Quercetin Nanoemulsion Ameliorates Neuronal Dysfunction in Experimental Alzheimer’s Disease Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design
2.2.2. Preparation of Quercetin Nanoemulsion
2.2.3. Induction of Alzheimer’s Disease
2.2.4. Tissue Homogenate
2.2.5. Measurement of Brain Oxidative Stress and Inflammatory Markers
2.2.6. HPLC Method for Neurotransmitters Estimation
2.2.7. Immunohistochemistry of Brain Cyclooxygenases
2.2.8. HPLC Condition
2.2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Z.S.; Spartano, N.; Beiser, A.; DeCarli, C.; Auerbach, S.H.; Vasan, R.S.; Seshadri, S. Physical Activity, Brain Volume, and Dementia Risk: The Framingham Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohandas, E.; Rajmohan, V.; Raghunath, B. Neurobiology of Alzheimer’s disease. Indian J. Psychiatr. 2009, 51, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Muirhead, K.E.; Borger, E.; Aitken, L.; Conway, S.J.; Gunn-Moore, F.J. The consequences of mitochondrial amyloid beta-peptide in Alzheimer’s disease. Biochem. J. 2010, 24, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Prema, A.; Thenmozhi, A.J.; Manivasagam, T.; Essa, M.M.; Akbar, M.D.; Akbar, M. Fenugreek Seed Powder Nullified Aluminium Chloride Induced Memory Loss, Biochemical Changes, Aβ Burden and Apoptosis via Regulating Akt/GSK3β Signaling Pathway. PLoS ONE 2016, 11, e01659552016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, T.; Falk, S.; Rohrbeck, A.; Georgii, S.; Herzog, C.; Wiegand, A.; Hotz, S.; Boschek, B.; Zorn, H.; Brunn, H. Migration of aluminum from food contact materials to food—A health risk for consumers? Part I of III: Exposure to aluminum, release of aluminum, tolerable weekly intake (TWI), toxicological effects of aluminum, study design, and methods. Environ. Sci. Eur. 2017, 29, 19. [Google Scholar] [CrossRef]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [Green Version]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of quercetin and quercetin glycosides from onion (Allium cepa L.) solid waste as an antioxidant, urease and xanthine oxidase inhibitors. Food Chem. 2017, 235, 119–126. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Read, M.A. Flavonoids: Naturally occurring anti-inflammatory agents. Am. J. Pathol. 1995, 147, 235–237. [Google Scholar]
- Rysz, J.; Franczyk, B.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Ageing, Age-Related Cardiovascular Risk and the Beneficial Role of Natural Components Intake. Int. J. Mol. Sci. 2021, 23, 183. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.M.; Zhao, B.; Luo, T.; Kaiser, C.; Cavender, G.; Hamilton-Reeves, J.; Sullivan, D.K.; Shay, N.F. Consumption of Quercetin and Quercetin-Containing Apple and Cherry Extracts Affects Blood Glucose Concentration, Hepatic Metabolism, and Gene Expression Patterns in Obese C57BL/6J High Fat-Fed Mice. J. Nutr. 2016, 146, 1001–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (CD44+/CD24-) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Verma, P.R.P.; Singh, S.K. Screening and preparation of quercetin doped nanoemulsion: Characterizations, antioxidant and anti-bacterial activities. LWT 2020, 124, 109141. [Google Scholar] [CrossRef]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot Essent Fat Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Liu, X.; Liu, M.; Chi, H.; Liu, J.; Han, H. Protective effects of quercetin and taraxasterol against H2O2-induced human umbilical vein endothelial cell injury in vitro. Exp. Ther. Med. 2015, 10, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Nisar, M.F.; Yousaf, M.; Saleem, M.; Khalid, H.; Niaz, K.; Yaqub, M.; Waqas, M.Y.; Ahmed, A.; Abaid-Ullah, M.; Chen, J.; et al. Development of Iron Sequester Antioxidant Quercetin@ZnO Nanoparticles with Photoprotective Effects on UVA-Irradiated HaCaT Cells. Oxid. Med. Cell Longev. 2021, 2021, 6072631. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Ma, Y.; Yu, A.; Cai, F.; Shao, W.; Zhai, G. Formulation optimization and in situ absorption in rat intestinal tract of quercetin-loaded microemulsion. Colloids Surf B Biointerfaces 2009, 71, 306–314. [Google Scholar] [CrossRef]
- Azuma, K.; Ippoushi, K.; Ito, H.; Higashio, H.; Terao, J. Combination of lipids and emulsifiers enhances the absorption of orally administered quercetin in rats. J. Agric. Food Chem. 2002, 50, 1706–1712. [Google Scholar] [CrossRef]
- Hussein, J.; El-Bana, M.; Refaat, E.; El-Naggar, M.E. Synthesis of carvacrol-based nanoemulsion for treating neurodegenerative disorders in experimental diabetes. J. Funct. Foods 2017, 37, 441–448. [Google Scholar] [CrossRef]
- Quintão, F.J.; Tavares, R.S.; Vieira-Filho, S.A.; Souza, G.H.B.; Santos, O. Hydroalcoholic extracts of Vellozia squamata: Study of its nanoemulsions for pharmaceutical or cosmetic applications. Rev. Bras. Farmacogn. 2013, 23, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.S.; Verma, P.R.P.; Singh, S.K. Quercetin-Loaded Nanomedicine as Nutritional Application. In Nanomedicine for Bioactives; Rahman, M., Beg, S., Kumar, V., Ahmad, F.J., Eds.; Springer: Singapore, 2020; pp. 259–301. [Google Scholar]
- Al-Hariri, M.; Alsunni, A.; Shaikh, M.H.; Eldin, T.G.; Ghamdi, K.A.; Alharbi, A.F.; Alhawaj, H.; Chathoth, S. Caffeic Acid Phenethyl Ester reduces Pro Inflammatory Cytokines in Moderate Swimming Test in Growing Rats Model. J. Inflamm. Res. 2021, 14, 5653–5657. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoaty, M.A.; Ibrahim, M.A.; Ahmed, N.S.; Abdelaziz, M.A. Confirmatory studies on the antioxidant and antidiabetic effect of quercetin in rats. Indian J. Clin. Biochem. 2010, 25, 188–192. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Choi, J.-S. Effects of quercetin on the pharmacokinetics of Etoposide after oral or intravenous administration of etoposide in rats. Anticancer Res. 2009, 29, 1411–1415. [Google Scholar]
- Arbain, N.H.; Salim, N.; Wui, W.T.; Basri, M.; Rahman, M.B.A. Optimization of Quercetin loaded Palm Oil Ester Based Nanoemulsion Formulation for Pulmonary Delivery. J. Oleo Sci. 2018, 67, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, M.; Ahmed, M.; Surapaneni, K.M.; Veeraraghavan, V.P.; Arulselvan, P. Neuroprotective effects of ononin against the aluminium chloride-induced Alzheimer’s disease in rats. Saudi J. Biol. Sci. 2021, 28, 4232–4239. [Google Scholar] [CrossRef]
- Al-Hariri, M.; Eldin, T.G.; Al-Harbi, M.; Hashim, T.; Ahmad, R. Effect of Propolis Administration on the Endocrine Functions and Histopathology of Pancreas in Streptozotocin-Induced Diabetic Rats. Adv. Sci. Eng. Med. 2019, 11, 1155–1160. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for improved antioxidant and antidepressant-like activity. Drug Deliv. 2012, 19, 378–391. [Google Scholar] [CrossRef]
- Mohamed, N.E.-S.; Abd El-Moneim, A.E. Ginkgo biloba extract alleviates oxidative stress and some neurotransmitters changes induced by aluminum chloride in rats. Nutrition 2017, 35, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hoozemans, J.J.M.; Rozemuller, J.M.; van Haastert, E.S.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase-1 and -2 in the different stages of Alzheimer’s disease pathology. Curr. Pharm. Des. 2008, 14, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Medhat, D.; El-Mezayen, H.A.; El-Naggar, M.E.; Farrag, A.R.; Abdelgawad, M.E.; Hussein, J.; Kamal, M.H. Evaluation of urinary 8-hydroxy-2-deoxyguanosine level in experimental Alzheimer’s disease: Impact of carvacrol nanoparticles. Mol. Biol. Rep. 2019, 46, 4517–4527. [Google Scholar] [CrossRef] [PubMed]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.A.; El-Tahawy, G. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in alzheimer’s disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, e3702518. [Google Scholar] [CrossRef]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative Role of Red Wine Polyphenols against Brain Pathology in Alzheimer’s and Parkinson’s Disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savale, S. Formulation and Evaluation of Quercetin Nanoemulsions for Treatment of Brain Tumor via Intranasal pathway. Asian J. Biomater. Res. 2017, 3, 28–32. [Google Scholar]
- Zhang, H.; Yu, D.; Sun, J.; Liu, X.; Jiang, L.; Guo, H.; Ren, F. Interaction of plant phenols with food macronutrients: Characterisation and nutritional-physiological consequences. Nutr. Res. Rev. 2014, 27, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Li, Z.; Percival, S.S.; Bonard, S.; Gu, L. Fabrication of nanoparticles using partially purified pomegranate ellagitannins and gelatin and their apoptotic effects. Mol. Nutr. Food Res. 2011, 55, 1096–1103. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, Z.A.; Langer, S.R.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Podolak, I. A Flavonoid on the Brain: Quercetin as a Potential Therapeutic Agent in Central Nervous System Disorders. Life 2022, 12, 591. [Google Scholar] [CrossRef] [PubMed]
- Gella, A.; Durany, N. Oxidative stress in Alzheimer disease. Cell Adh. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Yildirim, C.; Saglam, A.S.Y.; Guney, S.; Turan, B.; Ebegil, M.; Cevher, S.C.; Balabanli, B. Investigation Covering the Effect of Boron plus Taurine Application on Protein Carbonyl and Advanced Oxidation Protein Products Levels in Experimental Alzheimer Model. Biol. Trace. Elem. Res. 2022. [CrossRef]
- Kotilinek, L.A.; Westerman, M.A.; Wang, Q.; Panizzon, K.; Lim, G.P.; Simonyi, A.; Lesne, S.; Falinska, A.; Younkin, L.H.; Younkin, S.G.; et al. Cyclooxygenase-2 inhibition improves amyloid-β-mediated suppression of memory and synaptic plasticity. Brain 2008, 131, 651–664. [Google Scholar] [CrossRef]
- Qin, W.; Ho, L.; Pompl, P.N.; Peng, Y.; Zhao, Z.; Xiang, Z.; Robakis, N.K.; Shioi, J.; Suh, J.; Pasinetti, G.M. Cyclooxygenase (COX)-2 and COX-1 potentiate beta-amyloid peptide generation through mechanisms that involve gamma-secretase activity. J. Biol. Chem. 2003, 278, 50970–50977. [Google Scholar] [CrossRef] [Green Version]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Tan, X.; Reis, J.C.; Badr, M.Z.; Papasian, C.J.; Morrison, D.C.; Qureshi, N. Inhibition of nitric oxide in LPS-stimulated macrophages of young and senescent mice by δ-tocotrienol and quercetin. Lipids Health Dis. 2011, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, T.; Ogasawara, Y.; Ishii, K.; Takahashi, H.; Tanabe, S. Accumulation of aluminum in ferritin isolated from rat brain. Neurosci. Lett. 2004, 366, 264–267. [Google Scholar] [CrossRef]
- Pavun, L.; Dimitrić-Marković, J.; Đurđević, P.; Jelikić-Stankov, M.; Đikanović, D.; Ćirić, A.; Malešev, D. Development and validation of spectrofluorimetric and LC–MS/MS methods for the determination of hesperidin in human plasma and pharmaceutical forms. J. Serb. Chem. Soc. 2012, 77, 1625–1640. [Google Scholar] [CrossRef]
- Iriti, M.; Vitalini, S.; Fico, G.; Faoro, F. Neuroprotective herbs and foods from different traditional medicines and diets. Molecules 2010, 15, 3517–3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, W.A.; Jeyabalan, J.; Kichambre, S.; Gupta, R.C. Oxidatively generated DNA damage after Cu(II) catalysis of dopamine and related catecholamine neurotransmitters and neurotoxins: Role of reactive oxygen species. Free Radic. Biol. Med. 2011, 50, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Szutowicz, A. Aluminum, NO, and nerve growth factor neurotoxicity in cholinergic neurons. J. Neurosci. Res. 2001, 66, 1009–1018. [Google Scholar] [CrossRef]
- Jyoti, A.; Sethi, P.; Sharma, D. Bacopa monniera prevents from aluminium neurotoxicity in the cerebral cortex of rat brain. J. Ethnopharmacol. 2007, 111, 56–62. [Google Scholar] [CrossRef]
- Elreedy, H.; Elfiky, A.; Mahmoud, A.; Ebrahim, K.S.; Ghazy, M. Effect of Quercetin as Therapeutic And Protective Agent In Aluminum Chloride-Induced Alzheimer’s Disease Rats. Egypt. J. Chem. 2022, 65, 633–641. [Google Scholar] [CrossRef]
- Anwar, H.M.; Georgy, G.S.; Hamad, S.R.; Badr, W.K.; El Raey, M.A.; Abdelfattah, M.O.; Wink, M.; Sobeh, M.A. Leaf Extract of Harrisonia abyssinica Ameliorates Neurobehavioral, Histological and Biochemical Changes in the Hippocampus of Rats with Aluminum Chloride-Induced Alzheimer’s Disease. Antioxidants 2021, 11, 947. [Google Scholar] [CrossRef]
- Regitz, C.; Dußling, L.M.; Wenzel, U. Amyloid-beta (Aβ₁₋₄₂)-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol. Nutr. Food Res. 2014, 58, 1931–1940. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Jan, O.; Aneta, W. Antioxidant activity of the phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Moriwaki, M.; Timm, D.; Yamagata, H.; Maruyama, G.; Nisihara, Y.; Nakazawa, T.; Takata, S.; Nakamura, D. 13-Weeks subchronic toxicity of isoquercitrin-γ-cyclodextrin (IQC-γCD) molecular inclusion complex in Sprague-Dawley rats. Food Chem. Toxicol. 2021, 152, 112217. [Google Scholar] [CrossRef]
- Welton, A.F.; Tobias, L.D.; Fiedler-Nagy, C. Effect of flavonoids on arachidonic acid metabolism. Prog. Clin. Biol. Res. 1986, 213, 231–242. [Google Scholar] [PubMed]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F.; Rivera-Megret, F.; Blasina, F.; Arredondo, F.; Abin-Carriquiry, J.; Costa, G.; Echeverry, C.; Lafon, L.; Heizen, H.; Ferreira, M.; et al. Neuroprotection by flavonoids. Braz. J. Med. Res. 2003, 36, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Dajas, F.; Rivera, F.; Blasina, F.; Arredondo, F.; Echeverry, C.; Lafon, L.; Morquio, A.; Heinzen, H. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox. Res. 2003, 5, 377–384. [Google Scholar] [CrossRef]
- Dajas, F.; Costa, G.; Abin-Carriquiry, J.A.; Echeverry, C.; Martínez-Borges, A.; Dajas-Bailador, F. Antioxidant and cholinergic neuroprotective mechanisms in experimental parkinsonism. Funct. Neurol. 2001, 17, 37–44. [Google Scholar]


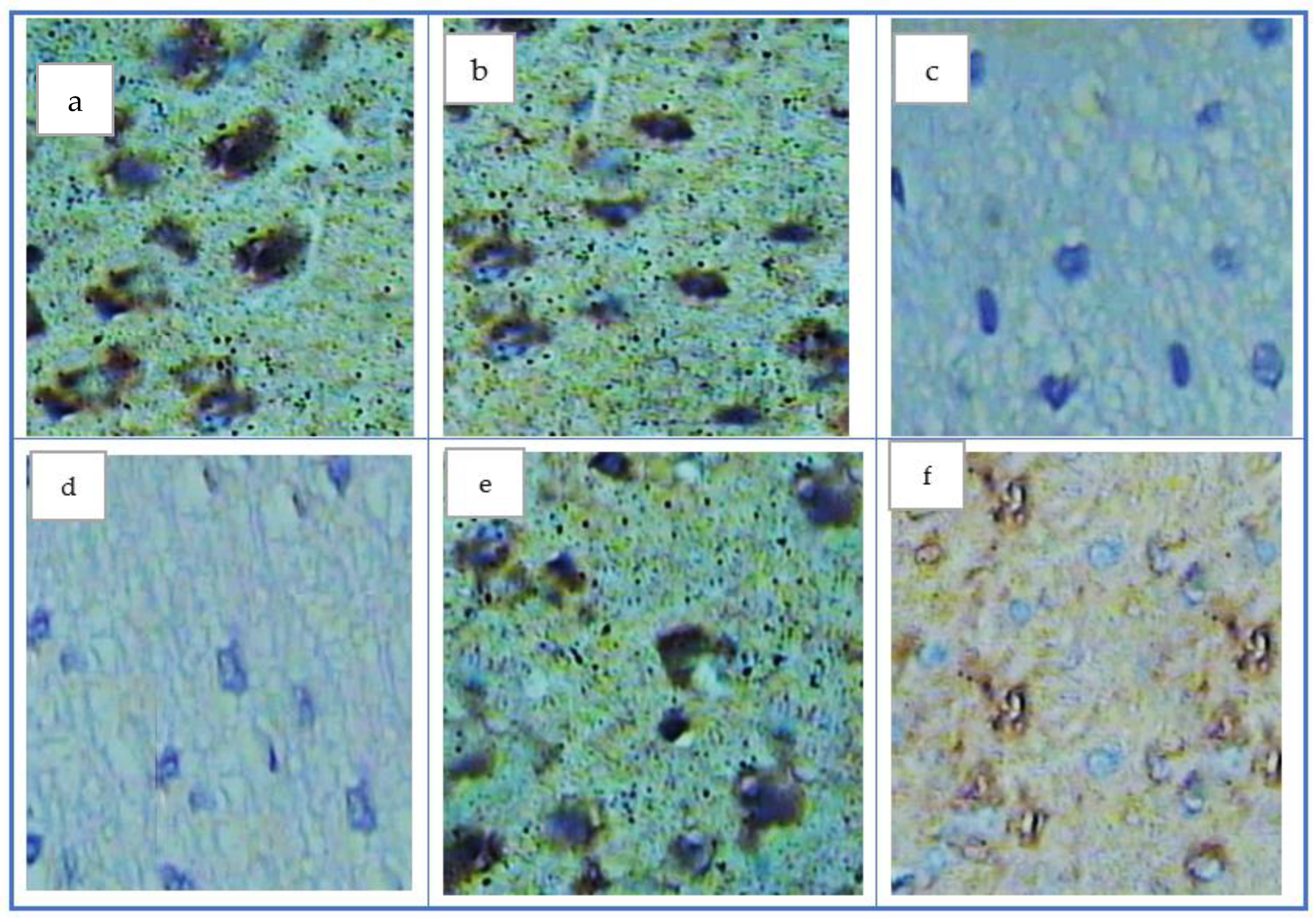
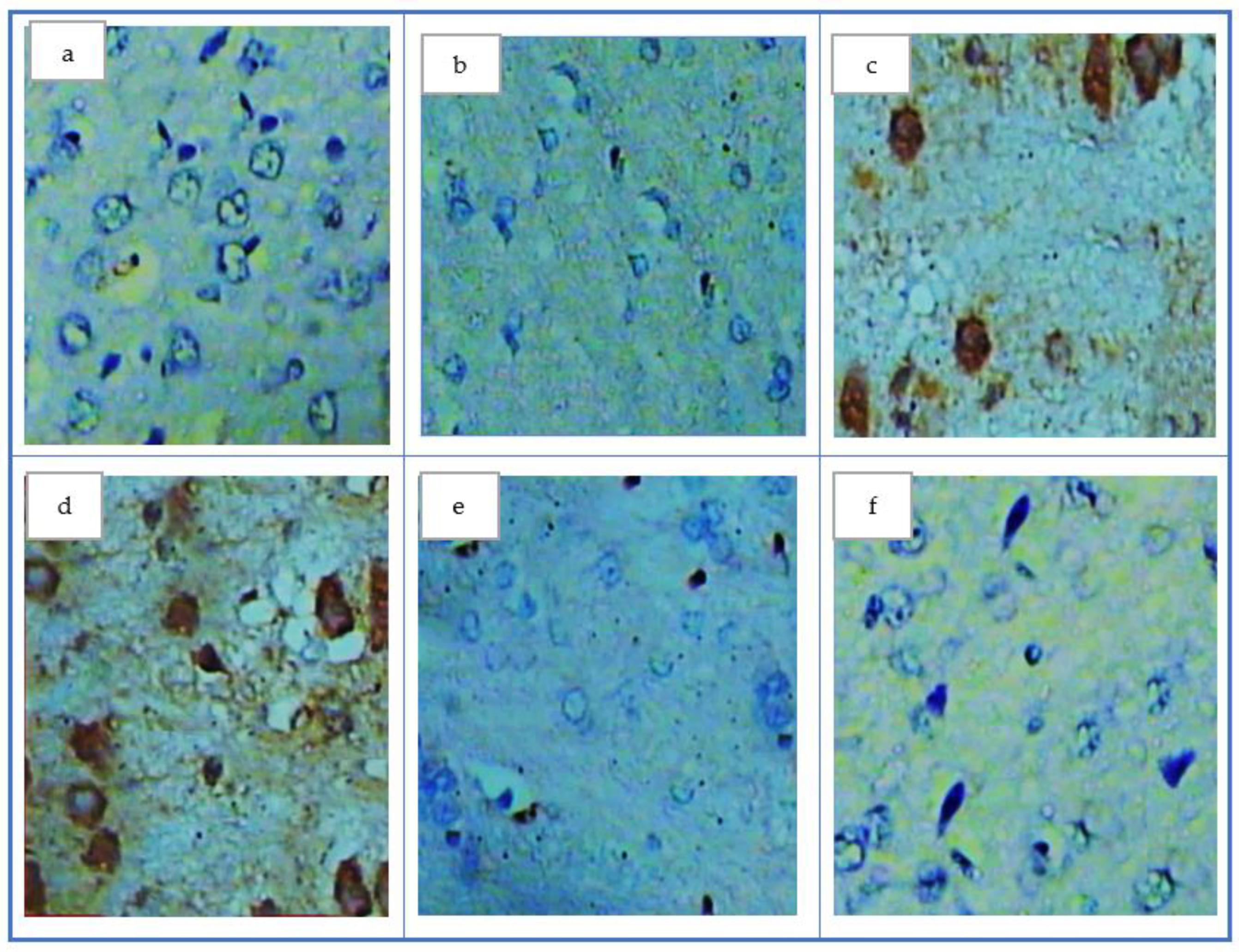
| Groups | SOD (U/g Tissue) | GSH (m mol/g Tissue) | MDA (nmol/g Tissue) | AOPP (ng/g Tissue) |
|---|---|---|---|---|
| Control group | 320.23 ± 1.21 | 44.38 ± 0.67 | 11.34 ± 0.22 | 3.62 ± 0.65 |
| Quercetin group | 314.65 ± 0.98 | 45.32 ± 0.73 | 10.31 ± 0.16 | 3.42 ± 0.48 |
| AD group | 211.24 ± 0.93 a | 22.01 ± 0.59 a | 42.09 ± 0.74 a | 21.71 ± 0.59 a |
| Treated group I | 214.84 ± 1.10 a | 23.20 ± 0.61 a | 43.02 ± 0.53 a | 3.51 ± 0.65 a |
| Treated group II | 275.03 ± 0.85 a,b | 37.34 ± 0.68 a,b | 31.21 ± 0.22 a,b | 14.29 ± 044 a,b |
| Treated group III | 313.36 ± 0.76 b,c | 42.87 ± 0.80 b,c | 22.82 ± 0.41 a,b,c | 7.23 ± 0.67 a,b,c |
| Groups | IL-1β (Pg/mL) | TNF-α (Pg/mL) | Adiponectin μg/g Tissue |
|---|---|---|---|
| Control group | 24.04 ± 0.99 | 26.83 ± 0.93 | 2.72 ± 0.23 |
| Quercetin group | 22.37 ± 1.34 | 25.71 ± 0.98 | 2.41 ± 0.25 |
| AD group | 57.32 ± 1.02 a | 56.28 ± 1.02 a | 8.72 ± 0.13 a |
| Treated group I | 56.63 ± 0.91 a | 57.11 ± 1.11 a | 8.43 ± 0.18 a |
| Treated group II | 43.22 ± 1.21 a,b | 40.12 ± 0.96 a,b | 6.50 ± 0.14 a,b |
| Treated group III | 31.01 ± 0.78 a,b,c | 28.02 ± 0.82 b,c | 3.12 ± 0.09 b,c |
| Groups | Dopamine | Norepinephrine | Serotonin |
|---|---|---|---|
| Control group | 4.12 ± 0.34 | 3.85 ± 0.23 | 3.11 ± 0.27 |
| Quercetin group | 4.03 ± 0.48 | 3.92 ± 0.26 | 3.23 ± 0.31 |
| AD group | 6.92 ± 0.37 a | 6.31 ± 0.28 a | 5.76 ± 0.25 a |
| Treated group I | 6.73 ±0.72 a | 6.33 ± 0.40 | 5.83 ± 0.32 |
| Treated group II | 5.93 ±0.60 a,b | 5.29 ± 0.24 a,b | 4.50 ± 0.30 a,b |
| Treated group III | 4.37 ± 0.36 b,c | 4.012 ± 0.41 b,c | 3.31 ± 0.46 b,c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaqeel, N.K.; AlSheikh, M.H.; Al-Hariri, M.T. Quercetin Nanoemulsion Ameliorates Neuronal Dysfunction in Experimental Alzheimer’s Disease Model. Antioxidants 2022, 11, 1986. https://doi.org/10.3390/antiox11101986
Alaqeel NK, AlSheikh MH, Al-Hariri MT. Quercetin Nanoemulsion Ameliorates Neuronal Dysfunction in Experimental Alzheimer’s Disease Model. Antioxidants. 2022; 11(10):1986. https://doi.org/10.3390/antiox11101986
Chicago/Turabian StyleAlaqeel, Nouf K., Mona H. AlSheikh, and Mohammed T. Al-Hariri. 2022. "Quercetin Nanoemulsion Ameliorates Neuronal Dysfunction in Experimental Alzheimer’s Disease Model" Antioxidants 11, no. 10: 1986. https://doi.org/10.3390/antiox11101986






