Vitamin E Lipid-Based Nanodevices as a Tool for Ovine Sperm Protection against Oxidative Stress: Impact on Sperm Motility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Media
2.2. Vitamin E Nanoemulsion Formulation
2.3. Animals
2.4. Semen Collection and Initial Evaluation
2.5. Experimental Design
2.6. Sperm Motility Analysis
2.7. Flow Cytometry Analysis
2.8. Fluorescence Probes
2.8.1. Sperm Viability and Apoptosis-like Changes
2.8.2. Assessment of Acrosome Integrity
2.8.3. Assessment of Mitochondrial Activity
2.8.4. Assessment of Lipid Peroxidation
2.8.5. Production of Reactive Oxygen Species
2.8.6. Sperm Chromatin Structure Assay
2.9. Confocal Laser Scanning Microscope Analysis
2.10. Statistical Analysis
3. Results
3.1. Vitamin E Nanoemulsions: Formulation, Characterization and Confocal Microscopy
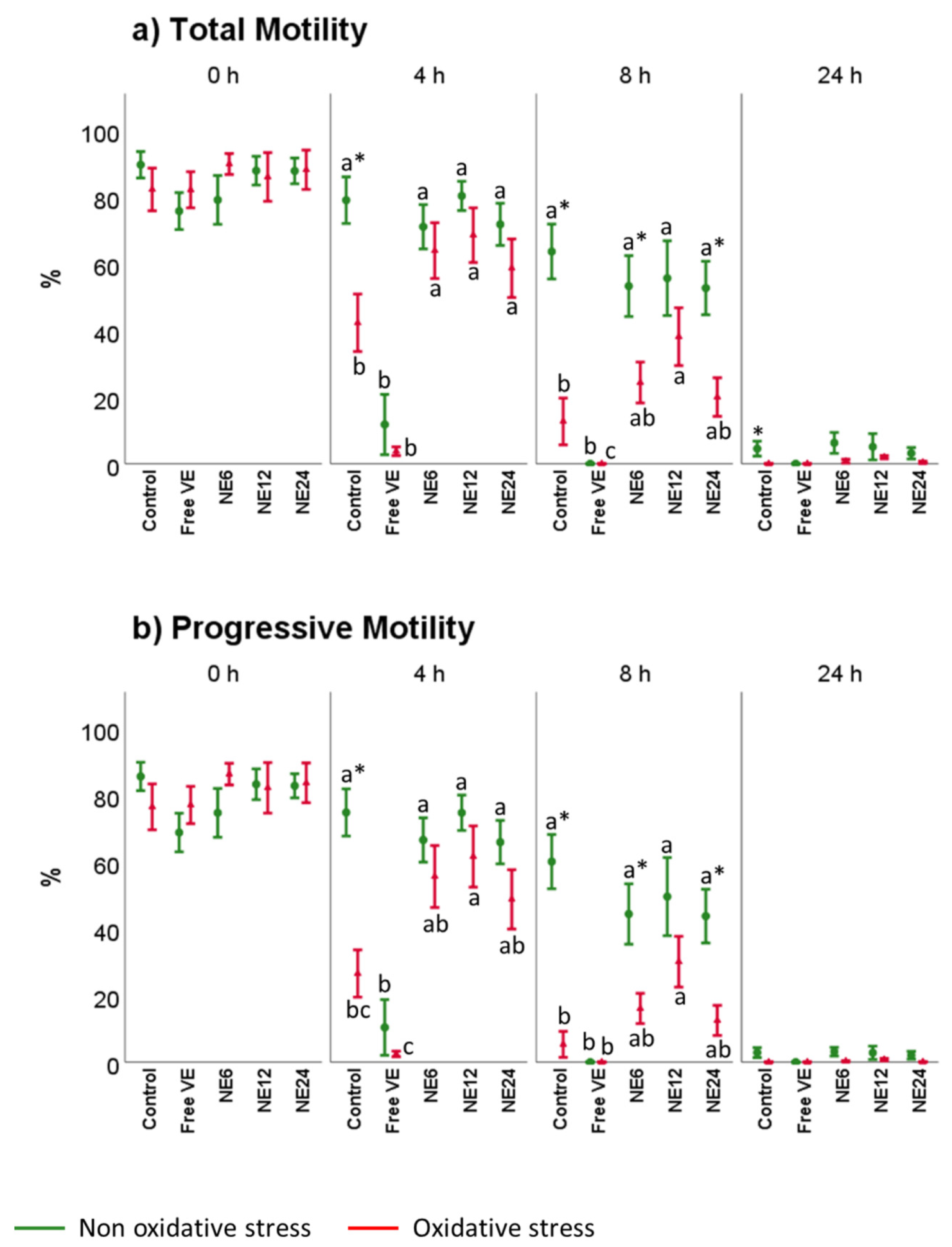
3.2. Vitamin E Nanoemulsions Effects on Sperm Motility Assessed by CASA
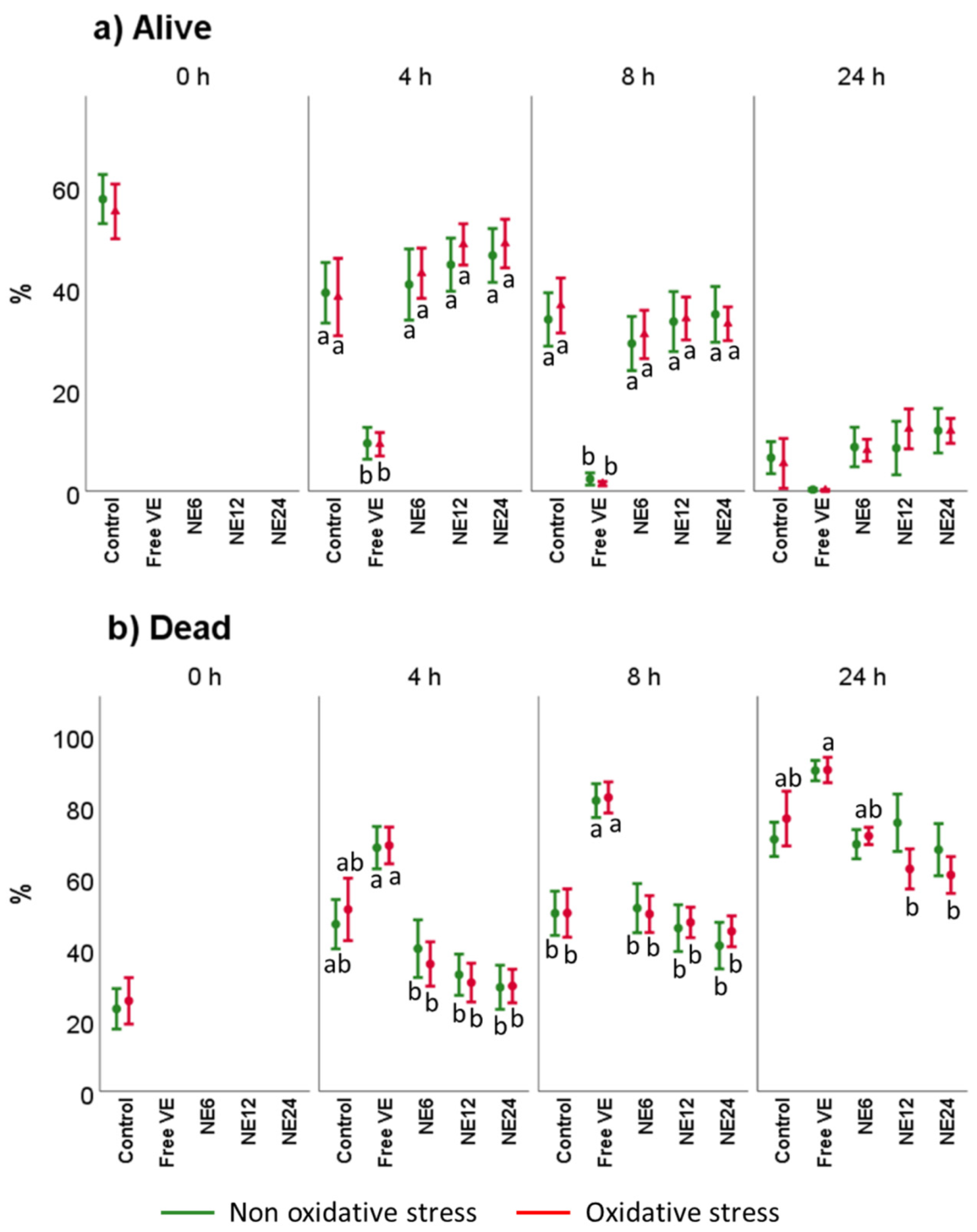
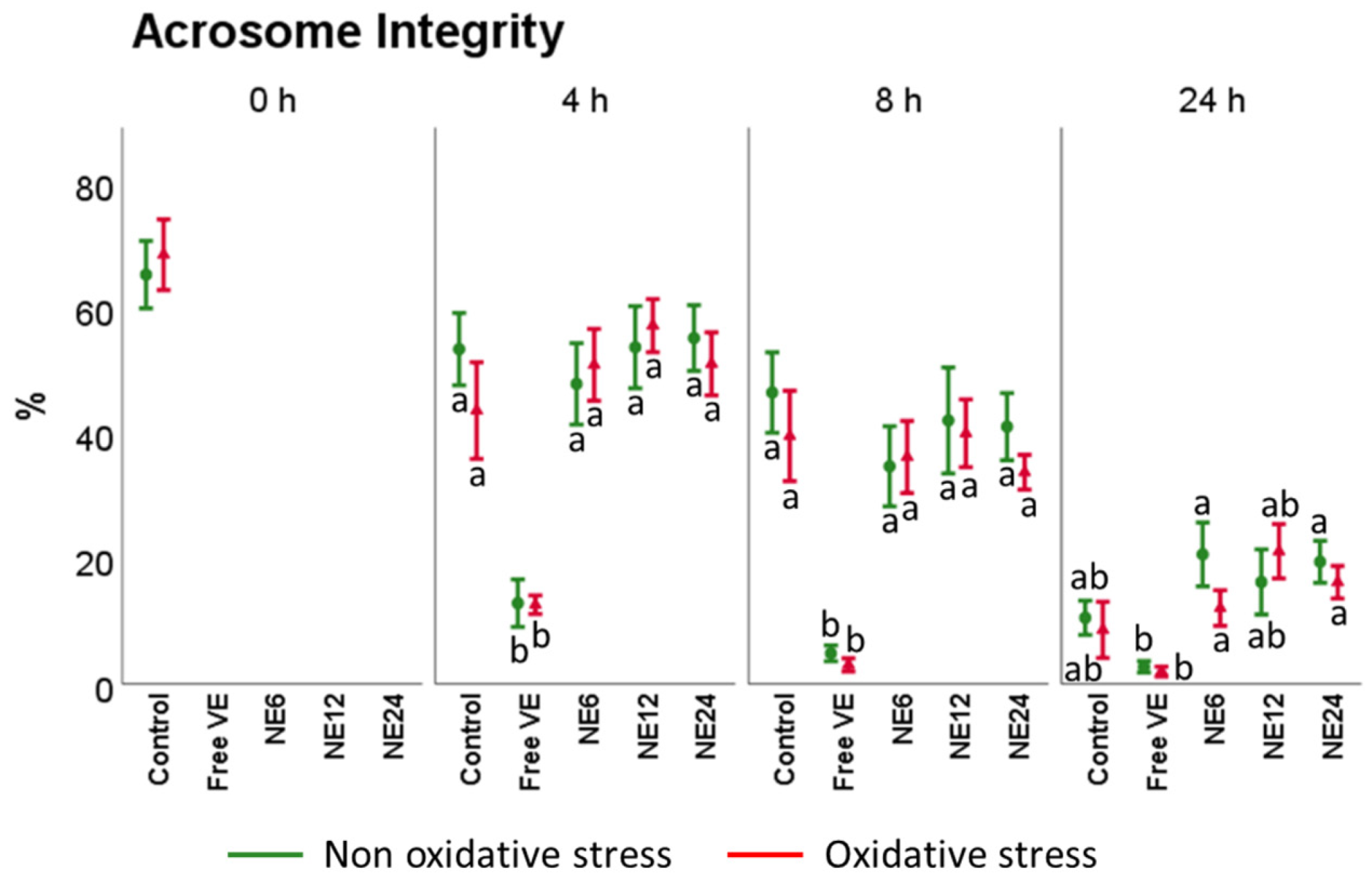
3.3. Vitamin E Nanoemulsions Effects on Sperm Viability and Acrosome Integrity
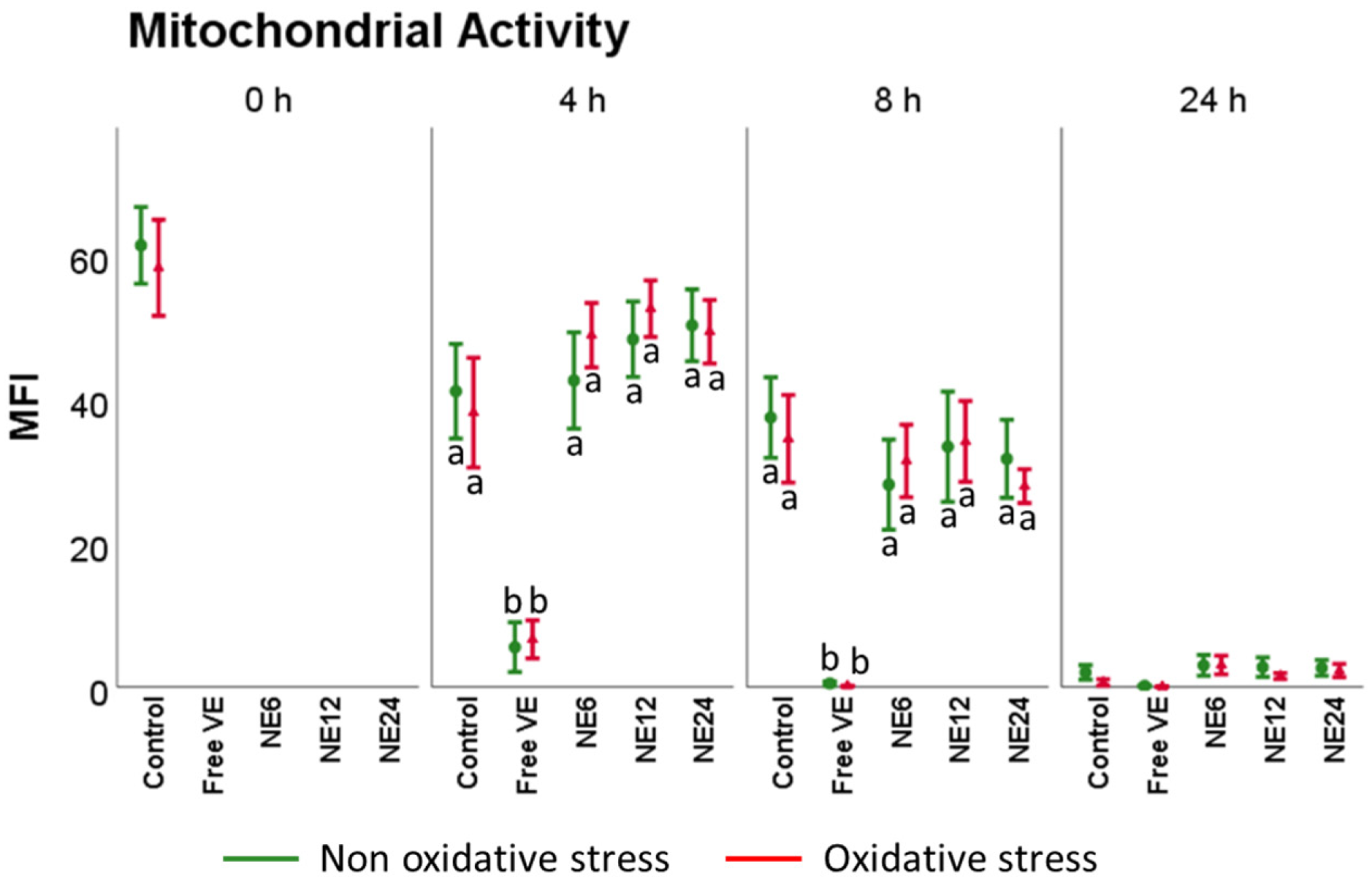
3.4. Vitamin E Nanoemulsions Effects on Mitochondrial Activity
3.5. Vitamin E Nanoemulsions Effects on Intracellular ROS Production and Lipid Peroxidation
3.6. Vitamin E Nanoemulsions Effects on DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Esposito, M.; Tosti, E.; Boni, R. Sperm Motility, Oxidative Status, and Mitochondrial Activity: Exploring Correlation in Different Species. Antioxidants 2021, 10, 1131. [Google Scholar] [CrossRef] [PubMed]
- St John, J.C. The transmission of mitochondrial DNA following assisted reproductive techniques. Theriogenology 2002, 57, 109–123. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell. Physiol. 2008, 217, 745–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [Green Version]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function--in sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef]
- Soria-Meneses, P.J.; Jurado-Campos, A.; Montoro, V.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.D.R. Ovine sperm DNA oxidation quantification using an 8-OHdG immunodetection assay. Reprod. Domest. Anim. 2019, 54 (Suppl. S4), 59–64. [Google Scholar] [CrossRef] [Green Version]
- Del Olmo, E.; Bisbal, A.; García-Álvarez, O.; Maroto-Morales, A.; Ramón, M.; Jiménez-Rabadán, P.; Anel-López, L.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.R. Free-radical production after post-thaw incubation of ram spermatozoa is related to decreased in vivo fertility. Reprod. Fertil. Dev. 2015, 27, 1187–1196. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hallap, T.; Nagy, S.; Jaakma, U.; Johannisson, A.; Rodriguez-Martinez, H. Mitochondrial activity of frozen-thawed spermatozoa assessed by MitoTracker Deep Red 633. Theriogenology 2005, 63, 2311–2322. [Google Scholar] [CrossRef]
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int. J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anel-López, L.; García-Álvarez, O.; Maroto-Morales, A.; Iniesta-Cuerda, M.; Ramón, M.; Soler, A.J.; Fernández-Santos, M.R.; Garde, J.J. Reduced glutathione addition improves both the kinematics and physiological quality of post-thawed red deer sperm. Anim. Reprod. Sci. 2015, 162, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anel-López, L.; García-Álvarez, O.; Parrilla, I.; Del Olmo, D.; Fernández-Santos, M.R.; Soler, A.J.; Maroto-Morales, A.; Ortiz, J.A.; Alkmin, D.V.; Tarantini, T.; et al. The Effect of Oxidative Stress on Thawed Bulk-Sorted Red Deer Sperm. Reprod. Domest. Anim. 2016, 51, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Anel-López, L.; Martínez-Rodríguez, C.; Soler, A.J.; Fernández-Santos, M.R.; Garde, J.J.; Morrell, J.M. Use of Androcoll-S after thawing improves the quality of electroejaculated and epididymal sperm samples from red deer. Anim. Reprod. Sci. 2015, 158, 68–74. [Google Scholar] [CrossRef]
- Domínguez-Rebolledo, A.E.; Fernández-Santos, M.R.; Bisbal, A.; Ros-Santaella, J.L.; Ramón, M.; Carmona, M.; Martínez-Pastor, F.; Garde, J.J. Improving the effect of incubation and oxidative stress on thawed spermatozoa from red deer by using different antioxidant treatments. Reprod. Fertil. Dev. 2010, 22, 856–870. [Google Scholar] [CrossRef]
- Domínguez-Rebolledo, A.E.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Garde, J.J.; Martínez-Pastor, F. Washing increases the susceptibility to exogenous oxidative stress in red deer spermatozoa. Theriogenology 2009, 72, 1073–1084. [Google Scholar] [CrossRef]
- Domínguez-Rebolledo, A.E.; Martínez-Pastor, F.; Bisbal, A.F.; Ros-Santaella, J.L.; García-Álvarez, O.; Maroto-Morales, A.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.R. Response of thawed epididymal red deer spermatozoa to increasing concentrations of hydrogen peroxide, and importance of individual male variability. Reprod. Domest. Anim. 2011, 46, 393–403. [Google Scholar] [CrossRef]
- Fernández-Santos, M.R.; Domínguez-Rebolledo, A.E.; Esteso, M.C.; Garde, J.J.; Martínez-Pastor, F. Refrigerated storage of red deer epididymal spermatozoa in the epididymis, diluted and with vitamin C supplementation. Reprod. Domest. Anim. 2009, 44, 212–220. [Google Scholar] [CrossRef]
- Fernández-Santos, M.R.; Martínez-Pastor, F.; García-Macías, V.; Esteso, M.C.; Soler, A.J.; Paz, P.; Anel, L.; Garde, J.J. Sperm characteristics and DNA integrity of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa frozen in the presence of enzymatic and nonenzymatic antioxidants. J. Androl. 2007, 28, 294–305. [Google Scholar] [CrossRef]
- Sánchez-Rubio, F.; Soria-Meneses, P.J.; Jurado-Campos, A.; Bartolomé-García, J.; Gómez-Rubio, V.; Soler, A.J.; Arroyo-Jimenez, M.M.; Santander-Ortega, M.J.; Plaza-Oliver, M.; Lozano, M.V.; et al. Nanotechnology in reproduction: Vitamin E nanoemulsions for reducing oxidative stress in sperm cells. Free Radic. Biol. Med. 2020, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rubio, F.; Fernández-Santos, M.R.; Castro-Vázquez, L.; García-Álvarez, O.; Maroto-Morales, A.; Soler, A.J.; Martínez-Pastor, F.; Garde, J.J. Cinnamtannin B-1, a novel antioxidant for sperm in red deer. Anim. Reprod. Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Mata-Campuzano, M.; Álvarez-Rodríguez, M.; del Olmo, E.; Fernández-Santos, M.R.; Garde, J.J.; Martínez-Pastor, F. Quality, oxidative markers and DNA damage (DNA) fragmentation of red deer thawed spermatozoa after incubation at 37 degrees C in presence of several antioxidants. Theriogenology 2012, 78, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Cimas, F.J.; Niza, E.; Juan, A.; Noblejas-López, M.; Bravo, I.; Lara-Sánchez, A.; Alonso-Moreno, C.; Ocaña, A. Controlled Delivery of BET-PROTACs: In Vitro Evaluation of MZ1-Loaded Polymeric Antibody Conjugated Nanoparticles in Breast Cancer. Pharmaceutics 2020, 12, 986. [Google Scholar] [CrossRef]
- Niza, E.; Ocaña, A.; Castro-Osma, J.; Bravo, I.; Alonso-Moreno, C. Polyester Polymeric Nanoparticles as Platforms in the Development of Novel Nanomedicines for Cancer Treatment. Cancers 2021, 13, 3387. [Google Scholar] [CrossRef]
- Rahimipour, M.; Talebi, A.R.; Anvari, M.; Sarcheshmeh, A.A.; Omidi, M. Effects of different doses of ethanol on sperm parameters, chromatin structure and apoptosis in adult mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 423–428. [Google Scholar] [CrossRef]
- Jurado-Campos, A.; Soria-Meneses, P.J.; Sánchez-Rubio, F.; Niza, E.; Bravo, I.; Alonso-Moreno, C.; Arenas-Moreira, M.; García-Álvarez, O.; Soler, A.J.; Garde, J.J.; et al. Vitamin E Delivery Systems Increase Resistance to Oxidative Stress in Red Deer Sperm Cells: Hydrogel and Nanoemulsion Carriers. Antioxidants 2021, 10, 1780. [Google Scholar] [CrossRef]
- Lozano, M.V.; Torrecilla, D.; Torres, D.; Vidal, A.; Dominguez, F.; Alonso, M.J. Highly efficient system to deliver taxanes into tumor cells: Docetaxel-loaded chitosan oligomer colloidal carriers. Biomacromolecules 2008, 9, 2186–2193. [Google Scholar] [CrossRef]
- Malo, A.F.; Garde, J.J.; Soler, A.J.; García, A.J.; Gomendio, M.; Roldan, E.R. Male fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. Biol. Reprod. 2005, 72, 822–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Pastor, F.; Aisen, E.; Fernández-Santos, M.R.; Esteso, M.C.; Maroto-Morales, A.; García-Álvarez, O.; Garde, J.J. Reactive oxygen species generators affect quality parameters and apoptosis markers differently in red deer spermatozoa. Reproduction 2009, 137, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, E.; García-Álvarez, O.; Maroto-Morales, A.; Ramón, M.; Jiménez-Rabadán, P.; Iniesta-Cuerda, M.; Anel-López, L.; Martínez-Pastor, F.; Soler, A.J.; Garde, J.J.; et al. Estrous sheep serum enables in vitro capacitation of ram spermatozoa while preventing caspase activation. Theriogenology 2016, 85, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P. The Sperm Chromatin Structure Assay (SCSA(R)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef] [Green Version]
- Evenson, D.P.; Wixon, R. Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology 2006, 65, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Falchi, L.; Bogliolo, L.; Galleri, G.; Ariu, F.; Zedda, M.T.; Pinna, A.; Malfatti, L.; Innocenzi, P.; Ledda, S. Cerium dioxide nanoparticles did not alter the functional and morphologic characteristics of ram sperm during short-term exposure. Theriogenology 2016, 85, 1274–1281.e1273. [Google Scholar] [CrossRef]
- Falchi, L.; Galleri, G.; Dore, G.M.; Zedda, M.T.; Pau, S.; Bogliolo, L.; Ariu, F.; Pinna, A.; Nieddu, S.; Innocenzi, P.; et al. Effect of exposure to CeO2 nanoparticles on ram spermatozoa during storage at 4 degrees C for 96 h. Reprod. Biol. Endocrinol. 2018, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Serrano, P.; Casares-Crespo, L.; Viudes-de-Castro, M.P. Chitosan-dextran sulphate nanoparticles for GnRH release in rabbit insemination extenders. Reprod. Domest. Anim. 2017, 52 (Suppl. S4), 72–74. [Google Scholar] [CrossRef] [Green Version]
- Holt, C.; Holt, W.V.; Moore, H.D.; Reed, H.C.; Curnock, R.M. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: Results of two fertility trials. J. Androl. 1997, 18, 312–323. [Google Scholar]
- Farrell, P.B.; Presicce, G.A.; Brockett, C.C.; Foote, R.H. Quantification of bull sperm characteristics measured by computer-assisted sperm analysis (CASA) and the relationship to fertility. Theriogenology 1998, 49, 871–879. [Google Scholar] [CrossRef]
- Robayo, I.; Montenegro, V.; Valdés, C.; Cox, J.F. CASA assessment of kinematic parameters of ram spermatozoa and their relationship to migration efficiency in ruminant cervical mucus. Reprod. Domest. Anim. 2008, 43, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Love, C.C. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology 2011, 76, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Vuthiphandchai, V.; Chomphuthawach, S.; Nimrat, S. Cryopreservation of red snapper (Lutjanus argentimaculatus) sperm: Effect of cryoprotectants and cooling rates on sperm motility, sperm viability, and fertilization capacity. Theriogenology 2009, 72, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Swathy, S.S.; Panicker, S.; Indira, M. Effect of exogenous selenium on the testicular toxicity induced by ethanol in rats. Indian J. Physiol. Pharmacol. 2006, 50, 215–224. [Google Scholar] [PubMed]
- Harikrishnan, R.; Abhilash, P.A.; Das, S.S.; Prathibha, P.; Rejitha, S.; John, F.; Kavitha, S.; Indira, M. Protective effect of ascorbic acid against ethanol-induced reproductive toxicity in male guinea pigs. Br. J. Nutr. 2013, 110, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Soares, S.L.; Brito, C.R.C.; Anciuti, A.N.; Gatti, N.C.; Corcini, C.D.; Varela, A.S., Jr.; Marques, M.G.; Fonseca, F.N.; Komninou, E.R.; Lucia, T.J. Nanocarried antioxidants in freezing extenders for boar spermatozoa. Andrologia 2021, 53, e14199. [Google Scholar] [CrossRef]
- Nasiri, A.H.; Towhidi, A.; Zeinoaldini, S. Combined effect of DHA and α-tocopherol supplementation during bull semen cryopreservation on sperm characteristics and fatty acid composition. Andrologia 2012, 44, 550–555. [Google Scholar] [CrossRef]
- Yuan, C.A.-O.; Wang, H.; Li, X.; Liu, H.; Zhao, J.; Lu, W.; Wang, J. Combined Effect of Flaxseed Oil and Vitamin E Supplementation During Bull Semen Cryopreservation on Sperm Characteristics. Biopreserv. Biobank 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/34919423/ (accessed on 24 July 2022).
- Pena, F.J.; Johannisson, A.; Wallgren, M.; Martinez, H.R. Antioxidant supplementation in vitro improves boar sperm motility and mitochondrial membrane potential after cryopreservation of different fractions of the ejaculate. Anim. Reprod. Sci. 2003, 78, 85–98. [Google Scholar] [CrossRef]
- Satorre, M.M.; Breininger, E.; Beconi, M.T.; Beorlegui, N.B. alpha-Tocopherol modifies tyrosine phosphorylation and capacitation-like state of cryopreserved porcine sperm. Theriogenology 2007, 68, 958–965. [Google Scholar] [CrossRef]
- Taylor, K.; Roberts, P.; Sanders, K.; Burton, P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online 2009, 18, 184–189. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurado-Campos, A.; Soria-Meneses, P.J.; Arenas-Moreira, M.; Alonso-Moreno, C.; Bravo, I.; Rodríguez-Robledo, V.; Sánchez-Ajofrín, I.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.d.R. Vitamin E Lipid-Based Nanodevices as a Tool for Ovine Sperm Protection against Oxidative Stress: Impact on Sperm Motility. Antioxidants 2022, 11, 1988. https://doi.org/10.3390/antiox11101988
Jurado-Campos A, Soria-Meneses PJ, Arenas-Moreira M, Alonso-Moreno C, Bravo I, Rodríguez-Robledo V, Sánchez-Ajofrín I, Soler AJ, Garde JJ, Fernández-Santos MdR. Vitamin E Lipid-Based Nanodevices as a Tool for Ovine Sperm Protection against Oxidative Stress: Impact on Sperm Motility. Antioxidants. 2022; 11(10):1988. https://doi.org/10.3390/antiox11101988
Chicago/Turabian StyleJurado-Campos, Alejandro, Pedro Javier Soria-Meneses, María Arenas-Moreira, Carlos Alonso-Moreno, Iván Bravo, Virginia Rodríguez-Robledo, Irene Sánchez-Ajofrín, Ana Josefa Soler, José Julián Garde, and María del Rocío Fernández-Santos. 2022. "Vitamin E Lipid-Based Nanodevices as a Tool for Ovine Sperm Protection against Oxidative Stress: Impact on Sperm Motility" Antioxidants 11, no. 10: 1988. https://doi.org/10.3390/antiox11101988
APA StyleJurado-Campos, A., Soria-Meneses, P. J., Arenas-Moreira, M., Alonso-Moreno, C., Bravo, I., Rodríguez-Robledo, V., Sánchez-Ajofrín, I., Soler, A. J., Garde, J. J., & Fernández-Santos, M. d. R. (2022). Vitamin E Lipid-Based Nanodevices as a Tool for Ovine Sperm Protection against Oxidative Stress: Impact on Sperm Motility. Antioxidants, 11(10), 1988. https://doi.org/10.3390/antiox11101988








