Natural Active Ingredients for Poly (Lactic Acid)-Based Materials: State of the Art and Perspectives
Abstract
:1. Introduction
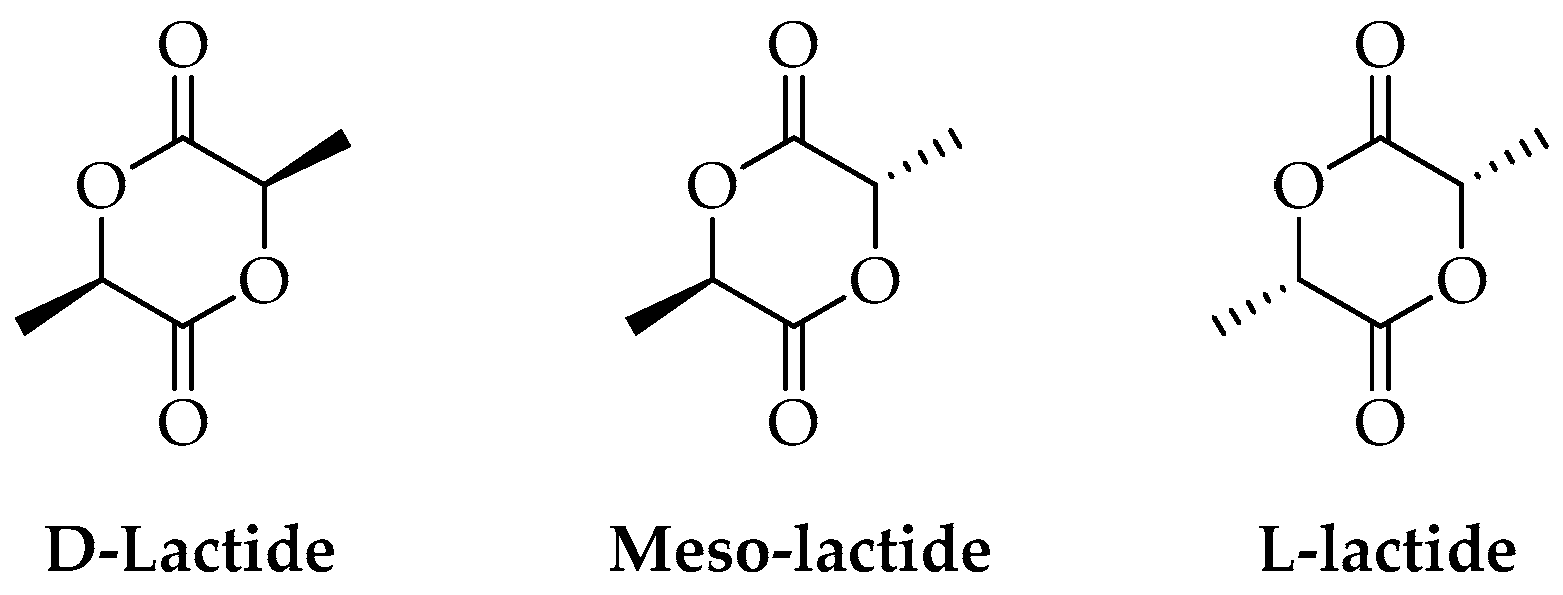
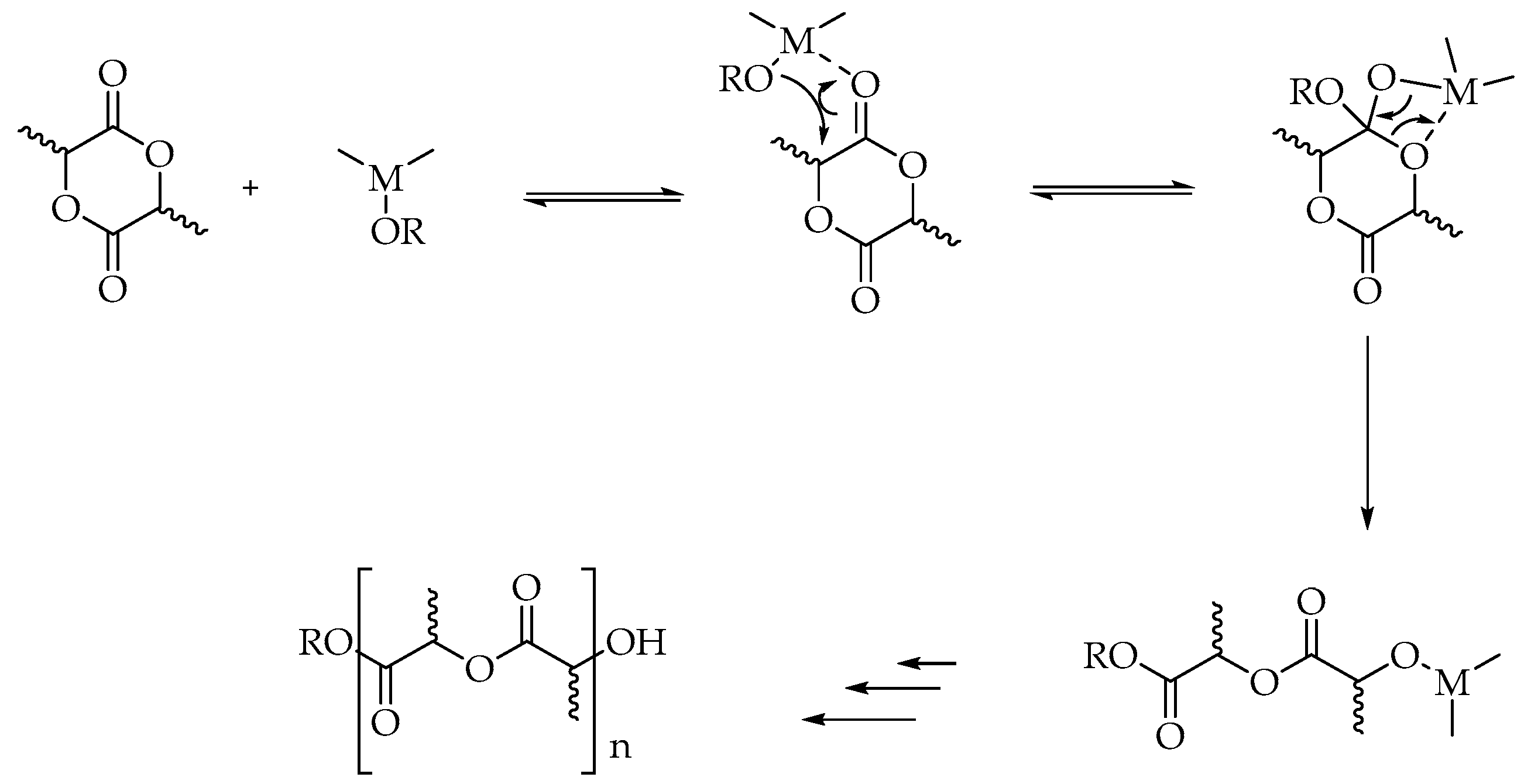
2. PLA Synthesis and Structure
3. Active Ingredients
3.1. Diffusion in Nature
3.2. Innovative Extraction Technologies from Natural Sources
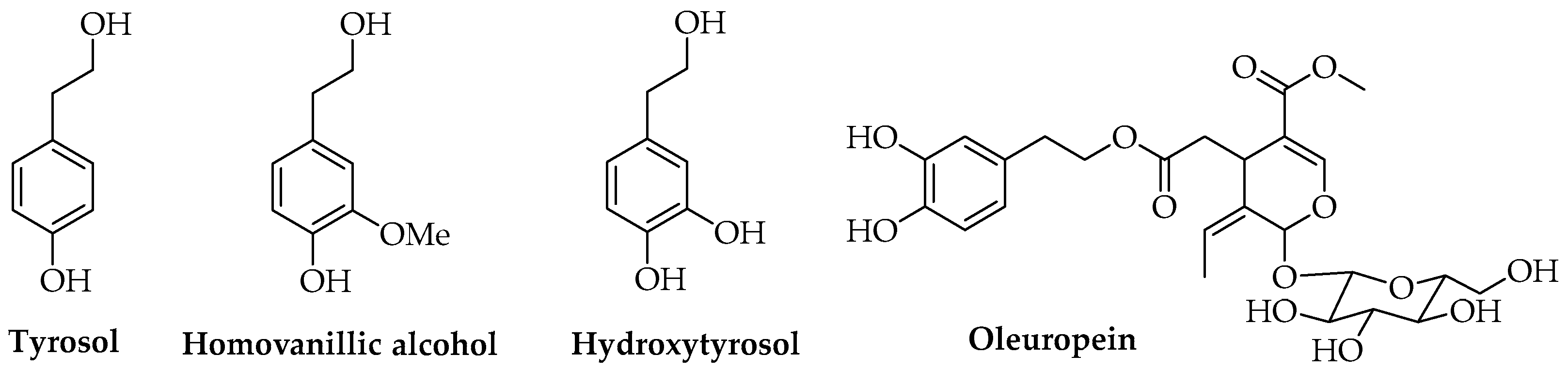
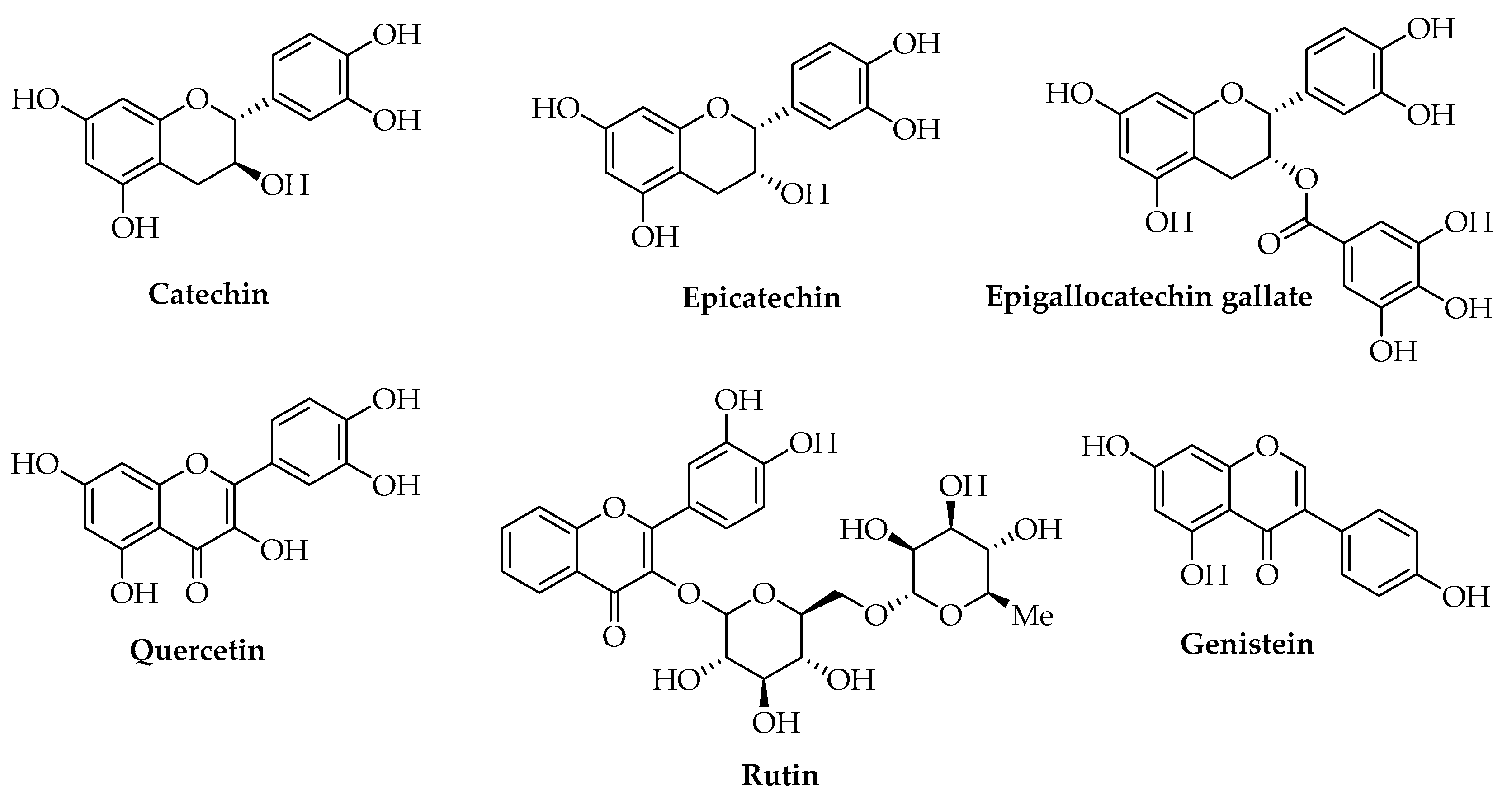
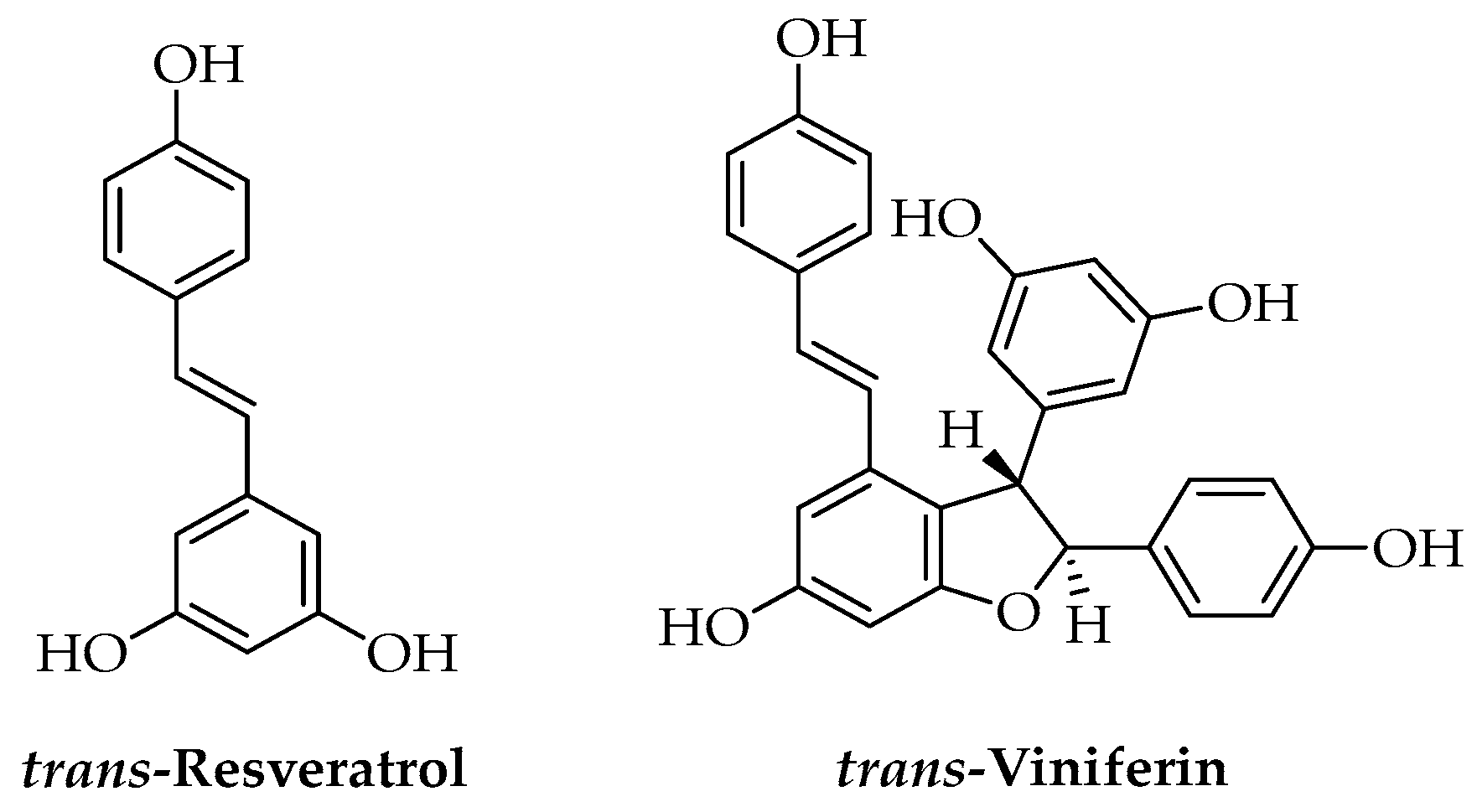
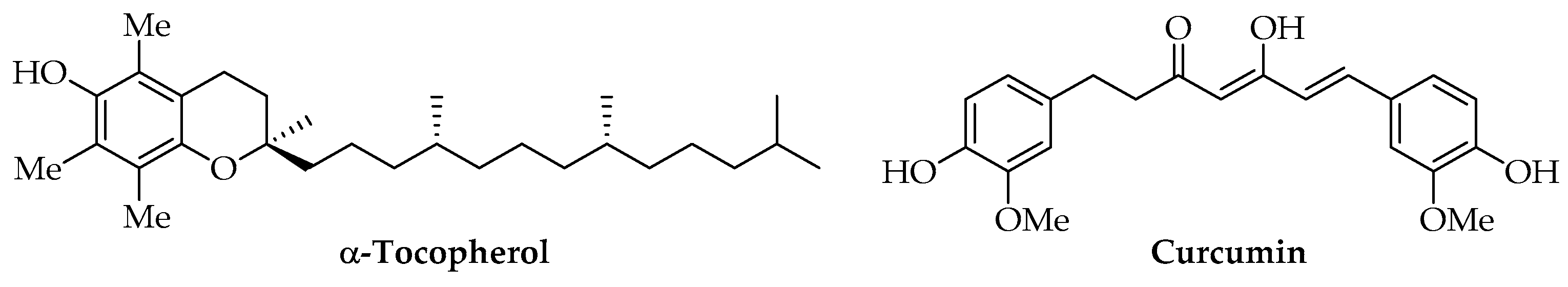
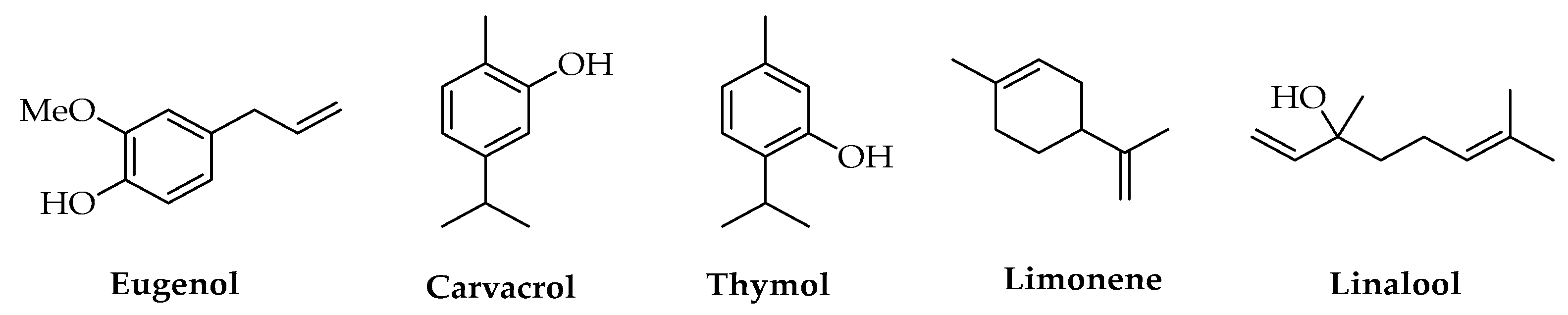
3.3. Phenols
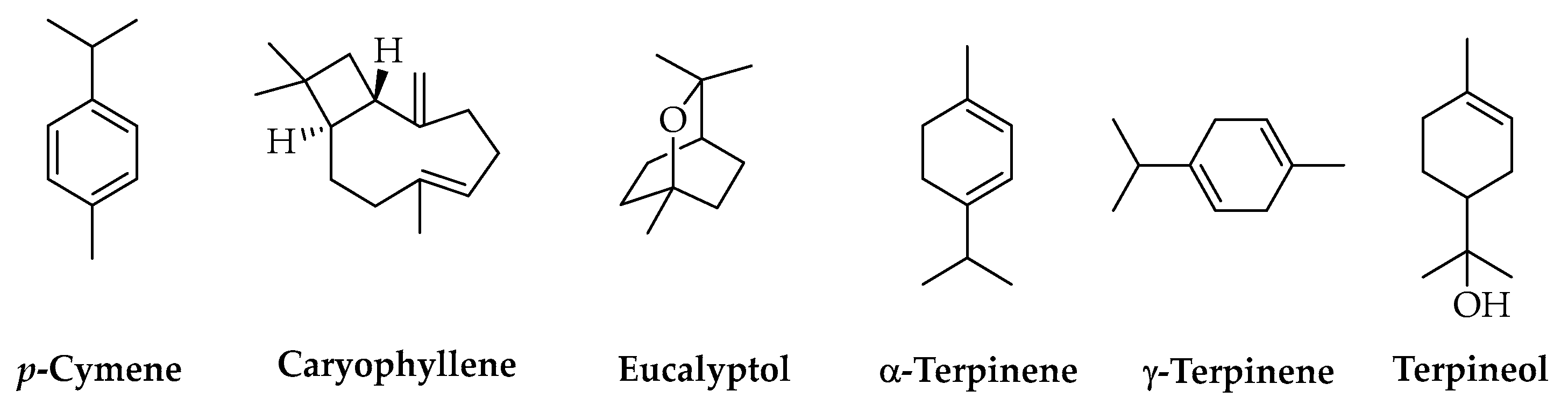
3.4. Terpenes
4. Active PLA-Based Materials
4.1. Processing Techniques
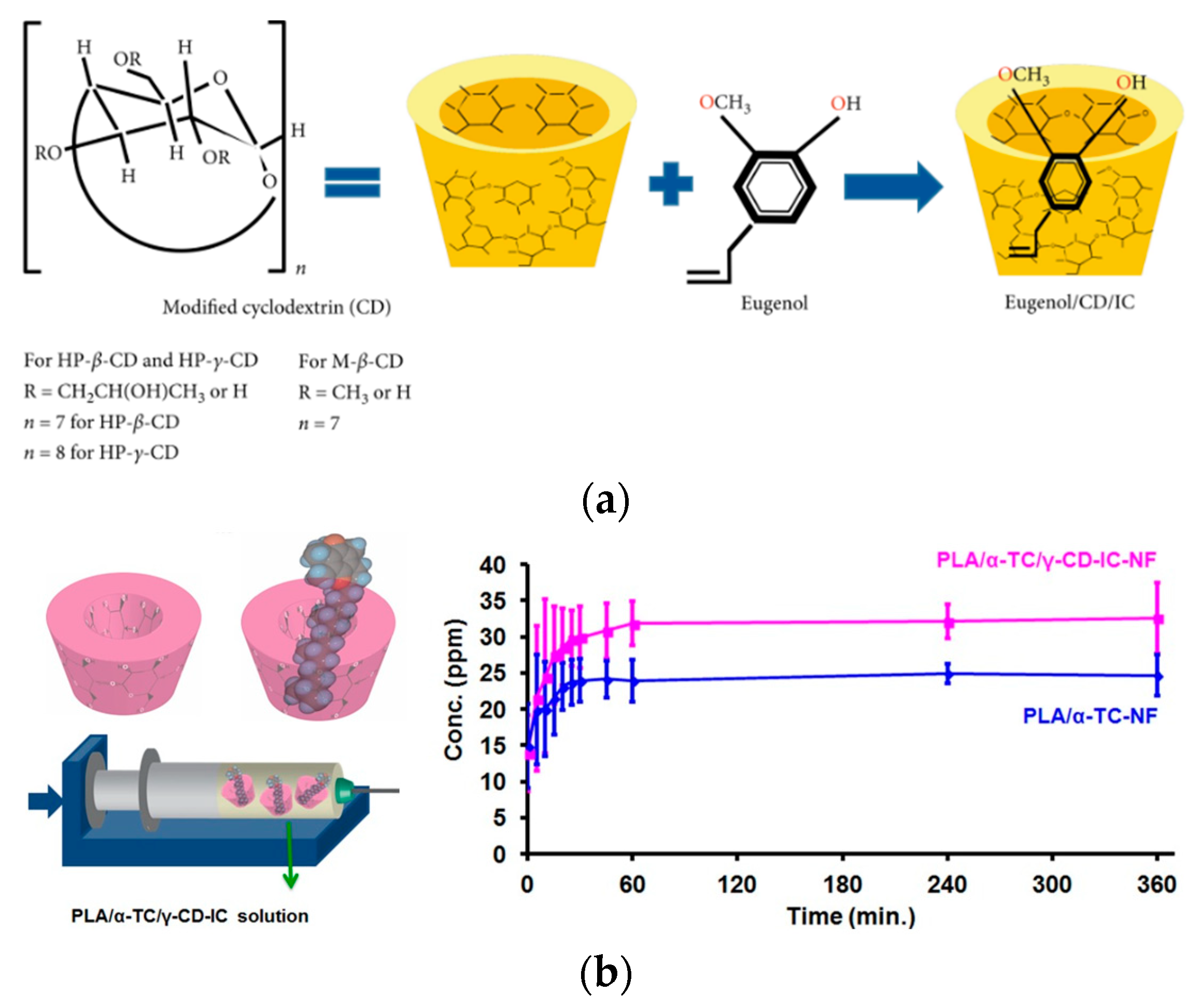
4.1.1. Encapsulation
4.1.2. Emulsification-Solvent Evaporation and sCO2 Impregnation
4.2. Properties
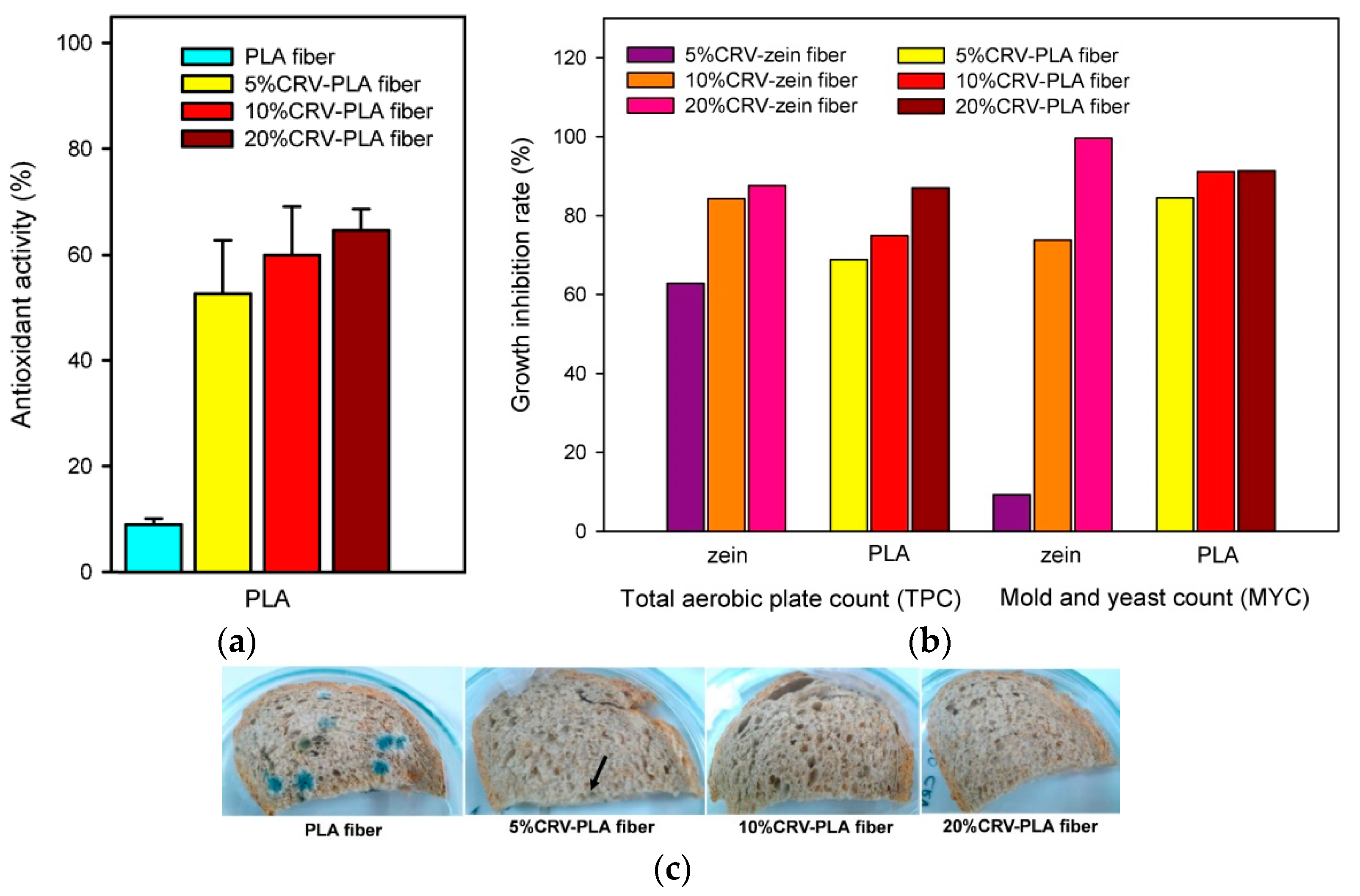
4.3. Antioxidant Activity
| Class | Compound | Source | AI Incorporation Technique | Activity | Application | Ref. |
|---|---|---|---|---|---|---|
| Curcuminoids | Curcumin | Curcuma longa L. | Solvent casting | Antioxidant Antimicrobial | Food | [134] |
| Curcumin | Curcuma longa L. | Solvent casting | Antioxidant Antimicrobial | Food | [135] | |
| Curcumin | Curcuma longa L. | Electrospinning | Antioxidant | Biomedical | [136] | |
| Curcumin | Curcuma longa L. | Encapsulation | Antioxidant | Biomedical | [137] | |
| Curcumin | Curcuma longa L. | Emulsification | Antioxidant | Biomedical | [138] | |
| solvent evaporation | Antimicrobial | |||||
| Flavonoids | Camellia sinensis L. (powder) | Electrospinning | Antimicrobial | Food | [96] | |
| Camellia sinensis L. (leaves) | Extrusion | Antioxidant | Food | [100] | ||
| Rosmarinus officinalis L. (leaves) | Melt mixing | Antioxidant Antimicrobial | Food | [101] | ||
| Allium ursinum L. (dried bulbs) | Solvent casting | Antimicrobial | Food | [113] | ||
| Catechin | Abies Nordmanniana (Steven) Spach (leaves) | Solvent casting | Antioxidant | Food | [97] | |
| Catechin | Commercial | Melt mixing | Antioxidant | [107] | ||
| Catechin | Commercial | Electrospinning | Antioxidant | Food | [109] | |
| Antimicrobial | ||||||
| Epicatechin | Liquidambar orientalis Mill. (leaves) | Extrusion | Antimicrobial | [99] | ||
| Epicatechin | Melt mixing | Antioxidant | [107] | |||
| Epigallocatechin gallate | Commercial | Thermo-compression molding | Antioxidant Antimicrobial | [110] | ||
| Genistein | Commercial | Electrospinning | Antioxidant | [108] | ||
| Quercetin | Commercial | Solvent casting | Antioxidant Antimicrobial | Food | [111] | |
| Rutin | Olea europaea L. (leaves) | Encapsulation | Antioxidant Antimicrobial | Cosmetic | [86] | |
| Phenethyl alcohols | Hydroxytyrosol | Olea europaea L. (by-products) | Solvent casting | Antioxidant | Food | [88] |
| Lipidic phenols | Cannabidiol | Linum usitatissimum L. (fibers) | Compression molding | Antioxidant Antimicrobial | Food | [162] |
| Cardanols | Anacardium occidentale L. (oil) | Melt extrusion | Antioxidant | Food | [210] | |
| α-Tocopherol | Commercial | Electrospinning | Antioxidant Antimicrobial | Food | [128] | |
| α-Tocopherol | Commercial | Emulsion solvent evaporation | Antioxidant | Food | [129] | |
| α-Tocopherol | Commercial | Melt blending | Antioxidant | Food | [130] | |
| Phenolic acids | 4-Hydrobenzoic acid | Linum usitatissimum L. | Compression molding | Antioxidant Antimicrobial | Food | [162] |
| Gallic acid | Commercial | Encapsulation Electrospinning | Antioxidant | Food | [186] | |
| Protocatechuic acid | Linum usitatissimum L. (leaves) | Extrusion | Antimicrobial | Food | [99] | |
| Rosmarinic acid | Commercial | Extrusion | Antioxidant Antimicrobial | Food | [17] | |
| Rosmarinic acid | Commercial | Encapsulation Supercritical emulsion extraction | Antioxidant | Biomedical | [102] | |
| Rosmarinic acid | Commercial | sCO2 impregnation | Antimicrobial | Food | [139] | |
| Rosmarinic acid | Commercial | Extrusion | Antimicrobial | Food | [142] | |
| Rosmarinic acid | Commercial | Melt blending | Antioxidant | Food | [145] | |
| Rosmarinic acid | Commercial | Impregnation | Antioxidant Antimicrobial | Food | [148] | |
| Rosmarinic acid | Commercial | Melt mixing Solvent casting | Antioxidant | Food | [149] | |
| Rosmarinic acid | Commercial | sCO2 impregnation | Antimicrobial | Food | [151] | |
| Rosmarinic acid | Commercial | sCO2 impregnation | Antioxidant | Food | [152] | |
| Tannic acid | Commercial | Layer-by-layer | Antioxidant Antimicrobial | Biomedical | [98] | |
| Phenolic extract | Abies Nordmanniana (Steven) Spach (leaves) | Solvent casting | Antioxidant | Food | [97] | |
| Aesculus hippocastanum L. (seeds) | Solvent casting | Antioxidant | Food | [97] | ||
| Camellia sinensis L. (leaves) | Extrusion | Antioxidant | Food | [100] | ||
| Rosmarinus officinalis L. (leaves) | Extrusion | Antioxidant Antimicrobial | Food | [101] | ||
| Durvillaea antarctica (Chamisso) Hariot (algae) (powder) | Solvent casting | Antioxidant Antimicrobial | Food | [109] | ||
| Allium ursinum L. (bulb) | Solvent casting | Antimicrobial | Food | [113] | ||
| Phenolic polymers | Lignin | Commercial | Extrusion | Antioxidant | Food | [118] |
| Lignin | Commercial | Solvent casting | Antioxidant | Biomedical | [119] | |
| Lignin | Commercial | Melt mixing | Antioxidant Antimicrobial | Biomedical | [120] | |
| Lignin | Commercial | Melt mixing | Antioxidant | [121] | ||
| Lignin | Commercial | Solvent casting | Antioxidant | Food | [122] | |
| Lignin | Commercial | Melt mixing | Antioxidant | Food | [123] | |
| Melatonin | Commercial | Nanoprecipitation | Antioxidant | Biomedical | [212] | |
| Procyanidins | Abies Nordmanniana (Steven) Spach (leaves) | Solvent casting | Antioxidant | Food | [97] | |
| Procyanidins | Aesculus hippocastanum L. (seeds) | Solvent casting | Antioxidant | Food | [97] | |
| Secoiridoids | Oleuropein | Olea europaea L. | Encapsulation | Antioxidant Antimicrobial | Cosmetic | [86] |
| Oleuropein | Olea europaea L. | Solvent casting | Antimicrobial | Food | [91] | |
| Stilbenes | trans-Resveratrol | Vitis vinifera L. (cane) | Melt mixing | Antimicrobial | Food | [117] |
| trans-Viniferin | Vitis vinifera L. (cane) | Melt mixing | Antimicrobial | Food | [117] | |
| Xanthonoids | Mangiferin | Mangifera indica L. (leaves) | sCO2 impregnation | Antioxidant | Biomedical | [213] |
| Class | Compound | Source | AI Incorporation Methodology | Activity | Application | Ref. |
|---|---|---|---|---|---|---|
| Benzaldeydes | Cinnamaldeyde | Commercial | Impregnation | Antimicrobial | Food | [139] |
| Cinnamaldeyde | Commercial | Melt mixing Solvent casting | Antioxidant | Food | [149] | |
| Vanillin | Olea europaea L. (leaves) | Encapsulation | Antioxidant Antimicrobial | Cosmetic | [86] | |
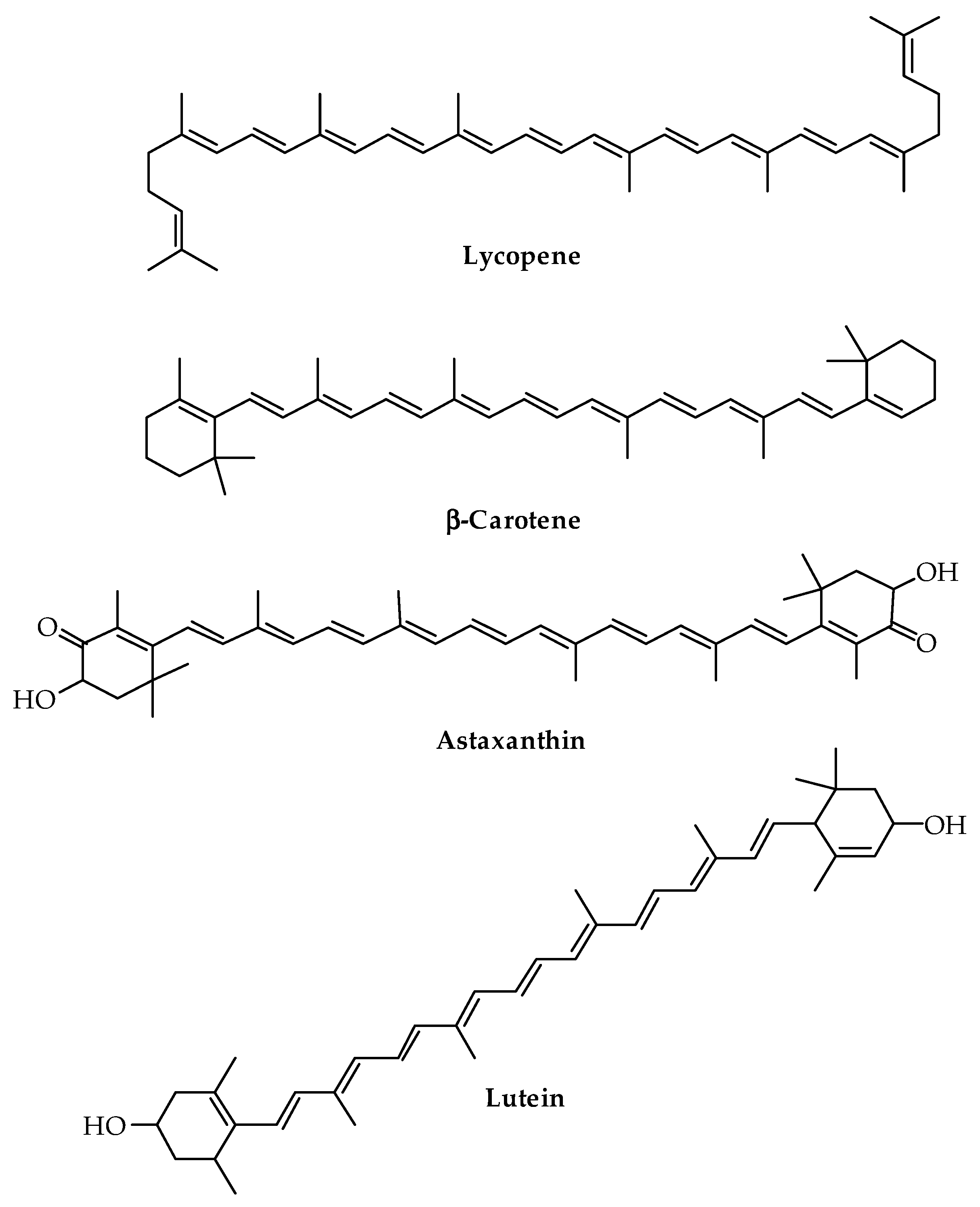
| Carotenoids | Astaxanthin | Tagetes erecta L. (flowers) | Extrusion | Antioxidant | Food | [163] |
| β-Carotene | Commercial | Encapsulation Supercritical emulsion extraction | Antioxidant | Biomedical | [102] | |
| Lycopene | Solanum lycopersicum L. (pulp) | Solvent casting | Antioxidant Antimicrobial | Food | [164] | |
| Lycopene | Commercial | Solvent casting | Antioxidant | Food | [165] | |
| Lutein | Linum usitatissimum L. (fibers) | Compression molding | Antioxidant Antimicrobial | Food | [162] | |
| Terpenes | Abietic acid | Abies Nordmanniana (Steven) Spach (leaves) | Solvent casting | Antioxidant | Food | [97] |
| β-Bisabolene | Tanacetum balsamita L. | Solvent casting | Antimicrobial | Food | [159] | |
| Carvacrol | Commercial | Extrusion | Antioxidant Antimicrobial | Food | [143] | |
| Carvacrol | Commercial | Electrospinning | Antioxidant Antimicrobial | Food | [144] | |
| Carvacrol | Commercial | Encapsulation | Antimicrobial | Food | [146] | |
| Carvacrol | Commercial | Electrospinning | Antioxidant Antimicrobial | Food | [147] | |
| Carvacrol | Commercial | Melt mixing Solvent casting | Antioxidant | Food | [149] | |
| Carvacrol | Commercial | sCO2 impregnation | Antioxidant | Food | [152] | |
| Carvacrol | Ziziphora clinopodioides Lam. | Solvent casting | Antioxidant Antimicrobial | Food | [156] | |
| Carvacrol | Zataria multiflora Boiss. | Solvent casting | Antimicrobial | Food | [161] | |
| β-Caryophyllene | Piper nigrum L. | Electrospinning | Antimicrobial | Biomedical | [160] | |
| Carvone | Tanacetum balsamita L. | Solvent casting | Antimicrobial | Food | [159] | |
| p-Cymene | Leptospermum scoparium (J.R.Forst. & G.Forst., 1776) | Electrospinning | Antimicrobial | Biomedical | [140] | |
| Eucalyptol | Melaleuca alterniflora (Maiden & Betche) Cheel, 1924 (oil) | Electrospinning | Antimicrobial | Biomedical | [140] | |
| Eucalyptol | Commercial | Flow-delay | Antioxidant Antimicrobial | Food | [150] | |
| Eugenol | Commercial | Impregnation | Antioxidant Antimicrobial | Food | [148] | |
| Geranial | Cymbopogon citratus (DC.) Stapf | Encapsulation | Antimicrobial | Food | [141] | |
| Limonene | Commercial | Melt mixing Solvent casting | Antioxidant | Food | [149] | |
| Limonene | Piper nigrum L. | Electrospinning | Antimicrobial | Biomedical | [160] | |
| Linalool | Commercial | Encapsulation | Antimicrobial | Food | [146] | |
| Linalool | Salvia sclarea L. | Electrospinning | Antimicrobial | Biomedical | [160] | |
| Linalool | Zataria multiflora Boiss. | Solvent casting | Antimicrobial | Food | [161] | |
| Linalyl acetate | Salvia sclarea L. | Electrospinning | Antimicrobial | Biomedical | [160] | |
| β-Myrcene | Cymbopogon citratus (DC.) Stapf | Encapsulation | Antimicrobial | Food | [141] | |
| Neral | Cymbopogon citratus (DC.) Stapf | Encapsulation | Antimicrobial | Food | [141] | |
| α-Pinene | Piper nigrum L. | Electrospinning | Antimicrobial | Biomedical | [160] | |
| β-Pinene | Piper nigrum L. | Electrospinning | Antimicrobial | Biomedical | [160] | |
| Sesquiterpenes | Leptospermum scoparium (J.R.Forst. & G.Forst., 1776) | Electrospinning | Antimicrobial | Biomedical | [140] | |
| α-Terpinene | Melaleuca alterniflora (Maiden & Betche) Cheel, 1924 | Electrospinning | Antimicrobial | Biomedical | [140] | |
| γ-Terpinene | Commercial | Electrospinning | Antimicrobial | Biomedical | [140] | |
| Terpineol | Melaleuca alterniflora (Maiden & Betche) Cheel, 1924 | Electrospinning | Antimicrobial | Biomedical | [140] | |
| Terpineol | Salvia sclarea L. | Electrospinning | Antimicrobial | Food | [160] | |
| Thymol | Thymus vulgaris L. (leaves) | Supercritical impregnation | Antimicrobial | Food | [151] | |
| Thymol | Ziziphora clinopodioides Lam. | Solvent casting | Antioxidant Antimicrobial | Food | [156] | |
| β-Thujone | Commercial | Electrospinning | Antioxidant Antimicrobial | Food | [96] | |
| β-Thujone | Zingiber officinale Roscoe | Melt blending | Antioxidant | Food | [130] | |
| β-Thujone | Thymus vulgaris L. | Solvent casting | Antimicrobial | Food | [154] | |
| β-Thujone | Origanum vulgare L. | Extrusion | Antioxidant Antimicrobial | Food | [158] | |
| β-Thujone | Tanacetum balsamita L. | Solvent casting | Antimicrobial | Food | [159] |
4.4. Antimicrobial Activity
4.5. Environmental Aspects
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Gkekaa, I.; Gioran, A.; Boziki, M.C.; Grigoriadis, N.; Chondrogianni, N.; Petrakis, S. Oxidative Stress and Neurodegeneration: Interconnected Processes in PolyQ Diseases. Antioxidants 2021, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Arfin, S.; Jha, N.K.; Kha, S.K.; Kesarim, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Pagan, L.U.; Gomes, M.J.; Gatto, M.; Mota, G.A.F.; Okoshi, K.; Okoshi, M.P. The Role of Oxidative Stress in the Aging Heart. Antioxidants 2022, 11, 336. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of Antioxidants in the Oxidation of Foods. Comp. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Nitrates/nitrites in food-risk for nitrosative stress and benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO estimates of the global burden of foodborne diseases. In Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Guidance on Core Indicators for Agrifood Systems—Measuring the Private Sector’s Contribution to the Sustainable Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). The State of Food and Agriculture-Moving forward on Food Loss and Waste Reduction; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; Available online: http://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 4 April 2022).
- Lee, S.Y.; Lee, S.J.; Choi, D.S.; Hur, S.J. Current topics in active and intelligent food packaging for preservation of fresh foods. J. Sci. Food. Agric. 2015, 95, 2799–2810. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 450/2009 of 29 May 2009 on Active and Intelligent Materials and Articles Intended to Come Into Contact with Food (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R0450 (accessed on 4 April 2022).
- Ramos, M.; Beltrán, A.; Fortunati, E.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic acid and quercetin as intelligent and active ingredients in poly (vinyl alcohol) films for food packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Lema, S.; Torres-Giner, S.; Quiles-Carrillo, L.; Gomez-Caturla, J.; Garcia-Garcia, D.; Balart, R. On the Use of Phenolic Compounds Present in Citrus Fruits and Grapes as Natural Antioxidants for Thermo-Compressed Bio-Based High-Density Polyethylene Films. Antioxidants 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Luzi, F.; Fanali, C.; Dugo, L.; Belluomo, M.G.; Torre, L.; Kenny, J.M.; Santi, L.; Bernini, R. Hydroxytyrosol as active ingredient in poly (vinyl alcohol) films for food packaging applications. Polym. Int. 2016, 65, 872–882. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Di Michele, A.; Pannucci, E.; Botticella, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R. Nanostructured starch combined with hydroxytyrosol in poly(vinyl alcohol) based ternary films as active packaging system. Carbohydr. Polym. 2018, 193, 239–248. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Clemente, M.; Grande, E.; Urciuoli, S.; Romani, A.; Torre, L.; Puglia, D.; Bernini, R.; Santi, L. Hydroxytyrosol and oleuropein-enriched extracts obtained from olive oil wastes and by-products as active antioxidant ingredients for poly (vinyl alcohol)-based films. Molecules 2021, 26, 2104. [Google Scholar] [CrossRef]
- Cerruti, P.; Malinconico, M.; Rychly, J.; Matisova-Rychla, L.; Carfagna, C. Effect of natural antioxidants on the stability of polypropylene films. Polym. Degrad. Stab. 2009, 94, 2095–2100. [Google Scholar] [CrossRef]
- Garcia, A.V.; Serrano, N.J.; Sanahuja, A.B.; Garrigós, M.C. Novel antioxidant packaging films based on poly(ε-caprolactone) and almond skin extract: Development and effect on the oxidative stability of fried almonds. Antioxidants 2020, 9, 629. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Stefaniak, K.; Masek, A. Green Copolymers Based on Poly(Lactic Acid) - Short Review. Materials 2021, 14, 5254. [Google Scholar] [CrossRef] [PubMed]
- Markets & Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/polylacticacid-387.html?gclid=Cj0KCQjwjbyYBhCdARIsAArC6LJ0OGab2pFwrXFJnGk8UAjTiHZGXxZmMZKHJ7Dfun7dvtaWv-7_9GMaAlrYEALw_wcB (accessed on 31 August 2022).
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Kvalvåg Pettersen, M.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Velásquez, E.; Vidal, C.P.; Guarda, A.; Galotto, M.J.; de Dicastillo, C.L. Active PLA Packaging Films: Effect of Processing and the Addition of Natural Antimicrobials and Antioxidants on Physical Properties, Release Kinetics, and Compostability. Antioxidants 2021, 10, 1976. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; Gaspar, P.D.; Lima, T.M.; Silva, P.D. What is the role of active packaging in the future of food sustainability? A systematic review. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A review of multilayer and composite films and coatings for active biodegradable packaging. npj Sci. Food 2022, 6, 1–16. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable Active Packaging with Controlled Release: Principles, Progress, and Prospects. ACS Food Sci. Technol. 2022, 2, 1166–1183. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(LA) fiber: An overview. Prog. Polym. Sci. 2007, 34, 455–482. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser-Saunders, I.; Jürgens, C.; Wolter, D. Polylactides-synthesis, characterization and medical application. Macromol. Symp. 1996, 103, 85–102. [Google Scholar] [CrossRef]
- Datta, R.; Henry, M. Lactic acid: Recent advances in products, processes and 10 technologies—A review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Richert, J. Synthesis, properties and applications of polylactide. Przetwórstwo Tworzyw 2009, 15, 192–199. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iniguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Hong, C.H.; Kim, S.H.; Seo, J.-Y.; Han, D.S. Development of Four Unit Processes for Biobased PLA Manifacturing. Int. Sch. Res. Not. 2012, 2012, 938261. [Google Scholar] [CrossRef] [Green Version]
- Ajioka, M.; Enomoto, K.; Suzuki, K.; Yamaguchi, A. Basic Properties of Polylactic Acid Produced by the Direct Condensation Polymerization of Lactic Acid. Bull. Chem. Soc. Jpn. 1995, 68, 2125–2131. [Google Scholar] [CrossRef]
- Ren, J. Synthesis and manufacture of PLA. In Biodegradable Poly(Lactic Acid): Synthesis, Modification, Processing and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 15–37. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, C.; Wang, Y. The synthesis of lactide catalyzed by ZnO-Sn(Oct)2. Polym. Mater. Sci. Eng. 2007, 23, 74–76. [Google Scholar]
- Jedlinski, Z.; Wałach, W.; Kurcok, P.; Adamus, G. Polymerization of lactones. XII, Polymerization of L-dilactide and L,D-dilactide in the presence of potassium methoxide. Macromol. Chem. Phys. 1991, 192, 2051–2057. [Google Scholar] [CrossRef]
- Kurcok, P.; Matuszowicz, A.; Jedliński, Z.; Kricheldorf, H.R.; Dubois, P.; Jérôme, R. Substituent effect in anionic polymerization of_-lactones initiated by alkali metal alkoxides. Macromol. Rapid Commun. 1995, 16, 513–519. [Google Scholar] [CrossRef]
- Jung, Y.K.; Lee, S.Y. Efficient production of polylactic acid and its copolymers by metabolically engineered Escherichia coli. J. Biotechnol. 2011, 151, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Chanfreau, S.; Mena, M.; Porras-Dominguez, J.R.; Ramirez-Gilly, M.; Gimeno, M.; Roquero, P.; Tecante, A.; Bàrzana, E. Enzymatic synthesis of poly-L-lactide and poly-L-lactide-co-glycolide in an ionic liquid. Bioprocess. Biosyst. Eng. 2010, 33, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Nutr. Food. Sci. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Suffredini, I.B.; Sader, H.S.; Gonçalves, A.G.; Reis, A.O.; Gales, A.C.; Varella, A.D.; Younes, R.N. Screening of antibacterial extracts from plants native to the brazilian amazon rain forest and atlantic forest. Braz. J. Med. Biol. Res. 2004, 37, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Durazzo, A.; Lucarini, M. Editorial: The State of Science and Innovation of Bioactive Research and Applications, Health, and Diseases. Front. Nutr. 2019, 6, 178. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Figueiredo, A.; Hugueney, P.; Durazzo, A. Recent Advances in Plant Metabolomics: From Metabolic Pathways to Health Impact. Biology 2022, 11, 238. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Osman, K.; Evangelopoulos, D.; Basavannacharya, C.; Gupta, A.; McHugh, T.D.; Bhakta, S.; Gibbons, S. An antibacterial from Hypericum acmosepalum inhibits ATP-dependent MurE ligase from Mycobacterium tuberculosis. Int. J. Antimicrob. Agents 2012, 39, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianch, A.; Chiavarini, M.; Impecciatore, M. Comparative screening of plant essential oil: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-based compounds from grape seeds: A biorefinery approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A.; Romani, A. Fruit Wastes as a Valuable Source of Value-Added Compounds: A Collaborative Perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef] [PubMed]
- Zuin, V.G.; Segatto, M.L.; Zanotti, K. Towards a green and sustainable fruit waste valorisation model in Brazil: Optimisation of homogenizer-assisted extraction of bioactive compounds from mango waste using a response surface methodology. Pure Appl. Chem. 2020, 92, 617–629. [Google Scholar] [CrossRef] [Green Version]
- Villacis-Chiriboga, J.; Vera, E.; Van Camp, J.; Ruales, J.; Elst, K. Valorization of byproducts from tropical fruits: A review, Part 2: Applications, economic, and environmental aspects of biorefinery via supercritical fluid extraction. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2305–2331. [Google Scholar] [CrossRef] [PubMed]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crisan, G.; Ferreira, I.C. Enzyme-assisted extractions of polyphenols-A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2020, 70, 105325. [Google Scholar] [CrossRef]
- Cai, H.; You, S.; Xu, Z.; Li, Z.; Guo, J.; Ren, Z.; Fu, C. Novel extraction methods and potential applications of polyphenols in fruit waste: A review. J. Food Meas. Charact. 2021, 15, 3250–3261. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, innovation and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef] [Green Version]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of hydroxytyrosol-enriched fractions from Olea europaea L. by-products and evaluation of the in vitro effects on a model of colorectal cancer cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef]
- Naviglio, D.; Pizzolongo, F.; Ferrara, L.; Aragon, A.; Santini, A. Extraction of pure lycopene from industrial tomato by-products in water using a new high-pressure process. J. Sci. Food. Agric. 2008, 88, 2414–2420. [Google Scholar] [CrossRef]
- Maric, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brncic, M.; Brncic, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols from Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Kingston, H.M.; Jessie, L.B. Introduction to Microwave Sample Preparation; American Chemical Society: Washington, DC, USA, 1998. [Google Scholar]
- Suzara, S.; Costa, D.A.; Gariepyb, Y.; Rochaa, S.C.S.; Raghavanb, V. Spilanthol extraction using microwave: Calibration curve for gas chromatography. Chem. Eng. Trans. 2013, 32, 1783–1788. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Caroleo, M.C.; Cione, E.; Plastina, P. Novel acrylic polymers for food packaging: Synthesis and antioxidant properties. Food Packag. Shelf Life 2017, 11, 84–90. [Google Scholar] [CrossRef]
- Kesente, M.; Kavetsou, E.; Roussaki, M.; Blidi, S.; Loupassaki, S.; Chanioti, S.; Siamandoura, P.; Stamatogianni, C.; Philippou, E.; Papaspyrides, C.; et al. Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering 2017, 4, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortenzi, M.A.; Gazzotti, S.; Marcos, B.; Antenucci, S.; Camazzola, S.; Piergiovanni, L.; Farina, H.; Di Silvestro, G.; Verotta, L. Synthesis of Polylactic Acid Initiated through Biobased Antioxidants: Towards Intrinsically Active Food Packaging. Polymers 2020, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Apicella, A.; Adiletta, G.; Albanese, D.; Di Matteo, M.; Incarnato, L. Biodegradable films based on poly(lactic acid) coatings and natural olive-wastewater extracts for active food packaging. Chem. Eng. Trans. 2021, 87, 85–90. [Google Scholar] [CrossRef]
- De Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Bellumori, M.; Cecchi, L.; Romani, A.; Mulinacci, N.; Innocenti, M. Recovery and stability over time of phenolic fractions by an industrial filtration system of olive mill wastewaters: A three-year study. J. Sci. Food Agric. 2018, 98, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Erdohan, Z.O.; Çam, B.; Turhan, K.N. Characterization of antimicrobial polylactic acid-based films. J. Food Eng. 2013, 119, 308–315. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-assisted extraction of phenolic compounds from olive leaves; a comparison with maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Naleini, N.; Rahimi, M.; Heydari, R. Oleuropein extraction using microfluidic system. Chem. Eng. Process. Process Intensif. 2015, 92, 1–6. [Google Scholar] [CrossRef]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Ulrih, N.P. Enhanced yield of oleuropein from olive leaves using ultra-sound-assisted extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Heydarid, R.; Rahimi, M.; Naleini, N. Optimization of oleuropein extraction from organic extracts using a micro-fluidic device and response surface methodology. Herb. Med. J. 2018, 3, 60–69. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havelt, T.; Brettschneider, S.; Schmitz, M. Evaluation of Practical Applicability and Synergistic Effects of Bio-Based Food Packaging Materials Combined with Plant-Based Stabilisers. Processes 2021, 9, 1838. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, Y.; Zhang, X.; Deng, Y.; Huang, D.; Huang, C.; Qu, Q. Quaternized chitin/tannic acid bilayers layer-by-layer deposited poly(lactic acid)/polyurethane nanofibrous mats decorated with photoresponsive complex and silver nanoparticles for antibacterial activity. Int. J. Biol. Macromol. 2022, 201, 448–457. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Yurttaş, E.; Durmus, A.; Özdemir, F.; Nagarajan, R.; Kalimuthu, M.; Kuzman, M.K. Properties of biocomposite films from PLA and thermally treated wood modifed with silver nanoparticles using leaf extracts of oriental sweetgum. J. Polym. Environ 2021, 29, 2409–2420. [Google Scholar] [CrossRef]
- Martins, C.; Vilarinhob, F.; Sanches Silvac, A.; Andrade, M.; Machado, A.V.; Conceição Castilho, M.; Sá, A.; Cunha, A.; Vaz, M.F.; Ramos, F. Active polylactic acid film incorporated with green tea extract: Development, characterization, and effectiveness. Ind. Crops Prod. 2018, 123, 100–110. [Google Scholar] [CrossRef]
- Darie-Niţa, R.N.; Vasile, C.; Stoleru, E.; Pamfil, D.; Zaharescu, T.; Tartau, L.; Tudorachi, N.; Brebu, M.A.; Pricope, G.M.; Dumitriu, R.P.; et al. Evaluation of the Rosemary Extract Effect on the Properties of Polylactic Acid-Based Materials. Materials 2018, 11, 1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez-Rota, C.; Palazzo, I.; Scognamiglio, M.R.; Mainar, A.; Reverchon, E.; Della Porta, G. β-Carotene, α-tocoferol and rosmarinic acid encapsulated within PLA/PLGA microcarriers by supercritical emulsion extraction: Encapsulation efficiency, drugs shelf-life and antioxidant activity. J. Supercrit. Fluids 2019, 146, 199–207. [Google Scholar] [CrossRef]
- Wang, D.; Lu, J.; Miao, A.; Xie, Z.; Yang, D. HPLC-DAD-ESI-MS/MS analysis of polyphenols and purine alkaloids in leaves of 22 tea cultivars in China. J. Food Compos. Anal. 2008, 21, 361–369. [Google Scholar] [CrossRef]
- Xia, T.; Shi, S.; Wan, X. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food Eng. 2006, 74, 557–560. [Google Scholar] [CrossRef]
- Tsubaki, S.; Iida, H.; Sakamoto, M.; Azuma, J.I. Microwave heating of tea residue yields polysaccharides, polyphenols, and plant biopolyester. J. Agric. Food Chem. 2008, 56, 11293–11299. [Google Scholar] [CrossRef]
- Jun, X.; Shuo, Z.; Bingbing, L.; Rui, Z.; Ye, L.; Deji, S.; Guofeng, Z. Separation of major catechins from green tea by ultrahigh pressure extraction. Int. J. Pharm. 2010, 386, 229–231. [Google Scholar] [CrossRef]
- Iniguez-Franco, F.; Soto-Valdez, H.; Peralta, E.; Ayala-Zavala, J.F.; Auras, R.; Gamez-Meza, N. Antioxidant activity and diffusion of catechin and epicatechin from antioxidant active films made of poly(L-lactic acid). J. Agric. Food Chem. 2012, 60, 6515–6523. [Google Scholar] [CrossRef]
- Buddhiranon, S.; Define, L.A.; Alexander, T.S.; Kyu, T. Genistein-modified poly(ethylene oxide)/poly(D,L-lactic acid) electrospun mats with improved antioxidant and anti-inflammatory properties. Biomacromolecules 2013, 14, 1423–1433. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López de Dicastillo, C.; Garrido, L.; Roa, K.; Galotto, M.J. Electrospun PVA fibers loaded with antioxidant fillers extracted from Durvillaea antarctica algae and their effect on plasticized PLA bionanocomposites. Eur. Polym. J. 2018, 103, 145–157. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; de la Caba, K.; Benjakul, S.; Prodpran, T. Properties and application of bilayer films based on poly (lactic acid) and fish gelatin containing epigallocatechin gallate fabricated by thermo-compression molding. Food Hydrocoll. 2020, 105, 105792. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. Fabrication of quercetin-loaded biopolymer films as functional packaging materials. ACS Appl. Polym. Mater. 2021, 3, 2131–2137. [Google Scholar] [CrossRef]
- Sobolewska, D.; Podolak, I.; Makowska-Wąs, J. Allium ursinum: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radusin, T.; Tomsik, A.; Saric, L.; Ristic, I.; Giacinti Baschetti, M.; Minelli, M.; Novakovic, A. Hybrid Pla/Wild Garlic Antimicrobial Composite Films for Food Packaging Application. Polym. Compos. 2019, 40, 893–900. [Google Scholar] [CrossRef]
- Riquelme, S.; Sáez, V.; Escobar, D.; Vergara, C.; Fuentealba, C.; Bustamante, L.; von Baer, D.; Jara, P.; Lamperti, L.; Mardones, C. Bench-scale extraction of stilbenoids and other phenolics from stored grape canes (Vitis vinifera): Optimization process, chemical characterization, and potential protection against oxidative damage. J. Chil. Chem. Soc. 2019, 64, 4414–4420. [Google Scholar] [CrossRef]
- Aliaño-González, M.J.; Richard, T.; Cantos-Villar, E. Grapevine cane extracts: Raw plant material, extraction methods, quantification, and applications. Biomolecules 2020, 10, 1195. [Google Scholar] [CrossRef]
- Billet, K.; Malinowska, M.A.; Munsch, T.; Unlubayir, M.; Bernonville, T.D.D.; Besseau, S.; Courdavault, V.; Oudin, A.; Pichon, O.; Clastre, M.; et al. Stilbenoid-enriched grape cane extracts for the biocontrol of grapevine diseases. In Plant Defence: Biological Control; Springer: Cham, Switzerland, 2020; pp. 215–239. [Google Scholar]
- Diaz-Galindo, P.E.; Nesic, A.; Cabrera-Barjas, G.; Dublan-García, O.; Ventura-Aguilar, R.I.; Vázquez-Armenta, F.J.; Aguilar-Montes de Oca, S.; Mardones, C.; Ayala-Zavala, J.F. Physico-chemical and antiadhesive properties of poly(lactic acid)/grapevine cane extract films against food pathogenic microorganisms. Polymers 2020, 12, 2967. [Google Scholar] [CrossRef]
- Domenek, S.; Louaifi, A.; Guinault, A.; Baumberger, S. Potential of lignins as antioxidant additive in active biodegradable packaging materials. J. Polym. Env. 2013, 21, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Kai, D.; Ren, W.; Tian, L.; Chee, P.L.; Liu, Y.; Ramakrishna, S.; Loh, X.J. Engineering poly(lactide)–lignin nanofibers with antioxidant activity for biomedical application. ACS Sustain. Chem. Eng. 2016, 4, 5268–5276. [Google Scholar] [CrossRef]
- Dominguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larrañeta, E. Antioxidant PLA composites containing lignin for 3D printing applications: A potential material for healthcare applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A biopolymer from forestry biomass for biocomposites and 3D printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef]
- Vaz Fontes, M.R.; Pereira da Rosa, M.; Fonseca, L.M.; Beck, P.H.; da Rosa Zavareze, E.; Guerra Dias, A.R. Thermal stability, hydrophobicity and antioxidant potential of ultrafine poly (lactic acid)/rice husk lignin fibers. Braz. J. Chem. Eng. 2020, 38, 133–144. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Huang, D.; Li, R.; Xu, P.; Li, T.; Deng, R.; Chen, S.; Zhang, Q. The cornerstone of realizing lignin value-addition: Exploiting the native structure and properties of lignin by extraction methods. Chem. Eng. J. 2020, 402, 126237. [Google Scholar] [CrossRef]
- Glasser, W.G.; Davé, V.; Frazier, C.E. Molecular weight distribution of (semi-) commercial lignin derivatives. J. Wood Chem. Technol. 1993, 13, 545–559. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuel Bioprod. Biorefin. 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Grilo, E.C.; Costa, P.N.; Gurgel, C.S.S.; Beserra, A.F.D.L.; Almeida, F.N.D.S.; Dimenstein, R. Alpha-tocopherol and gamma-tocopherol concentration in vegetable oils. Food Sci. Technol. 2014, 34, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/silver-NP/vitaminE bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanoparticle Res. 2014, 16, 2643. [Google Scholar] [CrossRef] [Green Version]
- Scarfato, P.; Avallone, E.; Galdi, M.S.; Di Maio, L.; Incarnato, L. Preparation, characterization, and oxygen scavenging capacity of biodegradable a-tocopherol/PLA microparticles for active food packaging applications. Polym. Comp. 2017, 38, 981–986. [Google Scholar] [CrossRef]
- Jiang, J.; Dong, Q.; Gao, H.; Han, Y.; Li, L. Enhanced mechanical and antioxidant properties of biodegradable poly (lactic) acid-poly(3-hydroxybutyrate-co-4-hydroxybutyrate) film utilizing α-tocopherol for peach storage. Packag. Technol. Sci. 2021, 34, 187–199. [Google Scholar] [CrossRef]
- Liu, K.; Chougnet, A.; Woggon, W.D. A short route to α-tocopherol. Angew. Chem. 2008, 120, 5911–5913. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yixuan, L.; Qaria, M.A.; Sivasamy, S.; Jianzhong, S.; Daochen, Z. Curcumin production and bioavailability: A comprehensive review of curcumin extraction, synthesis, biotransformation, and delivery systems. Ind. Crops Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Hanafi, S.; Sirait, M.; Irawan, C.; Rochaeni, H. Poly(Lactic Acid) packaging modified curcumin as bioactive substance in tea drink (Camelia sinensis). Asian J. Chem. 2018, 30, 145–147. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application. Intl. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Dhurai, B.; Saraswathy, N.; Maheswaran, R.; Sethypathu, P.; Vanitha, P.; Vigneshwarani, S.; Rameshbabu, V. Electrospinning of curcumin loaded chitosan/poly (lactic acid) nanofilm and evaluation of its medicinal characteristics. Front. Mat. Sci. 2013, 7, 350–361. [Google Scholar] [CrossRef]
- Rabanel, J.-M.; Faivre, J.; Djiokeng Paka, G.; Ramassamy, C.; Hildgen, P.; Banquy, X. Effect of polymer architecture on curcumin encapsulation and release from PEGylated polymer nanoparticles: Toward a drug delivery nanoplatform to the CNS. Eur. J. Pharm. Biopharm. 2015, 96, 409–420. [Google Scholar] [CrossRef] [Green Version]
- Alippilakkotte, S.; Sreejith, L. Pectin mediated synthesis of curcumin loaded poly (lactic acid) nanocapsules for cancer treatment. J. Drug. Deliv. Sci. Technol. 2018, 48, 66–74. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Compos. B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React. Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Antonioli, G.; Fontanella, G.; Echeverrigaray, S.; Longaray Delamare, A.P.; Pauletti, G.F.; Barcellos, T. Poly (lactic acid) nanocapsules containing lemongrass essential oil for postharvest decay control: In vitro and in vivo evaluation against phytopathogenic fungi. Food Chem. 2020, 326, 126997. [Google Scholar] [CrossRef] [PubMed]
- Del Nobile, M.A.; Conte, A.; Buonocore, G.G.; Incoronato, A.L.; Massaro, A.; Panza, O. Active packaging by extrusion processing of recyclable and biodegradable polymers. J. Food. Eng. 2009, 93, 1–6. [Google Scholar] [CrossRef]
- Burgos, N.; Armentano, I.; Fortunati, E.; Dominici, F.; Luzi, F.; Fiori, S.; Cristofaro, F.; Visai, L.; Jimenez, A.; Kenny, J.M. Functional properties of plasticized bio-based poly (lactic acid)-poly(hydroxybutyrate) (PLA-PHB) films for active food packaging. Food Bioprocess. Technol. 2017, 10, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol loaded electrospun fibrous films from zein and poly (lactic acid) for active food packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Ramos, M.; Fortunati, E.; Beltrán, A.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled release, disintegration, antioxidant, and antimicrobial properties of poly (Lactic Acid)/thymol/nanoclay composites. Polymers 2020, 12, 1878. [Google Scholar] [CrossRef]
- Campini, P.A.L.; de Oliveira, E.R.; Camani, P.H.; da Silva, C.G.; Della Coletta Yudice, E.; de Oliveira, S.A.; dos Santos Rosa, D. Assessing the efficiency of essential oil and active compounds/poly (lactic acid) microcapsules against common foodborne pathogens. Int. J. Biol. Macromol. 2021, 186, 702–713. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of eugenol loaded gelatin nanofibers by electrospinning technique as active packaging material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Masek, A.; Cichosz, S.; Piotrowska, M. Comparison of aging resistance and antimicrobial properties of ethylene–norbornene copolymer and poly (lactic acid) impregnated with phytochemicals embodied in thyme (Thymus vulgaris) and clove (Syzygium aromaticum). Int. J. Mol. Sci. 2021, 22, 13025. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Redhwi, H.H.; Tsagkalias, I.; Vouvoudi, E.C.; Achilias, D.S. Development of bio-composites with enhanced antioxidant activity based on poly (lactic acid) with thymol, carvacrol, limonene, or cinnamaldehyde for active food packaging. Polymers 2021, 13, 3652. [Google Scholar] [CrossRef]
- Chen, M.; Yan, X.; Cheng, M.; Zhao, P.; Wang, Y.; Zhang, R.; Wang, X.; Wang, X.J.; Chen, M. Preparation, characterization and application of poly (lactic acid)/corn starch/eucalyptus leaf essential oil microencapsulated active bilayer degradable film. Int. J. Biol. Macromol. 2022, 195, 264–273. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Intl. 2018, 107, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Fanovich, M.A.; Ivanovic, J.; Misic, D.; Alvarez, M.V.; Jaeger, P.; Zizovic, I.; Eggers, R. Development of polycaprolactone scaffold with antibacterial activity by an integrated supercritical extraction and impregnation process. J. Supercrit. Fluids 2013, 78, 42–53. [Google Scholar] [CrossRef]
- Ardjoum, N.; Chibani, N.; Boukerrou, A.; Djidjelli, H. Study of antimicrobial activities of thyme and propolis of PLA films. Macromol. Symp. 2021, 396, 200293. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Analyt. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Shavisi, N.; Khanjari, A.; Basti, A.A.; Misaghi, A.; Shahbazi, Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017, 124, 95–104. [Google Scholar] [CrossRef]
- Liu, D.; Li, H.; Jiang, L.; Chuan, Y.; Yuan, M.; Chen, H. Characterization of active packaging films made from poly (lactic acid)/poly(trimethylene carbonate) incorporated with oregano essential oil. Molecules 2016, 21, 695. [Google Scholar] [CrossRef] [Green Version]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermúdez, J.M.; Baños, A.; Núñez, C.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Development of PLA films containing oregano essential oil (Origanum vulgare L. virens) intended for use in food packaging. Food Addit. Contam. Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1374–1386. [Google Scholar] [CrossRef]
- Khodayari, M.; Basti, A.A.; Akhondzadeh, A.; Khanjari, A.; Misaghi, A.; Kamkar, A.; Shotorbani, P.M.; Hamedi, H. Effect of poly(lactic acid) films incorporated with different concentrations of Tanacetum balsamita essential oil, propolis ethanolic extract and cellulose nanocrystals on shelf life extension of vacuum-packed cooked sausages. Food Packag. Shelf Life 2019, 19, 200–209. [Google Scholar] [CrossRef]
- Wang, P.; Mele, E. Effect of antibacterial plant extracts on the morphology of electrospun poly(lactic acid) fibres. Materials 2018, 11, 923. [Google Scholar] [CrossRef] [Green Version]
- Rezaeigolestani, M.; Misaghi, A.; Khanjari, A.; Bastia, A.A.; Abdulkhanib, A.; Fayazfar, S. Antimicrobial evaluation of novel poly-lactic acid-based nanocomposites incorporated with bioactive compounds in-vitro and in refrigerated vacuumpacked cooked sausages. Int. J. Food Microbiol. 2017, 260, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wrobel-Kwiatkowska, M.; Czemplik, M.; Kulma, A.; Zuk, M.; Kaczmar, J.; Dyminska, L.; Hanuza, J.; Ptak, M.; Szopa, J. New biocomposites based on bioplastic flax fibers and biodegradable polymers. Biotechnol. Prog. 2012, 28, 5. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, H.; Soto-Valdez, H.; Auras, R. Poly(lactic acid) film incorporated with marigold flower extract (Tagetes erecta) intended for fatty-food application. Food Control 2014, 46, 55–56. [Google Scholar] [CrossRef]
- Asadi, S. Production of Biodegradable Film Based on Polylactic Acid, Modified with Lycopene Pigment and TiO2 and Studying Its Physicochemical Properties. J. Polym. Environ. 2019, 28, 433–444. [Google Scholar] [CrossRef]
- Pirsa, S.; Asadi, S. Innovative smart and biodegradable packaging for margarine based on a nanocomposite polylactic acid/lycopene film. Food Addit. Contam. Part A 2021, 38, 856–869. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and extraction of carotenoids produced by microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meleéndez-Martinez, J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Rodrigues Arruda, T.; Campos Bernardes, P.; Robledo Fialho e Moraes, A.; de Fátima Ferreira Soares, N. Natural bioactives in perspective: The future of active packaging based on essential oils and plant extracts themselves and those complexed by cyclodextrins. Food Res. Int. 2022, 156, 111160. [Google Scholar] [CrossRef]
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S. Advancements in biodegradable active films for food packaging: Effects of nano/microcapsule incorporation. Foods 2022, 11, 760. [Google Scholar] [CrossRef]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of promising bioactive compounds to improve their absorption, stability, functionality and the appearance of the final food products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, B.S.; Vasile, C. Encapsulation of natural bioactive compounds by electrospinning-applications in food storage and safety. Polymers 2021, 13, 3771. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Pinto, V.Z.; Göksen, G.; Alessandroni, L.; Lamri, M.; Dib, A.L.; Boukid, F. Electrospinning as a promising process to preserve the quality and safety of meat and meat products. Coatings 2022, 12, 644. [Google Scholar] [CrossRef]
- Alehosseini, A.; Ghorani, B.; Sarabi-Jamab, M.; Tucker, N. Principles of electrospraying: A new approach in protection of bioactive compounds in foods. Crit. Rev. Food Sci. Nutr. 2017, 58, 2346–2363. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel antimicrobial packaging film based on porous poly (lactic acid) nanofiber and polymeric coating for humidity-controlled release of thyme essential oil. LWT 2021, 135, 110034. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nanoparticles and natural nanocarriers for nanoencapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Ataei, S.; Azari, P.; Hassan, A.; Pingguan-Murphy, B.; Yahya, R.; Muhamad, F. Essential oils-loaded electrospun biopolymers: A future perspective for active food packaging. Adv. Polym. Tech. 2020, 2020, 9040535. [Google Scholar] [CrossRef]
- Silion, M.; Hritcu, D.; Lisa, G.; Popa, I.M. New hybrid materials based on layered double hydroxides and antioxidant compounds. Preparation, characterization and release kinetic studies. J. Porous Mater. 2012, 19, 267–276. [Google Scholar] [CrossRef]
- Pérez Amaro, L.; Cicogna, F.; Passaglia, E.; Morici, E.; Oberhauser, W.; Al-Malaika, S.; Tzankova Dintcheva, N.; Coiai, S. Thermo-oxidative stabilization of poly(lactic acid) with antioxidant intercalated layered double hydroxides. Polym. Degrad. Stab. 2016, 133, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Szente, L.; Fenyvesi, E. Cyclodextrin-enabled polymer composites for packaging (dagger). Molecules 2018, 23, 1556. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.R.; Marques, C.S.; Soares, N.F.F. Native cyclodextrins and their derivatives as potential additives for food packaging: A review. Polysaccharides 2021, 2, 825–842. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Xu, Y.; Huang, H.; Zhao, H.; Wang, J.; Wang, S. Synthesis and characterization of antibacterial polylactic acid film incorporated with cinnamaldehyde inclusions for fruit packaging. Int. J. Biol. Macromol. 2020, 164, 4547–4555. [Google Scholar] [CrossRef] [PubMed]
- Friné, V.C.; Hector, A.P.; Manuel, N.D.S.; Estrella, N.D.; Antonio, G.J. Biodegradable PLA food packaging hold Alternaria alternata. Polymers 2019, 11, 1720. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Contreras, F.; García-Caldera, N.; Padilla de la Rosa, J.D.; Martínez-Romero, D.; Núñez-Delicado, E.; Gabaldón, J.A. Effect of PLA active packaging containing monoterpene-cyclodextrin complexes on berries preservation. Polymers 2021, 13, 1399. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 231–239. [Google Scholar] [CrossRef]
- Aytac, Z.; Keskin, N.O.S.; Tekinay, T.; Uyar, T. Antioxidant α-tocopherol/γ-cyclodextrin–inclusion complex encapsulated poly (lactic acid) electrospun nanofibrous web for food packaging. J. Appl. Polym. Sci. 2017, 134, 44858. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Feng, K. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β -cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, A.; Fang, D.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Huang, C.; Li, W. Oregano essential oil/β-cyclodextrin inclusion compound polylactic acid/polycaprolactone electrospun nanofibers for active food packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric carriers designed for encapsulation of essential oils with biological activity. Pharmaceutics 2021, 13, 631. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Microencapsulation and nanoencapsulation using supercritical fluid (SCF) techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Zaphar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef]
- Liakos, I.L.; Grumezescu, A.M.; Holban, A.M.; Florin, I.; D’Autilia, F.; Carzino, R.; Bianchini, P.; Athanassiou, A. Polylactic acid-lemongrass essential oil nanocapsules with antimicrobial properties. Pharmaceuticals 2016, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Ibili, H.; Dasdemir, M.; Cankaya, I.T.; Orhan, M.; Gunesoglu, C.; Anul, S.A. Investigation of poly (lactic acid) nanocapsules containing the plant extract via coaxial electrospraying method for functional nonwoven applications. J. Ind. Text. 2021, 51, 5304S–5327S. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Villegas, C.; Garrido, L.; Roa, K.; Torres, A.; Galotto, M.J.; Rojas, A.; Romero, J. Modifying an Active Compound’s release kinetic using a supercritical impregnation process to incorporate an active agent into PLA electrospun mats. Polymers 2018, 10, 479. [Google Scholar] [CrossRef] [Green Version]
- Rojas, A.; Torres, A.; López de Dicastillo, C.; Velásquez, E.; Villegas, C.; Faba, S.; Rivera, P.; Guarda, A.; Romero, J.; Galotto, M.J. Foaming with scCO2 and impregnation with cinnamaldehyde of PLA nanocomposites for food packaging. Processes 2022, 10, 376. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly(lactic acid)/poly(butylene-succinate-co-adipate) (PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Richert, A.; Grabska-Zielińska, S.; Rudawska, A.; Bouaziz, M. Antibacterial films based on polylactide with the addition of quercetin and poly(ethylene glycol). Materials 2021, 14, 1643. [Google Scholar] [CrossRef] [PubMed]
- Yahyaoui, M.; Gordobil, O.; Herrera Díaz, R.; Abderrabba, M.; Labidi, J. Development of novel antimicrobial films based on poly(lactic acid) and essential oils. React. Funct. Polym. 2016, 109, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.-Y. Structure-activity relationships and rational design strategies for radical-scavenging antioxidants. Curr. Comput. Aided Drug Des. 2005, 1, 257–273. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No. 1935/2004 of 27 October 2004. On materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Communities 2004, 50, 4–17. [Google Scholar]
- UNE-EN 13130-1: 2005; Materials and Articles in Contact with Foodstuffs. Plastics Substances Subject to Limitation. Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and Food Simulants and the Determination of Substances in Plastics and the Selection of Conditions of Exposure to Food Simulants. Techstreet LLC: Ann Arbor, MI, USA, 2005.
- Bernini, R.; Barontini, M.; Cis, V.; Carastro, I.; Tofani, D.; Chiodo, R.A.; Lupattelli, P.; Incerpi, S. Synthesis and evaluation of the antioxidant activity of lipophilic phenethyl trifluoroacetate esters by in vitro ABTS, DPPH and in cell-culture DCF assays. Molecules 2018, 23, 208. [Google Scholar] [CrossRef] [Green Version]
- Bernini, R.; Carastro, I.; Santoni, F.; Clemente, M. Synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol. Antioxidants 2019, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Roma, E.; Mattoni, E.; Lupattelli, P.; Moeini, S.S.; Gasperi, T.; Bernini, R.; Incerpi, S.; Tofani, D. New dihydroxytyrosyl esters from dicarboxylic acids. Synthesis and evaluation of the antioxidant activity in vitro (ABTS) and in cell-cultures (DCF assay). Molecules 2020, 25, 3135. [Google Scholar] [CrossRef]
- Mele, G.; Bloise, E.; Cosentino, F.; Lomonaco, D.; Avelino, F.; Marciano, T.; Massaro, C.; Mazzetto, S.E.; Tammaro, L.; Scalone, A.G.; et al. Influence of cardanol oil on the properties of poly(lactic acid) films produced by melt extrusion. ACS Omega 2019, 4, 718–726. [Google Scholar] [CrossRef]
- Weisburger, J.H. Lycopene and tomato products in health promotion. Exp. Biol. Med. 2002, 227, 924–927. [Google Scholar] [CrossRef]
- Pandey, S.K.; Haldar, C.; Vishwas, D.K.; Maiti, P. Synthesis and in vitro evaluation of melatonin entrapped PLA nanoparticles: An oxidative stress and T-cell response using golden hamster. J. Biomed. Mater. Res. A 2015, 103A, 3034–3044. [Google Scholar] [CrossRef] [PubMed]
- Rosales, J.M.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Supercritical impregnation of PLA filaments with mango leaf extract to manufacture functionalized biomedical devices by 3D printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Enciu, A.-M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [Green Version]
- Diniz do Nascimento, L.; Moraes, A.A.B.d.; Costa, K.S.d.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G.d. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Bruenke, J.; Roschke, I.; Agarwal, S.; Riemann, T.; Greiner, A. Quantitative comparison of the antimicrobial efficiency of leaching versus nonleaching polymer materials. Macromol. Biosci. 2016, 16, 647–654. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, C.; Vo, A.; Ewald, A.; Remesch, M.; Kuever, J.; Bauer, J.; Griesheim, S.; Hauser, C.; Thielmann, J.; Tonndorf- Martini, S.; et al. Critical physiological factors influencing the outcome of antimicrobial testing according to ISO 22196/JIS Z 2801. PLoS ONE 2018, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2015, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SimÕes, D.; Miguela, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Tilesi, F.; Lombardi, A.; Mazzucato, A. Scientometric and methodological analysis of the recent literature on the health-related effects of tomato and tomato products. Foods 2021, 10, 1905. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable antimicrobial films for food packaging: Effect of antimicrobials on degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation assessment of poly (Lactic Acid) filled with functionalized titania nanoparticles (PLA/TiO2) under compost conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Atiweish, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental impact of bioplastic use: A review. Helyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Thelen, K.; Fronning, B.; Kravchenko, A.; Min, D.; Robertson, G. Integrating livestock manure with a corn–soybean bioenergy cropping system improves short-term carbon sequestration rates and net global warming potential. Biomass Bioenergy 2010, 34, 960–966. [Google Scholar] [CrossRef]
- Plevin, R.J.; Delucchi, M.A.; Creutzig, F. Using attributional life cycle assessment to estimate climate-change mitigation benefits misleads policy makers. J. Ind. Ecol. 2014, 18, 73–83. [Google Scholar] [CrossRef]
- Ingrao, C.; Tricase, C.; Cholewa-Wójcik, A.; Kawecka, A.; Rana, R.; Siracusa, V. Polylactic acid trays for fresh-food packaging: A Carbon Footprint assessment. Sci. Total Environ. 2015, 537, 385–398. [Google Scholar] [CrossRef]
- Blanc, S.; Massaglia, S.; Brun, F.; Peano, C.; Mosso, A.; Giuggioli, N.R. Use of bio-based plastics in the fruit supply chain: An integrated approach to assess environmental, economic, and social sustainability. Sustainability 2019, 11, 2475. [Google Scholar] [CrossRef] [Green Version]
- Rudeekit, Y.; Siriyota, P.; Intaraksa, P.; Chaiwutthinan, P.; Tajan, M.; Leejarkpai, T. Compostability and ecotoxicity of poly (lactic acid) and starch blends. Adv. Mat. Res. 2012, 506, 323–326. [Google Scholar] [CrossRef]
- Palsikowski, P.A.; Roberto, M.M.; Sommaggio, L.R.D.; Souza, P.M.S.; Morales, A.R.; Marin-Morales, M.A. Ecotoxicity evaluation of the biodegradable polymers PLA, PBAT and its blends using Allium cepa as test organism. J. Polym. Environ. 2018, 26, 938–945. [Google Scholar] [CrossRef]
- Piccardo, M.; Provenza, F.; Grazioli, E.; Anselmi, S.; Terlizzi, A.; Renzi, M. Impacts of plastic-made packaging on marine key species: Effects following water acidification and ecological implications. J. Mar. Sci. Eng. 2021, 9, 432. [Google Scholar] [CrossRef]
- Renzi, M.; Grazioli, E.; Blašković, A. Effects of different microplastic types and surfactant-microplastic mixtures under fasting and feeding conditions: A case study on Daphnia magna. Bull. Environ. Contam. Toxicol. 2019, 103, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, M.; Provenza, F.; Grazioli, E.; Cavallo, A.; Terlizzi, A.; Renzi, M. PET microplastics toxicity on marine key species is influenced by pH, particle size and food variations. Sci. Total Environ. 2020, 715, 136947. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Piccardo, M.; Terlizzi, A.; Renzi, M. Effects of polyethylene terephthalate (PET) microplastics and acid rain on physiology and growth of Lepidium sativum. Environ. Poll. 2021, 282, 116997. [Google Scholar] [CrossRef] [PubMed]
- Pignattelli, S.; Broccoli, A.; Piccardo, M.; Felline, S.; Terlizzi, A.; Renzi, M. Short-term physiological and biometrical responses of Lepidium sativum seedlings exposed to PET-made microplastics and acid rain. Ecotoxicol. Environ. Saf. 2021, 208, 111718. [Google Scholar] [CrossRef]
- Fastelli, P.; Renzi, M. Exposure of key marine species to sunscreens: Changing ecotoxicity as a possible indirect effect of global warming. Mar. Pollut. Bull. 2019, 149, 110517. [Google Scholar] [CrossRef]
- Piccardo, M.; Bertoli, M.; Pastorino, P.; Barcelo’, D.; Provenza, F.; Lesa, D.; Anselmi, S.; Elia, A.C.; Prearo, M.; Pizzul, E.; et al. Lethal and sublethal responses of Hydropsyche pellucidula (Insecta, Trichoptera) to commercial polypropylene microplastics after different preconditioning treatments. Toxics 2021, 9, 256. [Google Scholar] [CrossRef]
- Bogacka, M.; Salazar, G.L. Study on the effect of bio-based materials’ natural degradation in the environment. Sustainability 2022, 14, 4675. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Daliri, E.B.; Elahi, F.; Chelliah, R.; Lee, B.H.; Oh, D. New insights on the use of polyphenols as natural preservatives and their emerging safety concerns. Front. Sustain. Food. Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Etxabide, A.; Young, B.; Bremer, P.J.; Kilmartin, P.A. Non-permanent primary food packaging materials assessment: Identification, migration, toxicity, and consumption of substances. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4130–4145. [Google Scholar] [CrossRef]
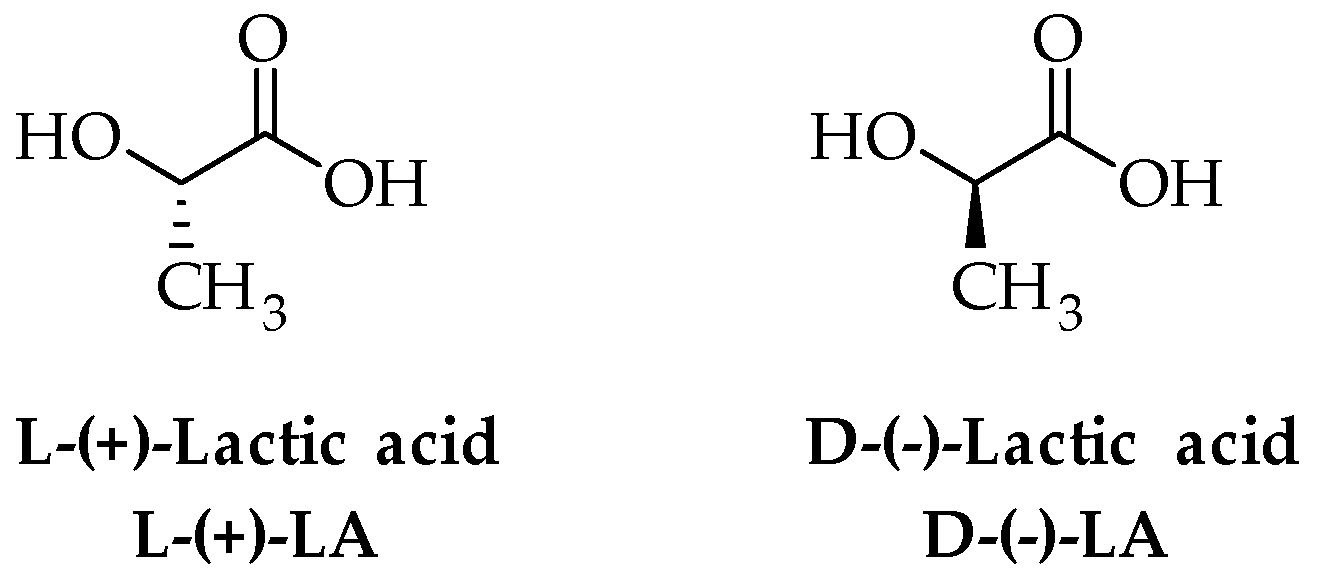

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, A.; Fochetti, A.; Vignolini, P.; Campo, M.; Durazzo, A.; Lucarini, M.; Puglia, D.; Luzi, F.; Papalini, M.; Renzi, M.; et al. Natural Active Ingredients for Poly (Lactic Acid)-Based Materials: State of the Art and Perspectives. Antioxidants 2022, 11, 2074. https://doi.org/10.3390/antiox11102074
Lombardi A, Fochetti A, Vignolini P, Campo M, Durazzo A, Lucarini M, Puglia D, Luzi F, Papalini M, Renzi M, et al. Natural Active Ingredients for Poly (Lactic Acid)-Based Materials: State of the Art and Perspectives. Antioxidants. 2022; 11(10):2074. https://doi.org/10.3390/antiox11102074
Chicago/Turabian StyleLombardi, Andrea, Andrea Fochetti, Pamela Vignolini, Margherita Campo, Alessandra Durazzo, Massimo Lucarini, Debora Puglia, Francesca Luzi, Marco Papalini, Monia Renzi, and et al. 2022. "Natural Active Ingredients for Poly (Lactic Acid)-Based Materials: State of the Art and Perspectives" Antioxidants 11, no. 10: 2074. https://doi.org/10.3390/antiox11102074
APA StyleLombardi, A., Fochetti, A., Vignolini, P., Campo, M., Durazzo, A., Lucarini, M., Puglia, D., Luzi, F., Papalini, M., Renzi, M., Cavallo, A., & Bernini, R. (2022). Natural Active Ingredients for Poly (Lactic Acid)-Based Materials: State of the Art and Perspectives. Antioxidants, 11(10), 2074. https://doi.org/10.3390/antiox11102074














