Environmental Exposure to Metals, Parameters of Oxidative Stress in Blood and Prostate Cancer: Results from Two Cohorts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Collection
2.3. Metal(loid)s Analyses
2.4. Oxidative Stress Parameters
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Prostate Cancer and Metal(loid)s Exposure
4.2. Prostate Cancer and Oxidative Stress Parameters
4.3. Impact of Metal(loid)s and Oxidative Stress on the PSA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. IARC 2021. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/27-prostate-fact-sheet.pdf (accessed on 29 March 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Croatia Fact Sheet Source: Globocan 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/191-croatia-fact-sheets.pdf (accessed on 29 March 2021).
- International Agency for Research on Cancer. Serbia Fact Sheet Source: Globocan 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/688-serbia-fact-sheets.pdf (accessed on 29 March 2021).
- Perdana, N.R.; Mochtar, C.A.; Umbas, R.; Hamid, A.R.A. The risk factors of prostate cancer and its prevention: A literature review. Acta Med. Indones. 2016, 48, 228–238. [Google Scholar] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannucci, E.; Liu, Y.; Platz, E.A.; Stampfer, M.J.; Willett, W.C. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int. J. Cancer 2007, 121, 1571–1578. [Google Scholar] [CrossRef] [Green Version]
- Karunasinghe, N.; Minas, T.Z.; Bao, B.-Y.; Lee, A.; Wang, A.; Zhu, S.; Masters, J.; Goudie, M.; Huang, S.-P.; Jenkins, F.J.; et al. Assessment of factors associated with psa level in prostate cancer cases and controls from three geographical regions. Sci. Rep. 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Macke, A.J.; Petrosyan, A. Alcohol and prostate cancer: Time to draw conclusions. Biomolecules 2022, 12, 375. [Google Scholar] [CrossRef]
- Pizent, A.; Čolak, B.; Kljaković Gašpić, Z.; Telišman, S. Prostate-specific antigen (PSA) in serum in relation to blood lead concentration and alcohol consumption in men. Arch. Ind. Hyg. Toxicol. 2009, 60, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Tarantino, G.; Crocetto, F.; Vito, C.D.; Martino, R.; Pandolfo, S.D.; Creta, M.; Aveta, A.; Buonerba, C.; Imbimbo, C. Clinical factors affecting prostate-specific antigen levels in prostate cancer patients undergoing radical prostatectomy: A retrospective study. Future Sci. OA 2021, 7, FSO643. [Google Scholar] [CrossRef]
- Wu, C.-C.; Pu, Y.S.; Wu, H.-C.; Yang, C.-Y.; Chen, Y.-C. Reversed association between levels of prostate specific antigen and levels of blood cadmium and urinary cadmium. Chemosphere 2011, 83, 1188–1191. [Google Scholar] [CrossRef]
- Wu, H.; Wang, M.; Raman, J.D.; McDonald, A.C. Association between urinary arsenic, blood cadmium, blood lead, and blood mercury levels and serum prostate-specific antigen in a population-based cohort of men in the united states. PLoS ONE 2021, 16, e0250744. [Google Scholar] [CrossRef]
- Agency for Toxic Substance and Disease Registry (ATSDR). Case Studies in Environmental Medicine (CSEM): Lead Toxicity. Available online: https://www.atsdr.cdc.gov/csem/lead/docs/CSEM-Lead_toxicity_508.pdf (accessed on 2 February 2022).
- World Health Organization. Action Is Needed on Chemicals of Major Public Health Concern 2010. Available online: http://www.who.int/ipcs/features/10chemicals_en.pdf (accessed on 20 April 2018).
- Baralić, K.; Javorac, D.; Marić, Đ.; Đukić-Ćosić, D.; Bulat, Z.; Antonijević Miljaković, E.; Anđelković, M.; Antonijević, B.; Aschner, M.; Buha Djordjevic, A. Benchmark dose approach in investigating the relationship between blood metal levels and reproductive hormones: Data set from human study. Environ. Int. 2022, 165, 107313. [Google Scholar] [CrossRef]
- Pizent, A.; Tariba, B.; Živković, T. Reproductive toxicity of metals in men. Arch. Ind. Hyg. Toxicol. 2012, 63, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Cui, J.; Chen, Q.; Zhou, N.; Zhou, Z.; Zhang, G.; Wu, W.; Yang, H.; Cao, J. Low-level lead exposure is associated with aberrant sperm quality and reproductive hormone levels in chinese male individuals: Results from the marhcs study low-level lead exposure is associated with aberrant sperm quality. Chemosphere 2020, 244, 125402. [Google Scholar] [CrossRef] [PubMed]
- Tariba Lovaković, B. Cadmium, Arsenic, and Lead: Elements Affecting Male Reproductive Health. Curr. Opin. Toxicol. 2020, 19, 7–14. [Google Scholar] [CrossRef]
- NTP National Toxicology Program. Report on Carcinogens, 15th ed.; Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, USA, 2016. Available online: https://ntp.niehs.nih.gov/ntp/roc/content/listed_substances_508.pdf (accessed on 2 February 2022).
- Buha-Đorđević, A.; Anđelković, M.; Kačavenda, E.; Javorac, D.; Antonijević-Miljaković, E.; Marić, Đ.; Baralić, K.; Đukić-Ćosić, D.; Ćurčić, M.; Antonijević, B.; et al. Cadmium levels in human breast tissue and estradiol serum levels: Is there a connection? Arhiv Farm. 2021, 71, 581–595. [Google Scholar] [CrossRef]
- Dyer, C.A. Heavy Metals as Endocrine-Disrupting Chemicals. In Endocrine-Disrupting Chemicals; Gore, A.C., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 111–133. ISBN 978-1-58829-830-0. [Google Scholar]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health Part B 2009, 12, 206–223. [Google Scholar] [CrossRef]
- Pizent, A. Developmental toxicity of endocrine-disrupting chemicals: Challenges and future directions. Arhiv Farm. 2021, 71, 544–564. [Google Scholar] [CrossRef]
- Benbrahim-Tallaa, L.; Webber, M.M.; Waalkes, M.P. Mechanisms of acquired androgen independence during arsenic-induced malignant transformation of human prostate epithelial cells. Environ. Health Perspect. 2007, 115, 243–247. [Google Scholar] [CrossRef]
- Vella, V.; Malaguarnera, R.; Lappano, R.; Maggiolini, M.; Belfiore, A. Recent views of heavy metals as possible risk factors and potential preventive and therapeutic agents in prostate cancer. Mol. Cell. Endocrinol. 2017, 457, 57–72. [Google Scholar] [CrossRef]
- Andreucci, A.; Mocevic, E.; Jönsson, B.A.; Giwercman, A.; Giwercman, Y.L.; Toft, G.; Lundh, T.; Bizzaro, D.; Specht, I.O.; Bonde, J.P. Cadmium may impair prostate function as measured by prostate specific antigen in semen: A cross-sectional study among european and inuit men. Reprod. Toxicol. 2015, 53, 33–38. [Google Scholar] [CrossRef]
- Buha, A.; Wallace, D.; Matovic, V.; Schweitzer, A.; Oluic, B.; Micic, D.; Djordjevic, V. Cadmium exposure as a putative risk factor for the development of pancreatic cancer: Three different lines of evidence. BioMed Res. Int. 2017, 2017, 1981837. [Google Scholar] [CrossRef]
- Mortoglou, M.; Manić, L.; Buha Djordjevic, A.; Bulat, Z.; Đorđević, V.; Manis, K.; Valle, E.; York, L.; Wallace, D.; Uysal-Onganer, P. Nickel’s role in pancreatic ductal adenocarcinoma: Potential involvement of MicroRNAs. Toxics 2022, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Mortoglou, M.; Wallace, D.; Buha Djordjevic, A.; Djordjevic, V.; Arisan, E.D.; Uysal-Onganer, P. MicroRNA-regulated signaling pathways: Potential biomarkers for pancreatic ductal adenocarcinoma. Stresses 2021, 1, 30–47. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.R.; Taalab, Y.M.; Heinze, S.; Tariba Lovaković, B.; Pizent, A.; Renieri, E.; Tsatsakis, A.; Farooqi, A.A.; Javorac, D.; Andjelkovic, M.; et al. Toxic-metal-induced alteration in mirna expression profile as a proposed mechanism for disease development. Cells 2020, 9, 901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Wang, Z.; Xu, Y.; Chen, S.; Han, Y.; Li, L.; Wang, M.; Jin, X.; Stockinger, H. Roles of reactive oxygen species in biological behaviors of prostate cancer. BioMed Res. Int. 2020, 2020, 1269124. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Conte, C.; Culig, Z. Oxidative stress-related aging: A role for prostate cancer? Biochim. Biophys. Acta (BBA)—Rev. Cancer 2009, 1795, 83–91. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Malins, D.C.; Johnson, P.M.; Barker, E.A.; Polissar, N.L.; Wheeler, T.M.; Anderson, K.M. Cancer-related changes in prostate DNA as men age and early identification of metastasis in primary prostate tumors. Proc. Natl. Acad. Sci. USA. 2003, 100, 5401–5406. [Google Scholar] [CrossRef] [Green Version]
- Miyake, H.; Hara, I.; Kamidono, S.; Eto, H. Oxidative DNA damage in patients with prostate cancer and its response to treatment. J. Urol. 2004, 171, 1533–1536. [Google Scholar] [CrossRef]
- Ohtake, S.; Kawahara, T.; Ishiguro, Y.; Takeshima, T.; Kuroda, S.; Izumi, K.; Miyamoto, H.; Uemura, H. Oxidative Stress Marker 8-Hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Mol. Clin. Oncol. 2018, 9, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Srivastava, J.K.; Shankar, E.; Kanwal, R.; Nawab, A.; Sharma, H.; Bhaskaran, N.; Ponsky, L.E.; Fu, P.; MacLennan, G.T.; et al. Oxidative stress and antioxidant status in high-risk prostate cancer subjects. Diagnostics 2020, 10, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Živković, T.; Tariba, B.; Pizent, A. Multielement analysis of human seminal plasma by octopole reaction cell ICP-MS. J. Anal. At. Spectrom. 2014, 29, 2114–2126. [Google Scholar] [CrossRef]
- Belsten, J.L.; Wright, A.J. European community—FLAIR common assay for whole-blood glutathione peroxidase (GSH-Px); Results of an inter-laboratory trial. Eur. J. Clin. Nutr. 1995, 49, 921–927. [Google Scholar] [PubMed]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Aycicek, A.; Erel, O. Total oxidant/antioxidant status in jaundiced newborns before and after phototherapy. J. Pediatr. 2007, 83, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Cui, Z.-G.; Ahmed, K.; Zaidi, S.F.; Muhammad, J.S. Ins and outs of cadmium-induced carcinogenesis: Mechanism and prevention. Cancer Treat. Res. Commun. 2021, 27, 100372. [Google Scholar] [CrossRef] [PubMed]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Hao, R.; Shao, M.; Luo, Y. Cadmium levels in tissue and plasma as a risk factor for prostate carcinoma: A meta-analysis. Biol. Trace Elem. Res. 2016, 172, 86–92. [Google Scholar] [CrossRef]
- Waalkes, M.P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000, 79, 241–244. [Google Scholar] [CrossRef]
- Zimta, A.-A.; Schitcu, V.; Gurzau, E.; Stavaru, C.; Manda, G.; Szedlacsek, S.; Berindan-Neagoe, I. Biological and molecular modifications induced by cadmium and arsenic during breast and prostate cancer development. Environ. Res. 2019, 178, 108700. [Google Scholar] [CrossRef]
- Ju-Kun, S.; Yuan, D.-B.; Rao, H.-F.; Chen, T.-F.; Luan, B.-S.; Xu, X.-M.; Jiang, F.-N.; Zhong, W.-D.; Zhu, J.-G. Association between cd exposure and risk of prostate cancer: A prisma-compliant systematic review and meta-analysis. Medicine 2016, 95, e2708. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xun, P.; Nishijo, M.; Carter, S.; He, K. Cadmium exposure and risk of prostate cancer: A meta-analysis of cohort and case-control studies among the general and occupational populations. Sci. Rep. 2016, 6, 25814. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-G.; Ryoo, J.-H.; Chang, S.-J.; Kim, C.-B.; Park, J.-K.; Koh, S.-B.; Ahn, Y.-S. Blood lead levels and cause-specific mortality of inorganic lead-exposed workers in South Korea. PLoS ONE 2015, 10, e0140360. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Zhang, B.; Liu, Y.; Lu, T.; Shi, Y.; Shan, G.; Dong, L. Urinary lead concentration is an independent predictor of cancer mortality in the U.S. general population. Front. Oncol. 2018, 8, 242. [Google Scholar] [CrossRef]
- Schober, S.E.; Mirel, L.B.; Graubard, B.I.; Brody, D.J.; Flegal, K.M. Blood lead levels and death from all causes, cardiovascular disease, and cancer: Results from the NHANES III mortality study. Environ. Health Perspect. 2006, 114, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Park, M.Y.; Kang, M.-Y.; Shin, I.-S.; An, S.; Kim, H.-R. Occupational lead exposure and brain tumors: Systematic review and meta-analysis. Int. J. Environ. Res. Public. Health 2020, 17, 3975. [Google Scholar] [CrossRef]
- Fu, H.; Boffetta, P. Cancer and occupational exposure to inorganic lead compounds: A meta-analysis of published data. Occup. Environ. Med. 1995, 52, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, L.M.; Friesen, M.C.; Xiang, Y.-B.; Cai, H.; Koh, D.-H.; Ji, B.-T.; Yang, G.; Li, H.-L.; Locke, S.J.; Rothman, N.; et al. Occupational lead exposure and associations with selected cancers: The Shanghai men’s and women’s health study cohorts. Environ. Health Perspect. 2016, 124, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Steenland, K.; Boffetta, P. Lead and cancer in humans: Where are we now? Am. J. Ind. Med. 2000, 38, 295–299. [Google Scholar] [CrossRef]
- Wynant, W.; Siemiatycki, J.; Parent, M.-É.; Rousseau, M.-C. Occupational exposure to lead and lung cancer: Results from two case-control studies in Montreal, Canada. Occup. Environ. Med. 2013, 70, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Doolan, G.; Benke, G.; Giles, G. An update on occupation and prostate cancer. Asian Pac. J. Cancer Prev. 2014, 15, 501–516. [Google Scholar] [CrossRef] [Green Version]
- Fritschi, L.; Glass, D.C.; Tabrizi, J.S.; Leavy, J.E.; Ambrosini, G.L. Occupational risk factors for prostate cancer and benign prostatic hyperplasia: A case-control study in Western Australia. Occup. Environ. Med. 2007, 64, 60–65. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Shah, M.H. Comparative study of trace elements in blood, scalp hair and nails of prostate cancer patients in relation to healthy donors. Biol. Trace Elem. Res. 2014, 162, 46–57. [Google Scholar] [CrossRef]
- Siddiqui, M.K.J.; Srivastava, S.; Mehrotra, P.K. Environmental exposure to lead as a risk for prostate cancer. Biomed. Environ. Sci. 2002, 15, 298–305. [Google Scholar]
- Kaba, M.; Pirincci, N.; Yuksel, M.B.; Gecit, I.; Gunes, M.; Ozveren, H.; Eren, H.; Demir, H. Serum levels of trace elements in patients with prostate cancer. Asian Pac. J. Cancer Prev. 2014, 15, 2625–2629. [Google Scholar] [CrossRef] [Green Version]
- Guzel, S.; Kiziler, L.; Aydemir, B.; Alici, B.; Ataus, S.; Aksu, A.; Durak, H. Association of Pb, Cd, and Se concentrations and oxidative damage-related markers in different grades of prostate carcinoma. Biol. Trace Elem. Res. 2012, 145, 23–32. [Google Scholar] [CrossRef]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between serum heavy metals and prostate cancer risk—A multiple metal analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, Metals, Fibres and Dusts; International Agency for Research on Cancer: Lyon, France, 2012; ISBN 978-92-832-0135-9.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. A Review of Human Carcinogens. Part C: Arsenic, Metals, Fibres, and Dusts. Vol 100C; International Agency for Research on Cancer: Lyon, France, 2012.
- Benbrahim-Tallaa, L.; Waalkes, M.P. Inorganic arsenic and human prostate cancer. Environ. Health Perspect. 2008, 116, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Boroje, I.J.; Ferdosi, H.; Kramer, Z.J.; Lamm, S.H. Prostate cancer incidence in U.S. counties and low levels of arsenic in drinking water. Int. J. Environ. Res. Public Health 2020, 17, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulka, C.M.; Jones, R.M.; Turyk, M.E.; Stayner, L.T.; Argos, M. Arsenic in drinking water and prostate cancer in Illinois Counties: An ecologic study. Environ. Res. 2016, 148, 450–456. [Google Scholar] [CrossRef] [Green Version]
- IARC. Agents Classified by the IARC Monographs, Volumes 1–130—IARC Monographs on the Identification of Carcinogenic Hazards to Humans. 2022. Available online: https://monographs.iarc.who.int/Agents-Classified-by-the-Iarc/ (accessed on 20 February 2022).
- Zefferino, R.; Piccoli, C.; Ricciardi, N.; Scrima, R.; Capitanio, N. Possible mechanisms of mercury toxicity and cancer promotion: Involvement of gap junction intercellular communications and inflammatory cytokines. Oxid. Med. Cell. Longev. 2017, 2017, 7028583. [Google Scholar] [CrossRef]
- Akintunde, J.K.; Babaita, A.K. Effect of PUFAs from Pteleopsis suberosa stem bark on androgenic enzymes, cellular ATP and prostatic acid phosphatase in mercury chloride—Exposed rat. Middle East Fertil. Soc. J. 2017, 22, 211–218. [Google Scholar] [CrossRef]
- Zaichick, V. Differences between 66 chemical element contents in normal and cancerous prostate. J. Anal. Oncol. 2017, 6, 37–56. [Google Scholar] [CrossRef]
- Foster, H.; Kennedy, G.; Maisonneuve, P.; Krewski, D.; Ghadiria, P. A case-control study of toenail selenium, mercury, arsenic and cadmium and cancer of the breast, colon and prostate in Montreal. Trends Cancer Res. 2008, 4, 15–18. [Google Scholar]
- Nurchi, V.M.; Buha Djordjevic, A.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic toxicity: Molecular targets and therapeutic agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Rambousková, J.; Krsková, A.; Slavíková, M.; Čejchanová, M.; Černá, M. Blood levels of lead, cadmium, and mercury in the elderly living in institutionalized care in the Czech Republic. Exp. Gerontol. 2014, 58, 8–13. [Google Scholar] [CrossRef]
- Cole, P.; Rodu, B. Epidemiologic studies of chrome and cancer mortality: A series of meta-analyses. Regul. Toxicol. Pharmacol. 2005, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.D.; Garcia, S.C.; Brucker, N.; Goethel, G.; Sauer, E.; Lacerda, L.M.; Oliveira, E.; Trombini, T.L.; Machado, A.B.; Pressotto, A.; et al. Occupational risk assessment of exposure to metals in chrome plating workers. Drug Chem. Toxicol. 2022, 45, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Zhang, X.; Wang, X.-C.; Jin, L.-F.; Yang, Z.-P.; Jiang, C.-X.; Chen, Q.; Ren, X.-B.; Cao, J.-Z.; Wang, Q.; et al. Chronic occupational exposure to hexavalent chromium causes DNA damage in electroplating workers. BMC Public Health 2011, 11, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krstev, S.; Knutsson, A. Occupational risk factors for prostate cancer: A meta-analysis. J. Cancer Prev. 2019, 24, 91–111. [Google Scholar] [CrossRef] [Green Version]
- Goulart, M.; Batoréu, M.C.; Rodrigues, A.S.; Laires, A.; Rueff, J. Lipoperoxidation products and thiol antioxidants in chromium exposed workers. Mutagenesis 2005, 20, 311–315. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, K.; Feng, Q.; Xu, Y.; Zhang, Z. Chromium(VI) promotes cell migration through targeting epithelial-mesenchymal transition in prostate cancer. Toxicol. Lett. 2019, 300, 10–17. [Google Scholar] [CrossRef]
- Nsonwu-Anyanwu, A.; Icha, B.; Nsonwu, M.; William, M.; Emughupogh, K.; Usoro, C. Assessment of essential and non-essential elements as risk evaluation indices in men with prostate cancer in Calabar South-South Nigeria. Middle East J. Cancer 2022, 13, 285–292. [Google Scholar] [CrossRef]
- Blanc-Lapierre, A.; Rhazi, M.; Richard, H.; Parent, M.-E. O22-3 Occupational Exposure to Chromium, Nickel and Cadmium, and Prostate Cancer Risk and in a Population-Based Case-Control Study in Montreal, Canada. Occup. Environ. Med. 2016, 73, A42. [Google Scholar] [CrossRef]
- Çelen, İ.; Müezzinoğlu, T.; Ataman, O.Y.; Bakırdere, S.; Korkmaz, M.; Neşe, N.; Şenol, F.; Lekili, M. Selenium, nickel, and calcium levels in cancerous and non-cancerous prostate tissue samples and their relation with some parameters. Environ. Sci. Pollut. Res. 2015, 22, 13070–13076. [Google Scholar] [CrossRef]
- Chang, W.-H.; Lee, C.-C.; Yen, Y.-H.; Chen, H.-L. Oxidative damage in patients with benign prostatic hyperplasia and prostate cancer co-exposed to phthalates and to trace elements. Environ. Int. 2018, 121, 1179–1184. [Google Scholar] [CrossRef]
- Guntupalli, J.N.R.; Padala, S.; Gummuluri, A.V.R.M.; Muktineni, R.K.; Byreddy, S.R.; Sreerama, L.; Kedarisetti, P.C.; Angalakuduru, D.P.; Satti, B.R.; Venkatathri, V.; et al. Trace elemental analysis of normal, benign hypertrophic and cancerous tissues of the prostate gland using the particle-induced x-ray emission technique. Eur. J. Cancer Prev. 2007, 16, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Yaman, M.; Atici, D.; Bakırdere, S.; Akdeniz, İ. Comparison of trace metal concentrations in malign and benign human prostate. J. Med. Chem. 2005, 48, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Zaichick, V.; Zaichick, S. Prostatic tissue levels of 43 trace elements in patients with prostate adenocarcinoma. Cancer Clin. Oncol. 2016, 5, 79. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Alexander, E.E.; Singh, R.; Shan, A.; Qian, J.; Santella, R.M.; Oberley, L.W.; Yan, T.; Zhong, W.; Jiang, X.; et al. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 2000, 89, 123–134. [Google Scholar] [CrossRef]
- Arsova-Sarafinovska, Z.; Eken, A.; Matevska, N.; Erdem, O.; Sayal, A.; Savaser, A.; Banev, S.; Petrovski, D.; Dzikova, S.; Georgiev, V.; et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin. Biochem. 2009, 42, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Arsova-Sarafinovska, Z.; Sayal, A.; Eken, A.; Erdem, O.; Erten, K.; Ozgök, Y.; Dimovski, A. Oxidative Stress and Antioxidant Status in Non-Metastatic Prostate Cancer and Benign Prostatic Hyperplasia. Clin. Biochem. 2006, 39, 176–179. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.K.; Bagatini, M.D.; Reetz, L.G.B.; Chiesa, J.; Battisti, I.E.; Gonçalves, J.F.; Duarte, M.M.F.; Schetinger, M.R.C.; Morsch, V.M. Oxidative stress and antioxidant status in prostate cancer patients: Relation to gleason score, treatment and bone metastasis. Biomed. Pharmacother. 2011, 65, 516–524. [Google Scholar] [CrossRef]
- Oh, B.; Figtree, G.; Costa, D.; Eade, T.; Hruby, G.; Lim, S.; Elfiky, A.; Martine, N.; Rosenthal, D.; Clarke, S.; et al. Oxidative Stress in Prostate Cancer Patients: A Systematic Review of Case Control Studies. Prostate Int 2016, 4, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk-Golec, K.; Tyloch, J.; Czuczejko, J. Antioxidant defense system in prostate adenocarcinoma and benign prostate hyperplasia of elderly patients. Neoplasma 2015, 62, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Tariba Lovaković, B.; Živković Semren, T.; Safner, T.; Gamulin, M.; Soče, M.; Pizent, A. Is low-level metal exposure related to testicular cancer? J. Environ. Sci. Health C Toxicol. Carcinog. 2021, 39, 87–107. [Google Scholar] [CrossRef]
- Gào, X.; Wilsgaard, T.; Jansen, E.H.J.M.; Xuan, Y.; Anusruti, A.; Brenner, H.; Schöttker, B. Serum total thiol levels and the risk of lung, colorectal, breast and prostate cancer: A prospective case–cohort study. Int. J. Cancer 2020, 146, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Henkler, F.; Brinkmann, J.; Luch, A. The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancers 2010, 2, 376–396. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Hartwig, A. Mechanisms in cadmium-induced carcinogenicity: Recent insights. Biometals 2010, 23, 951–960. [Google Scholar] [CrossRef]
- Zhu, Y.; Costa, M. Metals and molecular carcinogenesis. Carcinogenesis 2020, 41, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Damdimopoulou, P.; Stenius, U.; Halldin, K. Cadmium at nanomolar concentrations activates Raf–MEK–ERK1/2 MAPKs signaling via EGFR in human cancer cell lines. Chem.-Biol. Interact. 2015, 231, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Dasgupta, P.; Bhat, N.S.; Hashimoto, Y.; Saini, S.; Shahryari, V.; Yamamura, S.; Shiina, M.; Tanaka, Y.; Dahiya, R.; et al. Role of the PI3K/Akt pathway in cadmium induced malignant transformation of normal prostate epithelial cells. Toxicol. Appl. Pharmacol. 2020, 409, 115308. [Google Scholar] [CrossRef]
- Misra, U.K.; Gawdi, G.; Pizzo, S.V. Induction of mitogenic signalling in the 1LN prostate cell line on exposure to submicromolar concentrations of cadmium+. Cell. Signal. 2003, 15, 1059–1070. [Google Scholar] [CrossRef]
- Clarkson, T.W. The toxicology of mercury. Crit. Rev. Clin. Lab. Sci. 1997, 34, 369–403. [Google Scholar] [CrossRef]
- Fujimura, M.; Usuki, F. Methylmercury-mediated oxidative stress and activation of the cellular protective system. Antioxidants 2020, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, H.; Yin, Z.; Aschner, M.; Cai, J. Methylmercury toxicity and Nrf2-dependent detoxification in astrocytes. Toxicol. Sci. 2009, 107, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Buxton, S.; Garman, E.; Heim, K.E.; Lyons-Darden, T.; Schlekat, C.E.; Taylor, M.D.; Oller, A.R. Concise review of nickel human health toxicology and ecotoxicology. Inorganics 2019, 7, 89. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Jin, T.; Jiang, X.; Kong, Q.; Ye, T.; Nordberg, G.F. Effects on the prostate of environmental cadmium exposure—A cross-sectional population study in China. Biometals 2004, 17, 559–566. [Google Scholar] [CrossRef] [PubMed]
- van Wijngaarden, E.; Singer, E.A.; Palapattu, G.S. Prostate-specific antigen levels in relation to cadmium exposure and zinc intake: Results from the 2001–2002 National Health and Nutrition Examination Survey. Prostate 2008, 68, 122–128. [Google Scholar] [CrossRef]
- Matic, B.; Rakic, U.; Dejanovic, S.; Jovanovic, V.; Jevtic, M.; Djonovic, N. Industrially contaminated areas in Serbia as a potential public health threat to the exposed population. Tehnika 2017, 72, 441–447. [Google Scholar] [CrossRef]
- Halamić, J.; Miko, S. Geochemical atlas of the Republic of Croatia. Croat. Geol. Surv. 2009, 87, 823. [Google Scholar]
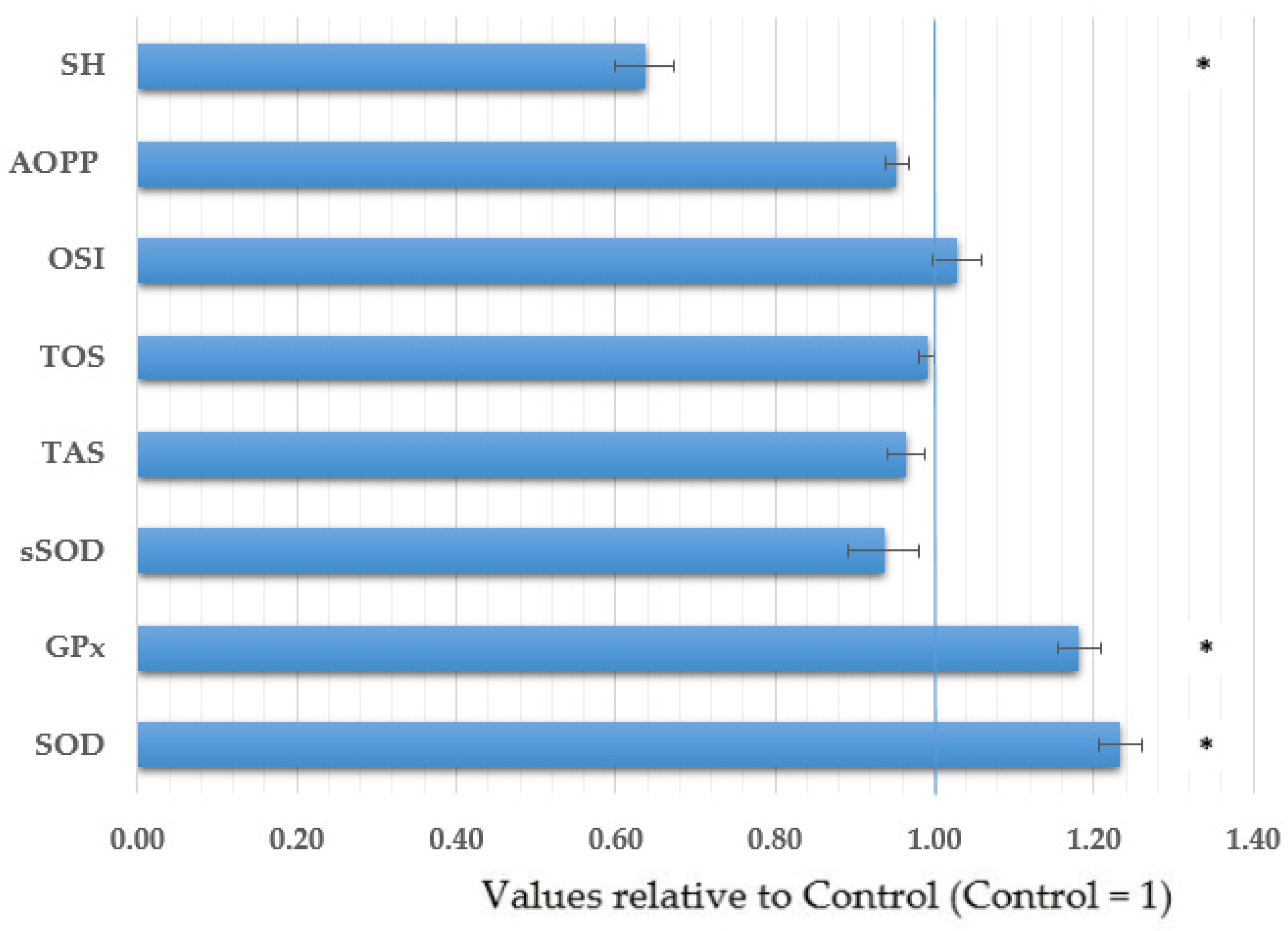
| Variable | Total | Croatian Cohort | Serbian Cohort | |||
|---|---|---|---|---|---|---|
| Cases (n = 103) | Control (n = 91) | Cases (n = 62) | Control (n = 30) | Cases (n = 41) | Control (n = 61) | |
| Age, years | 68 (44–81) | 45 (24–77) | 70 (54–81) | 54 (45–66) | 67 (44–78) | 38 (24–77) |
| BMI, kg/m2 | 26.7 (19.1–35.1) | 28.7 (20.1–40.1) | 26.5 (19.5–35.1) | 28.8 (23.8–37.4) | 26.8 (22.0–34.6) | 28.7 (20.1–40.1) |
| Active smoking status, N (%) | 17 (16.5) | 25 (27.5) | 9 (14.3) | 8 (25.8) | 8 (19) | 17 (27.4) |
| Current alcohol use, N (%) | 40 (38.8) | 27 (29.7) | 18 (28.6) | 8 (25.8) | 22 (52.4) | 19 (30.6) |
| PSA a, ng/mL | 11.99 (0.76–134.00) | 10.60 (2.34–134.00) | 13.81 (0.76–108.66) | |||
| Total | Croatian Cohort | Serbian Cohort | ||||
|---|---|---|---|---|---|---|
| Cases (n = 103) | Control (n = 91) | Cases (n = 62) | Control (n = 30) | Cases (n = 41) | Control (n = 61) | |
| Metal(loid) (µg/L) | ||||||
| Blood arsenic | ||||||
| median | 0.93 | 1.09 | 1.17 | 1.44 | 0.50 | 1.01 |
| IQR | 0.46–2.31 | 0.71–1.97 | 0.72–2.93 | 0.86–2.47 | 0.29–1.09 | 0.43–1.62 |
| p = 0.35 | p = 0.44 | p = 0.04 | ||||
| Blood cadmium | ||||||
| median | 0.80 | 0.80 | 0.41 | 0.40 | 1.30 | 1.00 |
| IQR | 0.32–1.36 | 0.49–1.30 | 0.27–0.77 | 0.24–0.83 | 1.10–1.90 | 0.80–1.40 |
| p = 0.66 | p = 0.88 | p = 0.0003 | ||||
| Blood chromium | ||||||
| median | 1.41 | 1.27 | 1.28 | 1.28 | 2.56 | 1.19 |
| IQR | 1.11–1.93 | 1.05–1.80 | 1.04–1.45 | 1.23–1.47 | 1.57–4.11 | 0.90–3.30 |
| p = 0.17 | p = 0.22 | p = 0.005 | ||||
| Blood lead | ||||||
| median | 20.75 | 32.00 | 23.35 | 22.34 | 15.70 | 37.70 |
| IQR | 11.77–37.56 | 21.2–47.24 | 14.95–44.13 | 15.60–31.94 | 7.90–21.65 | 28.00–53.90 |
| p = 0.0001 | p = 0.46 | p < 0.0001 | ||||
| Blood mercury | ||||||
| median | 7.10 | 2.92 | 3.49 | 1.15 | 13.51 | 3.72 |
| IQR | 2.58–14.33 | 1.08–4.74 | 1.75–8.10 | 0.48–2.40 | 7.66–20.76 | 2.16–5.82 |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | ||||
| Blood nickel | ||||||
| median | 10.56 | 17.57 | ||||
| IQR | 5.87–16.44 | 6.84–39.73 | ||||
| p = 0.03 | ||||||
| Serum nickel | ||||||
| median | 0.90 | 0.69 | ||||
| IQR | 0.65–1.10 | 0.63–0.75 | ||||
| p = 0.0006 | ||||||
| Parameter | BAs | BCd | BCr | BPb | BHg | BNi | SNi |
|---|---|---|---|---|---|---|---|
| (µg/L) | |||||||
| SOD (U/g Hb) | −0.040 | 0.113 | −0.126 | −0.067 | 0.365 | - | 0.219 |
| GPx (U/g Hb) | −0.099 | 0.012 | −0.149 | 0.050 | 0.102 | - | 0.125 |
| TAS (µmol/L) | 0.135 | 0.085 | −0.002 | 0.060 | −0.214 | −0.110 | - |
| TOS (µmol/L) | 0.077 | 0.057 | −0.062 | 0.049 | 0.016 | 0.006 | - |
| OSI | −0.113 | −0.073 | −0.035 | −0.034 | 0.223 | 0.090 | - |
| AOPP (µmol/L chloramine T equivalents) | −0.089 | −0.145 | −0.003 | 0.066 | −0.091 | −0.090 | - |
| SH (mmol/L) | 0.177 | −0.206 | −0.099 | 0.184 | −0.464 | 0.264 | - |
| sSOD (U/L) | 0.081 | 0.034 | −0.103 | 0.137 | −0.055 | 0.063 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizent, A.; Anđelković, M.; Tariba Lovaković, B.; Živković Semren, T.; Buha Djordjevic, A.; Gamulin, M.; Bonderović, V.; Aćimović, M.; Bulat, Z. Environmental Exposure to Metals, Parameters of Oxidative Stress in Blood and Prostate Cancer: Results from Two Cohorts. Antioxidants 2022, 11, 2044. https://doi.org/10.3390/antiox11102044
Pizent A, Anđelković M, Tariba Lovaković B, Živković Semren T, Buha Djordjevic A, Gamulin M, Bonderović V, Aćimović M, Bulat Z. Environmental Exposure to Metals, Parameters of Oxidative Stress in Blood and Prostate Cancer: Results from Two Cohorts. Antioxidants. 2022; 11(10):2044. https://doi.org/10.3390/antiox11102044
Chicago/Turabian StylePizent, Alica, Milena Anđelković, Blanka Tariba Lovaković, Tanja Živković Semren, Aleksandra Buha Djordjevic, Marija Gamulin, Vera Bonderović, Miodrag Aćimović, and Zorica Bulat. 2022. "Environmental Exposure to Metals, Parameters of Oxidative Stress in Blood and Prostate Cancer: Results from Two Cohorts" Antioxidants 11, no. 10: 2044. https://doi.org/10.3390/antiox11102044







