Bioaccessibility of Phenolic Compounds and Antioxidant Properties of Goat-Milk Powder Fortified with Grape-Pomace-Seed Extract after In Vitro Gastrointestinal Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SE and TME Powder
2.2. Simulated In Vitro Gastrointestinal Digestion
2.3. UHPLC-DAD MS/MS Analysis of Phenolic Compounds
2.4. Bioaccessibility
2.5. Electrophoretic Analysis
2.6. Total Phenolic Content and Antioxidant Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Composition of Digested TME Powder
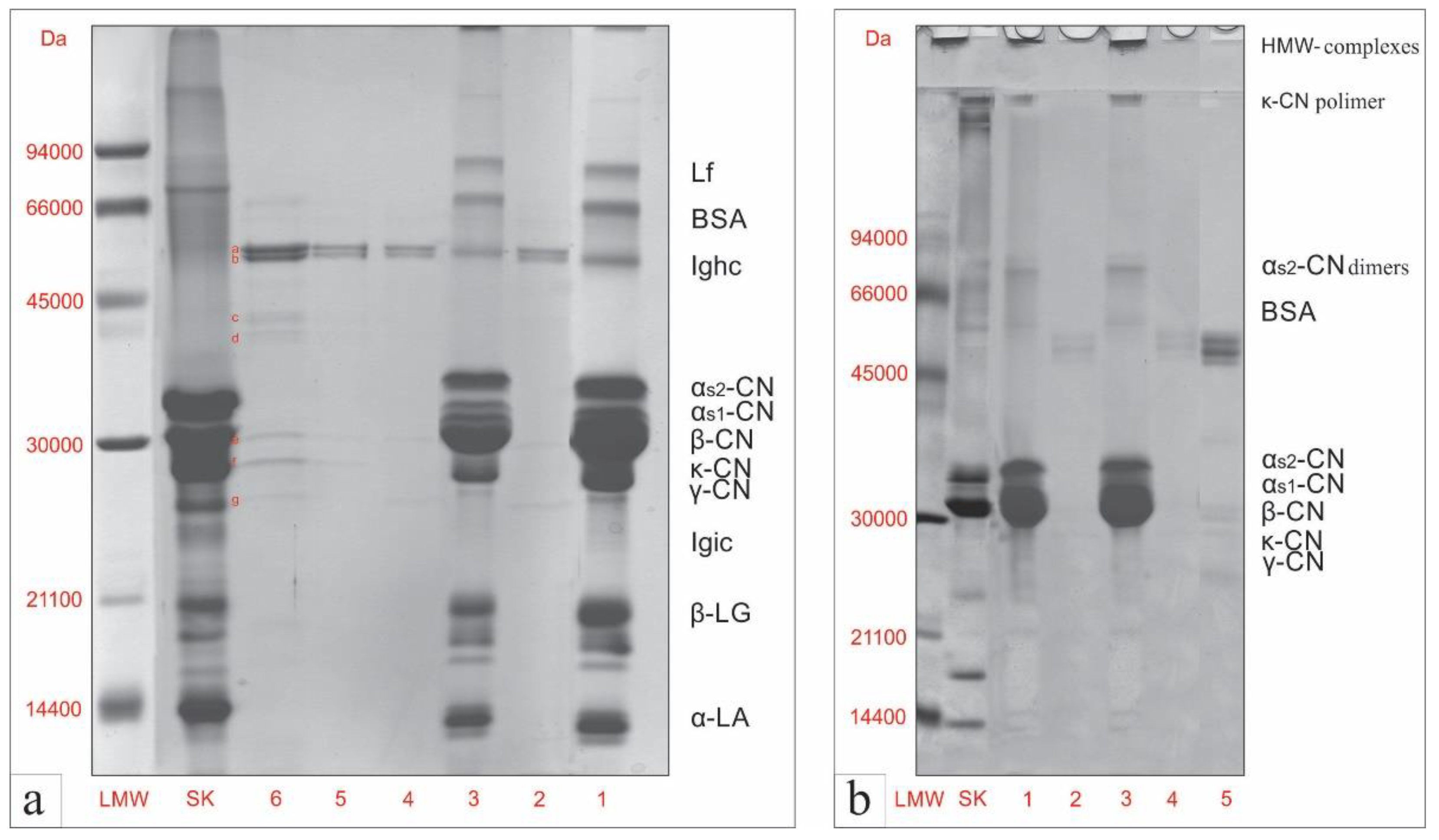
3.2. Electrophoretic Analysis of Milk Proteins after In Vitro Digestion
3.3. Total Phenolic Content and Antioxidant Properties of Digested Powders
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Xia, E.; He, X.; Li, H.; Wu, S.; Li, S.; Deng, G. Biological Activities of Polyphenols from Grapes. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 47–58. [Google Scholar]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Rabadán, A.; Nieto, R.; Bernabéu, R. Food Innovation as a Means of Developing Healthier and More Sustainable Foods. Foods 2021, 10, 2069. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Kandylis, P.; Dimitrellou, D.; Moschakis, T. Recent applications of grapes and their derivatives in dairy products. Trends Food Sci. Technol. 2021, 114, 696–711. [Google Scholar] [CrossRef]
- Kwon, H.C.; Bae, H.; Seo, H.G.; Han, S.G. Short communication: Chia seed extract enhances physiochemical and antioxidant properties of yogurt. J. Dairy Sci. 2019, 102, 4870–4876. [Google Scholar] [CrossRef]
- El, M.A.E.D.M.; Bahnasi, A.H.G.; Abd El, A.A.E.R. Physicochemical, Antioxidant, Microbiological And Sensory Characteristics Of Yoghurt Enriched With Garden Cress Seed Powder. NVEO-Nat. Volatiles Essent. Oils J. NVEO 2021, 8, 13416–13428. [Google Scholar]
- Chen, G.-L.; Chen, S.-G.; Chen, F.; Xie, Y.-Q.; Han, M.-D.; Luo, C.-X.; Zhao, Y.-Y.; Gao, Y.-Q. Nutraceutical potential and antioxidant benefits of selected fruit seeds subjected to an in vitro digestion. J. Funct. Foods 2016, 20, 317–331. [Google Scholar] [CrossRef]
- Fernández, K.; Labra, J. Simulated digestion of proanthocyanidins in grape skin and seed extracts and the effects of digestion on the angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2013, 139, 196–202. [Google Scholar] [CrossRef] [PubMed]
- José Jara-Palacios, M.; Gonçalves, S.; Hernanz, D.; Heredia, F.J.; Romano, A. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef]
- Laurent, C.; Besançon, P.; Caporiccio, B. Flavonoids from a grape seed extract interact with digestive secretions and intestinal cells as assessed in an in vitro digestion/Caco-2 cell culture model. Food Chem. 2007, 100, 1704–1712. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Tao, Y.; Zeng, M.; Zhang, S.; Tao, G.; Qin, F.; Chen, J. High pressure homogenization processing, thermal treatment and milk matrix affect in vitro bioaccessibility of phenolics in apple, grape and orange juice to different extents. Food Chem. 2016, 200, 107–116. [Google Scholar] [CrossRef]
- Lamothe, S.; Guérette, C.; Dion, F.; Sabik, H.; Britten, M. Antioxidant activity of milk and polyphenol-rich beverages during simulated gastrointestinal digestion of linseed oil emulsions. Food Res. Int. 2019, 122, 149–156. [Google Scholar] [CrossRef]
- Silva, F.A.; Queiroga, R.d.C.R.d.E.; de Souza, E.L.; Voss, G.B.; Borges, G.d.S.C.; Lima, M.d.S.; Pintado, M.M.E.; Vasconcelos, M.A.d.S. Incorporation of phenolic-rich ingredients from integral valorization of Isabel grape improves the nutritional, functional and sensory characteristics of probiotic goat milk yogurt. Food Chem. 2022, 369, 130957. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Stanisavljević, N.S.; Gašić, U.M.; Lević, S.; Kojić, M.O.; Tešić, Ž.L.; Nedović, V.; Barać, M.B.; Pešić, M.B. Polyphenol bioaccessibility and antioxidant properties of in vitro digested spray-dried thermally-treated skimmed goat milk enriched with pollen. Food Chem. 2021, 351, 129310. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.M.; Lević, S.; Stanojević, S.P.; Barać, M.B.; Tešić, Ž.L.; Nedović, V.; Pešić, M.B. Skimmed Goat’s Milk Powder Enriched with Grape Pomace Seed Extract: Phenolics and Protein Characterization and Antioxidant Properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesic, M.B.; Barac, M.B.; Stanojevic, S.P.; Ristic, N.M.; Macej, O.D.; Vrvic, M.M. Heat induced casein–whey protein interactions at natural pH of milk: A comparison between caprine and bovine milk. Small Rumin. Res. 2012, 108, 77–86. [Google Scholar] [CrossRef]
- Gođevac, D.; Tešević, V.; Velickovic, M.; Vujisica, L.; Vajs, V.; Milosavljevic, S. Polyphenolic compounds in seeds from some grape cultivars grown in Serbia. J. Serb. Chem. Soc. 2010, 75, 1641–1652. [Google Scholar] [CrossRef]
- Zdunić, G.; Gođevac, D.; Šavikin, K.; Krivokuća, D.; Mihailović, M.; Pržić, Z.; Marković, N. Grape Seed Polyphenols and Fatty Acids of Autochthonous Prokupac Vine Variety from Serbia. Chem. Biodivers. 2019, 16, e1900053. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Cilla, A.; González-Sarrías, A.; Tomás-Barberán, F.; Espín, J.C.; Barberá, R. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem. 2009, 114, 813–820. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D.; Verzelloni, E.; Conte, A. Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion. J. Funct. Foods 2014, 7, 506–516. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Ozdal, T.; Yalcinkaya, İ.; Toydemir, G.; Capanoglu, E. Polyphenol-Protein Interactions and Changes in Functional Properties and Digestibility. In Reference Module in Food Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Rahimi Yazdi, S.; Corredig, M. Heating of milk alters the binding of curcumin to casein micelles. A fluorescence spectroscopy study. Food Chem. 2012, 132, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Villalva, M.; Jaime, L.; Arranz, E.; Zhao, Z.; Corredig, M.; Reglero, G.; Santoyo, S. Nanoemulsions and acidified milk gels as a strategy for improving stability and antioxidant activity of yarrow phenolic compounds after gastrointestinal digestion. Food Res. Int. 2020, 130, 108922. [Google Scholar] [CrossRef] [PubMed]
- Kılıç Bayraktar, M.; Harbourne, N.B.; Fagan, C.C. Impact of heat treatment and acid gelation on polyphenol enriched milk samples. LWT 2019, 113, 108282. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. In vitro bioaccessibility of health-related compounds as affected by the formulation of fruit juice- and milk-based beverages. Food Res. Int. 2014, 62, 771–778. [Google Scholar] [CrossRef]
- Qie, X.; Cheng, Y.; Chen, Y.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; Li, W.; He, Z. In vitro phenolic bioaccessibility of coffee beverages with milk and soy subjected to thermal treatment and protein-phenolic interactions. Food Chem. 2022, 375, 131644. [Google Scholar] [CrossRef]
- Quan, W.; Qie, X.; Chen, Y.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effect of milk addition and processing on the antioxidant capacity and phenolic bioaccessibility of coffee by using an in vitro gastrointestinal digestion model. Food Chem. 2020, 308, 125598. [Google Scholar] [CrossRef]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef] [Green Version]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In Vitro Inhibitory Effect on Digestive Enzymes and Antioxidant Potential of Commonly Consumed Fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Dubiard, C.G.; Cheynier, V.; Meudec, E.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. In vitro digestion of dairy and egg products enriched with grape extracts: Effect of the food matrix on polyphenol bioaccessibility and antioxidant activity. Food Res. Int. 2016, 88, 284–292. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Bordenave, N.; Hamaker, B.R. Does flavor impact function? Potential consequences of polyphenol-protein interactions in delivery and bioactivity of flavan-3-ols from foods. Physiol. Behav. 2012, 107, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.; Chegeni, M.; Jones, O.G.; Liceaga, A.; Ferruzzi, M.G. The effect of milk proteins on the bioaccessibility of green tea flavan-3-ols. Food Res. Int. 2014, 66, 297–305. [Google Scholar] [CrossRef]
- Antunes-Ricardo, M.; Rodríguez-Rodríguez, C.; Gutiérrez-Uribe, J.A.; Cepeda-Cañedo, E.; Serna-Saldívar, S.O. Bioaccessibility, Intestinal Permeability and Plasma Stability of Isorhamnetin Glycosides from Opuntia ficus-indica (L.). Int. J. Mol. Sci. 2017, 18, 1816. [Google Scholar] [CrossRef] [Green Version]
- Razboršek, M.I.; Ivanović, M.; Kolar, M. Validated Stability-Indicating GC-MS Method for Characterization of Forced Degradation Products of Trans-Caffeic Acid and Trans-Ferulic Acid. Molecules 2021, 26, 2475. [Google Scholar] [CrossRef]
- Pérez-Ortiz, J.M.; Alguacil, L.F.; Salas, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; González-Martín, C. Antiproliferative and cytotoxic effects of grape pomace and grape seed extracts on colorectal cancer cell lines. Food Sci. Nutr. 2019, 7, 2948–2957. [Google Scholar] [CrossRef] [Green Version]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Egger, L.; Ménard, O.; Baumann, C.; Duerr, D.; Schlegel, P.; Stoll, P.; Vergères, G.; Dupont, D.; Portmann, R. Digestion of milk proteins: Comparing static and dynamic in vitro digestion systems with in vivo data. Food Res. Int. 2019, 118, 32–39. [Google Scholar] [CrossRef]
- Nehir El, S.; Karakaya, S.; Simsek, S.; Dupont, D.; Menfaatli, E.; Eker, A.T. In vitro digestibility of goat milk and kefir with a new standardised static digestion method (INFOGEST cost action) and bioactivities of the resultant peptides. Food Funct. 2015, 6, 2322–2330. [Google Scholar] [CrossRef]
- Park, H.; Lee, M.; Kim, K.-T.; Park, E.; Paik, H.-D. Antioxidant and antigenotoxic effect of dairy products supplemented with red ginseng extract. J. Dairy Sci. 2018, 101, 8702–8710. [Google Scholar] [CrossRef] [Green Version]
- Gad, A.; Abdelazim, H. The antioxidant properties of skim milk supplemented with rosemary and green tea extracts in response to pasteurisation, homogenisation and the addition of salts. Int. J. Dairy Technol. 2010, 63, 349–355. [Google Scholar] [CrossRef]
- Cilla, A.; Perales, S.; Lagarda, M.J.; Barberá, R.; Clemente, G.; Farré, R. Influence of storage and in vitro gastrointestinal digestion on total antioxidant capacity of fruit beverages. J. Food Compos. Anal. 2011, 24, 87–94. [Google Scholar] [CrossRef]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef]
- Shori, A.B. Storage quality and antioxidant properties of yogurt fortified with polyphenol extract from nutmeg, black pepper, and white pepper. Electron. J. Biotechnol. 2022, 57, 24–30. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Samardžić, J.; Janković, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef] [PubMed]

| SSF | SGF | SIF | |
|---|---|---|---|
| pH 7.0 | pH 3.0 | pH 7.0 | |
| Compound | Conc. in SSF mmol L−1 | Conc. in SGF mmol L−1 | Conc. in SSF mmol L−1 |
| KCl | 15.1 | 6.9 | 6.8 |
| KH2PO4 | 3.7 | 0.9 | 0.8 |
| NaHCO3 | 13.6 | 25 | 85 |
| NaCl | - | 47.2 | 38.4 |
| MgCl2·(H2O)6 | 0.15 | 0.1 | 0.33 |
| (NH4)2CO3 | 0.06 | 0.5 | - |
| NaOH | - | - | 8.4 |
| HCl | 1.1 | 15.6 | - |
| * CaCl2·(H2O)2 | 0.75 | 0.075 | 0.3 |
| Samples (µg/L) | SE | TM | DTM | TME | MBP % | DTME | QP % | Recovery % |
|---|---|---|---|---|---|---|---|---|
| Phenolic acid | ||||||||
| Gallic acid | 3444.81 ± 117.4 a | n.d. | n.d. | 645.68 ± 20.12 b | 81.26 | n.d. | 0 | 0 |
| Protocatehuic acid | 62.50 ± 1.99 a | n.d. | n.d. | 57.02 ± 2.46 b | 8.77 | n.d. | 0 | 0 |
| Caffeic acid | 73.28 ± 4.30 a | n.d. | n.d. | 42.82 ± 2.38 b | 41.56 | 41.89 ± 3.55 b | 97.83 | 57.17 |
| Σ | 3580.6 (27.63) | / | / | 745.52 (31.49) | 79.18 | 41.89 (1.78) | 5.62 | 1.17 |
| Flavan-3-ols | ||||||||
| Catechin | 8282.64 ± 246.89 a | n.d. | n.d. | 1444.85 ± 67.4 b | 82.56 | 2169.46 ± 81.50 c | 150.15 | 26.19 |
| Catechin gallate | 688.84 ± 36.32 a | n.d. | n.d. | 45.11 ± 0.113 b | 93.45 | 32.08 ± 1.43 c | 71.12 | 4.66 |
| Galocatechin | n.d. | n.d. | n.d. | n.d. | - | n.d. | - | - |
| Epigalocatechin | n.d. | n.d. | n.d. | n.d. | - | n.d. | - | - |
| Epigalocatechin-gallate | n.d. | n.d. | n.d. | n.d. | - | n.d. | - | - |
| Σ | 8971.5 (69.23) | / | / | 1490 (62.93) | 83.39 | 2201.5 (93.50) | 147.76 | 24.54 |
| Other detected phenolics | ||||||||
| Quercetin-3-glucoside | 131.25 ± 6.66 a | n.d. | n.d. | 64.48 ± 3.94 b | 50.87 | 26.75 ± 1.88 c | 41.48 | 20.38 |
| Isohramnetin-3-O-glucoside | 49.82 ± 4.40 | n.d. | n.d. | n.d. | 100 | n.d. | - | 0 |
| Kaempferol | 123.93 ± 7.49 | n.d. | n.d. | n.d. | 100 | n.d. | - | 0 |
| Apigenin-7-O-glucoside | 16.08 ± 0.94 a | n.d. | n.d. | n.d. | 100 | 15.66 ± 2.02 a | - | 97.43 |
| Naringenin | 40.08 ± 4.55 | n.d. | n.d. | n.d. | 100 | n.d. | - | 0 |
| Aeskuletin | 45.89 ± 3.34 a | n.d. | n.d. | 67.79 ± 3.45 b | - | 61.23 ± 1.47 c | 90.32 | 133.42 |
| Σ | 407.06 (3.14) | / | / | 132.28 (5.59) | 67.50 | 103.64 (4.42) | 78.35 | 25.46 |
| Total phenolic compounds | 12,959.12 | / | / | 2367.76 | 81.73 | 2347.1 | 99.13 | 18.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Gašić, U.M.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Bioaccessibility of Phenolic Compounds and Antioxidant Properties of Goat-Milk Powder Fortified with Grape-Pomace-Seed Extract after In Vitro Gastrointestinal Digestion. Antioxidants 2022, 11, 2164. https://doi.org/10.3390/antiox11112164
Milinčić DD, Stanisavljević NS, Kostić AŽ, Gašić UM, Stanojević SP, Tešić ŽL, Pešić MB. Bioaccessibility of Phenolic Compounds and Antioxidant Properties of Goat-Milk Powder Fortified with Grape-Pomace-Seed Extract after In Vitro Gastrointestinal Digestion. Antioxidants. 2022; 11(11):2164. https://doi.org/10.3390/antiox11112164
Chicago/Turabian StyleMilinčić, Danijel D., Nemanja S. Stanisavljević, Aleksandar Ž. Kostić, Uroš M. Gašić, Slađana P. Stanojević, Živoslav Lj. Tešić, and Mirjana B. Pešić. 2022. "Bioaccessibility of Phenolic Compounds and Antioxidant Properties of Goat-Milk Powder Fortified with Grape-Pomace-Seed Extract after In Vitro Gastrointestinal Digestion" Antioxidants 11, no. 11: 2164. https://doi.org/10.3390/antiox11112164








