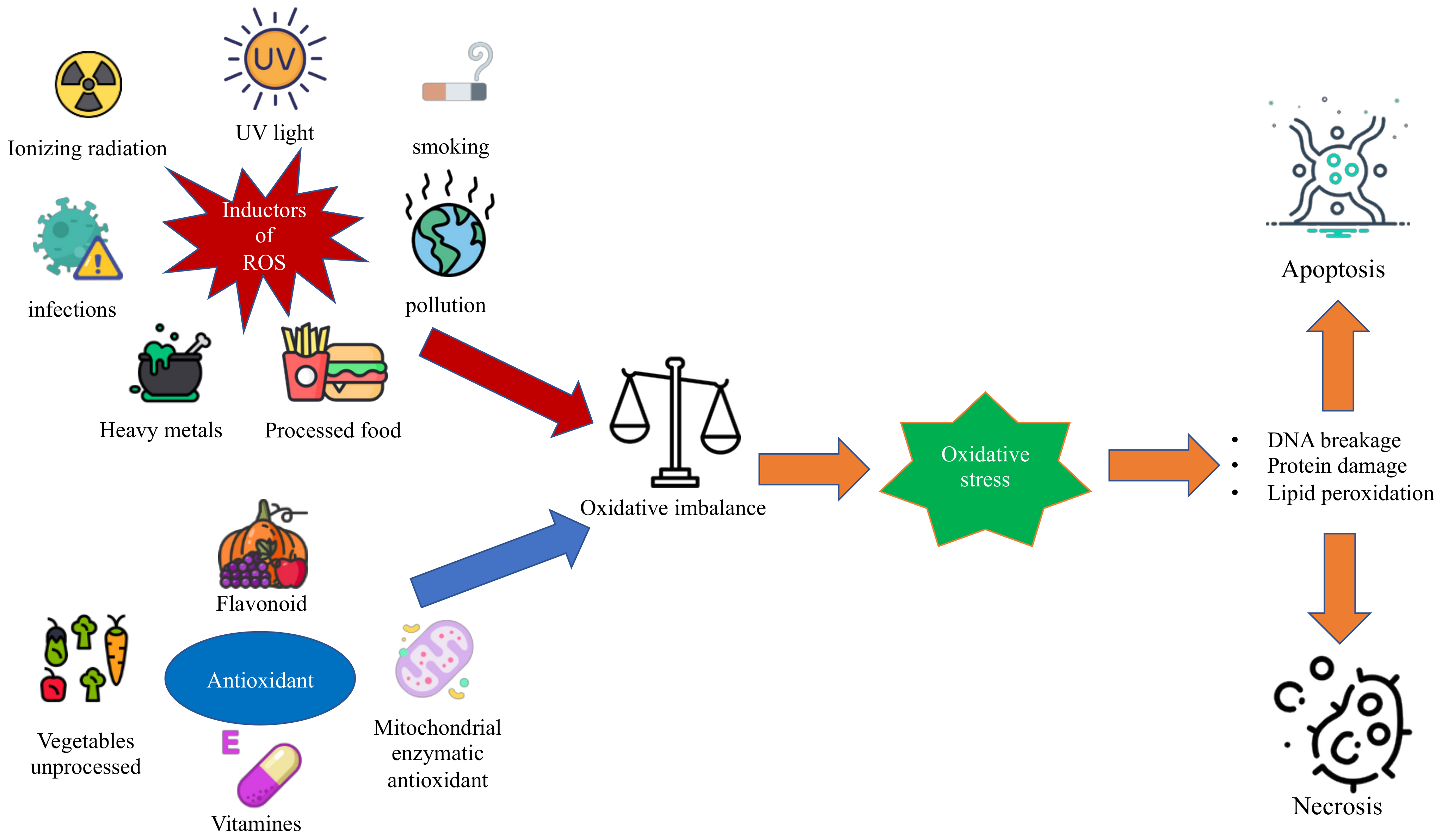
1. Introduction
1.1. Oxidative Stress and Reactive Oxygen Species
1.2. Antioxidant Substances
2. Pediatrics Syndromes Associated with Oxidative Stress
2.1. Fetal Alcohol Spectrum Disorders
Therapeutic Approach for Oxidative Stress in FASD
2.2. Williams-Beuren Syndrome
Therapeutic Approach for Oxidative Stress in Williams-Beuren Syndrome
2.3. Ataxia-Telangiectasia
Therapeutic Approach for Oxidative Stress in Ataxia-Telangiectasia
2.4. Down Syndrome
Down Syndrome Therapy
2.5. Marfan Syndrome
Therapeutic Approach for Oxidative Stress in MFS
2.6. Fanconi’s Anemia
Therapeutic Approach for Oxidative Stress in Fanconi’s Anemia
2.7. Autism Spectrum Disorders (ASD)
Therapeutic Approach for Oxidative Stress in ASD
2.8. Primitive Immunodeficiencies
2.8.1. Common Variable Immunodeficiency
2.8.2. Therapeutic Approach for Oxidative Stress in CVID
2.8.3. Severe Combined Immunodeficiency (Reticular Dysgenesia)
2.8.4. Therapeutic Approach for Oxidative Stress in RD
2.8.5. Chronic Granulomatous Disease
2.8.6. Therapeutic Approach for Oxidative Stress in CGD
2.9. Gaucher Disease
Therapeutic Approach for Oxidative Stress in GD
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 8-OH-G | 8-hydro(dexoxy)-guanosine |
| Aβ | beta-amyloid |
| AD-DS | Alzheimer’s disease-dementia |
| ADH | alcohol dehydrogenase |
| ALDH | acetaldehyde dehydrogenase |
| A-T | Ataxia telangiectasia |
| APP | Amyloid Beta A4 Precursor Protein |
| ATM | Ataxia Telangiectasia Mutated |
| BACH1 | BTB domain and CNC homolog 1 |
| CAT | Catalases |
| COPD | Chronic obstructive pulmonary disease |
| CYP2E1 | Cytochrome P450 2E1 |
| DDR | DNA damage response |
| DNA | Deoxyribonucleic acid |
| DS | Down Syndrome |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| ELN | Elastin |
| Ets-2 | ETS Protooncogene 2 |
| FAS | Fetal alcohol syndrome |
| FASD | Fetal alcohol spectrum disorder |
| GSTs | Glutathione transferase |
| GTPx | Glutathione peroxidase |
| H2O2 | Hydrogen peroxide |
| HOO | Hydroperoxide radical |
| HO2 | Hydroperoxide |
| HSA 21 | Human cromosome 21 |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| LPO | Lipid peroxidation |
| O2− | Superoxide anion |
| OS | Oxidative stress |
| MPO | Myeloperoxidase |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NADH | Nicotinamide adenine dinucleotide |
| NCF1 | Neutrophil cytosolic factor 1 |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| O2•− | Superoxide anion |
| •OH | Hydroxyl radical |
| NOX4 | NADPH oxidase |
| OX-LDL | Oxidized low-density lipoprotein |
| PFAS | Partial Fetal Alcohol Syndrome |
| PI3K | Phosphoinositide 3-kinase |
| Q | Ubiquinone |
| QH2 | Reduced ubiquinone |
| Q- | Semiquinone anion |
| ROO | Lipoperoxide radical |
| ROS | Radical oxygen species |
| S100β | S100 calcium-binding protein, beta |
| SOD-1 | Superoxide dismutase |
| TATA boxes | Thymine-adenine-thymine-adenine boxes |
| TNF-alpha | Tumor necrosis factor |
| UVA | Type A ultraviolet radiation |
| WBS | Williams–Beuren Syndrome |
| WBSCR | Williams–Beuren Syndrome critical region |
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239. [Google Scholar] [CrossRef]
- Sies, H. On the history of oxidative stress: Concept and some aspects of current development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Pallardó, F.V.; Lloret, A.; Lebel, M.; D’Ischia, M.; Cogger, V.C.; Le Couteur, D.G.; Gadaleta, M.N.; Castello, G.; Pagano, G. Mitochondrial dysfunction in some oxidative stress-related genetic diseases: Ataxia-Telangiectasia, Down Syndrome, Fanconi Anaemia and Werner Syndrome. Biogerontology 2010, 11, 401–419. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Mikoluc, B.; Pietrucha, B.; Heropolitanska-Pliszka, E.; Pac, M.; Motkowski, R.; Car, H. Oxidative stress, mitochondrial abnormalities and antioxidant defense in Ataxia-telangiectasia, Bloom syndrome and Nijmegen breakage syndrome. Redox Biol. 2017, 11, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Tarani, L.; Rasio, D.; Tarani, F.; Parlapiano, G.; Valentini, D.; Dylag, K.A.; Spalice, A.; Paparella, R.; Fiore, M. Pediatrics for Disability: A Comprehensive Approach to Children with Syndromic Psychomotor Delay. Curr. Pediatr. Rev. 2021, 18, 110–120. [Google Scholar] [CrossRef]
- Convertini, P.; Menga, A.; Andria, G.; Scala, I.; Santarsiero, A.; Castiglione Morelli, M.A.; Iacobazzi, V.; Infantino, V. The contribution of the citrate pathway to oxidative stress in Down syndrome. Immunology 2016, 149, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Tarani, L.; Micangeli, G.; Rasio, D.; Ottombrino, S.; Liberati, N.; de Angelis, D.; Carito, V.; Greco, A.; Ceccanti, M.; Fiore, M. Clinical and genetic approach to the dysmorphic child. Biomed. Rev. 2018, 29, 37–46. [Google Scholar] [CrossRef]
- Marreiro, D.D.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; Soares de Oliveira, A.R. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 55. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski 2020, 48, 124–127. [Google Scholar] [PubMed]
- Azadmanesh, J.; Borgstahl, G.E.O. A review of the catalytic mechanism of human manganese superoxide dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.F. Superoxide dismutases: Ancient enzymes and new insights. FEBS Lett. 2012, 586, 585–595. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. JAOCS J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Hahner, F.; Moll, F.; Schröder, K. NADPH oxidases in the differentiation of endothelial cells. Cardiovasc. Res. 2020, 116, 262–268. [Google Scholar] [CrossRef]
- Bradshaw, P.C. Cytoplasmic and mitochondrial NADPH-coupled Redox systems in the regulation of aging. Nutrients 2019, 11, 504. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Kanda, N.; Hsu, C.-C.; Sakaguchi, O. Lipid Peroxide Formation and Membrane Damage in Endotoxin-Poisoned Mice. Microbiol. Immunol. 1981, 25, 229–244. [Google Scholar] [CrossRef]
- Ferranti, C.S.; Cheng, J.; Thompson, C.; Zhang, J.; Rotolo, J.A.; Buddaseth, S.; Fuks, Z.; Kolesnick, R.N. Fusion of lysosomes to plasma membrane initiates radiation-induced apoptosis. J. Cell Biol. 2020, 219, 219. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Heinecke, J. Oxidative Stress and Antioxidants in Exercise. Curr. Med. Chem. 2012, 8, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Kasha, M. Singlet molecular oxygen in the Haber-Weiss reaction. Proc. Natl. Acad. Sci. USA 1994, 91, 12365–12367. [Google Scholar] [CrossRef] [PubMed]
- Karran, P.; Brem, R. Protein oxidation, UVA and human DNA repair. DNA Repair 2016, 44, 178–185. [Google Scholar] [CrossRef]
- Hadrava Vanova, K.; Kraus, M.; Neuzil, J.; Rohlena, J. Mitochondrial complex II and reactive oxygen species in disease and therapy. Redox Rep. 2020, 25, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, C.; Cao, S.; Sheng, T.; Dong, N.; Xu, Y. Fenton reactions drive nucleotide and ATP syntheses in cancer. J. Mol. Cell Biol. 2018, 10, 448–459. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef]
- Bleier, L.; Dröse, S. Superoxide generation by complex III: From mechanistic rationales to functional consequences. Biochim. Biophys. Acta—Bioenerg. 2013, 1827, 1320–1331. [Google Scholar] [CrossRef]
- Schäfer, K.; Künzler, P.; Klingl, A.; Eubel, H.; Carrie, C. The Plant Mitochondrial TAT Pathway Is Essential for Complex III Biogenesis. Curr. Biol. 2020, 30, 840–853. [Google Scholar] [CrossRef]
- Grimm, S. Respiratory chain complex II as general sensor for apoptosis. Biochim. Biophys. Acta—Bioenerg. 2013, 1827, 565–572. [Google Scholar] [CrossRef]
- Treberg, J.R.; Quinlan, C.L.; Brand, M.D. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). J. Biol. Chem. 2011, 286, 252502. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 1–28. [Google Scholar] [CrossRef]
- San Gabriel, P.T.; Liu, Y.; Schroder, A.L.; Zoellner, H.; Chami, B. The role of thiocyanate in modulating myeloperoxidase activity during disease. Int. J. Mol. Sci. 2020, 21, 6450. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxidants Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [PubMed]
- Kargapolova, Y.; Geißen, S.; Zheng, R.; Baldus, S.; Winkels, H.; Adam, M. The enzymatic and non-enzymatic function of myeloperoxidase (Mpo) in inflammatory communication. Antioxidants 2021, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J. The dual role of myeloperoxidase in immune response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Lim, S.; Caramori, G.; Chung, K.F.; Barnes, P.J.; Adcock, I.M. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001, 15, 1110–1112. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.F.; Lin, W.L.; Ma, Y.C. A study of reactive oxygen species in mainstream of cigarette. Indoor Air 2005, 15, 135–140. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Gebicki, J.M. Oxidative stress, free radicals and protein peroxides. Arch. Biochem. Biophys. 2016, 595, 33–39. [Google Scholar] [CrossRef]
- Henri, P.; Beaumel, S.; Guezennec, A.; Poumès, C.; Stoebner, P.E.; Stasia, M.J.; Guesnet, J.; Martinez, J.; Meunier, L. MC1R expression in HaCaT keratinocytes inhibits UVA-induced ROS production via NADPH Oxidase- and cAMP-dependent mechanisms. J. Cell. Physiol. 2012, 227, 2578–2585. [Google Scholar] [CrossRef]
- Brugè, F.; Tiano, L.; Astolfi, P.; Emanuelli, M.; Damiani, E. Prevention of UVA-induced oxidative damage in human dermal fibroblasts by new UV filters, assessed using a novel In Vitro experimental system. PLoS ONE 2014, 9, e83401. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta—Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Mailloux, R.J.; McBride, S.L.; Harper, M.E. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem. Sci. 2013, 38, 592–602. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The role of reactive oxygen species in arsenic toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef]
- Engwa, G.A.; Nweke, F.N.; Nkeh-Chungag, B.N. Free Radicals, Oxidative Stress-Related Diseases and Antioxidant Supplementation. Altern. Ther. Health Med. 2022, 28, 28. [Google Scholar]
- Obeng-Gyasi, E. Lead exposure and oxidative stress—A life course approach in U.S. adults. Toxics 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Siddiqui, M.K.J. Low level lead exposure and oxidative stress: Current opinions. Clin. Chim. Acta 2007, 383, 57–64. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, G.; Terracina, S.; Petrella, C.; Greco, A.; Minni, A.; Lucarelli, M.; Agostinelli, E.; Ralli, M.; de Vincentiis, M.; Raponi, G.; et al. Alcohol and Head and Neck Cancer: Updates on the Role of Oxidative Stress, Genetic, Epigenetics, Oral Microbiota, Antioxidants, and Alkylating Agents. Antioxidants 2022, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Messina, M.P.; Petrella, C.; D’Angelo, A.; Greco, A.; Ralli, M.; Ferraguti, G.; Tarani, L.; Vitali, M.; Ceccanti, M. Antioxidant properties of plant polyphenols in the counteraction of alcohol-abuse induced damage: Impact on the Mediterranean diet. J. Funct. Foods 2020, 71, 104012. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Hardivillé, S.; Banerjee, P.S.; Selen Alpergin, E.S.; Smith, D.M.; Han, G.; Ma, J.; Talbot, C.C.; Hu, P.; Wolfgang, M.J.; Hart, G.W. TATA-Box Binding Protein O-GlcNAcylation at T114 Regulates Formation of the B-TFIID Complex and Is Critical for Metabolic Gene Regulation. Mol. Cell 2020, 77, 1143–1152. [Google Scholar] [CrossRef]
- Patikoglou, G.A.; Kim, J.L.; Sun, L.; Yang, S.H.; Kodadek, T.; Burley, S.K. TATA element recognition by the TATA box-binding protein has been conserved throughout evolution. Genes Dev. 1999, 13, 3217–3230. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Maslov, L.N.; Naryzhnaya, N.V.; Boshchenko, A.A.; Popov, S.V.; Ivanov, V.V.; Oeltgen, P.R. Is oxidative stress of adipocytes a cause or a consequence of the metabolic syndrome? J. Clin. Transl. Endocrinol. 2019, 15, 1–5. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The role of vitamin E in immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Niki, E.; Naito, Y.; Yoshikawa, T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic. Res. 2013, 47, 869–880. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The pharmacokinetics of vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Edge, R.; McGarvey, D.J.; Truscott, T.G. The carotenoids as anti-oxidants—A review. J. Photochem. Photobiol. B Biol. 1997, 41, 189. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef] [PubMed]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Petrella, C.; Carito, V.; Carere, C.; Ferraguti, G.; Ciafrè, S.; Natella, F.; Bello, C.; Greco, A.; Ralli, M.; Mancinelli, R.; et al. Oxidative stress inhibition by resveratrol in alcohol-dependent mice. Nutrition 2020, 79–80, 110783. [Google Scholar] [CrossRef]
- Petrella, C.; Di Certo, M.G.; Gabanella, F.; Barbato, C.; Ceci, F.M.; Greco, A.; Ralli, M.; Polimeni, A.; Angeloni, A.; Severini1, C.; et al. Mediterranean Diet, Brain and Muscle: Olive Polyphenols and Resveratrol Protection in Neurodegenerative and Neuromuscular Disorders. Curr. Med. Chem. 2021, 28, 7595–7613. [Google Scholar] [CrossRef]
- Carito, V.; Ceccanti, M.; Tarani, L.; Ferraguti, G.; Chaldakov, G.N.; Fiore, M. Neurotrophins’ Modulation by Olive Polyphenols. Curr. Med. Chem. 2016, 23, 3189–3197. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, M.; Valentina, C.; Vitali, M.; Iannuzzi, S.; Tarani, L.; De Nicolo, S.; Ceccanti, M.M.; Ciafre, S.; Tirassa, P.; Capriglione, I.; et al. Serum BDNF and NGF Modulation by Olive Polyphenols in Alcoholics during Withdrawal. J. Alcohol. Drug Depend. 2015, 03, 2. [Google Scholar] [CrossRef]
- Carito, V.; Ceccanti, M.; Cestari, V.; Natella, F.; Bello, C.; Coccurello, R.; Mancinelli, R.; Fiore, M. Olive polyphenol effects in a mouse model of chronic ethanol addiction. Nutrition 2017, 33, 65–69. [Google Scholar] [CrossRef]
- Fiore, M.; Petrella, C.; Coriale, G.; Rosso, P.; Fico, E.; Ralli, M.; Greco, A.; De Vincentiis, M.; Minni, A.; Polimeni, A.; et al. Markers of Neuroinflammation in the Serum of Prepubertal Children with Fetal Alcohol Spectrum Disorders. CNS Neurol. Disord.—Drug Targets 2021, 20. [Google Scholar] [CrossRef]
- Ferraguti, G.; Merlino, L.; Battagliese, G.; Piccioni, M.G.; Barbaro, G.; Carito, V.; Messina, M.P.; Scalese, B.; Coriale, G.; Fiore, M.; et al. Fetus morphology changes by second-trimester ultrasound in pregnant women drinking alcohol. Addict. Biol. 2020, 25, e12724. [Google Scholar] [CrossRef] [PubMed]
- Coriale, G.; Fiorentino, D.; Lauro, F.D.I.; Marchitelli, R.; Scalese, B.; Fiore, M.; Maviglia, M.; Ceccanti, M. Fetal Alcohol Spectrum Disorder (FASD): Neurobehavioral profile, indications for diagnosis and treatment. Riv. Psichiatr. 2013, 48, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wilhoit, L.F.; Scott, D.A.; Simecka, B.A. Fetal Alcohol Spectrum Disorders: Characteristics, Complications, and Treatment. Community Ment. Health J. 2017, 53, 711–718. [Google Scholar] [CrossRef]
- Denny, L.A.; Coles, S.; Blitz, R. Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders. Am. Fam. Physician 2017, 96, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Mattson, S.N.; Crocker, N.; Nguyen, T.T. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol. Rev. 2011, 21, 81–101. [Google Scholar] [CrossRef]
- Goodlett, C.R.; Horn, K.H.; Zhou, F.C. Alcohol teratogenesis: Mechanisms of damage and strategies for intervention. Exp. Biol. Med. 2005, 230, 394–406. [Google Scholar] [CrossRef]
- Ciafrè, S.; Carito, V.; Ferraguti, G.; Greco, A.; Chaldakov, G.N.; Fiore, M.; Ceccanti, M. How alcohol drinking affects our genes: An epigenetic point of view. Biochem. Cell Biol. 2019, 97, 345–356. [Google Scholar] [CrossRef]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global prevalence of fetal alcohol spectrum disorder among children and youth: A systematic review and meta-analysis. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Banakar, M.K.; Kudlur, N.S.; George, S. Fetal alcohol spectrum disorder(FASD. Indian J. Pediatr. 2009, 76, 1173–1175. [Google Scholar] [CrossRef]
- Aragón, A.S.; Coriale, G.; Fiorentino, D.; Kalberg, W.O.; Buckley, D.; Phillip Gossage, J.; Ceccanti, M.; Mitchell, E.R.; May, P.A. Neuropsychological characteristics of Italian children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2008, 32, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Fiorentino, D.; Phillip Gossage, J.; Kalberg, W.O.; Eugene Hoyme, H.; Robinson, L.K.; Coriale, G.; Jones, K.L.; Del Campo, M.; Tarani, L.; et al. Epidemiology of FASD in a province in Italy: Prevalence and characteristics of children in a random sample of schools. Alcohol. Clin. Exp. Res. 2006, 30, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, G.; Ciolli, P.; Carito, V.; Battagliese, G.; Mancinelli, R.; Ciafrè, S.; Tirassa, P.; Ciccarelli, R.; Cipriani, A.; Messina, M.P.; et al. Ethylglucuronide in the urine as a marker of alcohol consumption during pregnancy: Comparison with four alcohol screening questionnaires. Toxicol. Lett. 2017, 275, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ciafrè, S.; Ferraguti, G.; Greco, A.; Polimeni, A.; Ralli, M.; Ceci, F.M.; Ceccanti, M.; Fiore, M. Alcohol as an early life stressor: Epigenetics, metabolic, neuroendocrine and neurobehavioral implications. Neurosci. Biobehav. Rev. 2020, 118, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.L.; Sokol, R.J. A Revised Conservative Estimate of the Incidence of FAS and its Economic Impact. Alcohol. Clin. Exp. Res. 1991, 15, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Stade, B.; Ali, A.; Bennett, D.; Campbell, D.; Johnston, M.; Lens, C.; Tran, S.; Koren, G. The burden of prenatal exposure to alcohol: Revised measurement of cost. Can. J. Clin. Pharmacol. 2009, 16, e91–e102. [Google Scholar]
- Gupta, K.K.; Gupta, V.K.; Shirasaka, T. An Update on Fetal Alcohol Syndrome-Pathogenesis, Risks, and Treatment. Alcohol. Clin. Exp. Res. 2016, 40, 1594–1602. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Grzelczyk, J.; Żukiewicz-Sobczak, W. Peripheral Oxidation Markers in Down Syndrome Patients: The Better and the Worse. Dis. Markers 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Davis-Anderson, K.L.; Wesseling, H.; Siebert, L.M.; Lunde-Young, E.R.; Naik, V.D.; Steen, H.; Ramadoss, J. Fetal regional brain protein signature in FASD rat model. Reprod. Toxicol. 2018, 76, 84–92. [Google Scholar] [CrossRef]
- Bhatia, S.; Drake, D.M.; Miller, L.; Wells, P.G. Oxidative stress and DNA damage in the mechanism of fetal alcohol spectrum disorders. Birth Defects Res. 2019, 111, 714–748. [Google Scholar] [CrossRef]
- Chater-Diehl, E.J.; Laufer, B.I.; Castellani, C.A.; Alberry, B.L.; Singh, S.M. Alteration of gene expression, DNA methylation, and histone methylation in free radical scavenging networks in adult mouse hippocampus following fetal alcohol exposure. PLoS ONE 2016, 11, e0154836. [Google Scholar] [CrossRef]
- Khalid, O.; Kim, J.J.; Kim, H.S.; Hoang, M.; Tu, T.G.; Elie, O.; Lee, C.; Vu, C.; Horvath, S.; Spigelman, I.; et al. Gene expression signatures affected by alcohol-induced DNA methylomic deregulation in human embryonic stem cells. Stem Cell Res. 2014, 12, 791–806. [Google Scholar] [CrossRef]
- Van Houten, B.; Woshner, V.; Santos, J.H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair 2006, 5, 145–152. [Google Scholar] [CrossRef]
- Memo, L.; Gnoato, E.; Caminiti, S.; Pichini, S.; Tarani, L. Fetal alcohol spectrum disorders and fetal alcohol syndrome: The state of the art and new diagnostic tools. Early Hum. Dev. 2013, 89, S40–S43. [Google Scholar] [CrossRef]
- Brocardo, P.S.; Gil-Mohapel, J.; Christie, B.R. The Role of Oxidative Stress in Fetal Alcohol Spectrum Disorders. Brain Res. Rev. 2011, 67, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, M.R.; Boutelet-Bochan, H.; Person, R.E.; Fantel, A.G.; Juchau, M.R. Catalytic activity and quantitation of cytochrome P-450 2E1 in prenatal human brain. J. Pharmacol. Exp. Ther. 1999, 289, 1648–1653. [Google Scholar] [PubMed]
- De La Monte, S.M.; Kril, J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014, 127, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Li, Y.; Peng, Y. A review of interventions against fetal alcohol spectrum disorder targeting oxidative stress. Int. J. Dev. Neurosci. 2018, 71, 140–145. [Google Scholar] [CrossRef]
- Zheng, D.; Li, Y.; He, L.; Tang, Y.; Li, X.; Shen, Q.; Yin, D.; Peng, Y. The protective effect of astaxanthin on fetal alcohol spectrum disorder in mice. Neuropharmacology 2014, 84, 13–18. [Google Scholar] [CrossRef]
- Davis, K.; Desrocher, M.; Moore, T. Fetal Alcohol Spectrum Disorder: A Review of Neurodevelopmental Findings and Interventions. J. Dev. Phys. Disabil. 2011, 23, 143–167. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 Fatty Acids, the Brain and Retina: Preface. World Rev. Nutr. Diet. 2009, 99, I–XVI. [Google Scholar] [CrossRef]
- Sarsilmaz, M.; Songur, A.; Özyurt, H.; Kuş, I.; Özen, O.A.; Özyurt, B.; Söǧüt, S.; Akyol, Ö. Potential role of dietary ω-3 essential fatty acids on some oxidant/antioxidant parameters in rats’ corpus striatum. Prostaglandins Leukot. Essent. Fat. Acids 2003, 69, 253–259. [Google Scholar] [CrossRef]
- Walton, J.R.; Martens, M.A.; Pober, B.R. The proceedings of the 15th professional conference on Williams Syndrome. Am. J. Med. Genet. Part A 2017, 173, 1159–1171. [Google Scholar] [CrossRef]
- Bódizs, R.; Gombos, F.; Gerván, P.; Szocs, K.; Réthelyi, J.M.; Kovács, I. Aging and sleep in Williams syndrome: Accelerated sleep deterioration and decelerated slow wave sleep decrement. Res. Dev. Disabil. 2014, 35, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Lenhoff, H.M.; Wang, P.P.; Greenberg, F.; Bellugi, U. Williams syndrome and the brain. Sci. Am. 1997, 277, 68–73. [Google Scholar] [CrossRef]
- Wan, E.S.; Pober, B.R.; Washko, G.R.; Raby, B.A.; Silverman, E.K. Pulmonary function and emphysema in Williams-Beuren syndrome. Am. J. Med. Genet. Part A 2010, 152, 653–656. [Google Scholar] [CrossRef]
- Ferrari, M.; Stagi, S. Oxidative stress in down and williams-beuren syndromes: An overview. Molecules 2021, 26, 3139. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Faury, G. NOX- and ROS-Driven Hypertension in Elastin Insufficiency. Function 2021, 2, zqab035. [Google Scholar] [CrossRef]
- Kozel, B.A.; Danback, J.R.; Waxler, J.L.; Knutsen, R.H.; De Las Fuentes, L.; Reusz, G.S.; Kis, E.; Bhatt, A.B.; Pober, B.R. Williams syndrome predisposes to vascular stiffness modified by antihypertensive use and copy number changes in NCF1. Hypertension 2014, 63, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol.—Hear. Circ. Physiol. 2003, 285, H2290-H2297. [Google Scholar] [CrossRef]
- Csiszar, A.; Lehoux, S.; Ungvari, Z. Hemodynamic forces, vascular oxidative stress, and regulation of BMP-2/4 expression. Antioxidants Redox Signal. 2009, 11, 1683–1697. [Google Scholar] [CrossRef]
- Lehoux, S. Redox signalling in vascular responses to shear and stretch. Cardiovasc. Res. 2006, 71, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Meroni, S.B.; Galardo, M.N.; Rindone, G.; Gorga, A.; Riera, M.F.; Cigorraga, S.B. Molecular mechanisms and signaling pathways involved in Sertoli cell proliferation. Front. Endocrinol. 2019, 10, 224. [Google Scholar] [CrossRef]
- Troia, A.; Knutsen, R.H.; Halabi, C.M.; Malide, D.; Yu, Z.X.; Wardlaw-Pickett, A.; Kronquist, E.K.; Tsang, K.M.; Kovacs, A.; Mecham, R.P.; et al. Inhibition of NOX1 Mitigates Blood Pressure Increases in Elastin Insufficiency. Function 2021, 2, zqab015. [Google Scholar] [CrossRef] [PubMed]
- Tebbenkamp, A.T.N.; Varela, L.; Choi, J.; Paredes, M.I.; Giani, A.M.; Song, J.E.; Sestan-Pesa, M.; Franjic, D.; Sousa, A.M.M.; Liu, Z.W.; et al. The 7q11.23 Protein DNAJC30 Interacts with ATP Synthase and Links Mitochondria to Brain Development. Cell 2018, 175, 1088–1104. [Google Scholar] [CrossRef]
- Mizumura, K.; Cloonan, S.M.; Nakahira, K.; Bhashyam, A.R.; Cervo, M.; Kitada, T.; Glass, K.; Owen, C.A.; Mahmood, A.; Washko, G.R.; et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Investig. 2014, 124, 3987–4003. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Zijlstra, G.J.; van der Toorn, M.; Hesse, L.; Gras, R.; Hacken, N.H.T.T.; Krysko, D.V.; Vandenabeele, P.; De Vries, M.; Van Oosterhout, A.J.M.; et al. Cigarette smoke-induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2016, 310, L377-L386. [Google Scholar] [CrossRef]
- Van Der Toorn, M.; Slebos, D.J.; De Bruin, H.G.; Leuvenink, H.G.; Bakker, S.J.L.; Gans, R.O.B.; Koëter, G.H.; Van Oosterhout, A.J.M.; Kauffman, H.F. Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2007, 292, L1156-L1162. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, S.; Bernard, K.; Thannickal, V.J. Mitochondrial dysfunction in pulmonary fibrosis. Ann. Am. Thorac. Soc. 2017, 14, S383–S388. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, E.; Davies, K.J.A. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Lichtenstein, A.H.; Howard, B.V.; Steinberg, D.; Witztum, J.L. Antioxidant vitamin supplements and cardiovascular disease. Circulation 2004, 110, 637–641. [Google Scholar] [CrossRef]
- Adams, G.N.; Schmaier, A.H. The williams-beuren syndrome-a window into genetic variants leading to the development of cardiovascular disease. PLoS Genet. 2012, 8, e1002479. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Bikineyeva, A.T.; Budzyn, K.; Nazarewicz, R.R.; McCann, L.; Lewis, W.; Harrison, D.G.; Dikalov, S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010, 107, 106–116. [Google Scholar] [CrossRef]
- Lavin, M.F.; Shiloh, Y. Ataxia-telangiectasia: A multifaceted genetic disorder associated with defective signal transduction. Curr. Opin. Immunol. 1996, 8, 459–464. [Google Scholar] [CrossRef]
- Lavin, M.F. Ataxia-telangiectasia: From a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell Biol. 2008, 9, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Derheimer, F.A.; Kastan, M.B. Multiple roles of ATM in monitoring and maintaining DNA integrity. FEBS Lett. 2010, 584, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Mutant ATM Withdraws Translation. Sci. STKE 2000, 2000, 2000. [CrossRef]
- Schubert, R.; Erker, L.; Barlow, C.; Yakushiji, H.; Larson, D.; Russo, A.; Mitchell, J.B.; Wynshaw-Boris, A. Cancer chemoprevention by the antioxidant tempol in Atm-deficient mice. Hum. Mol. Genet. 2004, 13, 1793–1802. [Google Scholar] [CrossRef]
- Liu, N.; Stoica, G.; Yan, M.; Scofield, V.L.; Qiang, W.; Lynn, W.S.; Wong, P.K.Y. ATM deficiency induces oxidative stress and endoplasmic reticulum stress in astrocytes. Lab. Investig. 2005, 85, 1471–1480. [Google Scholar] [CrossRef]
- Stagni, V.; Cirotti, C.; Barilà, D. Ataxia-telangiectasia mutated kinase in the control of oxidative stress, mitochondria, and autophagy in cancer: A maestro with a large orchestra. Front. Oncol. 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016, 24, 566–581. [Google Scholar] [CrossRef]
- Weyemia, U.; Redon, C.E.; Aziz, T.; Choudhuri, R.; Maeda, D.; Parekh, P.R.; Bonner, M.Y.; Arbiser, J.L.; Bonner, W.M. NADPH oxidase 4 is a critical mediator in Ataxia telangiectasia disease. Proc. Natl. Acad. Sci. USA 2015, 112, 2121–2126. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Chen, X. The human Nox4: Gene, structure, physiological function and pathological significance. J. Drug Target. 2015, 23, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luna, L.; Sørbø, J.G.; Alseth, I.; Johansen, R.F.; Backe, P.H.; Danbolt, N.C.; Eide, L.; Bjørås, M. Human OXR1 maintains mitochondrial DNA integrity and counteracts hydrogen peroxide-induced oxidative stress by regulating antioxidant pathways involving p21. Free Radic. Biol. Med. 2014, 77, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Semlitsch, M.; Shackelford, R.E.; Zirkl, S.; Sattler, W.; Malle, E. ATM protects against oxidative stress induced by oxidized low-density lipoprotein. DNA Repair 2011, 10, 848–860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuura, E.; Kobayashi, K.; Tabuchi, M.; Lopez, L.R. Oxidative modification of low-density lipoprotein and immune regulation of atherosclerosis. Prog. Lipid Res. 2006, 45, 466–486. [Google Scholar] [CrossRef]
- Mazière, C.; Mazière, J.C. Activation of transcription factors and gene expression by oxidized low-density lipoprotein. Free Radic. Biol. Med. 2009, 46, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Manuszak, R.P.; Johnson, C.D.; Hellrung, D.J.; Link, C.J.; Wang, S. Iron chelators increase the resistance of Ataxia telangeictasia cells to oxidative stress. DNA Repair 2004, 3, 1263–1272. [Google Scholar] [CrossRef]
- Green, M.H.L.; Marcovitch, A.J.; Harcourt, S.A.; Lowe, J.E.; Green, I.C.; Arlett, C.F. Hypersensitivity of ataxia-telangiectasia fibroblasts to a nitric oxide donor. Free Radic. Biol. Med. 1997, 22, 343–347. [Google Scholar] [CrossRef]
- Reliene, R.; Schiestl, R.H. Experimental antioxidant therapy in ataxia telangiectasia. Clin. Med. Oncol. 2008, 2, CMO-S535. [Google Scholar] [CrossRef]
- Reliene, R.; Fischer, E.; Schiestl, R.H. Effect of N-acetyl cysteine on oxidative DNA damage and the frequency of DNA deletions in Atm-deficient mice. Cancer Res. 2004, 64, 5148–5153. [Google Scholar] [CrossRef]
- Patterson, D. Molecular genetic analysis of Down syndrome. Hum. Genet. 2009, 126, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Petersen, M.B.; McInnis, M.G.; Adelsberger, P.A.; Schinzel, A.A.; Binkert, F.; Pangalos, C.; Raoul, O.; Slaugenhaupt, S.A.; Hafez, M.; et al. The meiotic stage of nondisjunction in trisomy 21: Determination by using DNA polymorphisms. Am. J. Hum. Genet. 1992, 50, 544. [Google Scholar]
- Asim, A.; Kumar, A.; Muthuswamy, S.; Jain, S.; Agarwal, S. Down syndrome: An insight of the disease. J. Biomed. Sci. 2015, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Lott, I.; Head, E.; Doran, E.; Busciglio, J. Beta-Amyloid, Oxidative Stress and Down Syndrome. Curr. Alzheimer Res. 2006, 3, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Muchova, J.; Zitnanova, I.; Durackova, Z. Oxidative stress and Down syndrome. Do antioxidants play a role in therapy? Physiol. Res. 2014, 63, 535–542. [Google Scholar] [CrossRef]
- Tarani, L.; Carito, V.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Messina, M.P.; Rasio, D.; De Luca, E.; Putotto, C.; et al. Neuroinflammatory Markers in the Serum of Prepubertal Children with down Syndrome. J. Immunol. Res. 2020, 2020, 6937154. [Google Scholar] [CrossRef]
- Lott, I.T.; Head, E. Dementia in Down syndrome: Unique insights for Alzheimer disease research. Nat. Rev. Neurol. 2019, 15, 135–147. [Google Scholar] [CrossRef]
- Perluigi, M.; Tramutola, A.; Pagnotta, S.; Barone, E.; Allan Butterfield, D. The bach1/Nrf2 axis in brain in down syndrome and transition to alzheimer disease-like neuropathology and dementia. Antioxidants 2020, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Butterfield, D.A. Oxidative stress and down syndrome: A route toward Alzheimer-like dementia. Curr. Gerontol. Geriatr. Res. 2012, 2012, 724904. [Google Scholar] [CrossRef]
- Groner, Y.; Lieman-Hurwitz, J.; Dafni, N.; Sherman, L.; Levanon, D.; Bernstein, Y.; Danciger, E.; Elroy-Stein, O. Molecular Structure and Expression of the Gene Locus on Chromosome 21 Encoding the Cu/Zn Superoxide Dismutase and Its Relevance to Down Syndrome. Ann. N. Y. Acad. Sci. 1985, 450, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Gulesserian, T.; Seidl, R.; Hardmeier, R.; Cairns, N.; Lubec, G. Superoxide dismutase SOD1, encoded on chromosome 21, but not SOD2 is overexpressed in brains of patients with Down Syndrome. J. Investig. Med. 2001, 49, 41–46. [Google Scholar] [CrossRef]
- Barone, E.; Arena, A.; Head, E.; Butterfield, D.A.; Perluigi, M. Disturbance of redox homeostasis in Down Syndrome: Role of iron dysmetabolism. Free Radic. Biol. Med. 2018, 114, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zana, M.; Janka, Z.; Kálmán, J. Oxidative stress: A bridge between Down’s syndrome and Alzheimer’s disease. Neurobiol. Aging 2007, 28, 648–676. [Google Scholar] [CrossRef] [PubMed]
- De Haan, J.B.; Cristiano, F.; Iannello, R.C.; Kola, I. Cu/Zn-superoxide dismutase and glutathione peroxidase during aging. Biochem. Mol. Biol. Int. 1995, 35, 1281–1297. [Google Scholar]
- Perluigi, M.; Di Domenico, F.; Buttterfield, D.A. Unraveling the complexity of neurodegeneration in brains of subjects with Down syndrome: Insights from proteomics. Proteomics—Clin. Appl. 2014, 8, 73–85. [Google Scholar] [CrossRef]
- Ma, K.; Li, F.; Yu, Y.; Li, H. Screening of potential biomarkers for prenatal diagnosis of trisomy 21. J. Obstet. Gynaecol. 2017, 37, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Barone, E.; Head, E.; Butterfield, D.A.; Perluigi, M. HNE-modified proteins in Down syndrome: Involvement in development of Alzheimer disease neuropathology. Free Radic. Biol. Med. 2017, 111, 262–269. [Google Scholar] [CrossRef]
- Di Domenico, F.; Pupo, G.; Mancuso, C.; Barone, E.; Paolini, F.; Arena, A.; Blarzino, C.; Schmitt, F.A.; Head, E.; Butterfield, D.A.; et al. Bach1 overexpression in down syndrome correlates with the alteration of the HO-1/BVR-A system: Insights for transition to alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 1107–1120. [Google Scholar] [CrossRef]
- Donato, R. Intracellular and extracellular roles of S100 proteins. Microsc. Res. Tech. 2003, 60, 540–551. [Google Scholar] [CrossRef]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s double life: Intracellular regulator and extracellular signal. Biochim. Biophys. Acta—Mol. Cell Res. 2009, 1793, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Helguera, P.; Pelsman, A.; Pigino, G.; Wolvetang, E.; Head, E.; Busciglio, J. ets-2 promotes the activation of a mitochondrial death pathway in down’s syndrome neurons. J. Neurosci. 2005, 25, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Fabbrini, F.; D’Agostino, P.; Negri, R.; Greco, D.; Genesio, R.; D’Armiento, M.; Olla, C.; Paladini, D.; Zannini, M.; et al. Altered expression of mitochondrial and extracellular matrix genes in the heart of human fetuses with chromosome 21 trisomy. BMC Genom. 2007, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Head, E.; Muggenburg, B.A.; Zicker, S.; Milgram, N.W. Brain aging in the canine: A diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol. Aging 2002, 23, 809–818. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef]
- Lockitch, G.; Puterman, M.; Godolphin, W.; Sheps, S.; Tingle, A.J.; Quigley, G. Infection and immunity in Down syndrome: A trial of long-term low oral doses of zinc. J. Pediatr. 1989, 114, 781–787. [Google Scholar] [CrossRef]
- Anneren, G.; Magnusson, C.G.; Nordvall, S.L. Increase in serum concentrations of IgG2 and IgG4 by selenium supplementation in children with Down’s syndrome. Arch. Dis. Child. 1990, 65, 1353–1355. [Google Scholar] [CrossRef]
- Lott, I.T.; Doran, E.; Nguyen, V.Q.; Tournay, A.; Head, E.; Gillen, D.L. Down syndrome and dementia: A randomized, controlled trial of antioxidant supplementation. Am. J. Med. Genet. Part A 2011, 155, 1939–1948. [Google Scholar] [CrossRef]
- Lott, I.T. Antioxidants in Down syndrome. Biochim. Biophys. Acta—Mol. Basis Dis. 2012, 1822, 657–663. [Google Scholar] [CrossRef]
- Ellis, J.M.; Hooi, K.T.; Gilbert, R.E.; Muller, D.P.R.; Henley, W.; Moy, R.; Pumphrey, R.; Ani, C.; Davies, S.; Edwards, V.; et al. Supplementation with antioxidants and folinic acid for children with Down’s syndrome: Randomised controlled trial. BMJ 2008, 336, 594–597. [Google Scholar] [CrossRef]
- Lockrow, J.; Prakasam, A.; Huang, P.; Bimonte-Nelson, H.; Sambamurti, K.; Granholm, A.C. Cholinergic degeneration and memory loss delayed by vitamin E in a Down syndrome mouse model. Exp. Neurol. 2009, 216, 278–289. [Google Scholar] [CrossRef]
- Shichiri, M.; Yoshida, Y.; Ishida, N.; Hagihara, Y.; Iwahashi, H.; Tamai, H.; Niki, E. α-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Radic. Biol. Med. 2011, 50, 1801–1811. [Google Scholar] [CrossRef]
- Bouzid, M.A.; Filaire, E.; Matran, R.; Robin, S.; Fabre, C. Lifelong Voluntary Exercise Modulates Age-Related Changes in Oxidative Stress. Int. J. Sports Med. 2018, 39, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.M.; Powers, S.K. Oxidative Stress, Antioxidant Status, and the Contracting Diaphragm. Can. J. Appl. Physiol. 1998, 23, 23–55. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Leeuwenburgh, C.; Leichtweis, S.; Gore, M.; Fiebig, R.; Hollander, J.; Bejma, J. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann. N. Y. Acad. Sci. 1998, 854, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, G.V.; Pereira, F.D.; Fernhall, B. Therapeutics and Clinical Risk Management Dovepress Reduced exercise capacity in persons with Down syndrome: Cause, effect, and management. Ther. Clin. Risk Manag. 2010, 6, 601. [Google Scholar] [CrossRef]
- Shields, N.; Downs, J.; de Haan, J.B.; Taylor, N.F.; Torr, J.; Fernhall, B.; Kingsley, M.; Mnatzaganian, G.; Leonard, H. What effect does regular exercise have on oxidative stress in people with Down syndrome? A systematic review with meta-analyses. J. Sci. Med. Sport 2018, 21, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M.; Jing, H. Marfan’s syndrome: An overview. Sao Paulo Med. J. 2010, 128, 360–366. [Google Scholar] [CrossRef]
- Dietz, H.C.; Loeys, B.; Carta, L.; Ramirez, F. Recent progress towards a molecular understanding of Marfan syndrome. Am. J. Med. Genet.—Semin. Med. Genet. 2005, 139C, 4–9. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Renard, M.; De Backer, J. FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene 2016, 592, 279–291. [Google Scholar] [CrossRef]
- Coelho, S.G.; Almeida, A.G. Marfan syndrome revisited: From genetics to the clinic. Rev. Port. Cardiol. 2020, 39, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, S.M.; Sloan, B.; Jones, J.A. Pathophysiology and Pathogenesis of Marfan Syndrome. Adv. Exp. Med. Biol. 2021, 1348. [Google Scholar]
- Hugar, B.S.; Praveen, S.; Kainoor, S.K.; Shetty, A.R.S. Sudden death in marfan syndrome. J. Forensic Sci. 2014, 59, 1126–1128. [Google Scholar] [CrossRef]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef]
- Yang, H.H.C.; van Breemen, C.; Chung, A.W.Y. Vasomotor dysfunction in the thoracic aorta of Marfan syndrome is associated with accumulation of oxidative stress. Vascul. Pharmacol. 2010, 52, 37–45. [Google Scholar] [CrossRef]
- Liaw, N.; Dolan Fox, J.M.; Siddiqui, A.H.; Meng, H.; Kolega, J. Endothelial nitric oxide synthase and superoxide mediate hemodynamic initiation of intracranial aneurysms. PLoS ONE 2014, 9, e101721. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Gluba-Brzózka, A.; Rokicki, R.; Franczyk, B. Oxidative stress-related susceptibility to aneurysm in marfan’s syndrome. Biomedicines 2021, 9, 1171. [Google Scholar] [CrossRef]
- Jiménez-Altayó, F.; Meirelles, T.; Crosas-Molist, E.; Sorolla, M.A.; del Blanco, D.G.; López-Luque, J.; Mas-Stachurska, A.; Siegert, A.M.; Bonorino, F.; Barberà, L.; et al. Redox stress in Marfan syndrome: Dissecting the role of the NADPH oxidase NOX4 in aortic aneurysm. Free Radic. Biol. Med. 2018, 118, 44–58. [Google Scholar] [CrossRef]
- Chung, A.W.Y.; Yang, H.H.C.; Au Yeung, K.; Van Breemen, C. Mechanical and pharmacological approaches to investigate the pathogenesis of marfan syndrome in the abdominal aorta. J. Vasc. Res. 2008, 45, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, C.; Becatti, M.; Attanasio, M.; Lucarini, L.; Nassi, N.; Evangelisti, L.; Porciani, M.C.; Nassi, P.; Gensini, G.F.; Abbate, R.; et al. Evidence for oxidative stress in plasma of patients with Marfan syndrome. Int. J. Cardiol. 2010, 145, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Prakash, S.K.; Ramirez, F. Therapeutics Targeting Drivers of Thoracic Aortic Aneurysms and Acute Aortic Dissections: Insights from Predisposing Genes and Mouse Models. Annu. Rev. Med. 2017, 68, 163–176. [Google Scholar] [CrossRef]
- Bunton, T.E.; Jensen Biery, N.; Myers, L.; Gayraud, B.; Ramirez, F.; Dietz, H.C. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ. Res. 2001, 88, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.G.; Peng, T.; Luo, Y.; Li, M.C.; Lin, Y.H. Resveratrol reestablishes spermatogenesis after testicular injury in rats caused by 2,5-hexanedione. Chin. Med. J. 2008, 121, 1204–1209. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, J.; Qu, W.; Peng, X.; Xin, P.; Yang, X.; Ying, C.; Sun, X.; Hao, L. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J. Nutr. Biochem. 2012, 23, 1410–1416. [Google Scholar] [CrossRef]
- Fakeye, T.O.; Adegoke, A.O.; Omoyeni, O.C.; Famakinde, A.A. Effects of water extract of Hibiscus sabdariffa, Linn (Malvaceae) “Roselle” on excretion of a diclofenac formulation. Phyther. Res. 2007, 21, 96–98. [Google Scholar] [CrossRef]
- Lin, W.L.; Hsieh, Y.J.; Chou, F.P.; Wang, C.J.; Cheng, M.T.; Tseng, T.H. Hibiscus protocatechuic acid inhibits lipopolysaccharide-induced rat hepatic damage. Arch. Toxicol. 2003, 77, 42–47. [Google Scholar] [CrossRef]
- Perez-Torres, I.; Ruiz-Ramirez, A.; Banos, G.; El-Hafidi, M. Hibiscus Sabdariffa Linnaeus (Malvaceae), Curcumin and Resveratrol as Alternative Medicinal Agents Against Metabolic Syndrome. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 25–37. [Google Scholar] [CrossRef]
- Soto, M.E.; Zuñiga-Muñoz, A.; Guarner Lans, V.; Duran-Hernández, E.J.; Pérez-Torres, I. Infusion of Hibiscus sabdariffa L. modulates oxidative stress in patients with marfan syndrome. Mediators Inflamm. 2016, 2016, 8625203. [Google Scholar] [CrossRef]
- Askari, M.; Mozaffari, H.; Darooghegi Mofrad, M.; Jafari, A.; Surkan, P.J.; Amini, M.R.; Azadbakht, L. Effects of garlic supplementation on oxidative stress and antioxidative capacity biomarkers: A systematic review and meta-analysis of randomized controlled trials. Phyther. Res. 2021, 35, 3032–3045. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Soto, M.E.; Manzano-Pech, L.; Díaz-Diaz, E.; Soria-Castro, E.; Rubio-Ruíz, M.E.; Guarner-Lans, V. Oxidative Stress in Plasma from Patients with Marfan Syndrome Is Modulated by Deodorized Garlic Preliminary Findings. Oxid. Med. Cell. Longev. 2022, 2022, 5492127. [Google Scholar] [CrossRef]
- Tsui, V.; Crismani, W. The Fanconi Anemia Pathway and Fertility. Trends Genet. 2019, 35, 199–214. [Google Scholar] [CrossRef]
- Liu, W.; Palovcak, A.; Li, F.; Zafar, A.; Yuan, F.; Zhang, Y. Fanconi anemia pathway as a prospective target for cancer intervention. Cell Biosci. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Kulanuwat, S.; Jungtrakoon, P.; Tangjittipokin, W.; Yenchitsomanus, P.T.; Plengvidhya, N. Fanconi anemia complementation group C protection against oxidative stress-induced ß-cell apoptosis. Mol. Med. Rep. 2018, 18, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.D. VACTERL/VATER association. Orphanet J. Rare Dis. 2011, 6, 56. [Google Scholar] [CrossRef]
- Niraj, J.; Färkkilä, A.; D’Andrea, A.D. The fanconi anemia pathway in cancer. Annu. Rev. Cancer Biol. 2019, 3, 2585–2588. [Google Scholar] [CrossRef] [PubMed]
- Palovcak, A.; Liu, W.; Yuan, F.; Zhang, Y. Maintenance of genome stability by Fanconi anemia proteins. Cell Biosci. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Adam, Z.; Rani, R.; Zhang, X.; Pang, Q. Oxidative stress in fanconi anemia hematopoiesis and disease progression. Antioxidants Redox Signal. 2008, 10, 1909–1921. [Google Scholar] [CrossRef]
- Pagano, G.; Talamanca, A.A.; Castello, G.; Pallardó, F.V.; Zatterale, A.; Degan, P. Oxidative stress in Fanconi anaemia: From cells and molecules towards prospects in clinical management. Biol. Chem. 2012, 393, 11–21. [Google Scholar] [CrossRef]
- Van Twest, S.; Murphy, V.J.; Hodson, C.; Tan, W.; Swuec, P.; O’Rourke, J.J.; Heierhorst, J.; Crismani, W.; Deans, A.J. Mechanism of Ubiquitination and Deubiquitination in the Fanconi Anemia Pathway. Mol. Cell 2017, 65, 247–259. [Google Scholar] [CrossRef]
- Cappelli, E.; Bertola, N.; Bruno, S.; Degan, P.; Regis, S.; Corsolini, F.; Banelli, B.; Dufour, C.; Ravera, S. A multidrug approach to modulate the mitochondrial metabolism impairment and relative oxidative stress in fanconi anemia complementation group a. Metabolites 2022, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.L. Childhood developmental disorders: An academic and clinical convergence point for psychiatry, neurology, psychology and pediatrics. J. Child Psychol. Psychiatry Allied Discip. 2009, 50, 87–98. [Google Scholar] [CrossRef]
- Courchesne, E.; Gazestani, V.H.; Lewis, N.E. Prenatal Origins of ASD: The When, What, and How of ASD Development. Trends Neurosci. 2020, 43, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021, 51, 4253–4270. [Google Scholar] [CrossRef]
- Manivasagam, T.; Arunadevi, S.; Essa, M.M.; SaravanaBabu, C.; Borah, A.; Thenmozhi, A.J.; Qoronfleh, M.W. Role of Oxidative Stress and Antioxidants in Autism. Adv. Neurobiol. 2020, 24. [Google Scholar]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative Stress in Autism Spectrum Disorder—Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Mousavinejad, E.; Ghaffari, M.A.; Riahi, F.; Hajmohammadi, M.; Tiznobeyk, Z.; Mousavinejad, M. Coenzyme Q10 supplementation reduces oxidative stress and decreases antioxidant enzyme activity in children with autism spectrum disorders. Psychiatry Res. 2018, 265, 62–69. [Google Scholar] [CrossRef]
- Nikoo, M.; Radnia, H.; Farokhnia, M.; Mohammadi, M.R.; Akhondzadeh, S. N-acetylcysteine as an adjunctive therapy to risperidone for treatment of irritability in autism: A randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Clin. Neuropharmacol. 2015, 38, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bent, S.; Hendren, R.L.; Zandi, T.; Law, K.; Choi, J.E.; Widjaja, F.; Kalb, L.; Nestle, J.; Law, P. Internet-based, randomized, controlled trial of omega-3 fatty acids for hyperactivity in autism. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 658–666. [Google Scholar] [CrossRef]
- Hendouei, F.; Sanjari Moghaddam, H.; Mohammadi, M.R.; Taslimi, N.; Rezaei, F.; Akhondzadeh, S. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: A double-blind and placebo-controlled randomized trial. J. Clin. Pharm. Ther. 2020, 45, 324–334. [Google Scholar] [CrossRef]
- Maffeis, M.; Notarangelo, L.D.; Schumacher, R.F.; Soncini, E.; Soresina, A.; Lanfranchi, A.; Porta, F. Primary immunodeficiencies and oncological risk: The experience of the Children’s Hospital of Brescia. Front. Pediatr. 2019, 7, 232. [Google Scholar] [CrossRef]
- Xiao, X.; Miao, Q.; Chang, C.; Gershwin, M.E.; Ma, X. Common variable immunodeficiency and autoimmunity—An inconvenient truth. Autoimmun. Rev. 2014, 13, 858–864. [Google Scholar] [CrossRef]
- Tanir Basaranoglu, S.; Cekic, S.; Kirhan, E.; Dirican, M.; Kilic, S.S. Oxidative stress in common variable immunodeficiency. Eur. J. Inflamm. 2021, 19, 20587392211002411. [Google Scholar] [CrossRef]
- Aukrust, P.; Berge, R.K.; Müller, F.; Ueland, P.M.; Svardal, A.M.; Frøland, S.S. Elevated plasma levels of reduced homocysteine in common variable immunodeficiency—A marker of enhanced oxidative stress. Eur. J. Clin. Investig. 1997, 27, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Andrade, I.G.A.; de Souza, F.I.S.; Fonseca, F.L.A.; Aranda, C.S.; Sarni, R.O.S. Selenium-related nutritional status in patients with common variable immunodeficiency: Association with oxidative stress and atherosclerosis risk. BMC Immunol. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Vieira, D.G.; Costa-Carvalho, B.T.; Hix, S.; Da Silva, R.; Correia, M.S.G.; Sarni, R.O.S. Higher Cardiovascular Risk in Common Variable Immunodeficiency and X-Linked Agammaglobulinaemia Patients. Ann. Nutr. Metab. 2015, 66, 237–241. [Google Scholar] [CrossRef]
- Pannicke, U.; Hönig, M.; Hess, I.; Friesen, C.; Holzmann, K.; Rump, E.M.; Barth, T.F.; Rojewski, M.T.; Schulz, A.; Boehm, T.; et al. Reticular dysgenesis (aleukocytosis) is caused by mutations in the gene encoding mitochondrial adenylate kinase 2. Nat. Genet. 2009, 41, 101–105. [Google Scholar] [CrossRef]
- Haddad, E.; Hoenig, M. Hematopoietic Stem Cell Transplantation for Severe Combined Immunodeficiency (SCID). Front. Pediatr. 2019, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Kranc, K.R.; Dzierzak, E. Relieving oxidative stress in immune cells. J. Exp. Med. 2015, 212, 1140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van der Burg, M.; Mahlaoui, N.; Gaspar, H.B.; Pai, S.Y. Universal Newborn Screening for Severe Combined Immunodeficiency (SCID). Front. Pediatr. 2019, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, M.; Christenson, K.; Björnsdottir, H.; Osla, V.; Karlsson, A.; Dahlgren, C.; Speert, D.P.; Fasth, A.; Brown, K.L.; Bylund, J. Elevated mitochondrial reactive oxygen species and cellular redox imbalance in human NADPH-oxidase-deficient phagocytes. Front. Immunol. 2017, 8, 1828. [Google Scholar] [CrossRef]
- Bylund, J.; Goldblatt, D.; Speert, D.P. Chronic granulomatous disease: From genetic defect to clinical presentation. Adv. Exp. Med. Biol. 2005, 568, 568. [Google Scholar] [CrossRef]
- Segal, B.H.; Leto, T.L.; Gallin, J.I.; Malech, H.L.; Holland, S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine 2000, 79, 170–200. [Google Scholar] [CrossRef]
- Heropolitanska-Pliszka, E.; Berk, K.; Maciejczyk, M.; Sawicka-Powierza, J.; Bernatowska, E.; Wolska-Kusnierz, B.; Pac, M.; Dabrowska-Leonik, N.; Piatosa, B.; Lewandowicz-Uszynska, A.; et al. Systemic redox imbalance in patients with chronic granulomatous disease. J. Clin. Med. 2020, 9, 1397. [Google Scholar] [CrossRef]
- Gutierrez-Mariscal, F.M.; de la Cruz-Ares, S.; Torres-Peña, J.D.; Alcalá-Diaz, J.F.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme q10 and cardiovascular diseases. Antioxidants 2021, 10, 906. [Google Scholar] [CrossRef]
- López, L.C.; Luna-Sánchez, M.; García-Corzo, L.; Quinzii, C.M.; Hirano, M. Pathomechanisms in coenzyme Q10-deficient human fibroblasts. Mol. Syndromol. 2014, 5, 163–169. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.H.; Yang, E.J.; Kim, J.K.; Kim, E.K.; Jung, K.; Jung, H.; Lee, K.; Lee, H.H.; Lee, B.I.; et al. Coenzyme Q10 Inhibits Th17 and STAT3 Signaling Pathways to Ameliorate Colitis in Mice. J. Med. Food 2017, 20, 821–829. [Google Scholar] [CrossRef]
- Bennett, L.L.; Mohan, D. Gaucher disease and its treatment options. Ann. Pharmacother. 2013, 47, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Burrow, T.A.; Sun, Y.; Prada, C.E.; Bailey, L.; Zhang, W.; Brewer, A.; Wu, S.W.; Setchell, K.D.R.; Witte, D.; Cohen, M.B.; et al. CNS, lung, and lymph node involvement in Gaucher disease type 3 after 11years of therapy: Clinical, histopathologic, and biochemical findings. Mol. Genet. Metab. 2015, 114, 233–241. [Google Scholar] [CrossRef]
- Sidransky, E. Gaucher disease: Complexity in a “simple” disorder. Mol. Genet. Metab. 2004, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, B.H.; Kim, G.H.; Jung, C.W.; Lee, J.; Choi, J.H.; Yoo, H.W. Clinical and genetic characteristics of Gaucher disease according to phenotypic subgroups. Korean J. Pediatr. 2012, 55, 48. [Google Scholar] [CrossRef] [PubMed]
- Kartha, R.V.; Terluk, M.R.; Brown, R.; Travis, A.; Mishra, U.R.; Rudser, K.; Lau, H.; Jarnes, J.R.; Cloyd, J.C.; Weinreb, N.J. Patients with Gaucher disease display systemic oxidative stress dependent on therapy status. Mol. Genet. Metab. Reports 2020, 25, 100667. [Google Scholar] [CrossRef]
- Zimran, A.; Elstein, D. Gaucher Disease and Related Lysosomal Storage Diseases. In Williams Hematology, 9th ed.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Gervas-Arruga, J.; Cebolla, J.J.; De Blas, I.; Roca, M.; Pocovi, M.; Giraldo, P. The influence of genetic variability and proinflammatory status on the development of bone disease in patients with Gaucher disease. PLoS ONE 2015, 10, e0126153. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.S.; Da Silva Garcia, C.; De Souza Machado, F.; Da Silva Medeiros, N.; Wohlenberg, M.F.; Marinho, J.P.; Dani, C.; Funchal, C.; Coelho, J.C. Oxidative stress parameters of Gaucher disease type I patients. Mol. Genet. Metab. Rep. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Cleeter, M.W.J.; Chau, K.Y.; Gluck, C.; Mehta, A.; Hughes, D.A.; Duchen, M.; Wood, N.W.; Hardy, J.; Mark Cooper, J.; Schapira, A.H. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem. Int. 2013, 62, 1–7. [Google Scholar] [CrossRef]
- Roversi, F.M.; Galdieri, L.C.; Grego, B.H.C.; Souza, F.G.; Micheletti, C.; Martins, A.M.; D’Almeida, V. Blood oxidative stress markers in Gaucher disease patients. Clin. Chim. Acta 2006, 364, 316–320. [Google Scholar] [CrossRef]
- Jawaid, S.; Strainic, J.P.; Kim, J.; Ford, M.R.; Thrane, L.; Karunamuni, G.H.; Sheehan, M.M.; Chowdhury, A.; Gillespie, C.A.; Rollins, A.M.; et al. Glutathione Protects the Developing Heart from Defects and Global DNA Hypomethylation Induced by Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2021, 45, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, a001651. [Google Scholar] [CrossRef] [PubMed]
| ION/RADICAL: [4,20,21,22] | REACTION: [4,23,24,25] | ENZYME/COFACTORS: [26,27,28] |
|---|---|---|
| SUPEROXIDE ION | O2 + e− → O2•− | |
| HYDROGEN PEROXIDE | 2O2•− + 2H+ ⇔ O2 + H2O2 | SUPEROXIDE DISMUTASE |
| HYDROXYL RADICAL | H2O2 + e− → HO− + •OH | FENTON REACTION |
| SINGLET OXYGEN | 2H+ + 3O2 → 1O2 + H2O2 | NADPH |
| NITRIC OXIDE | 2 L-arginine + 3 NADPH + 3 H+ + 4 O2 → 2 L-citrulline + 2 NO + 3 NADP+ + 4 H2O2 | NITRIC OXIDE SYNTHASE |
| PEROXYNITRITE RADICAL | NO + O2•− → NO(O2)− |
| Pediatric Syndromes: | Genetic Mutations: | Clinical Features: | Alterations of Genes and Proteins Involved in Oxidative Imbalance: | Clinical Manifestations: |
|---|---|---|---|---|
| Ataxia-telangiectasia | Loss of function of ATM gene on chromosome 11q22.3 | Telangiectasia, Cerebellar ataxia, Immunodeficiency, Insulin resistance, Radiosensitivity, T-lymphoid tumors | PI3K (Phosphoinositide 3-kinase) [139]: a protein involved in cell growth and survival, his loss causes alterations in DNA damage response NOX4 (NADPH oxidase 4) [142]: an enzyme involved in the production of ROS is higher express OxLDL (oxidized low-density lipoprotein) [146]: is a proinflammatory chemoattractant for macrophages and T-lymphocytes | Telangiectasia cerebellar ataxia Telangiectasia, accelerated aging, promoter of T-lymphoid tumors Prothrombotic alterations and atherosclerosis |
| Autism spectrum disorders (ASD) | The responsible genes have not been found | Deficits in social communication and interaction, Repetitiveness and sectorial in behavior, interests or activities | MT-3 (Metallothioneine-3) [228]: a protein with detoxifying activity expressed in the brain is reduced in patients with ASD determining neuronal toxicity SOD-1 (Cu/Zn superoxide dismutase 1) [162]: is a protein that acts as an antioxidant defense and transforms the superoxide radicals into H2O2, it is reduced in patients with ASD GTPx (Glutathione peroxidase) [261]): is an enzyme that uses glutathione to convert H2O2 into two water molecules and detoxifies the peroxidized lipids and glutathione transferase (GSTs), which inactivates reactive metabolites such as epoxides, aldehydes, and hydroperoxides, it is reduced in patients with ASD | Accelerated neuronal death Neurological alterations, cell death Neurological alterations Cell death |
| Chronic granulomatous disease (CGD) | Mutations of the genes encoding for NADPH-oxidase | Recurrent infections, Recurrent abscess in the liver, gastrointestinal tract, lymph nodes and lungs, HypergammaglobulinemiaAnemia | NADPH-oxidase [18]: this enzyme is reduced in these patients determining a reduction in the production of ROS from phagocytes Coenzyme Q10 [248]: this coenzyme produces antioxidant substances, it is reduced in patients with CGD | Recurrent bacterial and mycotic infections Recurrent bacterial and mycotic infections |
| Common variable immunodeficiency disease (CVID) | Unknown mutations in 90% of cases. In 10% of cases are related to TNFRSF13B gene | Hypogammaglobulinemia, Recurrent infections, Autoimmune diseases, Lymphoid malignancy, Enteropathy granulomatous disease | CAT (catalase [17]): this enzyme catalyzes the decomposition of H2O2 to H2O and O2, its level is reduced in patients with CVID determining an overproduction of ROS GTPx (Glutathione peroxidase) [261]: this enzyme uses glutathione to convert H2O2 into two water molecules and detoxifies the peroxidized lipids and glutathione transferase (GSTs), which inactivates reactive metabolites such as epoxides, aldehydes, and hydroperoxides, it is reduced in patients with CVID | Promote carcinogenesis Recurrent infections, promote carcinogenesis |
| Down syndrome (DS) | Trisomy of chromosome 21 | Intellectual disability, Facial dysmorphism, Congenital heart disease. Anticipated Alzheimer disease. Leukemia, Hypotonia, Neurodevelopmental disorders | SOD-1 (Cu/Zn superoxide dismutase 1) [162]: this proteins acts as an antioxidant defense and transforms the superoxide radicals into H2O2, in DS is higher than normal, but this augmentation is not accompanied by the increases in catalases (CAT). and glutathione peroxidase (GTPx) with an accumulation of H2O2 APP (Amyloid Beta A4 Precursor Protein) [155]: is a protein with an over-production in these patients that leads to an increase in beta-amyloid (Aβ) BACH1 (BTB domain and CNC homolog 1) [169]: inhibits genes involved in cell stress response. In DS is upregulated determining the production of ROS. S100β (S100 calcium-binding protein) [171]: is a Ca2+ binding protein produced from astrocytes modulating the activity of endothelial cells microglia, and neurons. It is overexpressed in DS. Ets-2 (ETS Protooncogene 2, Transcription Factor) [172]: is a transcription factor involved in bone growth, immune response, and cancer. It is overexpressed in DS with increased neuronal apoptosis. | Accelerated aging, neurological alterations Early onset of Alzheimer’s disease Accelerated aging, neuronal alterations, intellectual disability Accelerated amyloid deposition, early onset of Alzheimer’s disease Alzheimer’s disease, neurological alterations and disability |
| Fanconi’s anemia (FA): | Mutations in genes involved in DNA repair and genomic stability, 15 genes have been identified | Aplasia of the radius, Skin hyperpigmentation, Microphthalmia Nystagmus, Reduced vision, Cardiac, renal and urogenital defects short, Stature deafness, Hypogonadism | SOD-1 (Cu/Zn superoxide dismutase 1) [162]: is a protein that acts as an antioxidant defense and transforms the superoxide radicals into H2O2, it is reduced in patients with FA TNF-α (Tumor necrosis factor alpha) [219]: is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the acute phase reaction. It is increased in patients with FA and this would lead to an increase in the production of O2•− PRDX3 (Peroxiredoxin 3) [220]: is an enzyme localized in mitochondria with antioxidant function, it is reduced in patients with FA | Accelerated cell deathApoptosis and disruption of DNA, promote carcinogenesisOverproduction of ROS determining DNA damage |
| Fetal alcohol spectrum disorders (FASD) | Related to alcohol exposure of fetus during pregnancy | Facial anomalies, Growth deficiency, Neurobehavioral impairment, Deficient brain growth, Non-febrile seizures | NOX2 and NOX4 (NADPH-dependent enzymes) [118,143]: are a family of proteins that produce ROS they are expressed in microglia, neurons, astrocytes, and in brain vessels. They are overexpressed in FASD. CYP2E1 (Cytochrome P450 isoform 2E1) [104]: is an enzyme involved in the metabolism of ethanol, it is reduced in the fetus and this determines the overproduction of ROS | Neurological anomalies Neurological anomalies |
| Gaucher disease | Mutations in GBA1 | Anemia, Fatigue, Neurological deterioration, Organomegaly | ROS [262]: accumulation of toxic glycosphingolipids cells leads to the production of reactive oxygen species | Asthenia Fatigue |
| Marfan syndrome | Mutations in the gene for fibrillin-1 (FBN1) | Skeletal abnormalities (dolichostenomelia, arachnodactyly, scoliosis, chest wall deformity, tall stature, ligamentous laxity, abnormal joint mobility, and protrusio acetabulae, scoliosis), Ectopia lentis Mitral valve disease, Dilatation of the aortic root, Aortic dissection | H202 [32]: H2O2 directly produced by NOX4 and/or by the transmutation from O2•− by superoxide dismutase (SODs) Endothelial dysfunction [188]: it increases the inducible nitric oxide synthase (iNOS) pathway leading to an excess in nitric oxide (NO) | Aortic aneurysm Aortic aneurysm |
| Reticular dysgenesis | Viral infections such as pneumonia, Mycotic infections such as candidiasis, Chronic diarrhea | AK2 (Adenylate kinase 2) [240]: it is a mitochondrial intermembrane space enzyme that regulates energy metabolism, it is reduced in patients with RD leading to an overproduction of ROS. | Maturation impeding of myeloid cells at the promyeloid stage Accelerated apoptosis | |
| Williams–Beuren syndrome | Microdeletion 7q11.23 | Facial dysmorphism, Aortic stenosis, Neurodevelopmental delays, Accelerated aging, Cocktail party personality | ELN (Elastin) [119]: encodes for elastin an important protein detectable in the vascular wall, which provides recoil to elastic vessels. It is present also in the elastic fibers of the lungs. DNAJC30 (DnaJ Heat Shock Protein Family Hsp40 Member C30) [125]: encodes for a mitochondrial protein involved in the removal of damaged complex I subunits. His loss determines an alteration in ATP synthase resulting in increased cell apoptosis | Cardiovascular alterations, pulmonary emphysema and accelerated aging Chronic lung disease, alterations in neocortical pyramidal neurons and altered behaviors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micangeli, G.; Menghi, M.; Profeta, G.; Tarani, F.; Mariani, A.; Petrella, C.; Barbato, C.; Ferraguti, G.; Ceccanti, M.; Tarani, L.; et al. The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants 2022, 11, 1983. https://doi.org/10.3390/antiox11101983
Micangeli G, Menghi M, Profeta G, Tarani F, Mariani A, Petrella C, Barbato C, Ferraguti G, Ceccanti M, Tarani L, et al. The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants. 2022; 11(10):1983. https://doi.org/10.3390/antiox11101983
Chicago/Turabian StyleMicangeli, Ginevra, Michela Menghi, Giovanni Profeta, Francesca Tarani, Alessandro Mariani, Carla Petrella, Christian Barbato, Giampiero Ferraguti, Mauro Ceccanti, Luigi Tarani, and et al. 2022. "The Impact of Oxidative Stress on Pediatrics Syndromes" Antioxidants 11, no. 10: 1983. https://doi.org/10.3390/antiox11101983
APA StyleMicangeli, G., Menghi, M., Profeta, G., Tarani, F., Mariani, A., Petrella, C., Barbato, C., Ferraguti, G., Ceccanti, M., Tarani, L., & Fiore, M. (2022). The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants, 11(10), 1983. https://doi.org/10.3390/antiox11101983









