Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Solvents
2.3. Preparation of Extract
2.4. FT-IR Analyses of the Crude Extract
2.5. GC-MS Analysis of the P. glauca Extracts
2.6. Total Phenolic Content (TPC)
2.7. Antioxidant Activity
2.7.1. ABTS and CUPRAC Analysis
2.7.2. Chelating Activity of Fe2+ and Cu2+
2.7.3. Effect on Antioxidant Enzymes Activity
Effect on Catalase Activity (CAT)
Effect on Superoxide Dismutase Activity (SOD)
Effect on Glutathione Reductase (GR) Activity
Effect on Glutathione Peroxidase (GPx) Activity
2.8. Anticholinesterase Activity
2.8.1. Effect on Acetylcholinesterase (AChE) Activity
2.8.2. Effect on Butyrylcholinesterase (BChE) Activity
2.9. Molecular Docking on Cholinesterse Activity
2.10. Effect on Cyclooxygenase-2 (COX-2) Activity
2.11. Anti-Hyaluronidase Activity
2.12. Cell Culture and Media
2.12.1. In Vitro Cytotoxicity and Determination of IC50
2.12.2. Apoptosis Analysis
2.12.3. Cell Cycle Analysis
2.13. Statistical Analysis
3. Results
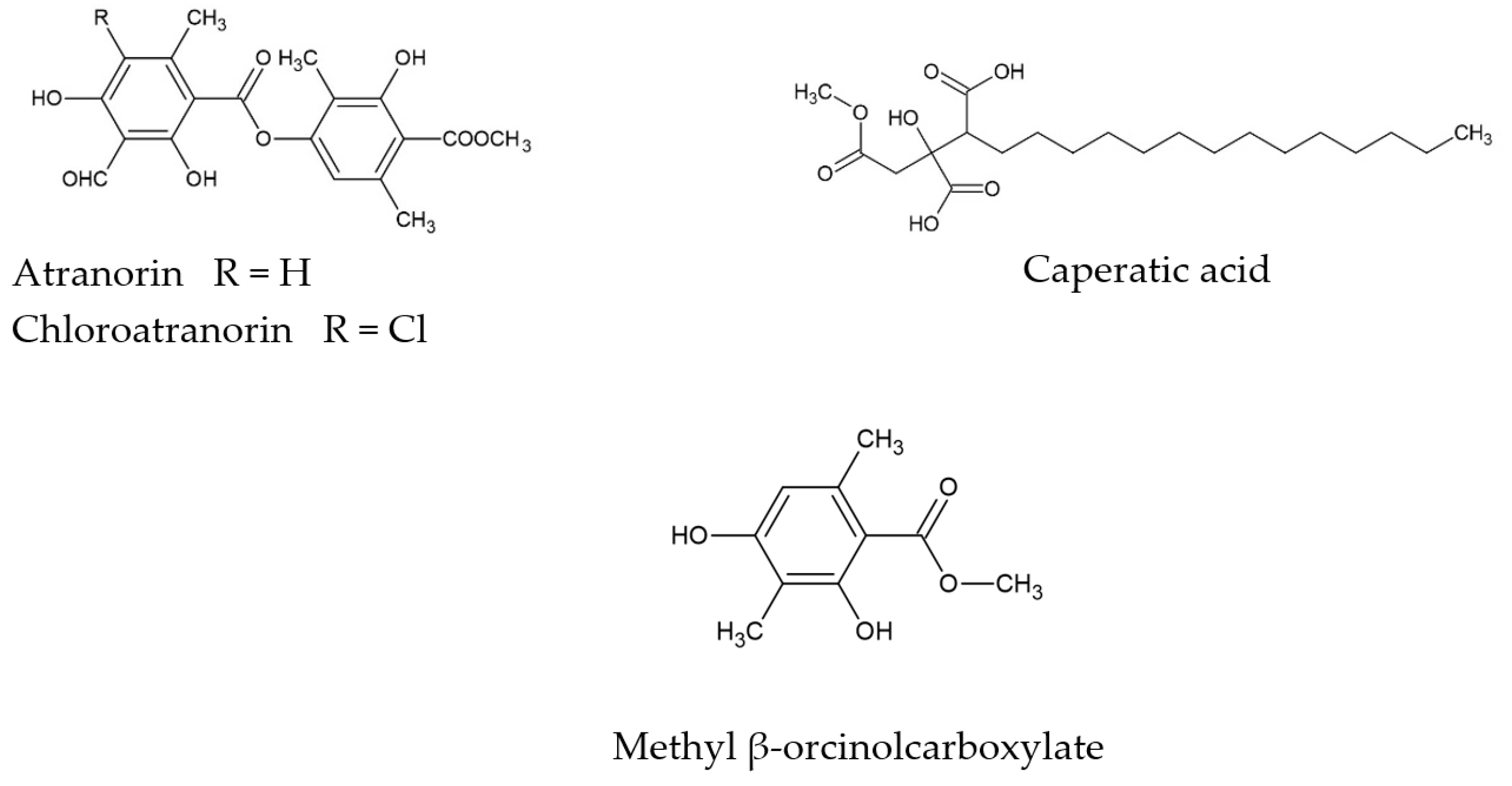
3.1. Phytochemicals Analysis of P. glauca
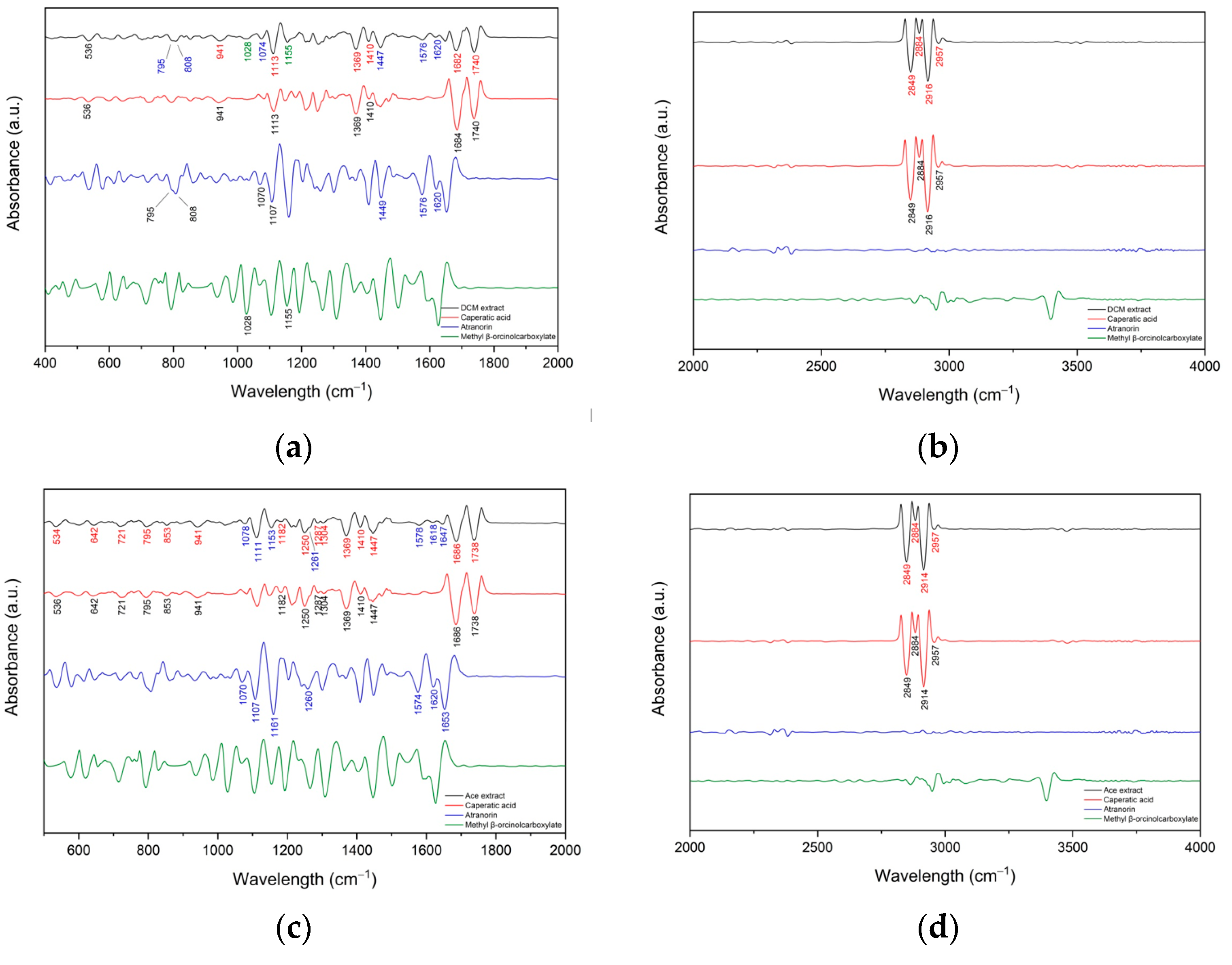
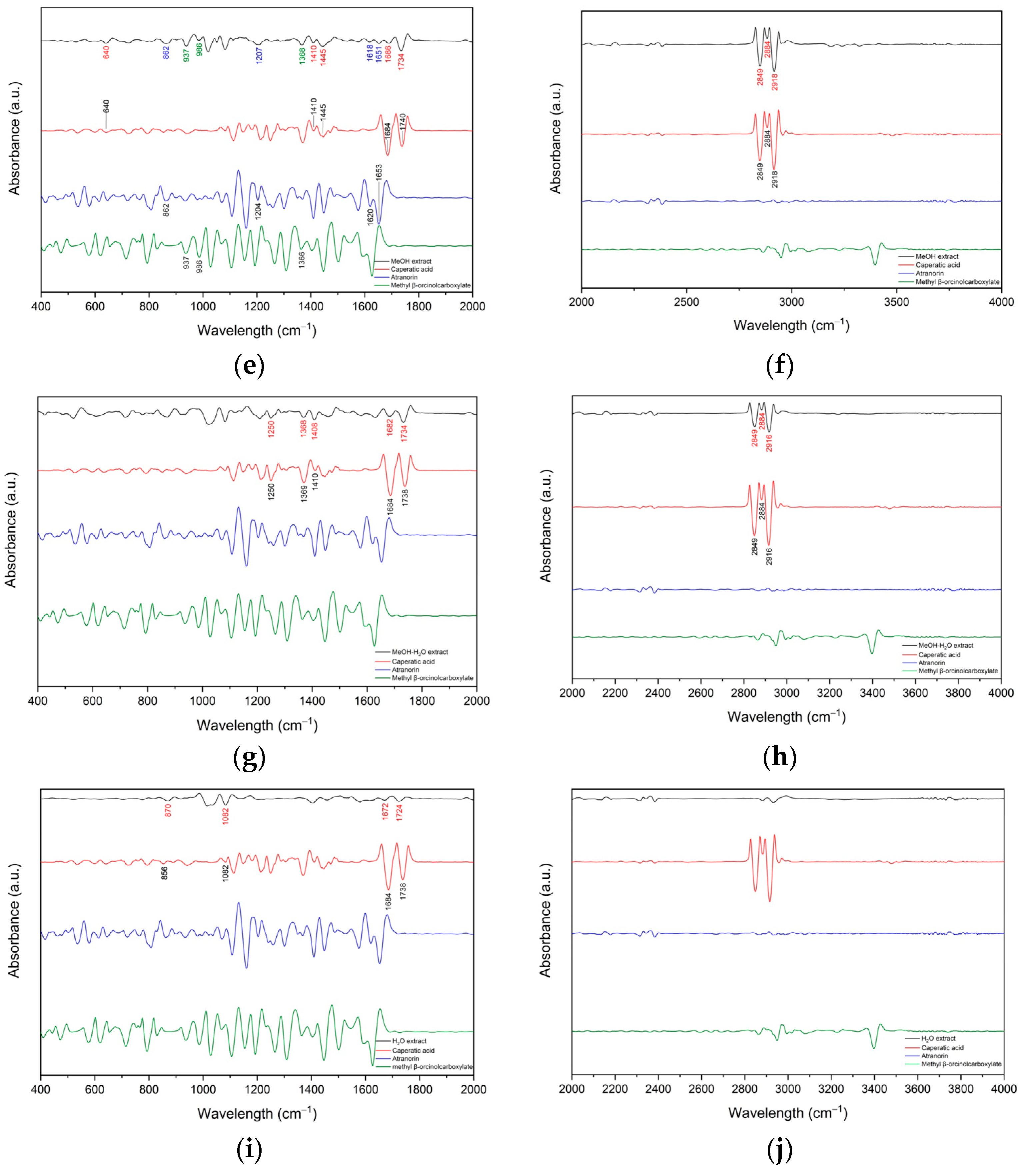
3.1.1. FT-IR Analyses
3.1.2. GC-MS Analysis of the P. glauca Extracts
3.1.3. Total Polyphenols Content
3.2. Biological activity
3.2.1. Antioxidant Activity of P. glauca
ABTS and CUPRAC Analysis
Cu2+ and Fe2+ Metal Chelating Ability
Impact on Reactive Oxygen Species (ROS) Homeostasis
3.2.2. Anticholinesterase Activity
AChE and BChE Inhibitory Effect
Molecular Docking on Cholinesterse
3.2.3. Anti-Inflammatory Activity
Inhibition of Cyclooxygenase-2 (COX-2)
Anti-Hyaluronidase Activity
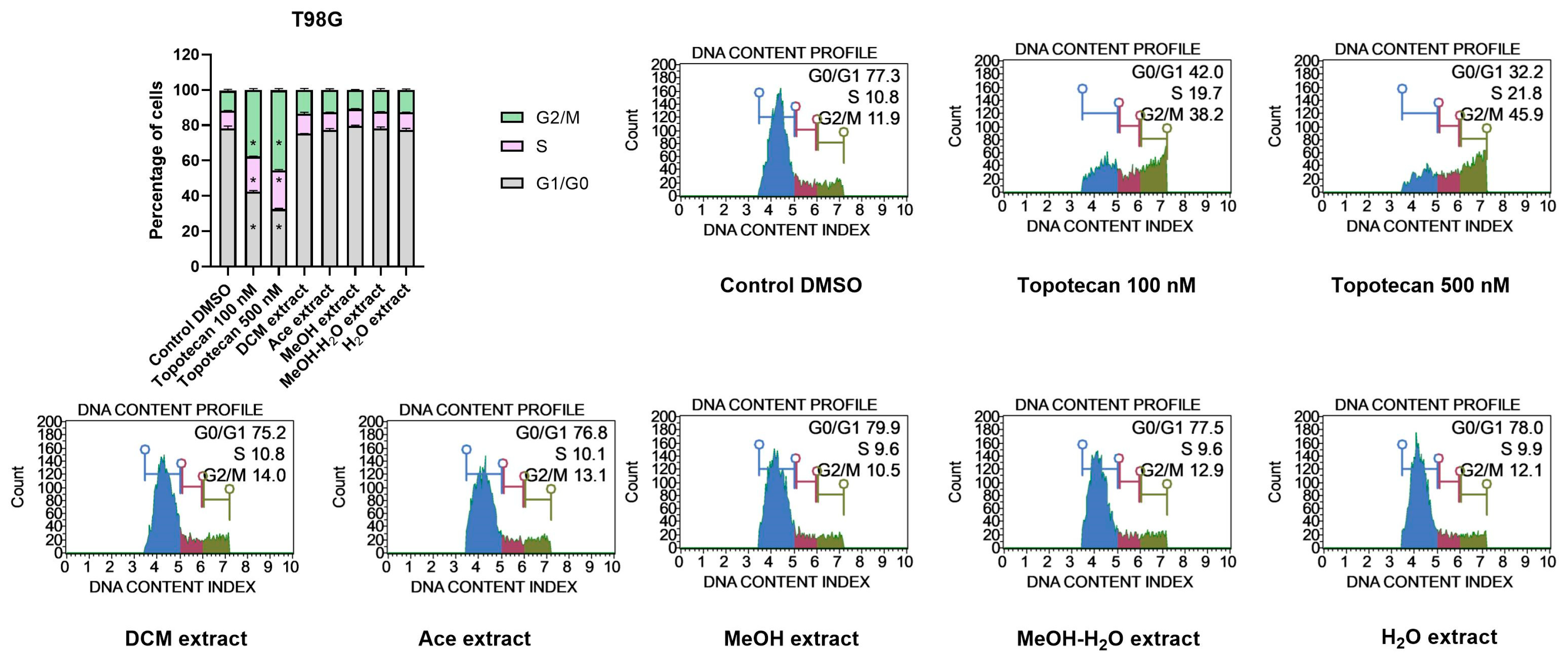
3.2.4. Anti-Tumour Activity
Cytotoxic Activity against GBM Cells
Pro-Apoptotic Activity
Impact on Cell Cycle Distribution of GBM Cells
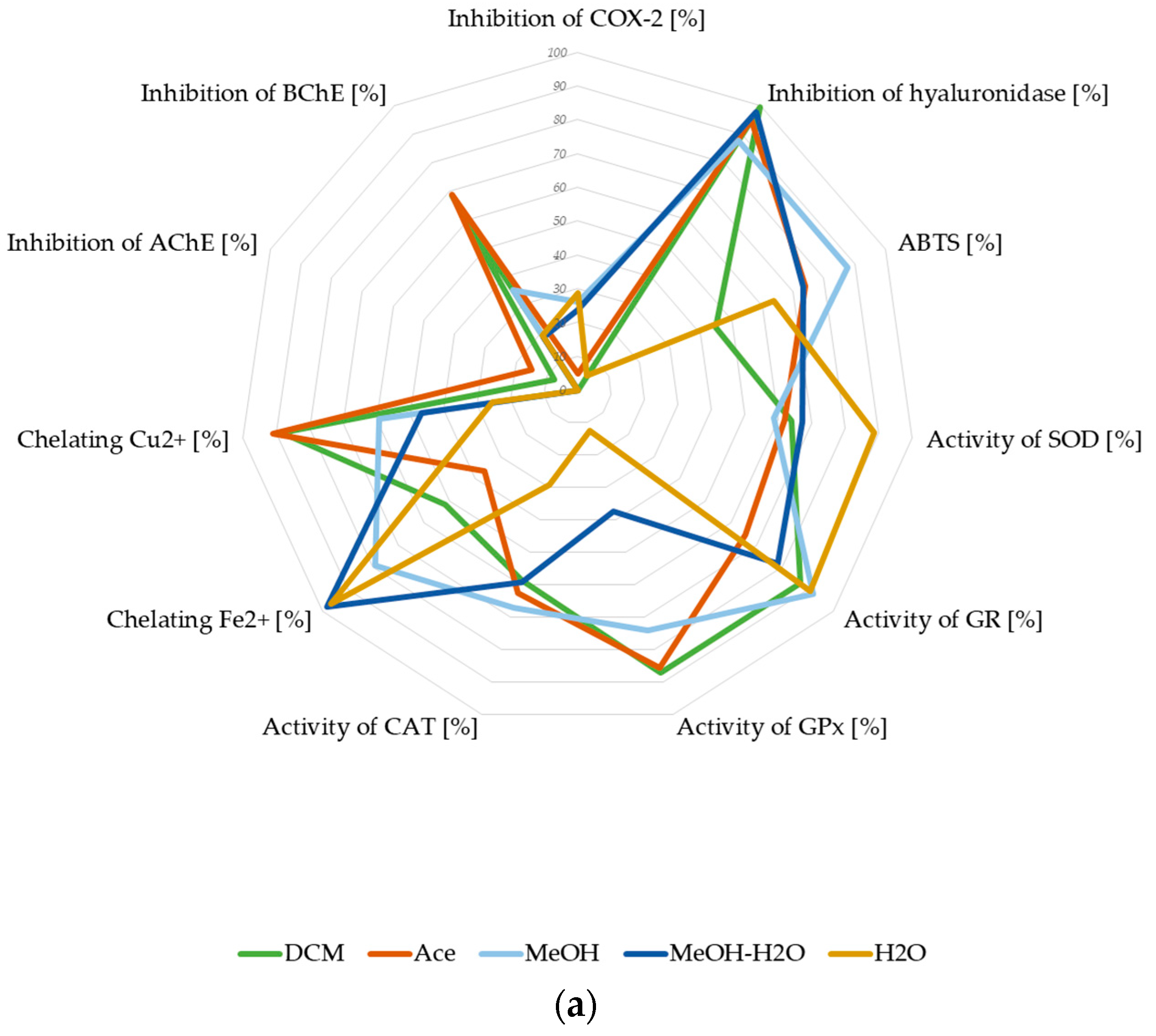
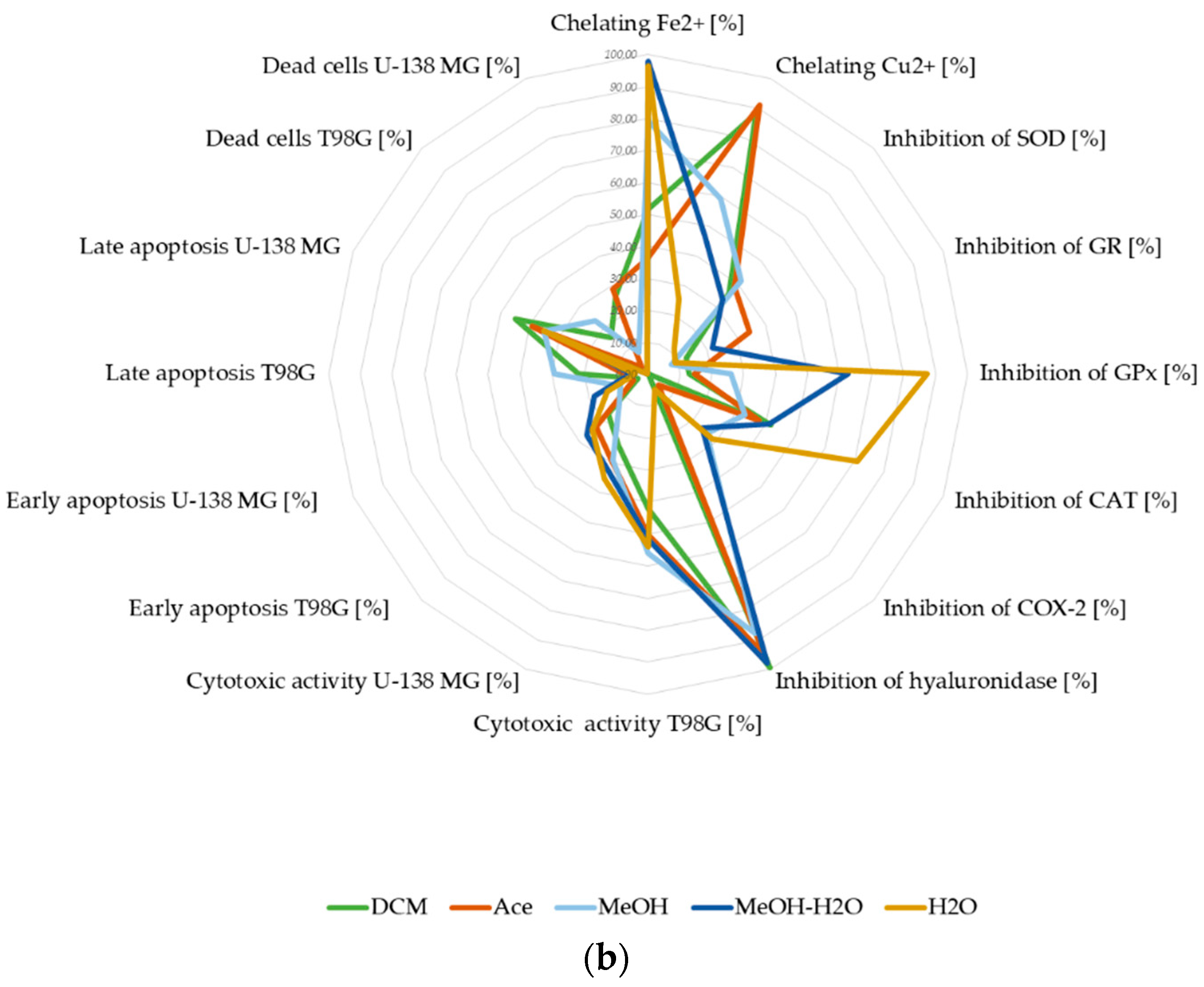
3.2.5. Summary of Biological Potential of Lichen-Derived Compounds and Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Javaid, S.F.; Giebel, C.; Khan, M.A.B.; Hashim, M.J. Epidemiology of Alzheimer’s disease and other dementias: Rising global burden and forecasted trends. F1000Research 2021, 10, 425. [Google Scholar] [CrossRef]
- Poddar, M.K.; Chakraborty, A.; Banerjee, S. Neurodegeneration: Diagnosis, prevention, and therapy. In Oxidoreductase; IntechOpen: London, UK, 2021; ISBN 1838809015. [Google Scholar]
- Teleanu, R.I.; Niculescu, A.-G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef] [PubMed]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s disease: Current therapeutic significance and future prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising incidence of glioblastoma multiforme in a well-defined population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Ge, H.; Lin, Y.; Kang, D. Glioblastoma vaccine tumor therapy research progress. Chin. Neurosurg. J. 2022, 8, 2. [Google Scholar] [CrossRef]
- Ogawa, K.; Kurose, A.; Kamataki, A.; Asano, K.; Katayama, K.; Kurotaki, H. Giant cell glioblastoma is a distinctive subtype of glioma characterized by vulnerability to DNA damage. Brain Tumor Pathol. 2020, 37, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, M.H.; Najda, A.; Albadrani, G.M.; Sayed, A.A. Natural bioactive molecules: An alternative approach to the treatment and control of glioblastoma multiforme. Biomed. Pharmacother. 2021, 141, 111928. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A. Impact of natural compounds on neurodegenerative disorders: From preclinical to pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [Green Version]
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular targets of natural compounds with anti-cancer properties. Int. J. Mol. Sci. 2021, 22, 13659. [Google Scholar] [CrossRef]
- Persano, F.; Gigli, G.; Leporatti, S. Natural Compounds as Promising Adjuvant Agents in the Treatment of Gliomas. Int. J. Mol. Sci. 2022, 23, 3360. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Kaproń, B.; Plech, T.; Żarowski, M.; Cielecka-Piontek, J. Lichen-Derived Compounds and Extracts as Biologically Active Substances with Anticancer and Neuroprotective Properties. Pharmaceuticals 2021, 14, 1293. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Celińska, A.; Kleszcz, R.; Studzińska-Sroka, E.; Łukaszyk, A.; Szoszkiewicz, A.; Stelcer, E.; Jopek, K.; Rucinski, M.; Cielecka-Piontek, J.; Krajka-Kuźniak, V. Lichen Secondary Metabolites Inhibit the Wnt/β-Catenin Pathway in Glioblastoma Cells and Improve the Anticancer Effects of Temozolomide. Cells 2022, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, T.; Stamenkovic, S.; Cvetkovic, V.; Radulovic, N.; Mladenovic, M.; Stankovic, M.; Topuzovic, M.; Radojevic, I.; Stefanovic, O.; Vasic, S.; et al. Platismatia glaucia and Pseudevernia furfuracea lichens as sources of antioxidant, antimicrobial and antibiofilm agents. EXCLI J. 2014, 13, 938–953. [Google Scholar]
- Ingelfinger, R.; Henke, M.; Roser, L.; Ulshöfer, T.; Calchera, A.; Singh, G.; Parnham, M.J.; Geisslinger, G.; Fürst, R.; Schmitt, I.; et al. Unraveling the Pharmacological Potential of Lichen Extracts in the Context of Cancer and Inflammation With a Broad Screening Approach. Front. Pharmacol. 2020, 11, 1322. [Google Scholar] [CrossRef]
- Obermayer, W.; Randlane, T. Morphological and chemical studies on Platismatia erosa (Parmeliaceae) from Tibet, Nepal and Bhutan. Bryologist 2012, 115, 51–60. [Google Scholar] [CrossRef]
- Abdallah, E.M. Evaluation of antimicrobial activity of a lichen used as a spice (Platismatia glauca). Adv. Life Sci. 2019, 6, 110–115. [Google Scholar]
- Gulluce, M.; Aslan, A.; Sokmen, M.; Sahin, F.; Adiguzel, A.; Agar, G.; Sokmen, A. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine 2006, 13, 515–521. [Google Scholar] [CrossRef]
- Šeklić, D.S.; Obradović, A.D.; Stanković, M.S.; Živanović, M.N.; Mitrović, T.L.; Stamenković, S.M.; Marković, S.D. Proapoptotic and antimigratory effects of Pseudevernia furfuracea and Platismatia glauca on colon cancer cell lines. Food Technol. Biotechnol. 2018, 56, 421. [Google Scholar] [CrossRef]
- Bézivin, C.; Tomasi, S.; Lohézic-Le Dévéhat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Paluszczak, J.; Kleszcz, R.; Studzińska-Sroka, E.; Krajka-Kuźniak, V. Lichen-derived caperatic acid and physodic acid inhibit Wnt signaling in colorectal cancer cells. Mol. Cell. Biochem. 2018, 441, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Chanaj-Kaczmarek, J.; Wysocki, M.; Karachitos, A.; Wojcińska, M.; Bartosz, G.; Matławska, I.; Kmita, H. Effects of plant extract antioxidative phenolic compounds on energetic status and viability of Saccharomyces cerevisiae cells undergoing oxidative stress. J. Funct. Foods 2015, 16, 364–377. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Galanty, A.; Gościniak, A.; Wieczorek, M.; Kłaput, M.; Dudek-Makuch, M.; Cielecka-Piontek, J. Herbal Infusions as a Valuable Functional Food. Nutrients 2021, 13, 4051. [Google Scholar] [CrossRef]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Watanabe, M.; de Moura Neiva, L.B.; da Costa Santos, C.X.; Laurindo, F.R.M.; Vattimo, M.d.F.F. Isoflavone and the heme oxygenase system in ischemic acute kidney injury in rats. Food Chem. Toxicol. 2007, 45, 2366–2371. [Google Scholar] [CrossRef]
- Szutowicz, A.; Kobes, R.D.; Orsulak, P.J. Colorimetric assay for monoamine oxidase in tissues using peroxidase and 2, 2′-azinodi (3-ethylbenzthiazoline-6-sulfonic acid) as chromogen. Anal. Biochem. 1984, 138, 86–94. [Google Scholar] [CrossRef]
- Parschat, K.; Canne, C.; Hüttermann, J.; Kappl, R.; Fetzner, S. Xanthine dehydrogenase from Pseudomonas putida 86: Specificity, oxidation–reduction potentials of its redox-active centers, and first EPR characterization. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 2001, 1544, 151–165. [Google Scholar] [CrossRef]
- Moreira, P.R.; Maioli, M.A.; Medeiros, H.C.D.; Guelfi, M.; Pereira, F.T.V.; Mingatto, F.E. Protective effect of bixin on carbon tetrachloride-induced hepatotoxicity in rats. Biol. Res. 2014, 47, 49. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Padmavathi, B.; Rao, A.R. Modulatory influence of Adhatoda vesica (Justicia adhatoda) leaf extract on the enzymes of xenobiotic metabolism, antioxidant status and lipid peroxidation in mice. Mol. Cell. Biochem. 2000, 213, 99–109. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- RCSB Protein Data Bank. Available online: https://www.rcsb.org (accessed on 7 January 2022).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 7 January 2022).
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Version 6.0; Semichem Inc.: Shawnee, KS, USA, 2016. [Google Scholar]
- UCSF Chimera. Available online: https://www.cgl.ucsf.edu/chimera/ (accessed on 8 January 2022).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Open Babel Program. Available online: http://openbabel.org (accessed on 8 January 2022).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Protein-Ligand Interaction Profiler. Available online: https://plip-tool.biotec.tu-dresden.de/ (accessed on 9 January 2022).
- Support|pymol.org. Available online: https://pymol.org/2/support.html? (accessed on 20 March 2022).
- Majchrzak-Celińska, A.; Zielińska-Przyjemska, M.; Wierzchowski, M.; Kleszcz, R.; Studzińska-Sroka, E.; Kaczmarek, M.; Paluszczak, J.; Cielecka-Piontek, J.; Krajka-Kuźniak, V. Methoxy-stilbenes downregulate the transcription of Wnt/β-catenin-dependent genes and lead to cell cycle arrest and apoptosis in human T98G glioblastoma cells. Adv. Med. Sci. 2021, 66, 6–20. [Google Scholar] [CrossRef]
- Chanaj-Kaczmarek, J.; Rosiak, N.; Szymanowska, D.; Rajewski, M.; Wender-Ozegowska, E.; Cielecka-Piontek, J. The Chitosan-Based System with Scutellariae baicalensis radix Extract for the Local Treatment of Vaginal Infections. Pharmaceutics 2022, 14, 740. [Google Scholar] [CrossRef]
- DeNoyer, L.K.; Dodd, J.G. Smoothing and derivatives in spectroscopy. In Handbook of Vibrational Spectroscopy; John Wiley Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Xu, C.-H.; Liu, S.-L.; Zhao, S.-N.; Li, S.-Z.; Sun, S.-Q. Unveiling ontogenesis of herbal medicine in plant chemical profiles by infrared macro-fingerprinting. Planta Med. 2013, 79, 1068–1076. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Ruiz, J.C.R.; Ordoñez, Y.B.M.; Basto, Á.M.; Campos, M.R.S. Antioxidant capacity of leaf extracts from two Stevia rebaudiana Bertoni varieties adapted to cultivation in Mexico. Nutr. Hosp. 2015, 31, 1163–1170. [Google Scholar]
- Winiarska-Mieczan, A.; Baranowska-Wójcik, E.; Kwiecień, M.; Grela, E.R.; Szwajgier, D.; Kwiatkowska, K.; Kiczorowska, B. The role of dietary antioxidants in the pathogenesis of neurodegenerative diseases and their impact on cerebral oxidoreductive balance. Nutrients 2020, 12, 435. [Google Scholar] [CrossRef] [Green Version]
- Sil, S.; Ghosh, T. Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer’s disease. J. Neuroimmunol. 2016, 291, 115–124. [Google Scholar] [CrossRef]
- Soria, F.N.; Paviolo, C.; Doudnikoff, E.; Arotcarena, M.-L.; Lee, A.; Danné, N.; Mandal, A.K.; Gosset, P.; Dehay, B.; Groc, L. Synucleinopathy alters nanoscale organization and diffusion in the brain extracellular space through hyaluronan remodeling. Nat. Commun. 2020, 11, 3440. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-G.; Jeong, Y.-I.; Jung, S.; Ryu, H.-H.; Jin, Y.-H.; Kim, I.-Y. The effect of hyaluronic acid on the invasiveness of malignant glioma cells: Comparison of invasion potential at hyaluronic acid hydrogel and matrigel. J. Korean Neurosurg. Soc. 2009, 46, 472. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, V.P.; Moura Neto, V.; Mentlein, R. Glioma infiltration and extracellular matrix: Key players and modulators. Glia 2018, 66, 1542–1565. [Google Scholar] [CrossRef] [PubMed]
- Millot, M.; Tomasi, S.; Articus, K.; Rouaud, I.; Bernard, A.; Boustie, J. Metabolites from the lichen Ochrolechia parella growing under two different heliotropic conditions. J. Nat. Prod. 2007, 70, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Neupane, B.P.; Malla, K.P.; Gautam, A.; Chaudhary, D.; Paudel, S.; Timsina, S.; Jamarkattel, N. Elevational trends in usnic acid concentration of lichen Parmelia flexilis in relation to temperature and precipitation. Climate 2017, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef] [Green Version]
- Marante, F.J.T.; Castellano, A.G.; Rosas, F.E.; Aguiar, J.Q.; Barrera, J.B. Identification and quantitation of allelochemicals from the lichen Lethariella canariensis: Phytotoxicity and antioxidative activity. J. Chem. Ecol. 2003, 29, 2049–2071. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Gómez-Serranillos, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [Green Version]
- Leuci, R.; Brunetti, L.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Importance of biometals as targets in medicinal chemistry: An overview about the role of Zinc (II) chelating agents. Appl. Sci. 2020, 10, 4118. [Google Scholar] [CrossRef]
- Bukhari, S.N.A. Dietary Polyphenols as Therapeutic Intervention for Alzheimer’s Disease: A Mechanistic Insight. Antioxidants 2022, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Suno, H.; Machida, M.; Dohi, T.; Ohmura, Y. Quantum chemical calculation studies toward microscopic understanding of retention mechanism of Cs radioisotopes and other alkali metals in lichens. Sci. Rep. 2021, 11, 8228. [Google Scholar] [CrossRef] [PubMed]
- Maslać, A.; Maslać, M.; Tkalec, M. The impact of cadmium on photosynthetic performance and secondary metabolites in the lichens Parmelia sulcata, Flavoparmelia caperata and Evernia prunastri. Acta Bot. Croat. 2016, 75, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Purvis, O.W. Adaptation and interaction of saxicolous crustose lichens with metals. Bot. Stud. 2014, 55, 23. [Google Scholar] [CrossRef] [Green Version]
- Hauck, M.; Huneck, S. Lichen substances affect metal adsorption in Hypogymnia physodes. J. Chem. Ecol. 2007, 33, 219–223. [Google Scholar] [CrossRef]
- Hauck, M. Metal homeostasis in Hypogymnia physodes is controlled by lichen substances. Environ. Pollut. 2008, 153, 304–308. [Google Scholar] [CrossRef]
- Hauck, M.; Willenbruch, K.; Leuschner, C. Lichen substances prevent lichens from nutrient deficiency. J. Chem. Ecol. 2009, 35, 71–73. [Google Scholar] [CrossRef] [Green Version]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy:‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Zalewska-Ziob, M.; Adamek, B.; Kasperczyk, J.; Romuk, E.; Hudziec, E. Chwali nska, E.; Dobija-Kubica, K.; Rogozinski, P.; Brulinski, K. Activity of Antioxidant Enzymes in the Tumor and Adjacent Noncancerous Tissues of Non-Small-Cell Lung Cancer. Oxid. Med. Cell. Longev. 2019, 2019, 2901840. [Google Scholar] [CrossRef] [Green Version]
- Backos, D.S.; Franklin, C.C.; Reigan, P. The role of glutathione in brain tumor drug resistance. Biochem. Pharmacol. 2012, 83, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhao, X.; Li, Y.; Li, G.; Liu, X. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease. Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Lan, J.; Shan, S.; Huang, E.; Bai, Y.; Guo, Y.; Jiang, D. Study of the antioxidant enzymes in human brain tumors. J. Neurooncol. 1996, 29, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Olivier, C.; Oliver, L.; Lalier, L.; Vallette, F.M. Drug resistance in glioblastoma: The two faces of oxidative stress. Front. Mol. Biosci. 2021, 7, 620677. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Gao, W.; Ma, S.; Liu, Y.; Lin, W. Observation of the elevation of cholinesterase activity in brain glioma by a near-infrared emission chemsensor. Anal. Chem. 2020, 92, 13405–13410. [Google Scholar] [CrossRef]
- Thompson, E.G.; Sontheimer, H. Acetylcholine receptor activation as a modulator of glioblastoma invasion. Cells 2019, 8, 1203. [Google Scholar] [CrossRef] [Green Version]
- Coelho dos Santos, T.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Antonio de Andrade Paes, M. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease. Ther. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121. [Google Scholar] [CrossRef] [Green Version]
- Reddy, R.G.; Veeraval, L.; Maitra, S.; Chollet-Krugler, M.; Tomasi, S.; Lohezic-Le Devehat, F.; Boustie, J.; Chakravarty, S. Lichen-derived compounds show potential for central nervous system therapeutics. Phytomedicine 2016, 23, 1527–1534. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of phytonutrient modulation of cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- Zhou, J.-R.; Kanda, Y.; Tanaka, A.; Manabe, H.; Nohara, T.; Yokomizo, K. Anti-hyaluronidase activity in vitro and amelioration of mouse experimental dermatitis by tomato saponin, Esculeoside A. J. Agric. Food Chem. 2016, 64, 403–408. [Google Scholar] [CrossRef]
- Dharmajaya, R.; Sari, D.K. Role and value of inflammatory markers in brain tumors: A case controlled study. Ann. Med. Surg. 2021, 63, 102107. [Google Scholar] [CrossRef] [PubMed]
- Monslow, J.; Govindaraju, P.; Puré, E. Hyaluronan—A functional and structural sweet spot in the tissue microenvironment. Front. Immunol. 2015, 6, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majchrzak-Celińska, A.; Misiorek, J.O.; Kruhlenia, N.; Przybyl, L.; Kleszcz, R.; Rolle, K.; Krajka-Kuźniak, V. COXIBs and 2,5-dimethylcelecoxib counteract the hyperactivated Wnt/β-catenin pathway and COX-2/PGE2/EP4 signaling in glioblastoma cells. BMC Cancer 2021, 21, 493. [Google Scholar] [CrossRef] [PubMed]
- Šeklić, D.S.; Jovanović, M.M. Platismatia glauca—Lichen species with suppressive properties on migration and invasiveness of two different colorectal carcinoma cell lines. J. Food Biochem. 2022, 46, e14096. [Google Scholar] [CrossRef]
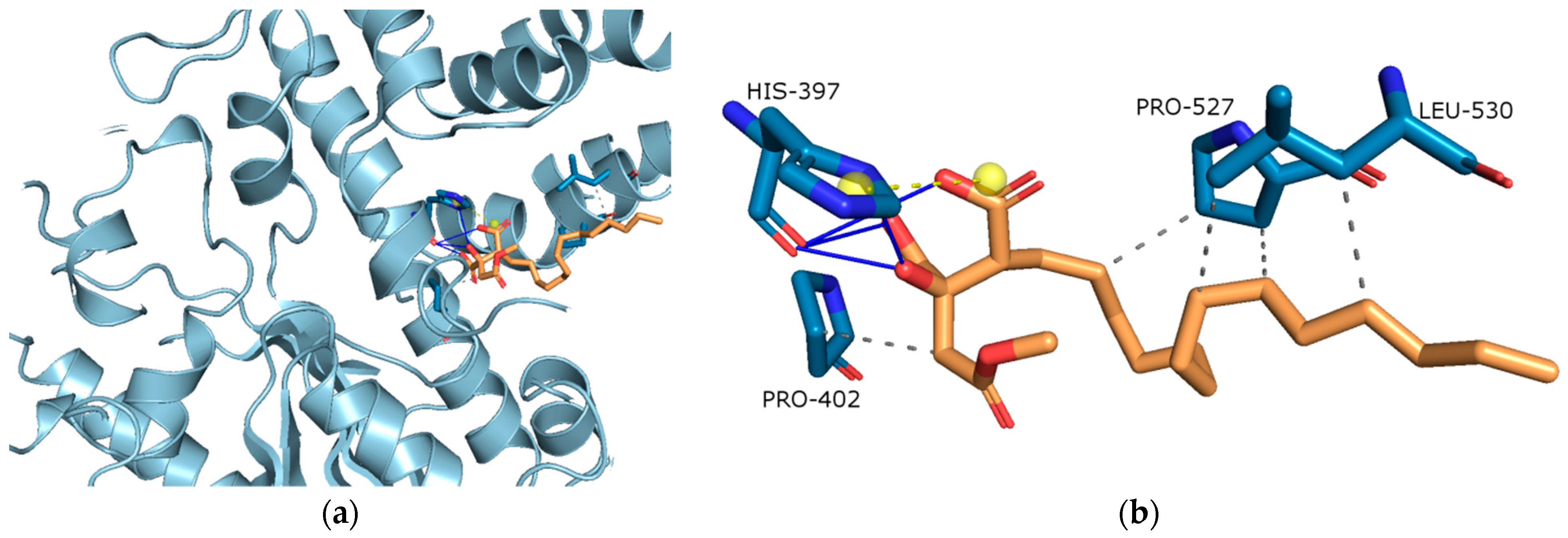
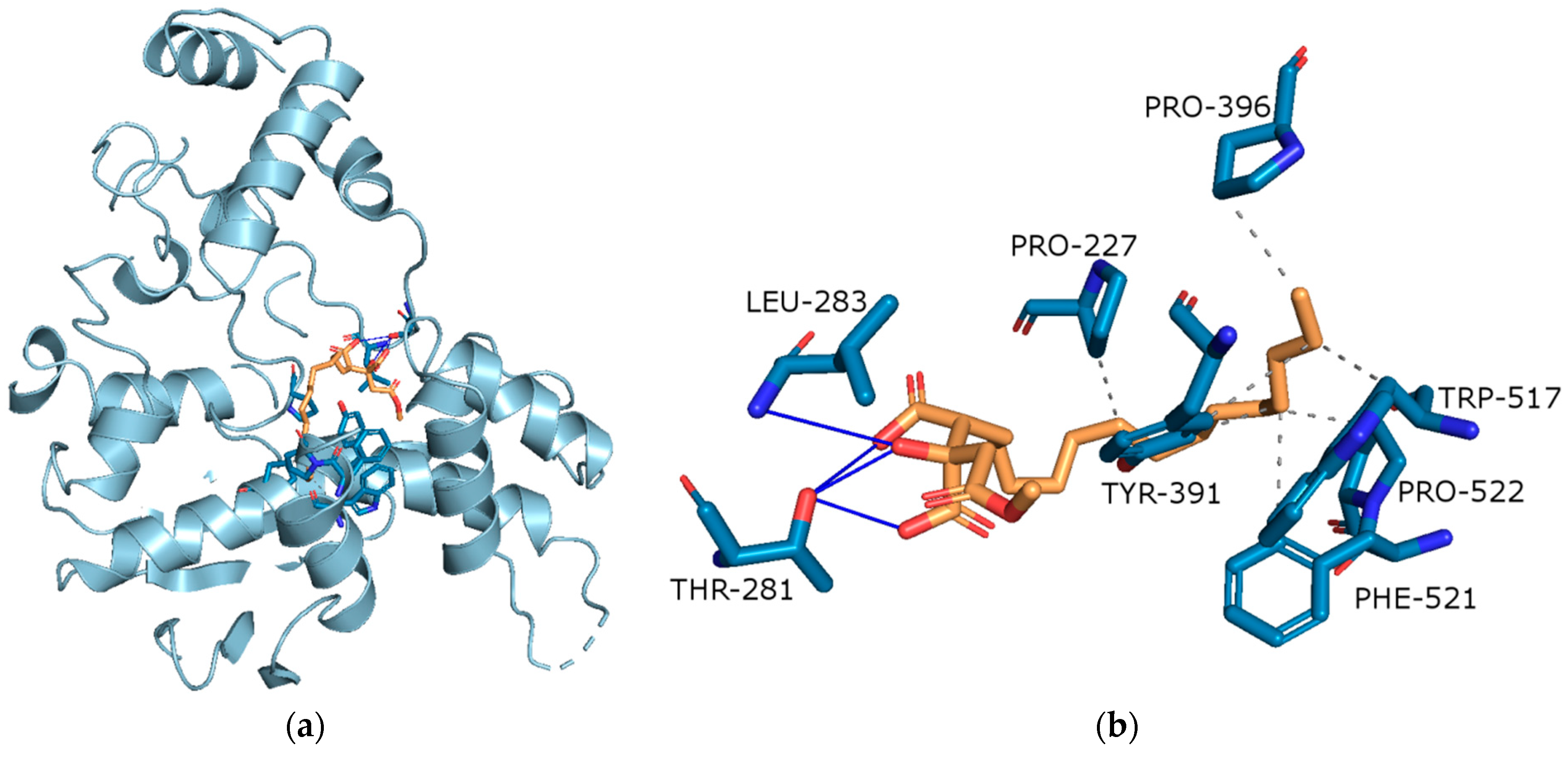
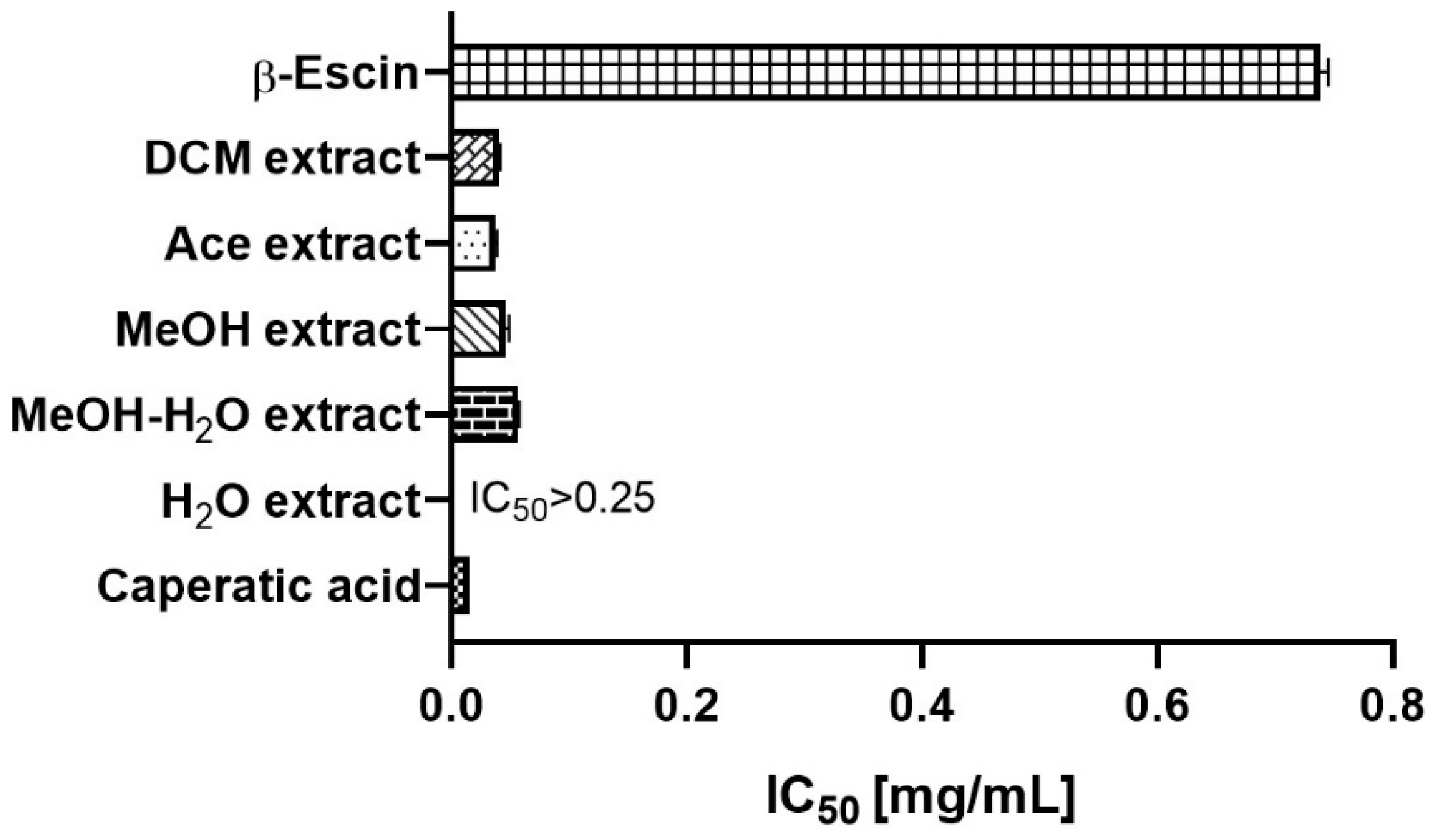

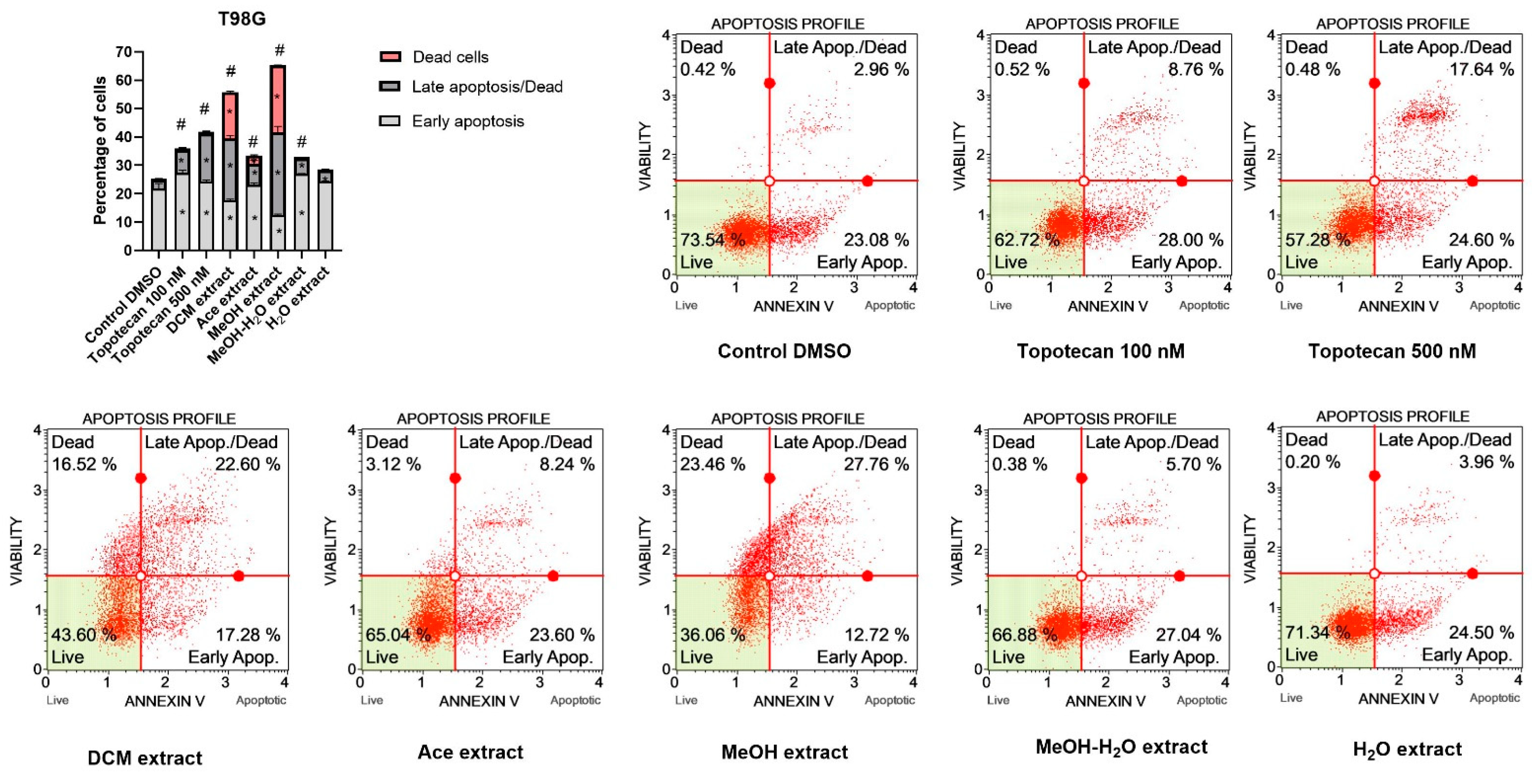


| Extract | Rt (min.) | Compounds | % of Total | MW | Formula |
|---|---|---|---|---|---|
| DCM extract | 6.305 | 2,2,6-trimethyloctane | 3.63 | 156.31 | C11H24 |
| 6.559 | 2,2,3,5-tetramethylheptane | 0.85 | 156.31 | C11H24 | |
| 6.886 | 2,3,3-trimethyloctane | 0.62 | 156.31 | C11H24 | |
| 6.928 | 3,3,5-trimethylheptane | 0.49 | 142.28 | C10H22 | |
| 7.043 | benzeneacetic acid, 3-tetradecyl ester | 0.24 | 332.50 | C22H36O2 | |
| 7.431 | 2,2,4,6,6-pentamethylheptane | 6.87 | 170.33 | C12H26 | |
| 7.659 | 2,3,6,7-tetramethyloctane | 4.55 | 170.33 | C12H26 | |
| 7.706 | 2,5,9-trimethyldecane | 1.07 | 184.37 | C13H28 | |
| 7.798 | 5,6-dimethyldecane | 0.21 | 170.33 | C12H26 | |
| 7.997 | 5-ethyl-2,2,3-trimethylheptane | 7.11 | 170.33 | C12H26 | |
| 8.034 | 2,6,7-trimethyldecane | 0.73 | 184.37 | C13H28 | |
| 8.324 | 5-nonanone | 60.01 | 142.24 | C9H18O | |
| 8.377 | decane | 2.72 | 142.28 | C10H22 | |
| 8.417 | 4-ethyl-2,2-dimethylhexane | 2.19 | 142.28 | C10H22 | |
| 8.545 | 3,7-dimethyldecane | 0.97 | 170.33 | C12H26 | |
| 8.626 | 2,2,4-trimethyl-5-hexen-3-ol | 0.71 | 142.24 | C9H18O | |
| 8.691 | 2,4-dimethylundecane | 0.47 | 184.37 | C13H28 | |
| 21.688 | 1,2,3,5,6,7-hexahydro-1,1,4,8-tetramethyl-s-indacene | 5.59 | 214.35 | C16H22 | |
| 25.641 | 9,9a-bis(acetyloxy)-1,1a,1b,4,4a,7a,7b,8,9,9a-decahydro-4a,7b-dihydroxy-1,1,6,8-tetramethyl-3-[(triphenylmethoxy)methyl]-, [1aR-(1aa,1bß,4aß,7aa,7ba,8a,9ß,9aa)]-5H-cyclopropa[3,4]benz[1,2-e]azulen-5-one | 0.97 | 690.83 | C43H46O8 | |
| Total | 100.00 | ||||
| Ace extract | 3.231 | triethyl borate | 12.24 | 145.99 | C6H15BO3 |
| 17.205 | benzoic acid, 2,4-dihydroxy-3,6-dimethylmethyl ester methyl ester | 46.50 | 196.20 | C10H12O4 | |
| 17.504 | atranorin | 4.34 | 374.35 374.35 374.35 | C19H18O8 | |
| 18.952 | 2-heptadecanone | 1.63 | 254.45 | C17H34O | |
| 19.174 | hexadecanoic acid, methyl ester | 0.54 | 270.45 | C17H34O2 | |
| 22.757 | E,E,Z-1,3,12-nonadecatriene-5,14-diol | 3.43 | 294.47 | C19H34O2 | |
| 23.057 | 12-methyl-E,E-2,13-octadecadien-1-ol | 2.75 | 280.49 | C19H36O | |
| 24.900 | spiculesporic acid | 27.17 | 328.40 | C17H28O6 | |
| 25.039 | ethyl iso-allocholate | 1.40 | 436.62 | C26H44O5 | |
| Total | 100.00 | ||||
| MeOH extract | 8.340 | acetophenone | 23.25 | 120.15 | C8H8O |
| 13.419 | (R)-α-methylbenzenemethanol | 0.36 | 122.16 | C8H10O | |
| 14.854 | 2,3,3-trimethyl-5-phenyl-1-pentene | 17.52 | 188.31 | C14H20 | |
| 18.797 | 3-phenylbutyrophenone | 22.46 | 224.30 | C16H16O | |
| 20.132 | 1,3-diphenyl-2-buten-1-one | 36.41 | 222.28 | C16H14O | |
| Total | 100.00 | ||||
| MeOH-H2O extract | 14.859 | 2,3,3-trimethyl-5-phenyl-1-pentene | 41.56 | 188.31 | C14H20 |
| 14.923 | (R*,R*)-5-nitro-1-phenyl-1-hexen-3-ol | 2.04 | 221.25 | C12H15NO3 | |
| 18.842 | 3-phenylbutyrophenone | 43.36 | 224.30 | C16H16O | |
| 20.219 | 1,3-diphenyl-2-buten-1-one | 9.65 | 222.28 | C16H14O | |
| 25.617 | 1,4-diol-1,4-diphenyl-2-butene | 3.39 | 240.30 | C16H16O2 | |
| Total | 100.00 | ||||
| H2O extract | 7.703 | 2-(2-(2-methoxyethoxy)ethoxy)ethyl 2-methylbutanoate | 5.66 | 248.32 | C12H24O5 |
| 9.653 | 4,5-dihydro-5-thioxo-1,2,4-triazin-3(2H)-one | 17.77 | 129.14 | C3H3N3OS | |
| 12.722 | 4,4,5,5-tetramethyl-2-phenyl-1,3,2-dioxaborolane | 14.28 | 204.07 | C12H17BO2 | |
| 13.531 | 4-octadecenal | 2.90 | 266.46 | C18H34O | |
| 14.003 | 9-oximino-2,7-diethoxyfluorene | 2.59 | 283.33 | C17H17NO3 | |
| 21.523 | triphenylphosphine | 56.81 | 262.29 | C18H15P | |
| Total | 100.00 |
| Extract/Substance | ABTS Antiradical Activity [%] | CUPRAC Activity | |||||
|---|---|---|---|---|---|---|---|
| Concentration [mg/mL] | IC50 [mg/mL] | IC0.5 [mg/mL] | |||||
| 0.024 | 0.048 | 0.095 | 0.190 | 0.381 | |||
| DCM extract | 25.88 ± 0.39 c | 35.87 ± 0.36 b | 41.71 ± 0.40 b | 42.56 ± 0.33 c | 44.61 ± 0.71 d | 0.381 f | 0.092 ± 0.00 a |
| Ace extract | 27.01 ± 0.44 a | 38.18 ± 0.26 a | 44.52 ± 0.18 a | 51.92 ± 0.22 b | 73.92 ± 0.28 b | 0.155 ± 0.004 c | 0.146 ± 0.002 c |
| MeOH extract | 19.32 ± 0.32 b | 28.79 ± 0.28 c | 41.78 ± 0.42 b | 63.23 ± 0.59 a | 87.64 ± 0.19 a | 0.129 ± 0.004 b | 0.245 ± 0.001 d |
| MeOH-H2O extract | 10.36 ± 0.27 c | 18.16 ± 0.74 d | 32.03 ± 0.50 c | 51.99 ± 1.27 b | 73.49 ± 0.65 b | 0.178 ± 0.013 d | 0.388 ± 0.002 f |
| H2O extract | 7.67 ± 0.19 d | 13.93 ± 0.24 e | 22.85 ± 0.49 d | 39.73 ± 0.31 d | 63.75 ± 0.82 c | 0.260 ± 0.007 e | 0.325 ± 0.001 e |
| Caperatic acid | 2.81 ± 0.93 e | 4.56 ± 0.35 f | 8.24 ± 0.14 e | 17.84 ±0.36 e | 31.67 ± 0.62 e | 0.381 f | 0.882 ± 0.051 g |
| Quercetin | nt | 0.002 ± 0.000 a | nt | ||||
| Vitamin C | nt | 0.006 ± 0.000 a | 0.012 ± 0.000 a | ||||
| Extracts/ Substance | Chelating Cu2+ [%] | IC50 [µg/mL] | |||||
|---|---|---|---|---|---|---|---|
| Concentration [mg/mL] | |||||||
| 0.005 | 0.01 | 0.02 | 0.04 | 0.08 | 0.16 | ||
| DCM extract | 18.7 ± 0.2 b | 35.1 ± 1.2 b | 58.8 ± 2.0 b | 88.4 ± 2.6 b | 96.0 ± 0.8 a | 94.7 ± 1.2 ab | 14.7 ± 0.6 a |
| Ace extract | 26.3 ± 1.5 a | 43.7 ± 0.6 a | 71.4 ± 0.3 a | 91.2 ± 1.3 ab | 96.9 ± 0.5 a | 96.1 ± 0.3 ab | 12.0 ± 0.8 a |
| MeOH extract | nt | 25.3 ± 1.4 c | 38.7 ± 4.6 c | 59.2 ± 4.4 c | 94.7 ± 0.5 a | 96.7 ± 0.1 ab | 29.8 ± 2.4 ba |
| MeOH-H2O extract | nt | 17.3 ± 0.1 d | 28.2 ± 1.3 d | 46.7 ± 1.6 d | 72.3 ± 3.6 b | 90.3 ± 0.7 b | 45.7 ± 3.1 b |
| H2O extracta | nt | 10.5 ± 0.2 e | 16.8 ± 1.8 e | 25.4 ± 3.1 e | 43.6 ± 0.9 c | 51.1 ± 1.6 c | 141.0 ± 17.4 c |
| Quercetin | 26.0 ± 2.0 a | 44.3 ± 0.5 a | 72.7 ± 0.9 a | 97.5 ± 1.0 a | 97.7 ± 0.1 a | 98.5 ± 5.2 a | 12.4 ± 0.4 a |
| Extracts/ Substance | Chelating Fe2+ [%] | IC50 [mg/mL] | ||||
|---|---|---|---|---|---|---|
| Concentration [mg/mL] | ||||||
| 0.08 | 0.16 | 0.8 | 1.6 | 4.0 | ||
| DCM extract | 7.9 ± 0.3 c | 13.8 ± 0.7 c | 35.6 ± 0.8 d | 51.7 ± 3.6 c | 61.4 ± 1.3 c | 1.83 ± 0.21 d |
| Ace extract | 6.5 ± 0.1 dc | 7.0 ± 1.3 d | 20.7 ± 0.2 e | 36.6 ± 5.6 d | 40.0 ± 3.2 d | 4.0 e |
| MeOH extract | 10.9 ± 0.5 b | 16.9 ± 1.5 c | 52.9 ± 2.7 b | 79.3 ± 2.2 b | 96.1 ± 0.3 a | 0.55 ± 0.02 b |
| MeOH-H2O extract | 36.0 ± 1.3 a | 64.2 ± 1.8 a | 90.9 ± 2.4 a | 98.0 ± 0.2 a | 100.2 ± 0.9 a | 0.10 ± 0.01 a |
| H2O extract | 34.4 ± 0.9 a | 48.2 ± 2.5 b | 92.8 ± 0.5 a | 96.5 ± 0.3 d | 99.2 ± 0.5 a | 0.15 ± 0.01 a |
| Quercetin | 5.2 ± 0.9 d | 16.2 ± 0.8 c | 42.5 ± 1.0 c | 54.2 ± 1.7 c | 72.6 ± 1.8 b | 1.17 ± 0.03 c |
| Extract/Substance | CAT Inhibition Under Reaction Conditions (%) | SOD Inhibition Under Reaction Conditions (%) | GR Inhibition under Reaction Conditions (%) | GPx Inhibition under Reaction Conditions (%) |
|---|---|---|---|---|
| DCM extract | 41.5 ± 3.5 b | 36.1 ± 2.2 b | 13.0 ± 2.1 c | 12.8 ± 2.4 e |
| Ace extract | 37.5 ± 4.4 bc | 38.3 ± 2.5 ab | 34.5 ±1.0 a | 14.5 ± 3.9 e |
| MeOH extract | 32.8 ± 3.1 bc | 41.3 ± 3.0 a | 7.9 ±1.4 d | 26.0 ± 4.1 d |
| MeOH-H2O extract | 40.9 ± 1.6 b | 33.0 ± 1.1 c | 21.7 ± 0.9 b | 62.5 ± 2.0 c |
| H2O extract | 70.8 ± 3.0 a | 11.5 ± 1.9 e | 9.2 ± 2.1 d | 87.5 ± 3.2 a |
| Caperatic acid | 30.7 ± 5.3 bc | 24.2 ± 0.8 d | 19.4 ± 2.3 b | 79.5 ± 3.8 b |
| Extract/ Substances | AChE Inhibition [%] | BChE Inhibition [%] | ||||
|---|---|---|---|---|---|---|
| Concentration [mg/mL] | IC50 [mg/mL] | Concentration [mg/mL] | IC50 [mg/mL] | |||
| 0.4 | 0.8 | 0.4 | 0.8 | |||
| DCM extract | na | 7.65 ± 1.35 c | nc | 50.57 ± 0.81 a | 68.56 ± 1.45 a | 0.405 ± 0.009 b |
| Ace extract | na | 14.82 ± 0.96 b | nc | 37.69 ± 0.68 b | 68.58 ± 0.22 a | 0.503 ± 0.057 c |
| MeOH extract | na | na | nc | 21.70 ± 1.22 d | 35.21 ± 1.27 b | 0.800 e |
| MeOH-H2O extract | na | na | nc | 6.64 ± 1.50 f | 18.64 ± 1.32 c | 0.800 e |
| H2O extract | na | na | nc | 11.60 ± 1.60 e | 19.13 ± 0.91 c | 0.800 e |
| Caperatic acid | 3.04 ± 0.72 | 30.98 ± 2.29 a | nc | 31.11 ± 0.88 c | 69.11 ± 1.93 a | 0.610 ± 0.013 d |
| Galantamine | nt | 0.00046 ± 0.00004 | nt | 0.004 ± 0.000 a | ||
| Extract/Substance | Equivalent Concentration of Acetylsalicylic Acid [mg/mL] | COX-2 Inhibition under Reaction Conditions [%] |
|---|---|---|
| DCM extract | na | na |
| Ace extract | 3.39 ± 0.01 e | 4.8 ± 1.0 d |
| MeOH extract | 18.64 ± 0.00 b | 26.2 ± 0.2 b |
| MeOH-H2O extract | 16.95 ± 0.01 d | 23.8 ± 0.2 c |
| H2O extract | 22.03 ± 0.02 a | 28.6 ± 0.3 a |
| Caperatic acid | 18.25 ± 0.02 c | 25.9 ± 0.1 b |
| Extracts/ Substance | T98G | U-138 MG |
|---|---|---|
| IC50 (µg/mL) | ||
| DCM | 65.8 ± 18.9 a | 72.8 ± 2.0 a |
| Ace | 44.8 ± 10.0 a | 65.7 ± 5.0 a |
| MeOH | 31.8 ± 10.4 a | 70.6 ± 11.9 a |
| MeOH-H2O | 57.8 ± 19.9 a | IC50 100 a |
| H2O | 46.8 ± 11.0 a | 88.2 ± 15.2 a |
| Caperatic acid | 100 * | 100 * |
| Extract | DCM | Ace | MeOH | MeOH-H2O | H2O |
|---|---|---|---|---|---|
| Neuroprotective potential [%] | 59.74 | 60.02 | 60.66 | 54.57 | 41.78 |
| Anti-GBM potential [%] | 33.74 | 33.67 | 35.17 | 36.43 | 31.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System? Antioxidants 2022, 11, 2069. https://doi.org/10.3390/antiox11102069
Studzińska-Sroka E, Majchrzak-Celińska A, Bańdurska M, Rosiak N, Szwajgier D, Baranowska-Wójcik E, Szymański M, Gruszka W, Cielecka-Piontek J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System? Antioxidants. 2022; 11(10):2069. https://doi.org/10.3390/antiox11102069
Chicago/Turabian StyleStudzińska-Sroka, Elżbieta, Aleksandra Majchrzak-Celińska, Monika Bańdurska, Natalia Rosiak, Dominik Szwajgier, Ewa Baranowska-Wójcik, Marcin Szymański, Wojciech Gruszka, and Judyta Cielecka-Piontek. 2022. "Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System?" Antioxidants 11, no. 10: 2069. https://doi.org/10.3390/antiox11102069
APA StyleStudzińska-Sroka, E., Majchrzak-Celińska, A., Bańdurska, M., Rosiak, N., Szwajgier, D., Baranowska-Wójcik, E., Szymański, M., Gruszka, W., & Cielecka-Piontek, J. (2022). Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System? Antioxidants, 11(10), 2069. https://doi.org/10.3390/antiox11102069