Exogenously-Sourced Salicylic Acid Imparts Resilience towards Arsenic Stress by Modulating Photosynthesis, Antioxidant Potential and Arsenic Sequestration in Brassica napus Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Cultivation, and Treatments
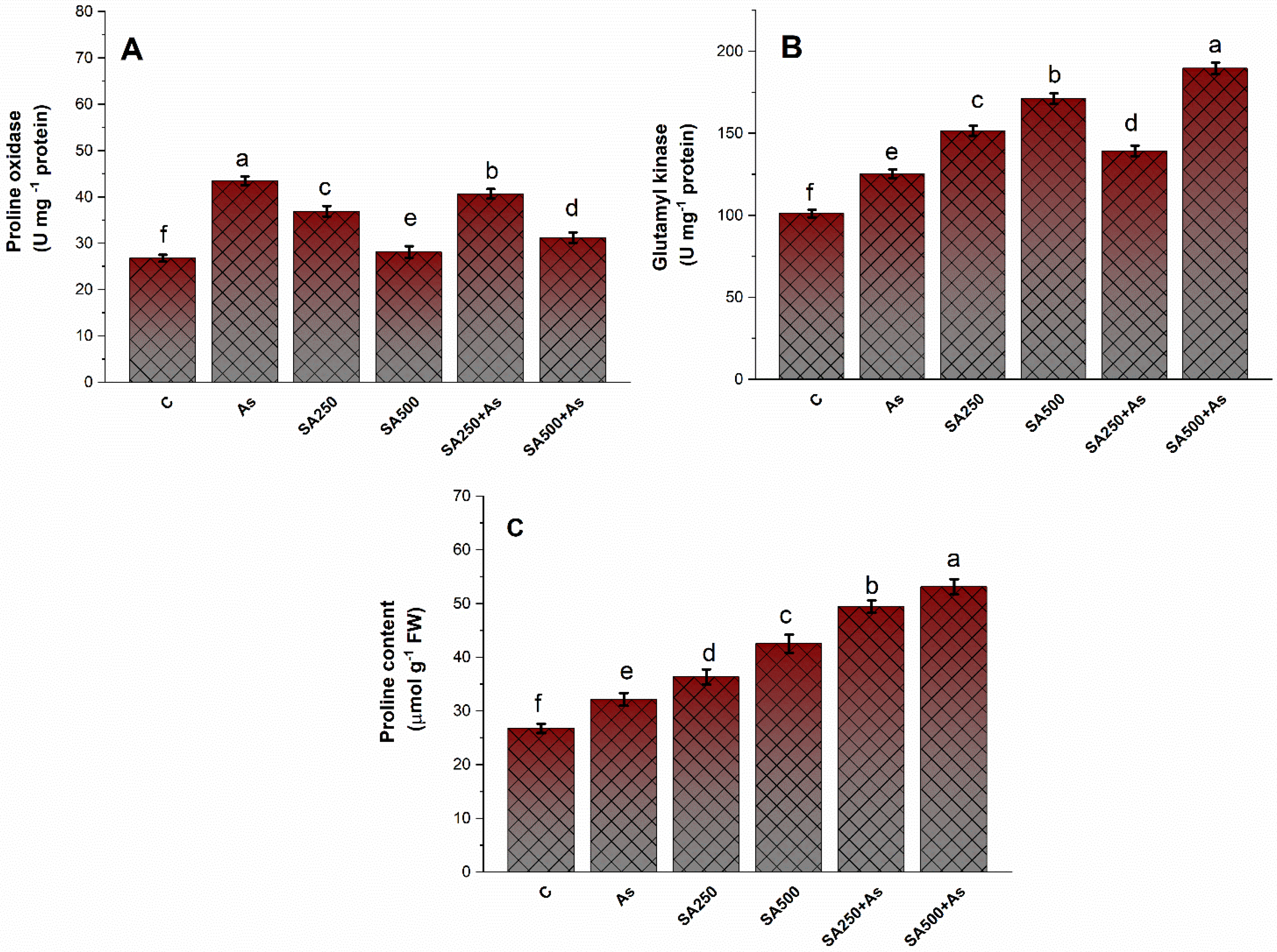
2.2. Proline Determination
2.3. Determination of Oxidative Stress of Plants
2.4. Electrolyte Leakage Determination
2.5. Determination of Antioxidant Process
2.6. Determination of Photosynthetic Characteristics and Growth Characteristics
2.7. Determination of Arsenic and Sulfur Content
2.8. Determination of Non-Protein Thiols (NPTs) and Total Phytochelatins (PCs)
2.9. Determination of Carbohydrates
2.10. Studying Root Cell Viability with Confocal Laser Microscopy
2.11. Physiological Measurement of Guard Cells
2.12. Statistical Analysis
3. Results
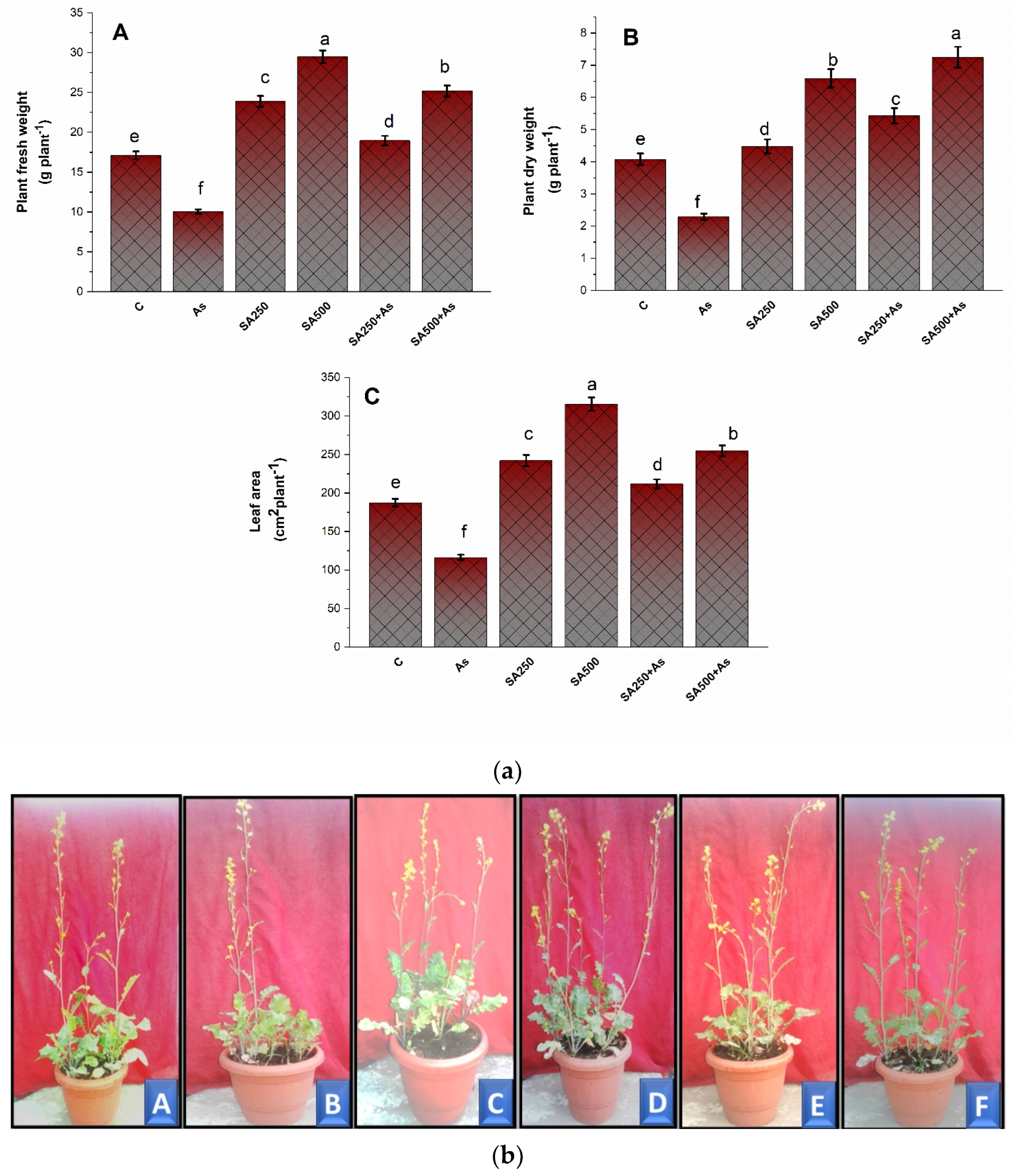
3.1. Impact of Salicylic Acid on Growth Metrics during As Stress
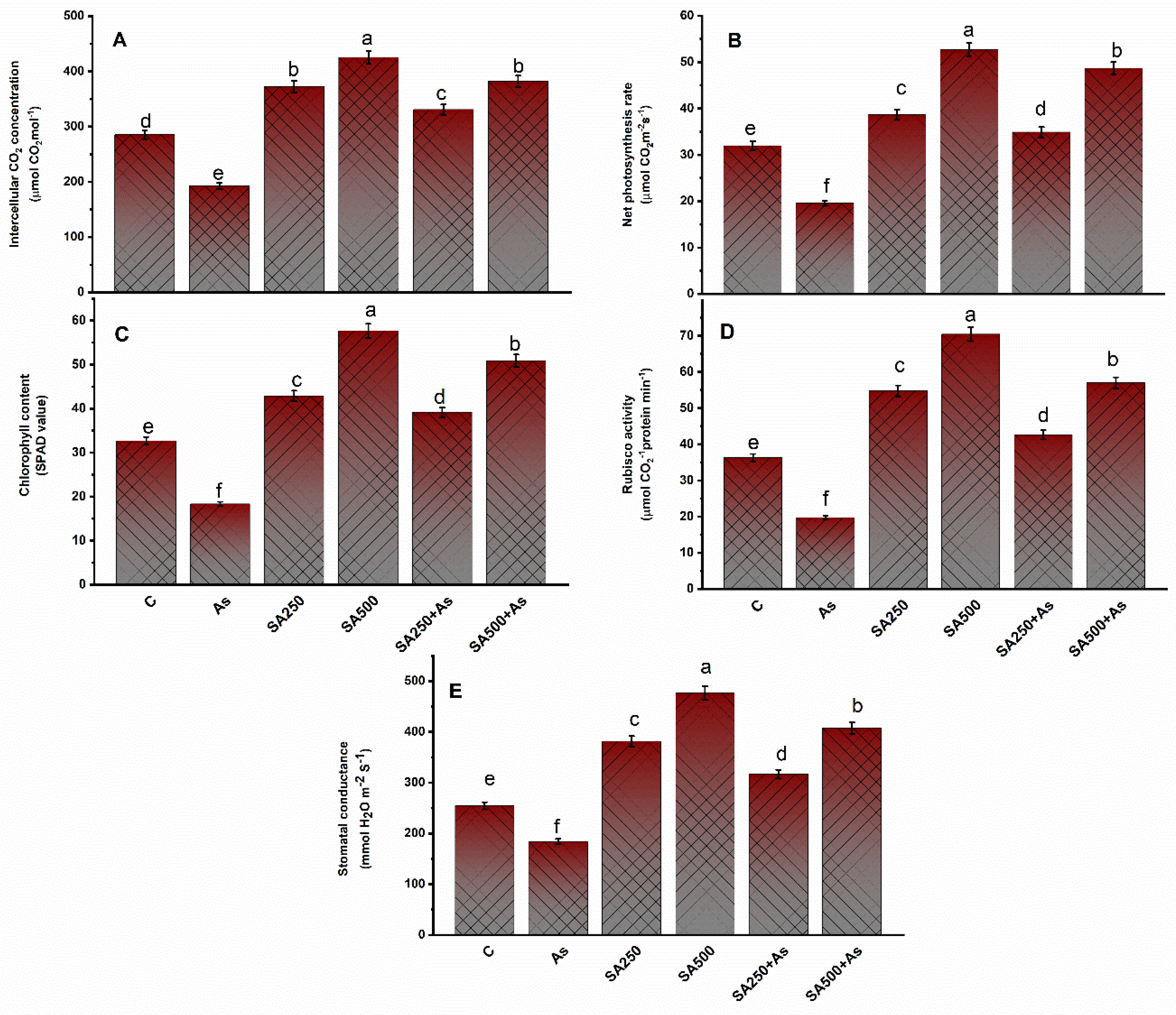
3.2. Impact of SA on Photosynthesis Parameters under As Stress
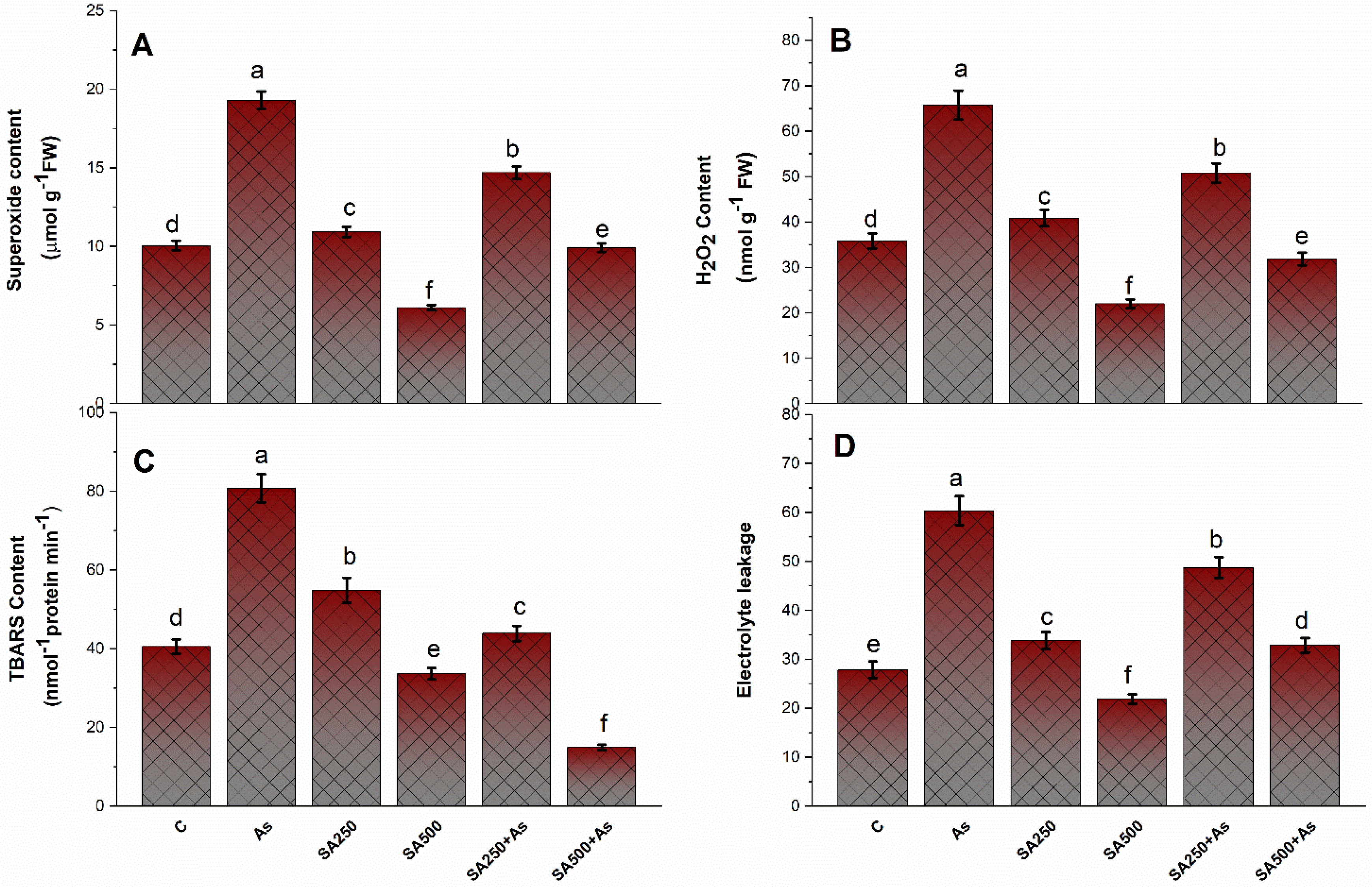
3.3. SA Reduces Oxidative Stress in Brassica napus Plants under As Stress
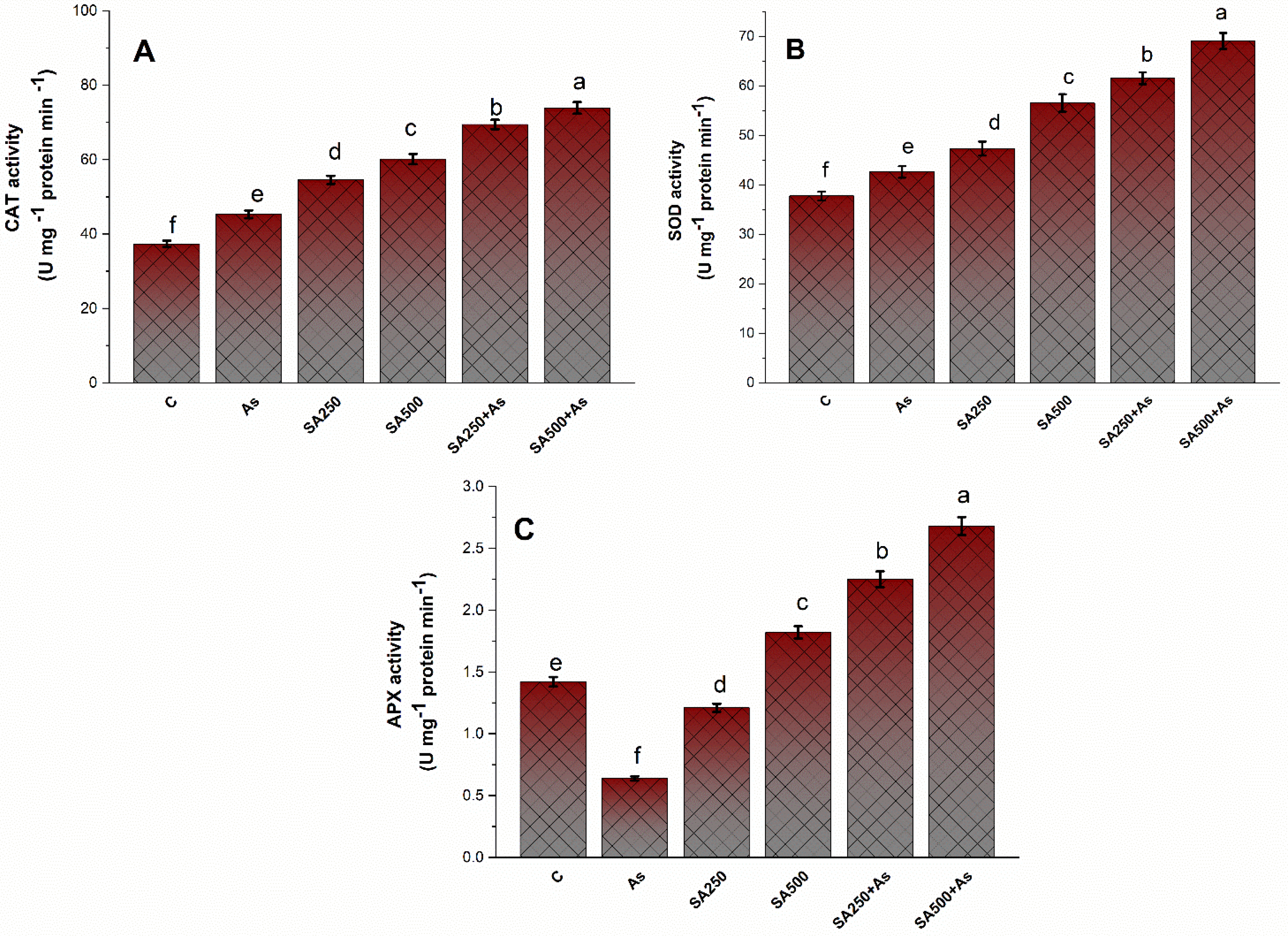
3.4. SA Up-Regulates the Antioxidant Metabolisms in B. napus under As Stress
3.5. Impact of Salicylic Acid on the Accumulation of Proline under As-Stress
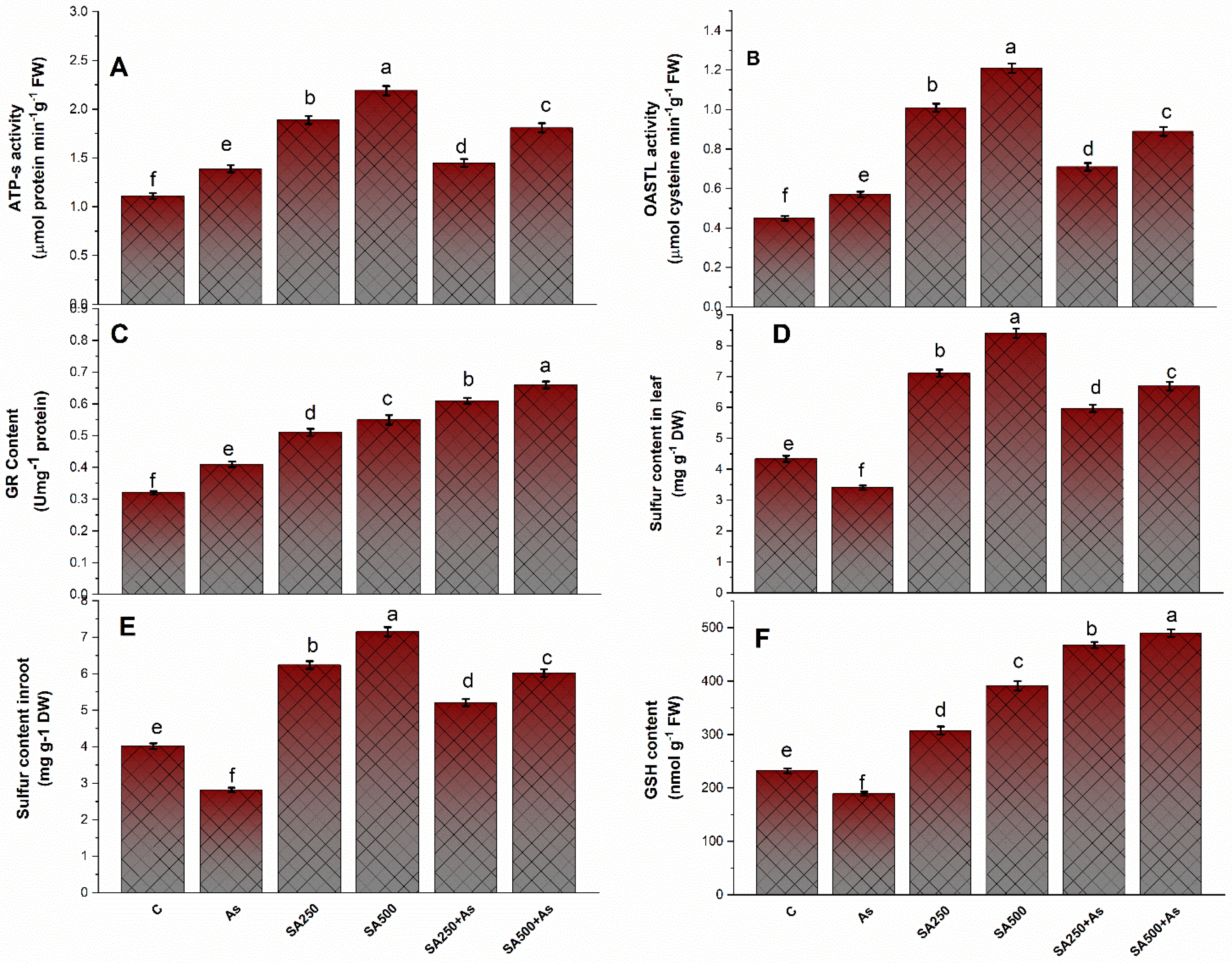
3.6. Impact of Salicylic Acid on S-Assimilation during As Stress
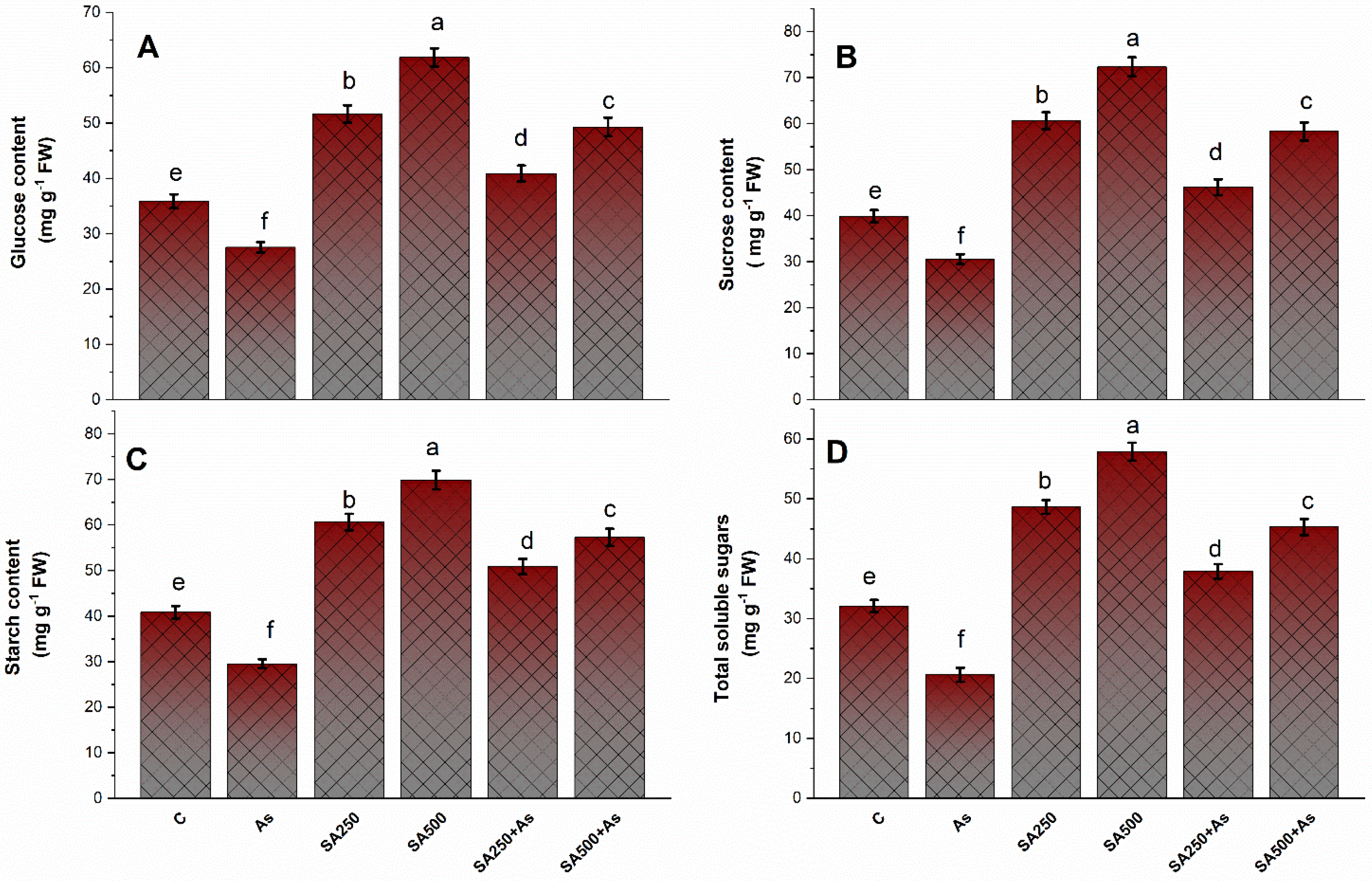
3.7. Impact of SA on the Carbohydrate’s Metabolism under As Stress
3.8. Impact of SA on Arsenic Accumulation in Roots and Leaves
3.9. Effect of SA on Cell Viability and Stomatal Studies under As-Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrakar, V.; Pandey, N.; Keshavkant, S. Plant responses to arsenic toxicity: Morphology and physiology. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 27–48. [Google Scholar]
- Chen, H.; Liu, G.; Qiao, N.; Kang, Z.; Hu, L.; Liao, J.; Li, Y. Toxic effects of arsenic trioxide on spermatogonia are associated with oxidative stress, mitochondrial dysfunction, autophagy and metabolomic alterations. Ecotoxicol. Environ. Saf. 2020, 190, 110063. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gill, R.A.; Ali, B.; Wang, J.; Islam, F.; Ali, S.; Zhou, W. Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology 2016, 25, 350–366. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, P.; Chen, W. Arsenic toxicity: The effects on plant metabolism. Front. Physiol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Wang, J.; Song, W.Y. Arsenic uptake and translocation in plants. Plant. Cell. Physiol. 2016, 57, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef] [Green Version]
- Kostecka-Gugała, A.; Latowski, D. Arsenic-induced oxidative stress in plants. In Mechanisms of Arsenic Toxicity and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 79–104. [Google Scholar]
- Garg, N.; Singla, P. Arsenic toxicity in crop plants: Physiological effects and tolerance mechanisms. Environ. Chem. Lett. 2011, 9, 303–321. [Google Scholar] [CrossRef]
- Verma, S.; Verma, P.K.; Chakrabarty, D. Arsenic Bio-volatilization by engineered yeast promotes rice growth and reduces arsenic accumulation in grains. Int. J. Environ. Res. 2019, 13, 475–485. [Google Scholar] [CrossRef]
- Mir, I.R.; Rather, B.A.; Masood, A.; Majid, A.; Sehar, Z.; Anjum, N.A.; Khan, N.A. Soil Sulfur Sources Differentially Enhance Cadmium Tolerance in Indian Mustard (Brassica juncea L.). Soil Syst. 2021, 5, 29. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Dixit, G.; Mishra, S.; Dwivedi, S.; Tiwari, M.; Mallick, S.; Tripathi, R.D. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front. Plant Sci. 2015, 6, 340. [Google Scholar] [CrossRef] [Green Version]
- Chandrakar, V.; Dubey, A.; Keshavkant, S. Modulation of antioxidant enzymes by salicylic acid in arsenic exposed Glycine max L. J. Soil Sci. Plant Nut. 2016, 16, 662–676. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma 2018, 255, 139–152. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Landi, M. The role of salicylic acid in plants exposed to heavy metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazar, R.; Iqbal, N.; Syeed, S.; Khan, N.A. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J. Plant Physiol. 2011, 168, 807–815. [Google Scholar] [CrossRef]
- Aoyama, K.; Nakaki, T. Glutathione in cellular redox homeostasis: Association with the excitatory amino acid carrier 1 (EAAC1). Molecules 2015, 20, 8742–8758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouari, M.; Hassena, A.B.; Trabelsi, L.; Rouina, B.B.; Decou, R.; Labrousse, P. Exogenous proline-mediated abiotic stress tolerance in plants: Possible mechanisms. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–121. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Emics Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 155–166. [Google Scholar]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline accumulation and its defensive role under diverse stress condition in plants: An Overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Joseph, E.A.; Radhakrishnan, V.V.; Mohanan, K.V. A study on the accumulation of proline-an osmoprotectant amino acid under salt stress in some native rice cultivars of North Kerala, India. Univers. J. Agric. Res. 2015, 3, 15–22. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Differential blockage of photosynthetic electron flow in young and mature leaves of Arabidopsis thaliana by exogenous proline. Photosynthetica 2015, 53, 471–477. [Google Scholar] [CrossRef]
- Stein, H.; Honig, A.; Miller, G.; Erster, O.; Eilenberg, H.; Csonka, L.N.; Zilberstein, A. Elevation of free proline and proline-rich protein levels by simultaneous manipulations of proline biosynthesis and degradation in plants. Plant Sci. 2011, 181, 140–150. [Google Scholar] [CrossRef]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.P.; Van Den Ende, W. Sugars as hydroxyl radical scavengers: Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef]
- Kofronova, M.; Maskova, P.; Lipavska, H. Two facets of world arsenic problem solution: Crop poisoning restriction and enforcement of phytoremediation. Planta 2018, 248, 19–35. [Google Scholar] [CrossRef]
- Kofroňová, M.; Hrdinová, A.; Mašková, P.; Tremlová, J.; Soudek, P.; Petrová, Š.; Lipavská, H. Multi-component antioxidative system and robust carbohydrate status, the essence of plant arsenic tolerance. Antioxidants 2020, 9, 283. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, H.; Sami, F.; Hayat, S. Glucose: Sweet or bitter effects in plants—A review on current and future perspective. Carbohydr. Res. 2020, 487, 107884. [Google Scholar] [CrossRef]
- Jha, A.B.; Dubey, R.S. Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J. Plant Physiol. 2004, 161, 867–872. [Google Scholar] [CrossRef]
- Li, C.x.; Feng, S.l.; Shao, Y.; Jiang, L.n.; Lu, X.y.; Hou, X.l. Effects of arsenic on seed germination and physiological activities of wheat seedlings. J. Environ. Sci. 2007, 19, 725–732. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernandez-Zapata, J.C.; Martinez Nicolas, J.J.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Mourato, M.P.; Moreira, I.N.; Leitão, I.; Pinto, F.R.; Sales, J.R.; Martins, L.L. Effect of heavy metals in plants of the genus Brassica. Int. J. Mol. Sci. 2015, 16, 17975–17998. [Google Scholar] [CrossRef]
- Lacalle, R.G.; Gómez-Sagasti, M.T.; Artetxe, U.; Garbisu, C.; Becerril, J.M. Brassica napus has a key role in the recovery of the health of soils contaminated with metals and diesel by rhizoremediation. Sci. Total Environ. 2018, 618, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Protective mechanisms of sulfur against arsenic phytotoxicity in Brassica napus by regulating thiol biosynthesis, sulfur-assimilation, photosynthesis, and antioxidant response. Plant Physiol. Biochem. 2022, 188, 1–11. [Google Scholar] [CrossRef]
- Bates, L.E.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hayzer, D.J.; Leisinger, T.H. The gene–enzyme relationships of proline biosynthesis in Escherichia coli. J. Gen. Microbiol. 1980, 118, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, A.H.C.; Cavalieri, A.J. Proline oxidase and water stress induced proline accumulation in spinach leaves. Plant Physiol. 1979, 63, 531–535. [Google Scholar] [CrossRef] [Green Version]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T. A Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Okuda, T.; Masuda, Y.; Yamanka, A.; Sagisaka, S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1934; Volume 105, pp. 121–126. [Google Scholar]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–211. [Google Scholar] [CrossRef]
- Usuda, H. The activation state of ribulose 1, 5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985, 26, 1455–1463. [Google Scholar]
- Chesnin, L.; Yien, C.H. Turbidimetric determination of available sulfates. Soil. Sci. Soc. Am. Proc. 1950, 15, 149–151. [Google Scholar] [CrossRef]
- Lou, L.; Kang, J.; Pang, H.; Li, Q.; Du, X.; Wu, W.; Lv, J. Sulfur protects pakchoi (Brassica chinensis L.) seedlings against cadmium stress by regulating ascorbate-glutathione 596 metabolism. Int. J. Mol. Sci. 2017, 18, 1628. [Google Scholar] [CrossRef] [Green Version]
- Irigoyen, J.; Einerich, D.; Sanchez-Diaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant 1992, 84, 55–60. [Google Scholar] [CrossRef]
- McCready, R.; Guggolz, J.; Silviera, V.; Owens, H. Determination of starch and amylose in vegetables. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Krishnaveni, S.; Balasubramanian, T.; Sadasivam, S. Sugar distribution in sweet stalk sorghum. Food Chem. 1984, 15, 229–232. [Google Scholar] [CrossRef]
- Jones, M.G.; Outlaw, W.H., Jr.; Lowry, O.H. Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol. 1977, 60, 379–383. [Google Scholar] [CrossRef] [Green Version]
- Sabagh, A.E.; Hossain, A.; Islam, M.S.; Iqbal, M.A.; Amanet, K.; Mubeen, M.; Erman, M. Prospective role of plant growth regulators for tolerance to abiotic stresses. In Plant Growth Regulators; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–38. [Google Scholar]
- Hernández, J.A.; Diaz-Vivancos, P.; Barba-Espín, G.; Clemente-Moreno, M.J. On the role of salicylic acid in plant responses to environmental stresses. In Salicylic Acid: A Multifaceted Hormone; Springer: Berlin/Heidelberg, Germany, 2017; pp. 17–34. [Google Scholar]
- Nazar, R.; Iqbal, N.; Khan, N.A. Salicylic Acid: A Multifaceted Hormone; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Rahman, M.A.; Hasegawa, H.; Rahman, M.M.; Islam, M.N.; Miah, M.M.; Tasmen, A. Effect of arsenic on photosynthesis, growth and yield of five widely cultivated rice (Oryza sativa L.) varieties in Bangladesh. Chemosphere 2007, 67, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- Tokar, E.J.; Benbrahim-Tallaa, L.; Ward, J.M.; Lunn, R.; Sams, R.L.; Waalkes, M.P. Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit. Rev. Toxicol. 2010, 40, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Iqbal, N.; Irfan, M.; Khan, N.A. Crosstalk of plant growth regulators protects photosynthetic performance from arsenic damage by modulating defense systems in rice. Ecotoxicol. Environ. Saf. 2021, 222, 112535. [Google Scholar] [CrossRef] [PubMed]
- Saidi, I.; Yousfi, N.; Borgi, M.A. Salicylic acid improves the antioxidant ability against arsenic-induced oxidative stress in sunflower (Helianthus annuus) seedling. J. Plant Nutr. 2017, 40, 2326–2335. [Google Scholar] [CrossRef]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic acid affects root meristem patterning via auxin distribution in a concentrationdependent manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef]
- Habibi, G. Exogenous salicylic acid alleviates oxidative damage of barley plants under drought stress. Acta Biol. Szeged. 2012, 56, 57–63. [Google Scholar]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of exogenous salicylic acid and nitric oxide on growth, photosynthesis, and ascorbate-glutathione cycle in salt stressed Vigna angularis. Biomolecules 2019, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Nazar, R.; Umar, S.; Khan, N.A. Exogenous salicylic acid improves photosynthesis and growth through increase in ascorbate-glutathione metabolism and S assimilation in mustard under salt stress. Plant. Signal. Behave. 2015, 10, e1003751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zengin, F. Effects of exogenous salicylic acid on growth characteristics and biochemical content of wheat seeds under arsenic stress. J. Environ. Biol. 2015, 36, 249. [Google Scholar] [PubMed]
- Naeem, M.; Sadiq, Y.; Jahan, A.; Nabi, A.; Aftab, T.; Khan, M.M.A. Salicylic acid restrains arsenic induced oxidative burst in two varieties of Artemisia annua L. by modulating antioxidant defence system and artemisinin production. Ecotoxicol. Environ. Saf. 2020, 202, 110851. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B.J.B.P. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Nazar, R.; Umar, S.; Khan, N.A.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar] [CrossRef]
- Slewinski, T.L. Non-structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. J. Exp. Bot. 2012, 63, 4647–4670. [Google Scholar] [CrossRef] [PubMed]
- Van Den Ende, W.; Valluru, R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: Scavenging and salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodary, S.E.A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Gupta, P.; Seth, C.S. Nitrate supplementation attenuates As (V) toxicity in Solanum lycopersicum L. cv Pusa Rohini: Insights into As (V) sub-cellular distribution, photosynthesis, nitrogen assimilation, and DNA damage. Plant Physiol. Biochem. 2019, 139, 44–55. [Google Scholar] [CrossRef]
- Garg, N.; Bharti, A. Salicylic acid improves arbuscular mycorrhizal symbiosis, and chickpea growth and yield by modulating carbohydrate metabolism under salt stress. Mycorrhiza 2018, 28, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I. Arsenic induced oxidative stress in plants. Biologia 2012, 67, 447–453. [Google Scholar] [CrossRef]
- Zahra, S.; Mahmood, S.; Noreen, S.; Akrem, A. Independent and combined nickel and cadmium induced lipid peroxidation of biological membranes and its mitigation through antioxidant enzymes in Grewia asiatica L. Pak. J. Life Soc. Sci. 2018, 16, 48–54. [Google Scholar]
- Shakirova, F.M.; Allagulova, C.R.; Maslennikova, D.R.; Klyuchnikova, E.O.; Avalbaev, A.M.; Bezrukova, M.V. Salicylic acid-induced protection against cadmium toxicity in wheat plants. Environ. Exp. Bot. 2016, 122, 19–28. [Google Scholar] [CrossRef]
- Noriega, G.; Caggiano, E.; Lecube, M.L.; Santa Cruz, D.; Batlle, A.; Tomaro, M.; Balestrasse, K.B. The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 2012, 25, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Alam, M.M.; Nahar, K.; Ahamed, K.U.; Fujita, M. Exogenous salicylic acid alleviates salt stress-induced oxidative damage in Brassica napus by enhancing the antioxidant defense and glyoxalase systems. Aust. J. Crop Sci. 2014, 8, 631–639. [Google Scholar]
- Poór, P. Effects of salicylic acid on the metabolism of mitochondrial reactive oxygen species in plants. Biomolecules 2020, 10, 341. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Gill, S.S. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capaldi, F.R.; Gratao, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur metabolism and stress defense responses in plants. Trop. Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, C.; Tan, Q.; Liu, J.; Sun, X. Effects of sulfur application on sulfur and arsenic absorption by rapeseed in arsenic-contaminated soil. Plant Soil. Environ. 2011, 57, 429–434. [Google Scholar] [CrossRef]
- Hausladen, A.; Alscher, R.G. Glutathione. In Antioxidants in Higher Plants; Alscher, R.G., Hess, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 1–30. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bano, K.; Kumar, B.; Alyemeni, M.N.; Ahmad, P. Exogenously-Sourced Salicylic Acid Imparts Resilience towards Arsenic Stress by Modulating Photosynthesis, Antioxidant Potential and Arsenic Sequestration in Brassica napus Plants. Antioxidants 2022, 11, 2010. https://doi.org/10.3390/antiox11102010
Bano K, Kumar B, Alyemeni MN, Ahmad P. Exogenously-Sourced Salicylic Acid Imparts Resilience towards Arsenic Stress by Modulating Photosynthesis, Antioxidant Potential and Arsenic Sequestration in Brassica napus Plants. Antioxidants. 2022; 11(10):2010. https://doi.org/10.3390/antiox11102010
Chicago/Turabian StyleBano, Koser, Bharty Kumar, Mohammed Nasser Alyemeni, and Parvaiz Ahmad. 2022. "Exogenously-Sourced Salicylic Acid Imparts Resilience towards Arsenic Stress by Modulating Photosynthesis, Antioxidant Potential and Arsenic Sequestration in Brassica napus Plants" Antioxidants 11, no. 10: 2010. https://doi.org/10.3390/antiox11102010
APA StyleBano, K., Kumar, B., Alyemeni, M. N., & Ahmad, P. (2022). Exogenously-Sourced Salicylic Acid Imparts Resilience towards Arsenic Stress by Modulating Photosynthesis, Antioxidant Potential and Arsenic Sequestration in Brassica napus Plants. Antioxidants, 11(10), 2010. https://doi.org/10.3390/antiox11102010






