Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants
Abstract
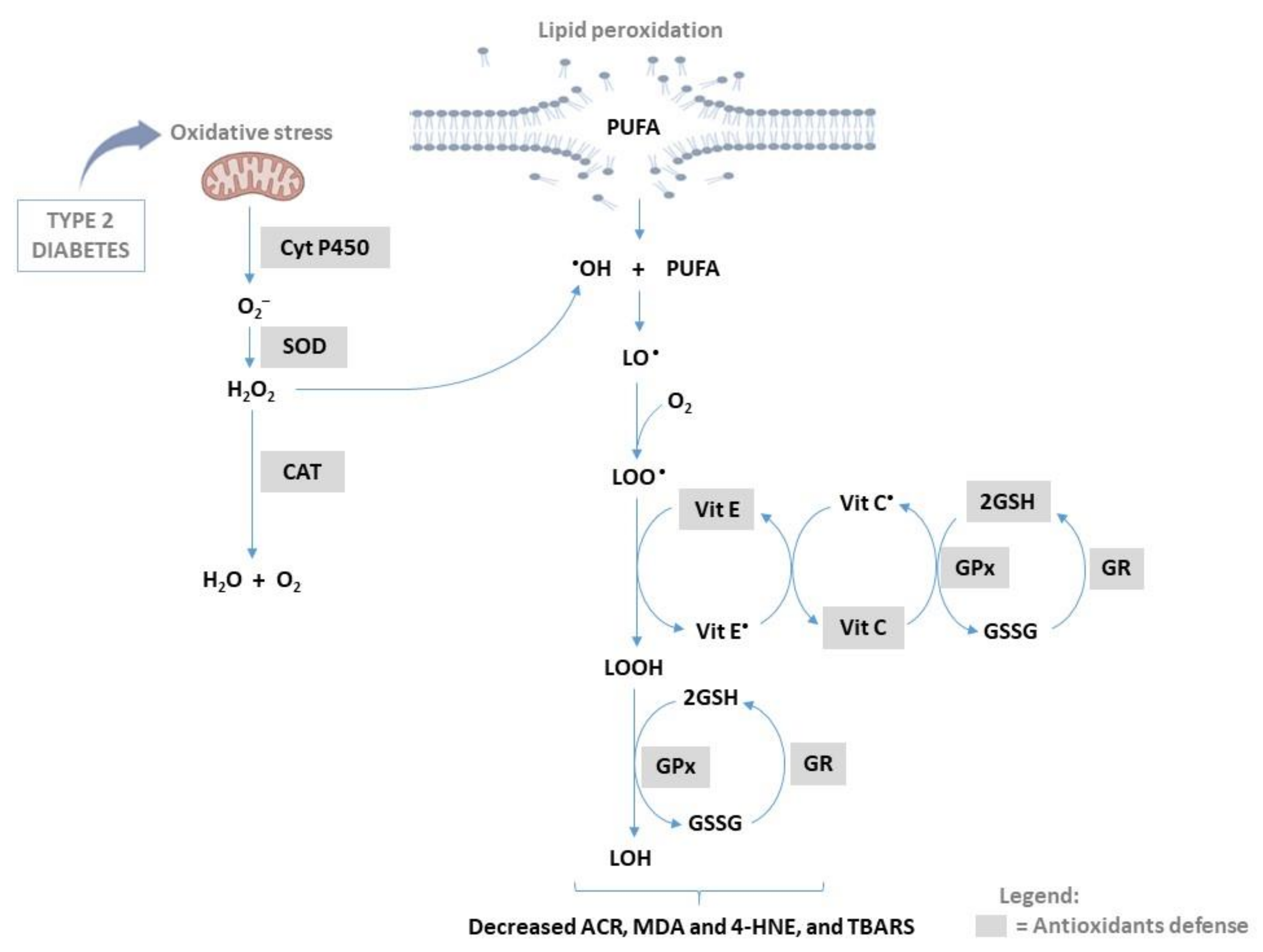
:1. Introduction
2. Prominent Mechanisms Implicated in Generation of Lipid Peroxidation Products
2.1. Non-Enzymatic Process in Lipid Peroxidation
2.2. Enzymatic Process in Lipid Peroxidation
3. Lipid Peroxidation Biomarkers
3.1. Malondialdehyde (MDA) as a Major Biomarker of Lipid Peroxidation
3.2. 4-Hydroxynonenal (HNE) as a Major Biomarker of Lipid Peroxidation
4. Lipid Peroxidation in Type 2 Diabetes
5. Antioxidants and Their Capacity to Terminate Lipid Peroxidation Products
5.1. Catalase and Its Effect on Lipid Peroxidation in Type 2 Diabetes
5.2. Coenzyme Q10 (CoQ10) and Its Effect on Lipid Peroxidation in Type 2 Diabetes
5.3. Glutathione (GSH) and Its Effect on Lipid Peroxidation in Type 2 Diabetes
5.4. Superoxide Dismutase (SOD) and Its Effect on Lipid Peroxidation in Type 2 Diabetes
5.5. Vitamins C and E and Their Effect on Lipid Peroxidation in Type 2 Diabetes
6. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Hao, M. Lipid Trafficking in Cells. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1289–1296. [Google Scholar] [CrossRef]
- Ewers, H.; Helenius, A. Lipid-mediated endocytosis. Cold Spring Harb. Perspect. Biol. 2011, 3, a004721–004735. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119–101140. [Google Scholar] [CrossRef]
- Shichiri, M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014, 54, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramana, K.V.; Srivastava, S.; Singhal, S.S. Lipid Peroxidation Products in Human Health and Disease 2019. Oxidative Med. Cell. Longev. 2019, 2019, 7147235–7147237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njie-Mbye, Y.F.; Chitnis, M.; Opere, C.; Ohia, S. Lipid peroxidation: Pathophysiological and pharmacological implications in the eye. Front. Physiol. 2003, 4, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Fatani, S.H.; Babakr, A.T.; NourEldin, E.M.; Almarzouki, A.A. Lipid peroxidation is associated with poor control of type-2 diabetes mellitus, Diabetes & Metabolic Syndrome. Clin. Res. Rev. 2016, 10, S64–S67. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438–3604368. [Google Scholar] [CrossRef] [Green Version]
- Niki, E.; Yoshida, Y.; Saito, Y.; Noguchi, N. Lipid peroxidation: Mechanisms, inhibition, and biological effects. Biochem. Biophys. Res. Commun. 2005, 338, 668–676. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941–108946. [Google Scholar] [CrossRef]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.M.; Sevinc, A.; Camci, C.; Cigli, A.; Buyukberber, S.; Savli, H.; Bayraktar, N. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol. 2002, 39, 117–122. [Google Scholar] [CrossRef]
- de Souza Bastos, A.; Graves, D.T.; de Melo Loureiro, A.P.; Júnior, C.R.; Corbi, S.C.T.; Frizzera, F.; Scarel-Caminaga, R.M.; Câmara, N.O.; Andriankaja, O.M.; Hiyane, M.I.; et al. Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. J. Diabetes Complicat. 2016, 30, 1593–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.Y.; Xu, X.; Li, X.C. Cardiovascular diseases: Oxidative damage and antioxidant protection. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3091–3096. [Google Scholar] [PubMed]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12, 820968–820972. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An Epigenetic Role of Mitochondria in Cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.I.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, J.; Roman, R.J.; Fan, F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? GeroScience 2022, 44, 1879–1883. [Google Scholar] [CrossRef]
- Choi, S.W.; Ho, C.K. Antioxidant properties of drugs used in Type 2 diabetes management: Could they contribute to, confound or conceal effects of antioxidant therapy? Redox Rep. Commun. Free. Radic. Res. 2018, 23, 1–24. [Google Scholar] [CrossRef]
- Davì, G.A.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid. Redox Signal. 2005, 7, 256–268. [Google Scholar] [CrossRef]
- Tangvarasittichai, O.; Tangvarasittichai, S. Oxidative stress, ocular disease and diabetes retinopathy. Curr. Pharm. Des. 2018, 24, 4726–4741. [Google Scholar] [CrossRef] [PubMed]
- Kesavulu, M.M.; Rao, B.K.; Giri, R.; Vijaya, J.; Subramanyam, G.; Apparao, C. Lipid peroxidation and antioxidant enzyme status in Type 2 diabetics with coronary heart disease. Diabetes Res. Clin. Pract. 2001, 53, 33–39. [Google Scholar] [CrossRef]
- Gunawardena, H.P.; Silva, R.; Sivakanesan, R.; Ranasinghe, P.; Katulanda, P. Poor Glycaemic Control Is Associated with Increased Lipid Peroxidation and Glutathione Peroxidase Activity in Type 2 Diabetes Patients. Oxidative Med. Cell. Longev. 2019, 2019, 9471697–9471707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Diabetes Federation (IDF). IDF Diabetes Atlas Tenth Edition. Available online: https://diabetesatlas.org/ (accessed on 20 August 2022).
- Mete, O.; Asa, S.L.; Butany, J. The evolving landscape of endocrine pathology practice. Semin. Diagn. Pathol. 2013, 30, 157. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Kelly, K.A.; Havrilla, C.M.; Brady, T.C.; Abramo, K.H.; Levin, E.D. Oxidative stress in toxicology: Established mammalian and emerging piscine model systems. Environ. Health Perspect. 1998, 106, 375–384. [Google Scholar] [CrossRef]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism. Biol. Implic. Anal. Determ. 2012, 106, 7. [Google Scholar] [CrossRef]
- Oranje, W.A.; Wolffenbuttel, B.H. Lipid peroxidation and atherosclerosis in type II diabetes. J. Lab. Clin. Med. 1999, 134, 19–32. [Google Scholar] [CrossRef]
- Ganjifrockwala, F.; Joseph, J.; George, G. Decreased total antioxidant levels and increased oxidative stress in South African type 2 diabetes mellitus patients. J. Endocrinol. Metab. Diabetes South Afr. 2017, 22, 21–25. [Google Scholar] [CrossRef]
- Hajeyah, A.A.; Griffiths, W.J.; Wang, Y.; Finch, A.J.; O’Donnell, V.B. The biosynthesis of enzymatically oxidized lipids. Front. Endocrinol. 2020, 11, 591819. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.C.; Abdel-Rahman, E.; Soliman, S.; Kamel, W.A.; Souka, S.; Lowe, J.E.; Abdel-Aleem, S. Depletion of total antioxidant capacity in type 2 diabetes. Metabolism 1999, 48, 1414–1417. [Google Scholar] [CrossRef]
- Caro, A.A.; Cederbaum, A.I. Role of cytochrome P450 in phospholipase A2- and arachidonic acid-mediated cytotoxicity. Free Radic. Biol. Med. 2006, 40, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and biochemistry of lipid peroxidation products. Free. Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Bilska-Wilkosz, A.; Iciek, M.; Górny, M. Chemistry and Biochemistry Aspects of the 4-Hydroxy-2,3-trans-nonenal. Biomolecules 2022, 12, 145. [Google Scholar] [CrossRef]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756–3085770. [Google Scholar] [CrossRef] [Green Version]
- Dias, I.H.; Griffiths, H.R. Oxidative stress in diabetes-circulating advanced glycation end products, lipid oxidation and vascular disease. Ann. Clin. Biochem. 2014, 51, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Zikova, N.; Komarc, M.; Fenclova, Z.; Vlckova, S.; Schwarz, J.; Makeš, O.; Syslova, K.; et al. Markers of lipid oxidative damage in the exhaled breath condensate of nano TiO(2) production workers. Nanotoxicology 2017, 11, 52–63. [Google Scholar] [CrossRef]
- Kartavenka, K.; Panuwet, P.; Yakimavets, V.; Jaikang, C.; Thipubon, K.; D’Souza, P.E.; Barr, D.B.; Ryan, P.B. LC-MS Quantification of Malondialdehyde-Dansylhydrazine Derivatives in Urine and Serum Samples. J. Anal. Toxicol. 2020, 44, 470–481. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Darrah, E.; Rosen, A. Chapter 56—Autoantibodies in Rheumatoid Arthritis. In Kelley and Firestein’s Textbook of Rheumatology, 10th ed.; Firestein, G.S., Budd, R.C., Gabriel, S.E., McInnes, I.B., O’Dell, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 5, pp. 831–845. [Google Scholar]
- Holvoet, P.; Perez, G.; Zhao, Z.; Brouwers, E.; Bernar, H.; Collen, D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J. Clin. Investig. 1995, 95, 2611–2619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuraman, A.; Rishitha, N.; Paramakrishnan, N.; Mahendran, B.; Ramesh, M. Role of Lipid Peroxidation Process in Neurodegenerative Disorders. Neurodegener. Disord. 2019, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Priyanka, B.; Prashanta, B.; Madhusnata, D. Lipid peroxidation: A biomarker of oxidative stress in type 2 diabetes mellitus. Malays. J. Med. Res. 2018, 2, 61–66. Available online: https://ejournal.lucp.net/index.php/mjmr/article/view/250 (accessed on 15 July 2022).
- Folden, D.V.; Gupta, A.; Sharma, A.C.; Li, S.Y.; Saari, J.T.; Ren, J. Malondialdehyde inhibits cardiac contractile function in ventricular myocytes via a p38 mitogen-activated protein kinase-dependent mechanism. Br. J. Pharmacol. 2003, 139, 1310–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Wang, F.; Yu, D.F.; Wu, P.F.; Chen, J.-G. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur. J. Pharmacol. 2011, 650, 184–194. [Google Scholar] [CrossRef]
- Khalili, J.; Biloklytska, H.F. Salivary malondialdehyde levels in clinically healthy and periodontal diseased individuals. Oral Dis. 2008, 14, 754–760. [Google Scholar] [CrossRef]
- Lorente, L.M.; Martín, M.; Abreu-González, P.; Domínguez-Rodriguez, A.; Labarta, L.; Díaz, C.; Solé-Violán, J.; Ferreres, J.; Cabrera, J.; Igeño, J.C.; et al. Sustained high serum malondialdehyde levels are associated with severity and mortality in septic patients. Crit. Care 2013, 17, R290. [Google Scholar] [CrossRef] [Green Version]
- Altun, H.; Şahin, N.; Kurutaş, E.B.; Karaaslan, U.; Sevgen, F.H.; Fındıklı, E. Assessment of malondialdehyde levels, superoxide dismutase, and catalase activity in children with autism spectrum disorders. Psychiatry Clin. Psychopharmacol. 2018, 28, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Asbaghi, O.; Naeini, F.; Ashtary-Larky, D.; Kaviani, M.; Rezaei Kelishadi, M.; Eslampour, E.; Moradi, S.; Mirzadeh, E.; Clark, C.C.T.; Naeini, A.A. Effects of chromium supplementation on blood pressure, body mass index, liver function enzymes and malondialdehyde in patients with type 2 diabetes: A systematic review and dose-response meta-analysis of randomized controlled trials. Complement. Ther. Med. 2021, 60, 102566–102755. [Google Scholar] [CrossRef] [PubMed]
- Gheflati, A.; Bashiri, R.; Ghadiri-Anari, A.; Reza, J.Z.; Kord, M.T.; Nadjarzadeh, A. The effect of apple vinegar consumption on glycemic indices, blood pressure, oxidative stress, and homocysteine in patients with type 2 diabetes and dyslipidemia: A randomized controlled clinical trial. Clin. Nutr. 2019, 33, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free. Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Al-Fawaeir, S.; Akgül, E.; Caycı, T.; Demirin, H.; Kurt, Y.; Aydin, I.; Ağıllı, M.; Özkan, E.; Yaman, H.; Çakır, E.; et al. Comparison of two Methods for Malondialdehyde Measurement. J. Clin. Anal. Med. 2011, 2, 2–4. [Google Scholar] [CrossRef]
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 826–838. [Google Scholar] [CrossRef] [Green Version]
- Chapple, S.J.; Cheng, X.; Mann, G.E. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013, 1, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Jaganjac, M.; Tirosh, O.; Cohen, G.; Sasson, S.; Zarkovic, N. Reactive aldehydes--second messengers of free radicals in diabetes mellitus. Free. Radic. Res. 2013, 47, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, W.A.; Kong, N. Anti-oxidative stress regulator NF-E2-related factor 2 mediates the adaptive induction of antioxidant and detoxifying enzymes by lipid peroxidation metabolite 4-hydroxynonenal. Cell Biosci. 2012, 2, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Milkovic, L.; Cipak Gasparovic, A.; Zarkovic, N. Overview on major lipid peroxidation bioactive factor 4-hydroxynonenal as pluripotent growth-regulating factor. Free. Radic. Res. 2015, 49, 850–860. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Guéraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, P.; Sharma, R.; Sharma, A.; Vatsyayan, R.; Yadav, S.; Singhal, S.S.; Rauniyar, N.; Prokai, L.; Awasthi, S.; Awasthi, Y.C. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry 2010, 49, 6263–6275. [Google Scholar] [CrossRef] [PubMed]
- Sasson, S. 4-Hydroxyalkenal-activated PPARδ mediates hormetic interactions in diabetes. Biochimie 2017, 136, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.D.; Prabhu, K.S.; Thompson, J.T.; Reddy, P.S.; Peters, J.M.; Peterson, B.R.; Reddy, C.C.; Vanden Heuvel, J.P. The oxidative stress mediator 4-hydroxynonenal is an intracellular agonist of the nuclear receptor peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). Free. Radic. Biol. Med. 2007, 42, 1155–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P.; Haber, E.P.; Hirabara, S.M.; Rebelato, E.L.O.; Procopio, J.; Morgan, D.; Oliveira-Emilio, H.C.A.; Carpinelli, A.R.; Curi, R. Diabetes associated cell stress and dysfunction: Role of mitochondrial and non-mitochondrial ROS production and activity. J. Physiol. 2007, 583, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Duerr, G.D.; Heinemann, J.C.; Arnoldi, V.; Feisst, A.; Kley, J.; Ghanem, A.; Welz, A.; Dewald, O. Cardiomyocyte specific peroxisome proliferator-activated receptor-α overexpression leads to irreversible damage in ischemic murine heart. Life Sci. 2014, 102, 88–97. [Google Scholar] [CrossRef]
- Zhi, S.M.; Fang, G.X.; Xie, X.M.; Liu, L.H.; Yan, J.; Liu, D.B.; Yu, H.Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, S.H. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: How are they interlinked?: Oxidative stress and diabetes mellitus. J. Cell. Biochem. 2017, 118, 3577–3585. [Google Scholar] [CrossRef]
- Manju, V.; Kalaivani Sailaja, J.; Nalini, N. Circulating lipid peroxidation and antioxidant status in cervical cancer patients: A case-control study. Clin. Biochem. 2002, 35, 621–625. [Google Scholar] [CrossRef]
- Hou, Y.M.; Lin, M.; Qiu, X.; He, M.; Zhang, Y.; Guo, F. Effect of Type-2 Diabetes Mellitus in Retinopathy Patients on MDA, SOD Activity and its Correlation with HbA1c. Braz. Arch. Biol. Technol. 2022, 64, e21200075–e21200082. [Google Scholar] [CrossRef]
- Mahreen, R.; Mohsin, M.; Nasreen, Z.; Siraj, M.; Ishaq, M. Significantly increased levels of serum malonaldehyde in type 2 diabetics with myocardial infarction. Int. J. Diabetes Dev. Ctries. 2010, 30, 49–51. [Google Scholar]
- Lou, B.; Boger, M.; Bennewitz, K.; Sticht, C.; Kopf, S.; Morgenstern, J.; Fleming, T.; Hell, R.; Yuan, Z.; Nawroth, P.P.; et al. Elevated 4-hydroxynonenal induces hyperglycaemia via Aldh3a1 loss in zebrafish and associates with diabetes progression in humans. Redox Biol. 2020, 37, 101723. [Google Scholar] [CrossRef] [PubMed]
- Miwa, I.; Ichimura, N.; Sugiura, M.; Hamada, Y.; Taniguchi, S. Inhibition of glucose-induced insulin secretion by 4-hydroxy-2-nonenal and other lipid peroxidation products. Endocrinology 2000, 141, 2767–2772. [Google Scholar] [CrossRef]
- Dham, D.; Roy, B.; Gowda, A.; Pan, G.; Sridhar, A.; Zeng, X.; Thandavarayan, R.A.; Palaniyandi, S.S. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: Challenges and opportunities. Free. Radic. Res. 2021, 55, 547–561. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83–101. [Google Scholar] [CrossRef] [Green Version]
- Buschmann, K.; Gramlich, Y.; Chaban, R.; Oelze, M.; Hink, U.; Münzel, T.; Treede, H.; Daiber, A.; Duerr, G.D. Disturbed Lipid Metabolism in Diabetic Patients with Manifest Coronary Artery Disease Is Associated with Enhanced Inflammation. Int. J. Environ. Res. Public Health 2021, 18, 892. [Google Scholar] [CrossRef]
- Naito, R.; Miyauchi, K. Coronary Artery Disease and Type 2 Diabetes Mellitus. Int. Heart J. 2017, 58, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Nuhu, F.; Bhandari, S. Oxidative Stress and Cardiovascular Complications in Chronic Kidney Disease, the Impact of Anaemia. Pharmaceuticals 2018, 11, 103. [Google Scholar] [CrossRef] [Green Version]
- De Geest, B.; Mishra, M. Role of Oxidative Stress in Diabetic Cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Muller, C.J.; Joubert, E.; Louw, J.; Essop, M.F.; Gabuza, K.B.; Ghoor, S.; Huisamen, B.; Johnson, R. Aspalathin Protects the Heart against Hyperglycemia-Induced Oxidative Damage by Up-Regulating Nrf2 Expression. Molecules 2017, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107–101113. [Google Scholar] [CrossRef]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Mishra, P.; Paital, B.; Jena, S.; Swain, S.S.; Kumar, S.; Yadav, M.K.; Chainy, G.B.N.; Samanta, L. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019, 9, 7408–7423. [Google Scholar] [CrossRef] [Green Version]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162–1190. [Google Scholar] [CrossRef] [Green Version]
- Dludla, P.V.; Joubert, E.; Muller, C.J.F.; Louw, J.; Johnson, R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-D-glucoside. Nutr. Metab. 2017, 14, 45–62. [Google Scholar] [CrossRef] [Green Version]
- Sabbatinelli, J.; Orlando, P.; Galeazzi, R.; Silvestri, S.; Cirilli, I.; Marcheggiani, F.; Dludla, P.V.; Giuliani, A.; Bonfigli, A.R.; Mazzanti, L.; et al. Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients 2020, 12, 1098. [Google Scholar] [CrossRef] [Green Version]
- Sözmen, B.; Delen, Y.; Girgin, F.K.; Sözmen, E.Y. Catalase and paraoxonase in hypertensive type 2 diabetes mellitus: Correlation with glycemic control. Clin. Biochem. 1999, 32, 423–427. [Google Scholar] [CrossRef]
- Góth, L. Reactive oxygen species, hydrogen peroxide, catalase and diabetes mellitus, Redox report: Communications. Free. Radic. Res. 2006, 11, 281–282. [Google Scholar] [CrossRef]
- Góth, L. Catalase deficiency and type 2 diabetes. Diabetes Care 2008, 31, e93. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, M.; Sharma, T.K.; Singh, I.; Singh, N.; Ghalaut, V.S.; Vardey, S.K.; Shankar, V. Antioxidant Enzymes and Lipid Peroxidation in Type 2 Diabetes Mellitus Patients with and without Nephropathy. N. Am. J. Med. Sci. 2013, 5, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Najafi, A.; Pourfarzam, M.; Zadhoush, F. Oxidant/antioxidant status in Type-2 diabetes mellitus patients with metabolic syndrome. J. Res. Med. Sci. 2016, 26, 20–25. [Google Scholar]
- Cirilli, I.; Damiani, E.; Dludla, P.V.; Hargreaves, I.; Marcheggiani, F.; Millichap, L.E.; Orlando, P.; Silvestri, S.; Tiano, L. Role of Coenzyme Q(10) in Health and Disease: An Update on the Last 10 Years (2010–2020). Antioxidants 2021, 10, 1325. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Clinical aspects of coenzyme Q10: An update. Nutrition 2010, 26, 250–254. [Google Scholar] [CrossRef]
- Noh, Y.H.; Kim, K.Y.; Shim, M.S.; Choi, S.H.; Choi, S.; Ellisman, M.H.; Weinreb, R.N.; Perkins, G.A.; Ju, W.K. Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis. 2013, 4, e820–e831. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Casado, M.E.; Quiles, J.L.; Barriocanal-Casado, E.; González-García, P.; Battino, M.; López, L.C.; Varela-López, A. The Paradox of Coenzyme Q(10) in Aging. Nutrients 2019, 11, 2221. [Google Scholar] [CrossRef] [Green Version]
- Samimi, F.; Baazm, M.; Eftekhar, E.; Rajabi, S.; Goodarzi, M.T.; Jalali Mashayekhi, F. Possible antioxidant mechanism of coenzyme Q10 in diabetes: Impact on Sirt1/Nrf2 signaling pathways. Res. Pharm. Sci. 2019, 14, 524–533. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Lin, C.P.; Huang, P.H.; Li, S.Y.; Chen, J.S.; Lin, F.Y.; Chen, J.W.; Lin, S.J. Coenzyme Q10 Attenuates High Glucose-Induced Endothelial Progenitor Cell Dysfunction through AMP-Activated Protein Kinase Pathway. J. Diabetes Res. 2016, 2016, 6384759–6384773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-ghoroury, E.A.; Raslan, H.M.; Badawy, E.A.; El-Saaid, G.S.; Agybi, M.H.; Siam, I.; Salem, S.I. Malondialdehyde and coenzyme Q10 in platelets and serum in type 2 diabetes mellitus: Correlation with glycemic control. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2009, 20, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Ates, O.; Bilen, H.; Keles, S.; Alp, H.H.; Keleş, M.S.; Yıldırım, K.; Ondaş, O.; Pınar, L.C.; Civelekler, M.; Baykal, O. Plasma coenzyme Q10 levels in type 2 diabetic patients with retinopathy. Int. J. Ophthalmol. 2013, 6, 675–679. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nyambuya, T.M.; Orlando, P.; Silvestri, S.; Mxinwa, V.; Mokgalaboni, K.; Nkambule, B.B.; Louw, J.; Muller, C.J.F.; Tiano, L. The impact of coenzyme Q(10) on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2020, 3, e00118–e00129. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Orlando, P.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Nyambuya, T.M.; Mxinwa, V.; Mokgalaboni, K.; Nkambule, B.B.; Johnson, R.; et al. Coenzyme Q(10) Supplementation Improves Adipokine Levels and Alleviates Inflammation and Lipid Peroxidation in Conditions of Metabolic Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2020, 21, 3247. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Clinical aspects of coenzyme Q10: An update. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 641–646. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71–92. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Boyland, E.; Chasseaud, L.F. The role of glutathione and glutathione S-transferases in mercapturic acid biosynthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969, 32, 173–219. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ye, R.; He, Y.; Li, Y.; Qiu, X. Protective effect of Jiaweibugan decoction against diabetic peripheral neuropathy. Neural Regen. Res. 2013, 8, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; McFarlane-Anderson, N.; Gordon-Strachan, G.M.; Wright-Pascoe, R.A.; Jahoor, F.; Boyne, M.S. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE 2018, 13, e0198626–e0198637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çolak, N.G.; Eken, N.T.; Frary, A.; Doğanlar, S. Chromatographic Analysis for Targeted Metabolomics of Antioxidant and Flavor-Related Metabolites in Tomato. Bio-Protocol 2021, 11, 110393–110417. [Google Scholar]
- Alataş, O.; Sahin, A.; Colak, O.; Inal, M.; Köken, T.; Yaşar, B.; Karahüseyinoglu, E. Beneficial effects of allopurinol on glutathione levels and glutathione peroxidase activity in rat ischaemic acute renal failure. J. Int. Med. Res. 1996, 24, 33–39. [Google Scholar] [CrossRef]
- Goyal, R.; Singhai, M.; Faizy, A.F. Glutathione peroxidase activity in obese and nonobese diabetic patients and role of hyperglycemia in oxidative stress. J. Mid-Life Health 2011, 2, 72–76. [Google Scholar] [CrossRef]
- Dworzański, J.; Strycharz-Dudziak, M.; Kliszczewska, E.; Kiełczykowska, M.; Dworzańska, A.; Drop, B.; Polz-Dacewicz, M. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS ONE 2020, 15, e0230374–e0230383. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Shan, W.; Zhai, G.; Qiu, C.; Liu, Y.; Xu, Y.; Yang, X. Association between glutathione peroxidase-3 activity and carotid atherosclerosis in patients with type 2 diabetes mellitus. Brain Behav. 2020, 10, e01773–e01780. [Google Scholar] [CrossRef] [PubMed]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Madi, M.; Babu, S.; Kumari, S.; Shetty, S.; Achalli, S.; Madiyal, A.; Bhat, M. Status of Serum and Salivary Levels of Superoxide Dismutase in Type 2 Diabetes Mellitus with Oral Manifestations: A Case Control Study. Ethiop. J. Health Sci. 2016, 26, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Tavares, A.M.; Silva, J.H.; Bensusan, C.O.; Ferreira, A.C.F.; Matos, L.P.L.; KLA, E.S.; Cardoso-Weide, L.C.; Taboada, G.F. Altered superoxide dismutase-1 activity and intercellular adhesion molecule 1 (ICAM-1) levels in patients with type 2 diabetes mellitus. PLoS ONE 2019, 14, e0216256–e0216265. [Google Scholar] [CrossRef] [Green Version]
- Longo-Mbenza, B.; Mvitu Muaka, M.; Masamba, W.; Muizila Kini, L.; Longo Phemba, I.; Kibokela Ndembe, D.; Tulomba Mona, D. Retinopathy in non diabetics, diabetic retinopathy and oxidative stress: A new phenotype in Central Africa? Int. J. Ophthalmol. 2014, 7, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Biju, T.; Shabeer, M.M.; Amitha, R.; Rajendra, B.P.; Suchetha, K. Comparative evaluation of serum superoxide dismutase and glutathione levels in periodontally diseased patients: An interventional study. Indian J. Dent. Res. 2014, 25, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019, 49, e13036–e13066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhusari, S.S.; Dobosy, J.R.; Fu, V.; Almassi, N.; Oberley, T.; Jarrard, D.F. Superoxide dismutase 1 knockdown induces oxidative stress and DNA methylation loss in the prostate. Epigenetics 2010, 5, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, Y. Lipid peroxides and superoxide dismutase (SOD) induction in skin inflammatory diseases, and treatment with SOD preparations. Dermatologica 1989, 179 (Suppl. 1), 101–106. [Google Scholar] [CrossRef]
- Lewandowski, Ł.; Urbanowicz, I.; Kepinska, M.; Milnerowicz, H. Concentration/activity of superoxide dismutase isozymes and the pro-/antioxidative status, in context of type 2 diabetes and selected single nucleotide polymorphisms (genes: INS, SOD1, SOD2, SOD3) - Preliminary findings. Biomed. Pharmacother. 2021, 137, 111396. [Google Scholar] [CrossRef]
- Bandeira Sde, M.; Guedes Gda, S.; da Fonseca, L.J.; Pires, A.S.; Gelain, D.P.; Moreira, J.C.; Rabelo, L.A.; Vasconcelos, S.M.; Goulart, M.O. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: Increase in lipid peroxidation and SOD activity. Oxidative Med. Cell. Longev. 2012, 2012, 819310–819323. [Google Scholar] [CrossRef] [Green Version]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free. Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, F.E. Vitamin C: An Antioxidant Agent. In Vitamin C; Hamza, A.H., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Naziroğlu, M.; Simşek, M.; Simşek, H.; Aydilek, N.; Ozcan, Z.; Atilgan, R. The effects of hormone replacement therapy combined with vitamins C and E on antioxidants levels and lipid profiles in postmenopausal women with Type 2 diabetes. Clin. Chim. Acta Int. J. Clin. Chem. 2004, 344, 63–71. [Google Scholar] [CrossRef]
- Rafighi, Z.; Shiva, A.; Arab, S.; Mohd Yousof, R. Association of dietary vitamin C and e intake and antioxidant enzymes in type 2 diabetes mellitus patients. Glob. J. Health Sci. 2013, 5, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant effects of vitamins in type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2018, 10, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Balbi, V.; Volpe, C.; Varricchio, G.; Gambardella, A.; Saccomanno, F.; Ammendola, S.; Varricchio, M.; D’Onofrio, F. Metabolic benefits deriving from chronic vitamin C supplementation in aged non-insulin dependent diabetics. J. Am. Coll. Nutr. 1995, 14, 387–392. [Google Scholar] [CrossRef]
- Plantinga, Y.; Ghiadoni, L.; Magagna, A.; Giannarelli, C.; Franzoni, F.; Taddei, S.; Salvetti, A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am. J. Hypertens. 2007, 20, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Hoffman, W.H.; Carl, G.F.; Khichi, M.; Cornwell, P.E. Lipid peroxidation and antioxidant vitamins prior to, during, and after correction of diabetic ketoacidosis. J. Diabetes Its Complicat. 2002, 16, 294–300. [Google Scholar] [CrossRef]
- Ibuki, F.K.; Bergamaschi, C.T.; da Silva Pedrosa, M.; Nogueira, F.N. Effect of vitamin C and E on oxidative stress and antioxidant system in the salivary glands of STZ-induced diabetic rats. Arch. Oral Biol. 2020, 116, 104765–104772. [Google Scholar] [CrossRef]
- Vinson, J.; Hsu, C.; Possanza, C.; Drack, A.; Pane, D.; Davis, R.; Klock, C.; Graser, K.; Wang, X. Lipid peroxidation and diabetic complications: Effect of antioxidant vitamins C and E. Adv. Exp. Med. Biol. 1994, 366, 430–432. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Masarwa, R.; Brunetti, V.C.; Aloe, S.M.; Henderson, R.W. Platt, K.B. Filion, Efficacy and Safety of Metformin for Obesity: A Systematic Review. Pediatrics 2021, 147, e20201610–e20201622. [Google Scholar] [CrossRef]
- Malik, A.; Morya, R.K.; Saha, S.; Singh, P.K.; Bhadada, S.K.; Rana, S.V. Oxidative stress and inflammatory markers in type 2 diabetic patients. Eur. J. Clin. Investig. 2020, 50, e13238–e13251. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732–9152744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Glantzounis, G.K.; Salacinski, H.J.; Yang, W.; Davidson, B.R.; Seifalian, A.M. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: A review. Liver Transplant. 2005, 11, 1031–1047. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797–7432806. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750–162766. [Google Scholar] [CrossRef] [Green Version]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Orlando, P.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Ziqubu, K.; Ndevahoma, F.; et al. Tea consumption and its effects on primary and secondary prevention of coronary artery disease: Qualitative synthesis of evidence from randomized controlled trials. Clin. Nutr. ESPEN 2021, 41, 77–87. [Google Scholar] [CrossRef]
- Miller III, E.R.; Appel, L.J.; Risby, T.H. Effect of dietary patterns on measures of lipid peroxidation: Results from a randomized clinical trial. Circulation 1998, 98, 2390–2395. [Google Scholar] [CrossRef] [Green Version]
- Freese, R.; Alfthan, G.; Jauhiainen, M.; Basu, S.; Erlund, I.; Salminen, I.; Aro, A.; Mutanen, M. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. Am. J. Clin. Nutr. 2002, 76, 950–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikail, M.A.; Ahmed, I.A.; Ibrahim, M.; Hazali, N.; Abdul Rasad, M.S.; Abdul Ghani, R.; Hashim, R.; Abdul Wahab, R.; Jahuari Arief, S.; Md Isa, M.L.; et al. Baccaurea angulata fruit inhibits lipid peroxidation and induces the increase in antioxidant enzyme activities. Eur. J. Nutr. 2016, 55, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Åsgård, R.; Rytter, E.; Basu, S.; Abramsson-Zetterberg, L.; Möller, L.; Vessby, B. High intake of fruit and vegetables is related to low oxidative stress and inflammation in a group of patients with type 2 diabetes. Scand. J. Food Nutr. 2007, 51, 149–158. [Google Scholar] [CrossRef]
- Yim, S.; Malhotra, A.; Veves, A. Antioxidants and CVD in diabetes: Where do we stand now. Curr. Diabetes Rep. 2007, 7, 8–13. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. https://doi.org/10.3390/antiox11102071
Shabalala SC, Johnson R, Basson AK, Ziqubu K, Hlengwa N, Mthembu SXH, Mabhida SE, Mazibuko-Mbeje SE, Hanser S, Cirilli I, et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants. 2022; 11(10):2071. https://doi.org/10.3390/antiox11102071
Chicago/Turabian StyleShabalala, Samukelisiwe C., Rabia Johnson, Albertus K. Basson, Khanyisani Ziqubu, Nokulunga Hlengwa, Sinenhlanhla X. H. Mthembu, Sihle E. Mabhida, Sithandiwe E. Mazibuko-Mbeje, Sidney Hanser, Ilenia Cirilli, and et al. 2022. "Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants" Antioxidants 11, no. 10: 2071. https://doi.org/10.3390/antiox11102071
APA StyleShabalala, S. C., Johnson, R., Basson, A. K., Ziqubu, K., Hlengwa, N., Mthembu, S. X. H., Mabhida, S. E., Mazibuko-Mbeje, S. E., Hanser, S., Cirilli, I., Tiano, L., & Dludla, P. V. (2022). Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants, 11(10), 2071. https://doi.org/10.3390/antiox11102071







