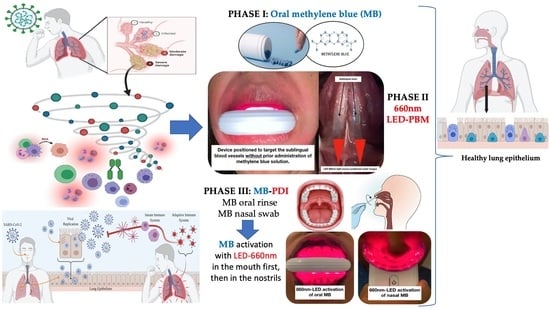
A Novel Approach of Combining Methylene Blue Photodynamic Inactivation, Photobiomodulation and Oral Ingested Methylene Blue in COVID-19 Management: A Pilot Clinical Study with 12-Month Follow-Up
Abstract
:1. Introduction
1.1. Oral Methylene Blue (MB)
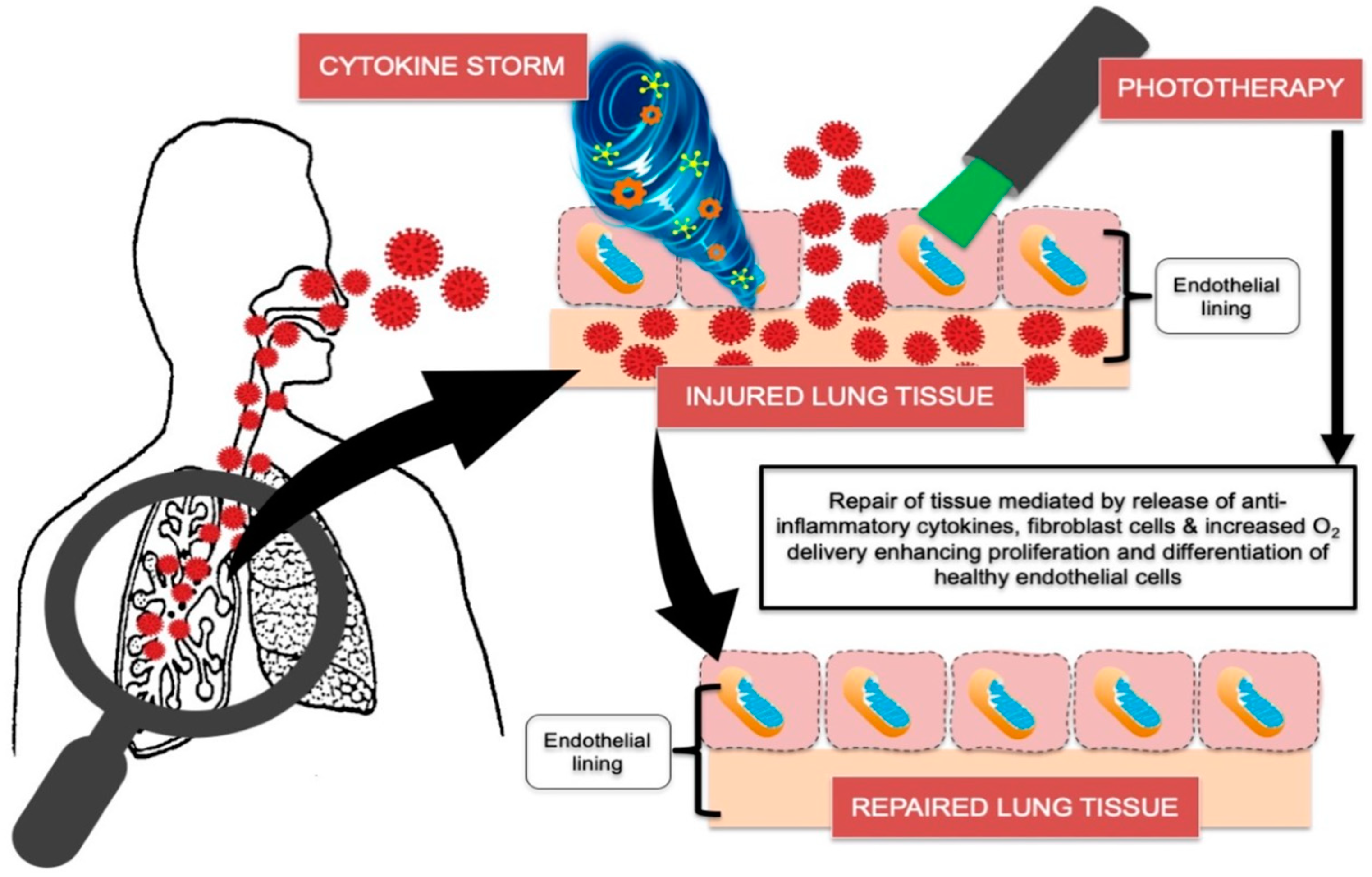
1.2. Photobiomodulation (PBM)
1.3. Local Photodynamic Viral Inactivation
1.4. Pilot Study Aims and Objectives
- To confirm the reduction in viral load with the use of serial quantitative polymerase chain reaction (PCR) tests (cycle threshold (CT) measurements).
- To evaluate symptom progression and recovery time over follow up time points.
- To appraise the feasibility and convenience of the home-based device and treatment of COVID-19 disease.
- To assess the long-term outcomes following treatment.
2. Materials and Methods
2.1. Pilot Study Design and Ethical Approval
2.1.1. Eligibility Criteria
Inclusion Criteria
- 1.
- Subjects who were residents of Nassau, Bahamas, presenting with COVID-19 symptoms of 10 days or less with a positive test for COVID-19, using reverse transcription polymerase chain reaction (RT-PCR) test.
- 2.
- Subjects were at least 18 years of age, of both genders, and of any ethnicity.
- 3.
- Subjects with or without systemic diseases (ASA I and II).
Exclusion Criteria
- 1.
- Concomitant therapy: patients who were taking any FDA/MHRA/EMA/WHO approved anti-viral drugs. (Note: as Ivermectin, hydroxychloroquine and N-acetyl cysteine were not formally approved or recognized COVID-19 treatments at the time this pilot study was conducted, patients were not excluded if they were already taking these medications/supplements. However, it was clearly annotated if any of these medications/supplements were administered, thereby a response with and without these agents could be assessed).
- 2.
- Any enrolled subjects who needed urgent medical care, such as O2 saturation < 90% or severe hypertension (i.e., subjects medically compromised).
- 3.
- Subjects who were unable to properly comply with the treatment instructions and complete the course of the treatment protocols.
2.2. Assessment Tools of Clinical Outcomes
2.2.1. Cycle Threshold Tests
2.2.2. Patient Clinical Data
2.2.3. Home Delivery of Treatment Supplies
2.2.4. Description of the Treatment Device
2.2.5. Patient Education
2.3. Description of the Treatment Protocols
2.3.1. Phase I: Oral-MB
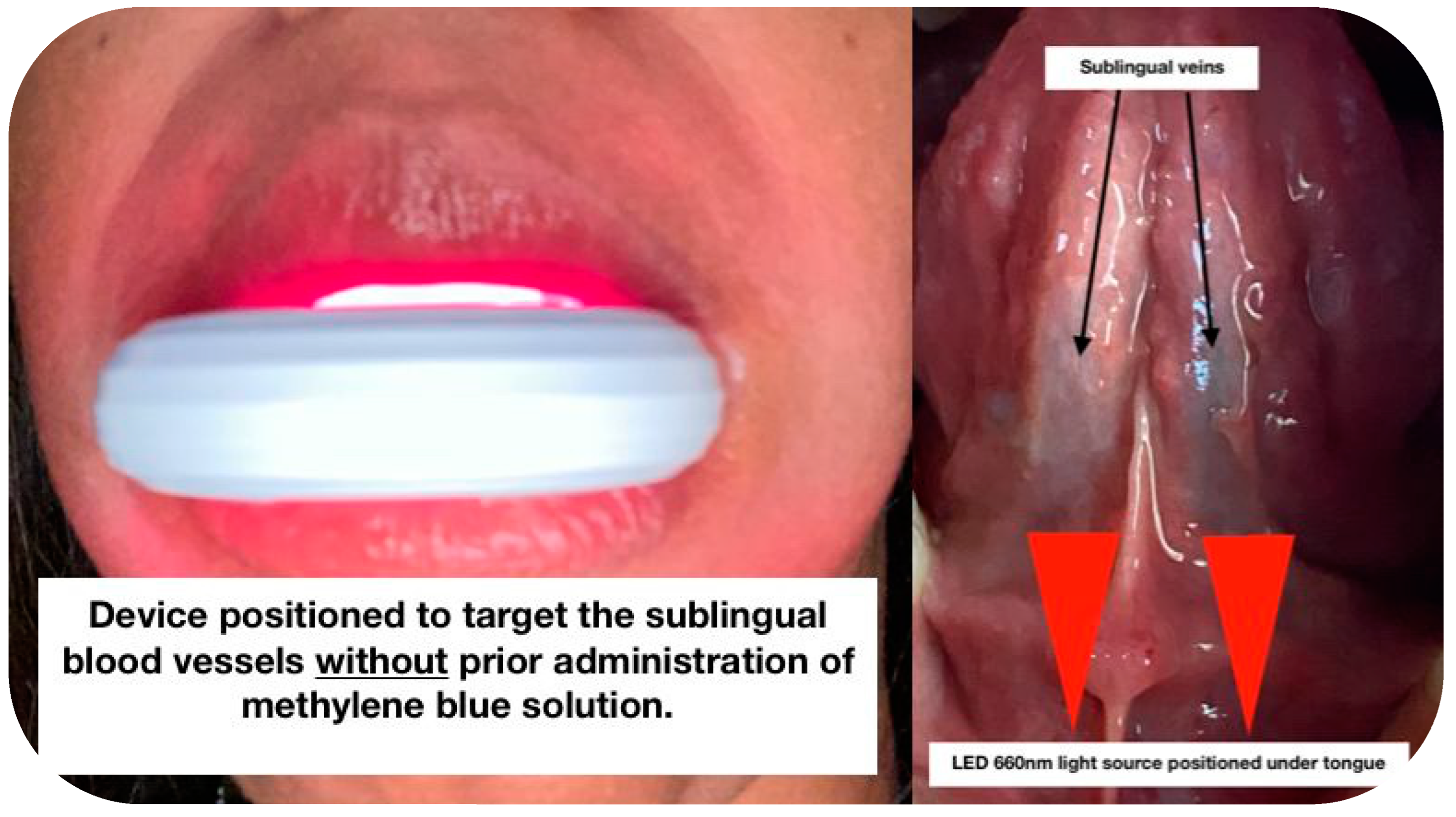
2.3.2. Phase II: Sublingual LED-PBM Therapy
2.3.3. Phase III: Local Oral/Nasal PDI (ONPDI)
2.4. One-Year Follow Up
- Have you had any persistent symptoms after your initial COVID-19 infection which lasted for a period 1–3 months or greater?
- Have you had any recurrence of COVID-19 symptoms after your initial infection?
- Have you had a positive COVID-19 test after your initial infection?
3. Case Description and Results
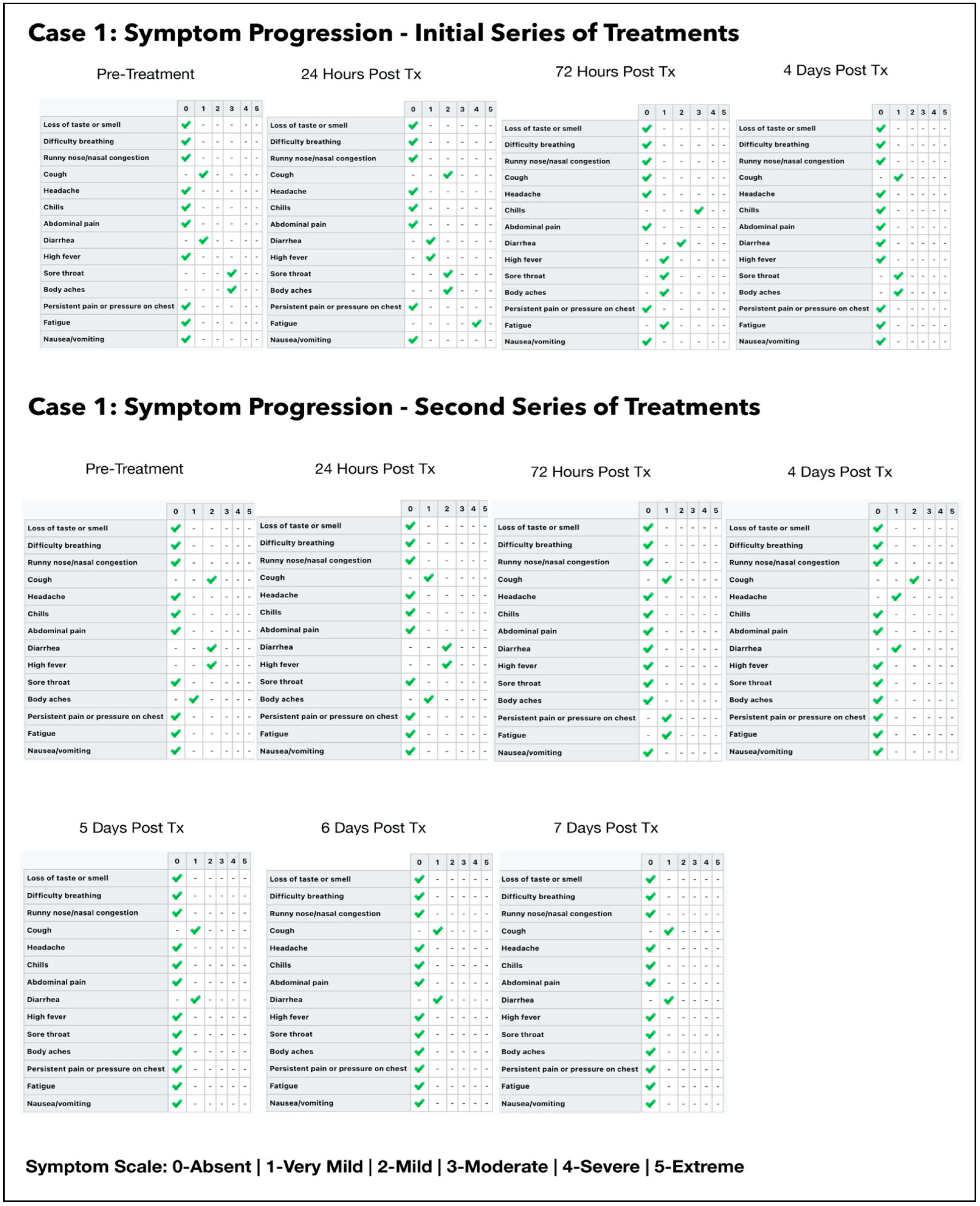
3.1. Subject #1
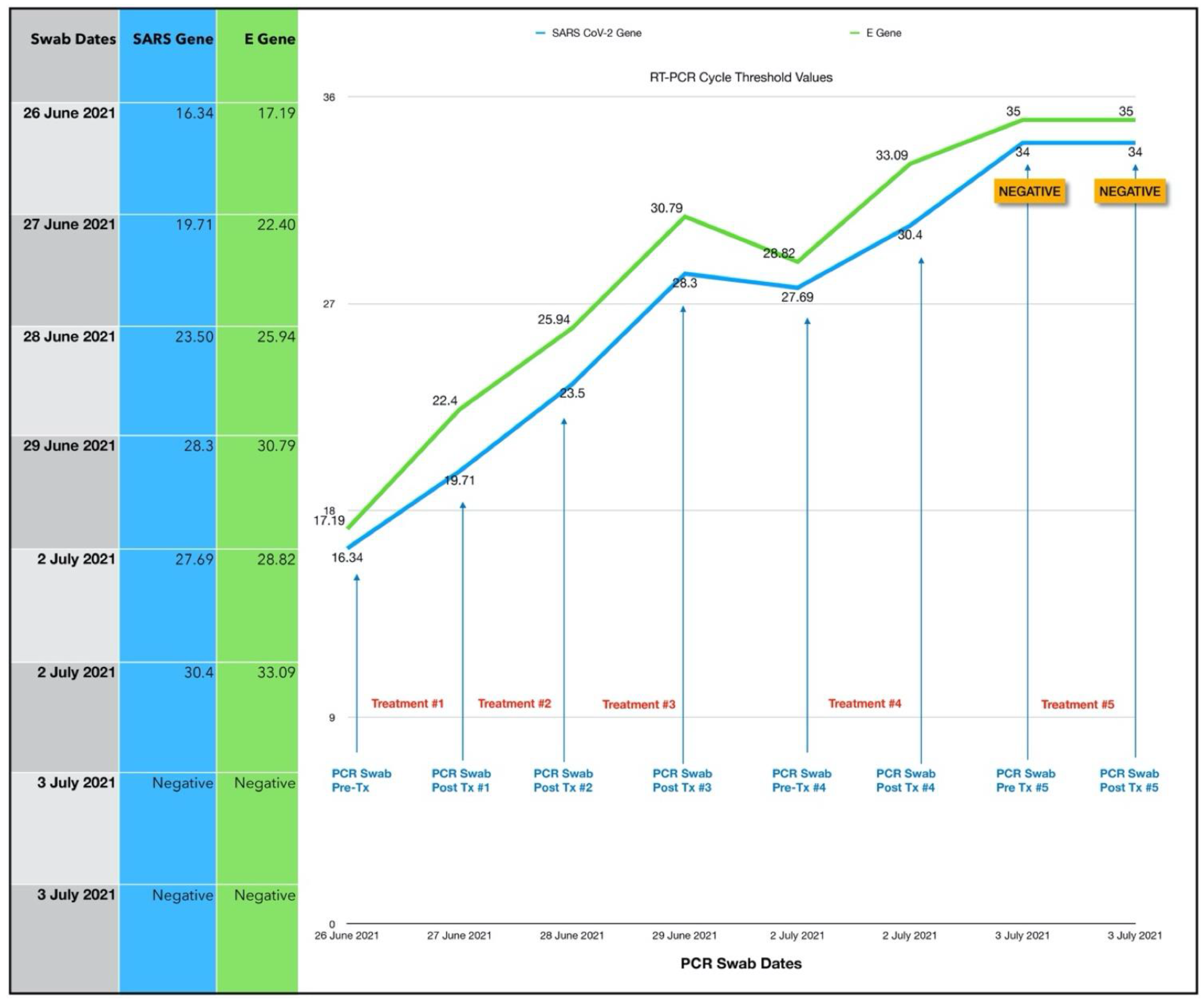
3.1.1. Results of the Initial Treatment Series
3.1.2. Results of the Second Treatment Series
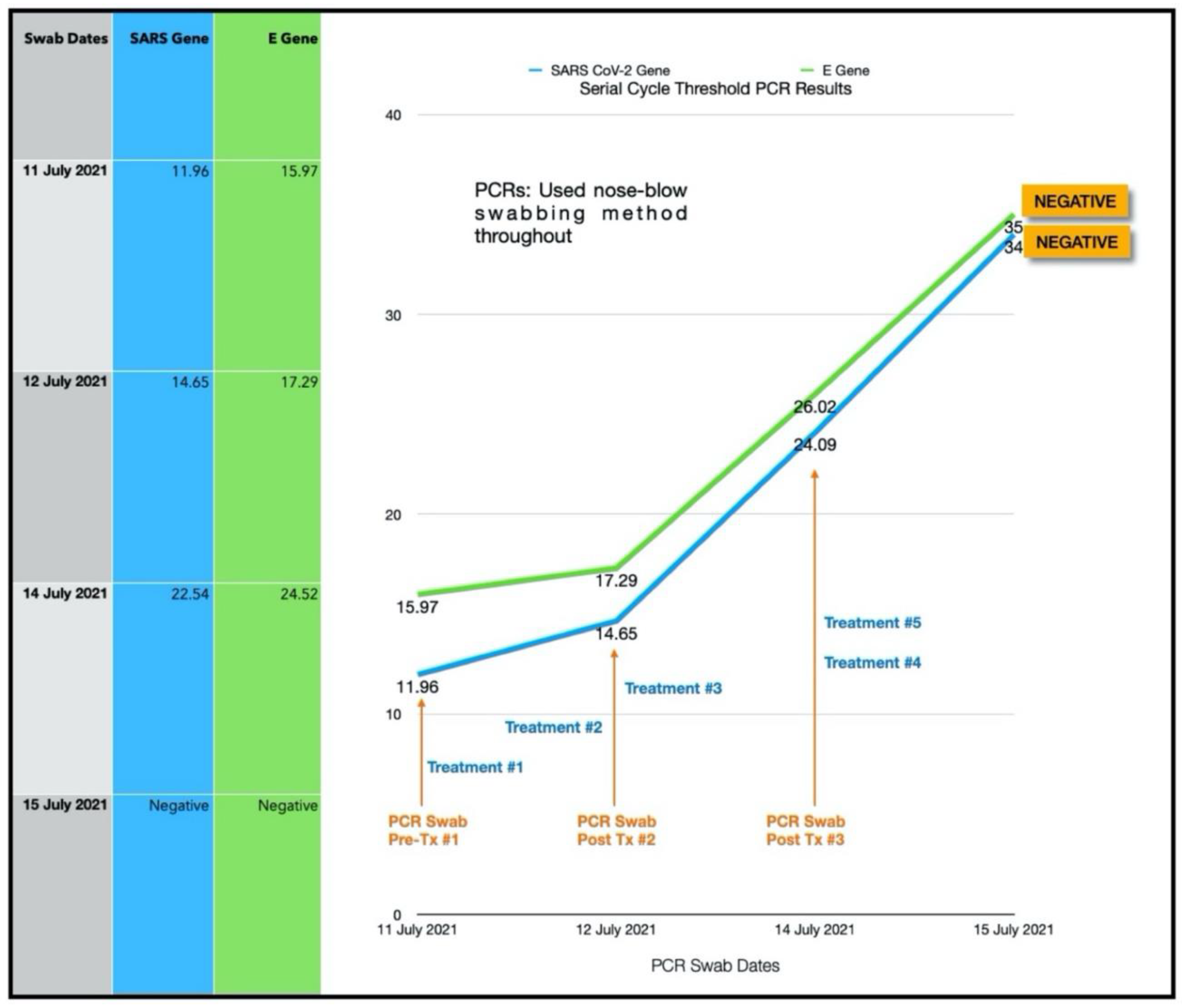
3.1.3. Estimated Viral Load Reduction (EVLR)
3.1.4. One Year Follow Up
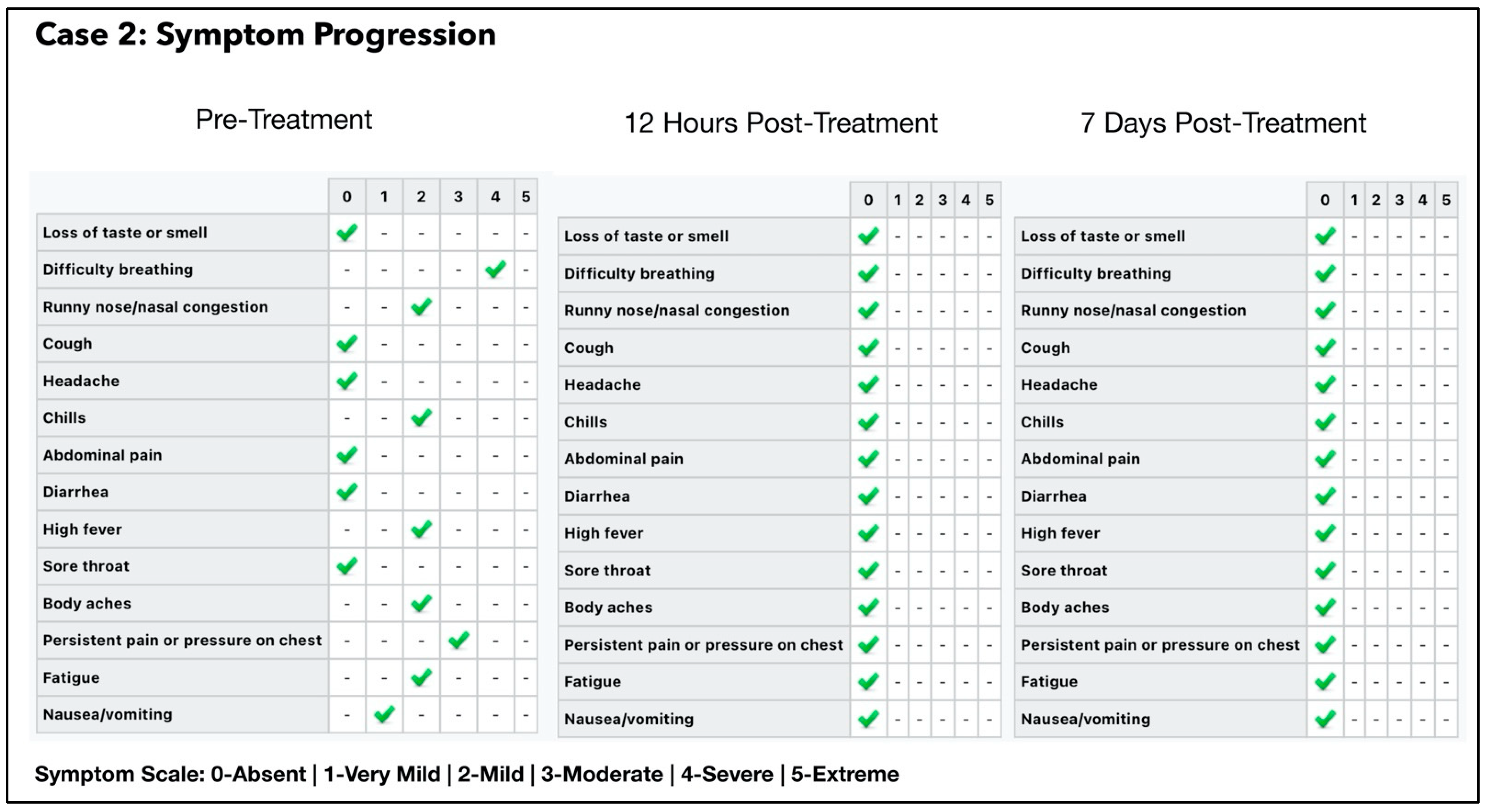
3.2. Subject #2
One-Year Follow Up
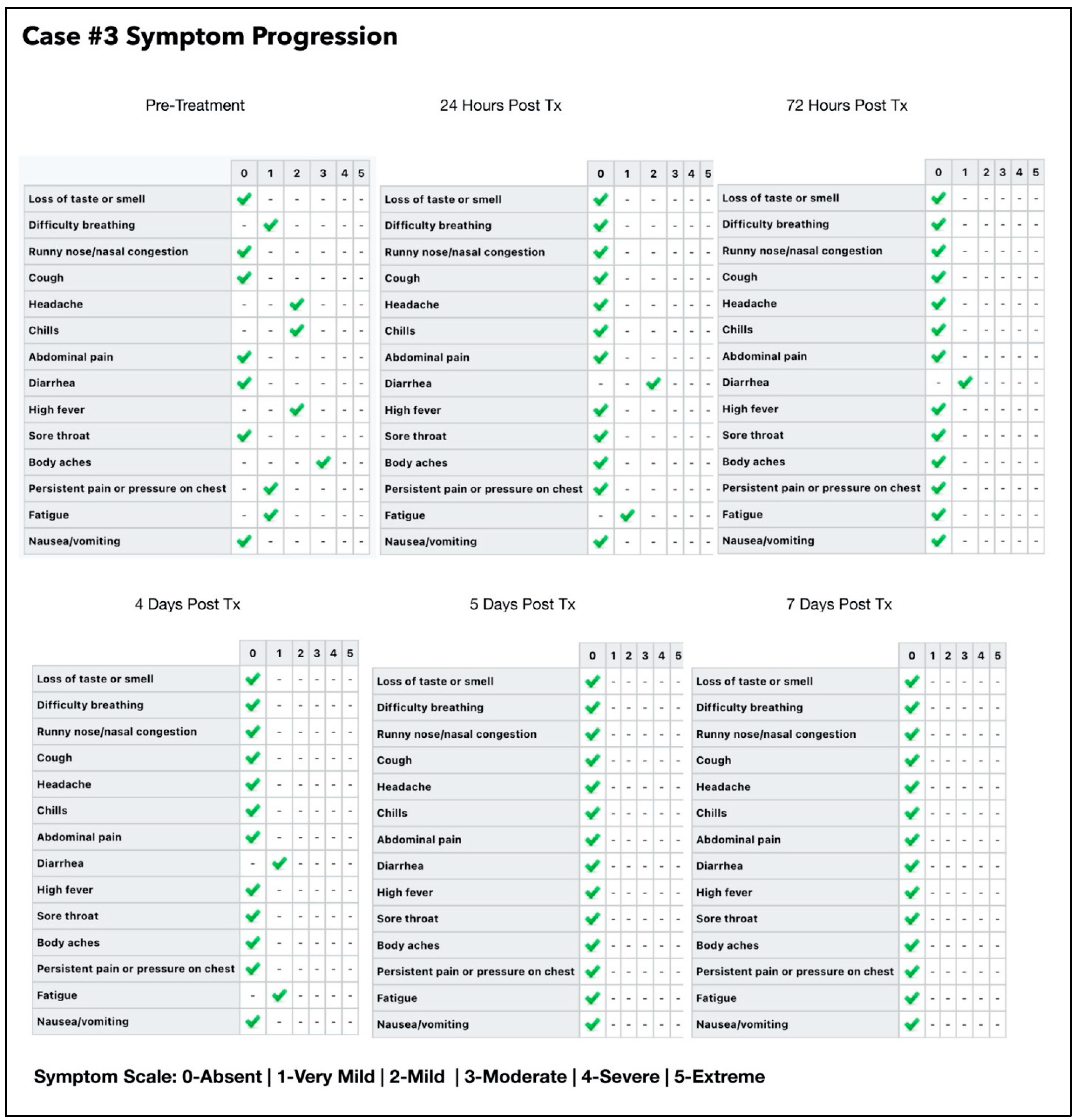
3.3. Subject #3
- One session per day of ONPDI: on Days 0 to 2.
- Three sessions of ONPDI on Day 3.
- Oral-MB was administered from 6 July to 10 July 2021 and PBM therapy was administered from 7 July to 10 July 2021.
3.3.1. Estimated Viral Load Reduction (EVLR)
3.3.2. One Year Follow-Up
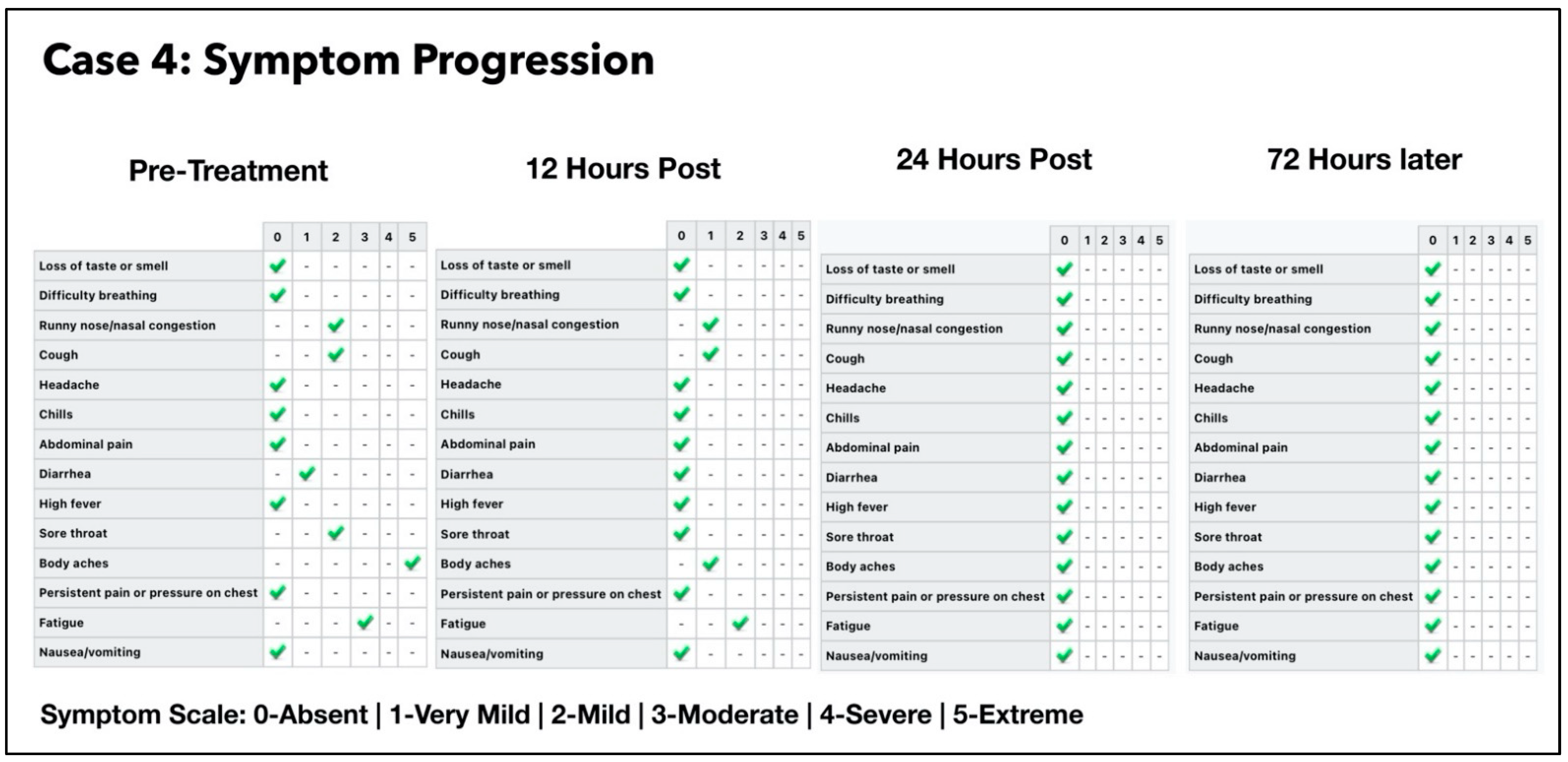
3.4. Subject #4
- Three sessions of topical oral/nasal PDT on Days 0 and 1.
- Two sessions of topical oral/nasal PDT on Day 3.
- Oral MB and sublingual PBM 8-hourly Day 0 to Day 2.
3.4.1. Estimated Viral Load Reduction (EVLR)
3.4.2. One Year Follow-Up
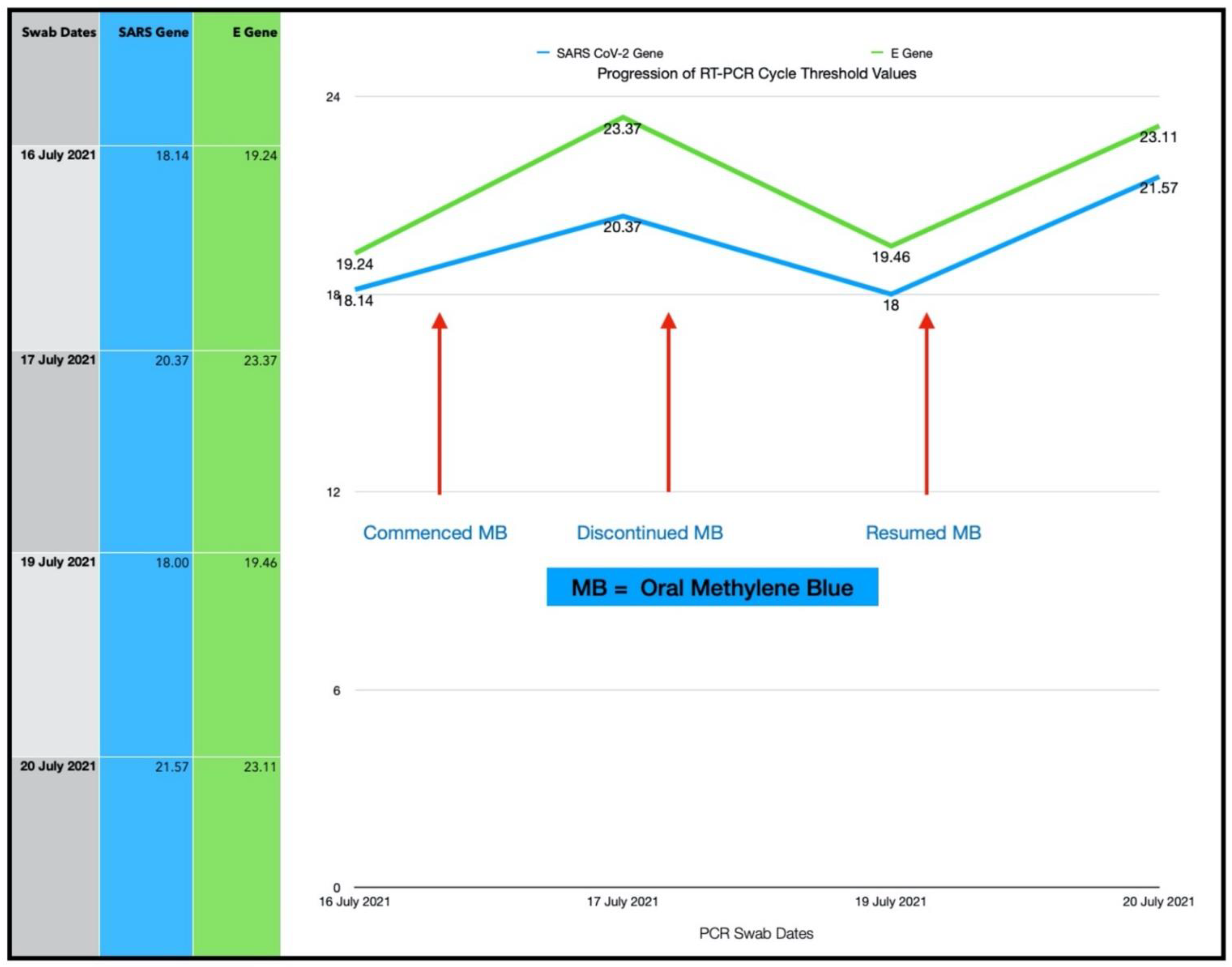
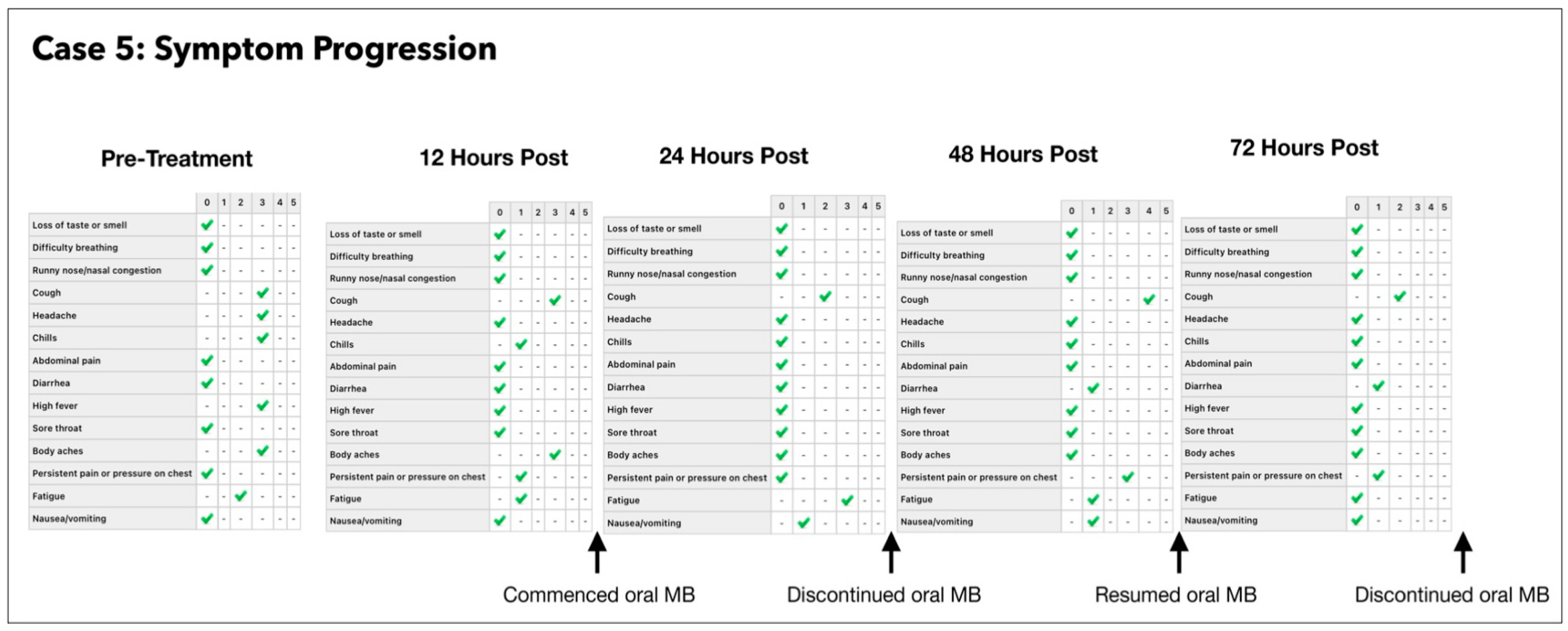
3.5. Subject #5
- Number of ONPDI treatments prescribed 8-hourly.
- Oral MB-prescribed 8-hourly, but this treatment schedule was not maintained.
One Year Follow-Up
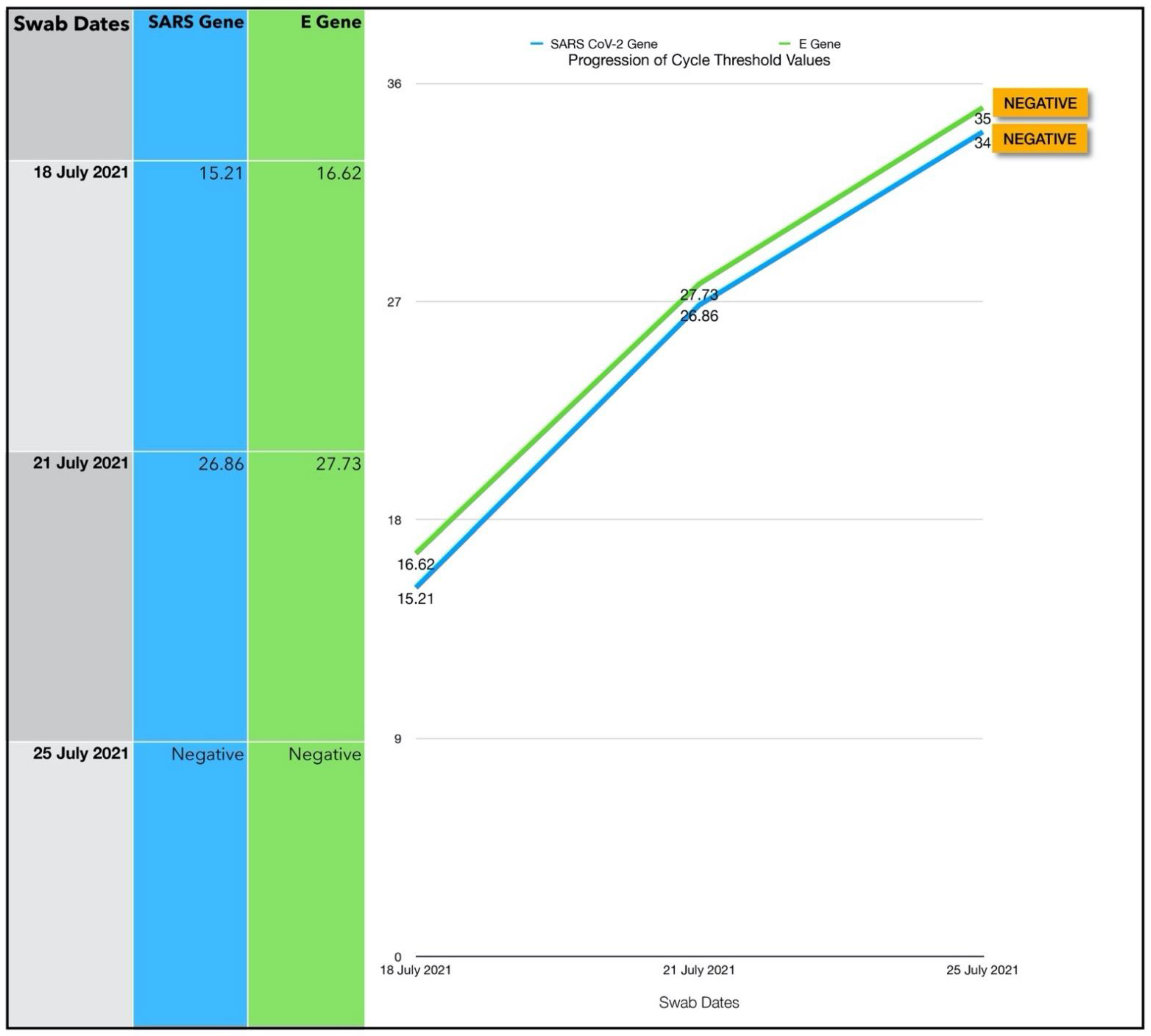
3.6. Subject #6
- 1.
- ONPDI:
- First course of eight treatments; Day 0 to Day 2.
- Second course of eight treatments; Day 4 to Day 6.
- 2.
- Sublingual PBM; Day 4 to Day 7.
- 3.
- No oral-MB was administered as the patient was pregnant.
3.6.1. Estimated Viral Load Reduction (EVLR)
3.6.2. One Year Follow-Up
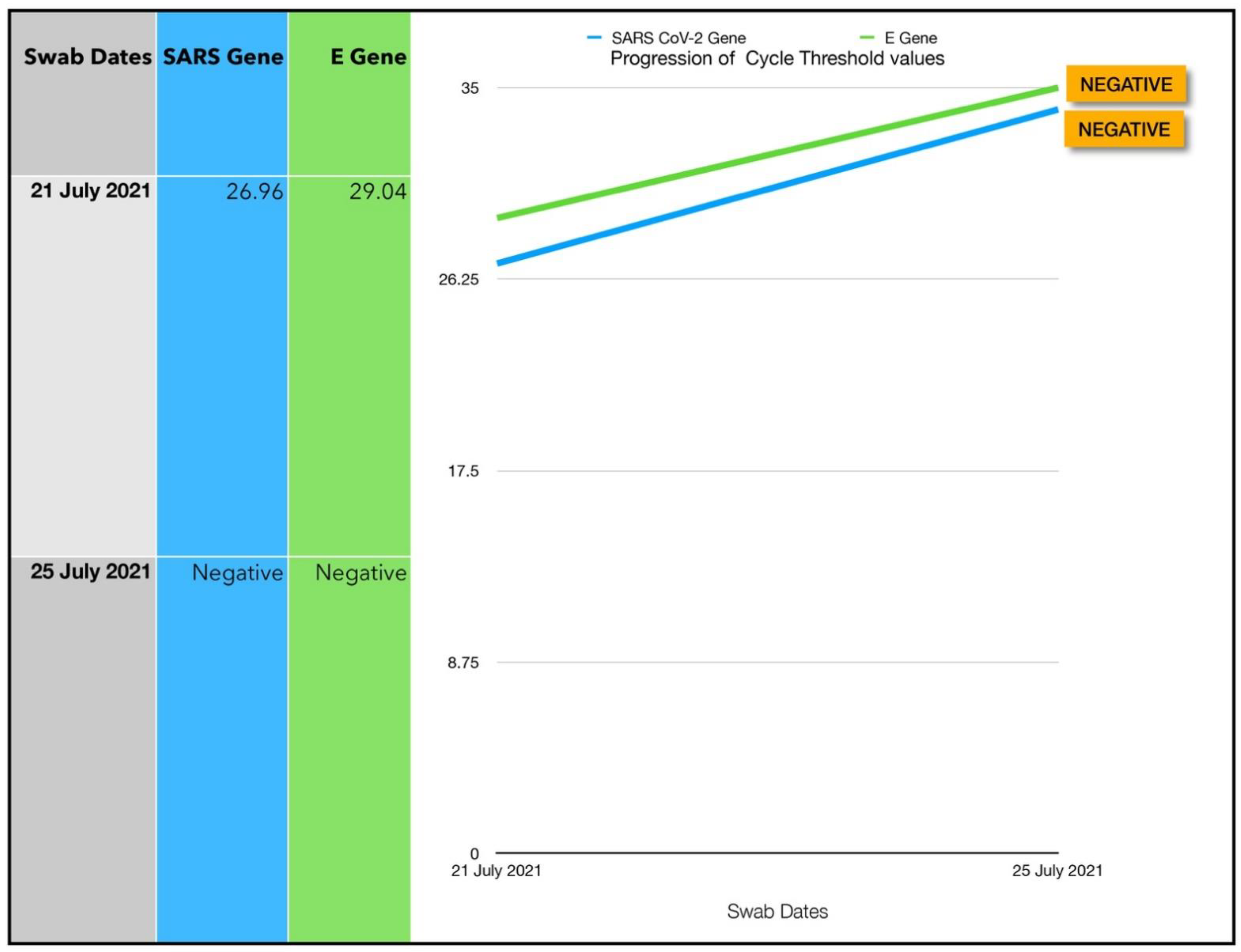
3.7. Subject #7
- Topical ONPDI-course of eight treatments, Day 0 to Day 2
- No oral-MB administered as patient presented early and had mild symptoms
3.7.1. Estimated Viral Load Reduction (EVLR)
3.7.2. One Year Follow-Up
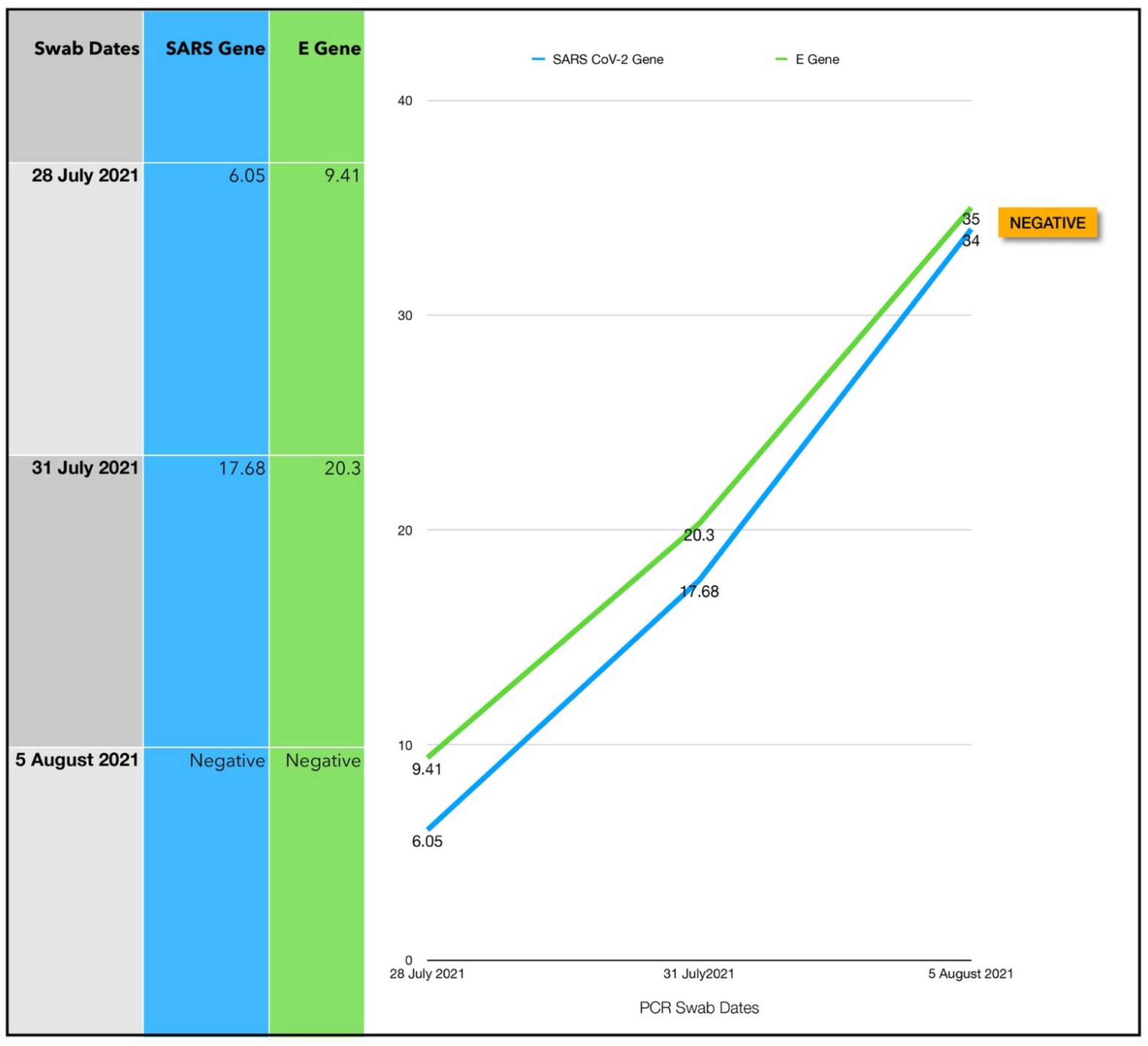
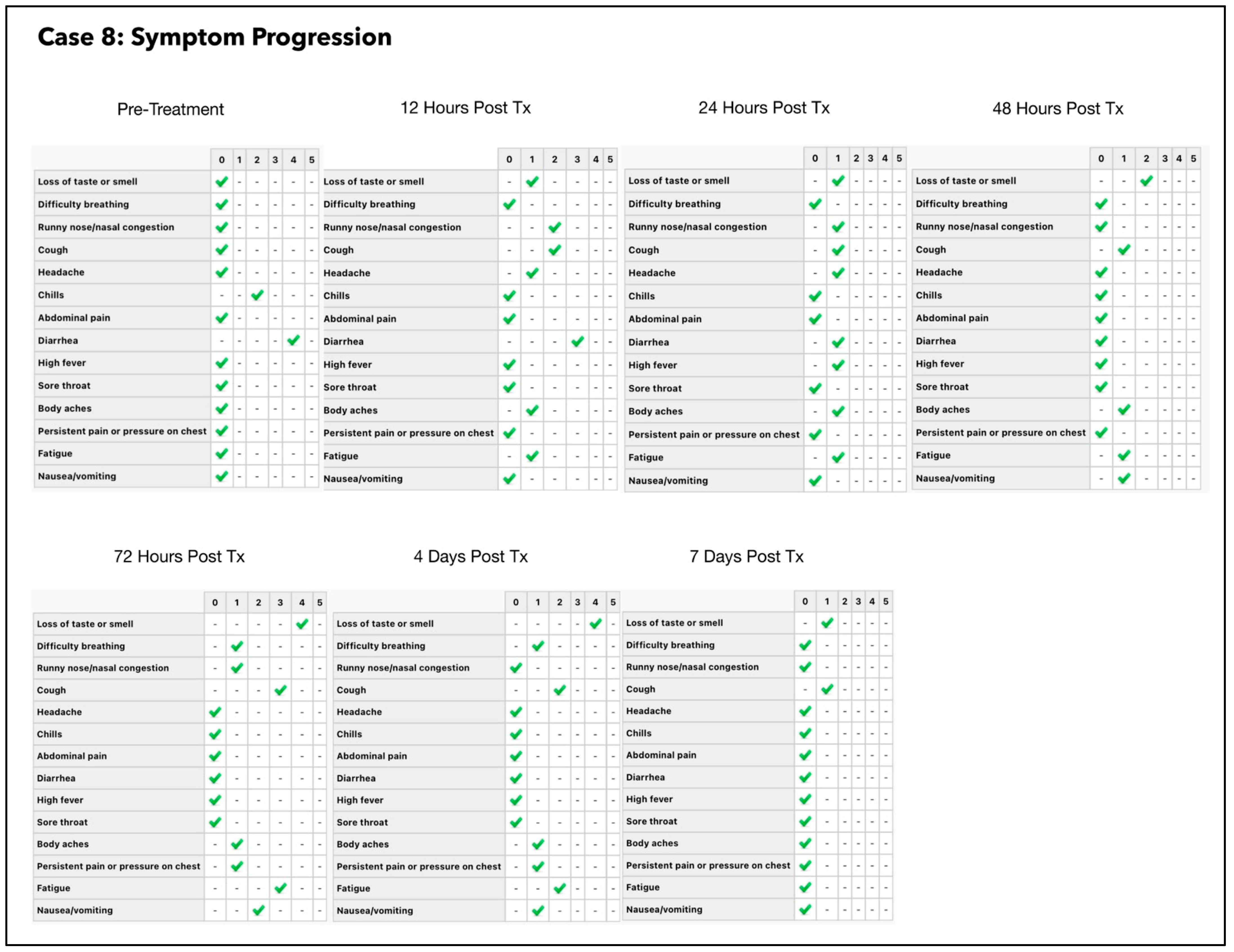
3.8. Subject #8
- Oral-MB capsules.
- Sublingual PBM.
- ONPDI.
3.8.1. Estimated Viral Load Reduction (EVLR)
3.8.2. One Year Follow-Up
4. Discussion
4.1. Assessment of Treatment Response and Outcomes
4.2. Adverse Effects
4.3. Long-Term Follow-Up
4.4. Logistical Considerations
4.5. Evaluation of Related-Published Clinical Studies
4.6. Study Limitations
4.7. Future Potential
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Li, Q.; Liang, Z.; Li, T.; Liu, S.; Cui, Q.; Nie, J.; Wu, Q.; Qu, X.; Huang, W.; et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg. Microbes. Infect. 2022, 11, 1–5. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Rezusta, A.; Juarranz, A.; Hamblin, M.R. Editorial: Antimicrobial Photodynamic Therapy: A New Paradigm in the Fight Against Infections. Front. Med. 2021, 8, 788888. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Sălăgean, T.; Bordea, I.R.; Benedicenti, S. Phototherapy as a Rational Antioxidant Treatment Modality in COVID-19 Management; New Concept and Strategic Approach: Critical Review. Antioxidants 2020, 9, 875. [Google Scholar] [CrossRef]
- Ghahestani, S.M.; Shahab, E.; Karimi, S.; Madani, M.H. Methylene blue may have a role in the treatment of COVID-19. Med. Hypotheses 2020, 144, 110163. [Google Scholar] [CrossRef]
- Dabholkar, N.; Gorantla, S.; Dubey, S.K.; Alexander, A.; Taliyan, R.; Singhvi, G. Repurposing methylene blue in the management of COVID-19: Mechanistic aspects and clinical investigations. Biomed. Pharmacother. 2021, 142, 112023. [Google Scholar] [CrossRef]
- Duicu, O.M.; Privistirescu, A.; Wolf, A.; Petruş, A.; Dănilă, M.D.; Raţiu, C.D.; Muntean, D.M.; Sturza, A. Methylene blue improves mitochondrial respiration and decreases oxidative stress in a substrate-dependent manner in diabetic rat hearts. Can. J. Physiol. Pharmacol. 2017, 95, 1376–1382. [Google Scholar] [CrossRef] [Green Version]
- Tucker, D.; Lu, Y.; Zhang, Q. From Mitochondrial Function to Neuroprotection-an Emerging Role for Methylene Blue. Mol. Neurobiol. 2018, 55, 5137–5153. [Google Scholar] [CrossRef]
- Rojas, J.C.; Bruchey, A.K.; Gonzalez-Lima, F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog. Neurobiol. 2012, 96, 32–45. [Google Scholar] [CrossRef] [Green Version]
- Auchter, A.; Williams, J.; Barksdale, B.; Monfils, M.H.; Gonzalez-Lima, F. Therapeutic benefits of methylene blue on cognitive impairment during chronic cerebral hypoperfusion. J. Alzheimers Dis. 2014, 42, S525–S535. [Google Scholar] [CrossRef]
- Chuang, S.T.; Papp, H.; Kuczmog, A.; Eells, R.; Condor Capcha, J.M.; Shehadeh, L.A.; Jakab, F.; Buchwald, P. Methylene Blue Is a Nonspecific Protein-Protein Interaction Inhibitor with Potential for Repurposing as an Antiviral for COVID-19. Pharmaceuticals 2022, 15, 621. [Google Scholar] [CrossRef]
- Cagno, V.; Medaglia, C.; Cerny, A.; Tapparel, C. Methylene Blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro. Sci. Rep. 2021, 11, 14295. [Google Scholar] [CrossRef]
- Ahn, H.; Kang, S.G.; Yoon, S.I.; Ko, H.J.; Kim, P.H.; Hong, E.J.; An, B.S.; Lee, E.; Lee, G.S. Methylene blue inhibits NLRP3, NLRC4, AIM2, and non-canonical inflammasome activation. Sci. Rep. 2017, 7, 2409. [Google Scholar] [CrossRef]
- Kast, R.E. Inhibiting the NLRP3 Inflammasome With Methylene Blue as Treatment Adjunct in Myelodysplasia. Front. Oncol. 2018, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Lösche, W.; Bosia, A.; Heller, R.; Pescarmona, G.P.; Arese, P.; Till, U. Methylene blue inhibits the arachidonic acid metabolism in human blood platelets. Biomed. Biochim. Acta 1988, 47, S100-3. [Google Scholar]
- McCullough, P.A.; Alexander, P.E.; Armstrong, R.; Arvinte, C.; Bain, A.F.; Bartlett, R.P.; Berkowitz, R.L.; Berry, A.C.; Borody, T.J.; Brewer, J.H.; et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev. Cardiovasc. Med. 2020, 21, 517–530. [Google Scholar] [CrossRef]
- Bojadzic, D.; Alcazar, O.; Buchwald, P. Methylene blue inhibits the SARS-CoV-2 spike–ACE2 protein-protein interaction–a mechanism that can contribute to its antiviral activity against COVID-19. Front. Pharmacol. 2020, 11, 600372. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ohnishi, S. Uncoating of Influenza Virus in Endosomes. J. Virol. 1984, 51, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Gorshkov, K.; Chen, C.Z.; Bostwick, R.; Rasmussen, L.; Tran, B.N.; Cheng, Y.S.; Xu, M.; Pradhan, M.; Henderson, M.; Zhu, W.; et al. The SARS-CoV-2 Cytopathic Effect Is Blocked by Lysosome Alkalizing Small Molecules. ACS Infect. Dis. 2021, 7, 1389–1408. [Google Scholar] [CrossRef]
- te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Fenn, A.M.; Skendelas, J.P.; Moussa, D.N.; Muccigrosso, M.M.; Popovich, P.G.; Lifshitz, J.; Eiferman, D.S.; Godbout, J.P. Methylene Blue Attenuates Traumatic Brain Injury-Associated Neuroinflammation and Acute Depressive-Like Behavior in Mice. J. Neurotrauma 2015, 32, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Zhi-Hang, L.; Si-Yuan, W.; Li-Li, C.; Jia-Yuan, Z.; Qing-Feng, K.; Dan-Rui, X.; Wen-Ping, L. Methylene Blue Mitigates Acute Neuroinflammation after Spinal Cord Injury through Inhibiting NLRP3 Inflammasome Activation in Microglia. Front. Cell. Neurosci. 2017, 11, 391. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, H.; Yu, J.; Chen, X.; Yang, L. Methylene Blue Attenuates Diabetic Retinopathy by Inhibiting NLRP3 Inflammasome Activation in STZ-Induced Diabetic Rats. Ocul. Immunol. Inflamm. 2019, 27, 836–843. [Google Scholar] [CrossRef]
- Thesnaar, L.; Bezuidenhout, J.J.; Petzer, A.; Petzer, J.P.; Cloete, T.T. Methylene blue analogues: In vitro antimicrobial minimum inhibitory concentrations and in silico pharmacophore modelling. Eur. J. Pharm. Sci. 2021, 157, 105603. [Google Scholar] [CrossRef]
- Bragulat, M.R.; Abarca, M.L.; Bruguera, M.T.; Cabañes, F.J. Dyes as fungal inhibitors: Effect on colony diameter. Appl. Environ. Microbiol. 1991, 57, 2777–2780. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, E. Prevention of cytopathic effect and propagation of poliovirus by methylene blue. Z. Nat. B 1960, 15, 588–592. [Google Scholar]
- Shafran, N.; Shafran, I.; Ben-Zvi, H.; Sofer, S.; Sheena, L.; Krause, I.; Shlomai, A.; Goldberg, E.; Sklan, E.H. Secondary bacterial infection in COVID-19 patients is a stronger predictor for death compared to influenza patients. Sci. Rep. 2021, 11, 12703. [Google Scholar] [CrossRef]
- Ng, B.K.; Cameron, A.J. The role of methylene blue in serotonin syndrome: A systematic review. Psychosomatics 2010, 51, 194–200. [Google Scholar] [CrossRef]
- Alda, M.; McKinnon, M.; Blagdon, R.; Garnham, J.; MacLellan, S.; O’Donovan, C.; Hajek, T.; Nair, C.; Dursun, S.; MacQueen, G. Methylene blue treatment for residual symptoms of bipolar disorder: Randomised crossover study. Br. J. Psychiatry 2017, 210, 54–60. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration. FDA Drug Safety Communication: Updated Information about the Drug Interaction Between Methylene Blue (Methylthioninium Chloride) and Serotonergic Psychiatric Medications. FDA. 2011. Available online: http://www.fda.gov/Drugs/DrugSafety/ucm276119.htm. (accessed on 15 August 2022).
- Lu, G.; Nagbanshi, M.; Goldau, N.; Mendes Jorge, M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Müller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Sălăgean, T.; Pop, I.D.; Bordea, I.R.; Benedicenti, S. Understanding COVID-19 Pandemic: Molecular Mechanisms and Potential Therapeutic Strategies. An Evidence-Based Review. J. Inflamm. Res. 2021, 14, 13–56. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Oliveira, M.C., Jr.; Greiffo, F.R.; Rigonato-Oliveira, N.C.; Custódio, R.W.; Silva, V.R.; Damaceno-Rodrigues, N.R.; Almeida, F.M.; Albertini, R.; Lopes-Martins, R.Á.; de Oliveira, L.V.; et al. Low level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J. Photochem. Photobiol. B 2014, 134, 57–63. [Google Scholar] [CrossRef] [PubMed]
- de Lima, F.M.; Villaverde, A.B.; Albertini, R.; Corrêa, J.C.; Carvalho, R.L.; Munin, E.; Araújo, T.; Silva, J.A.; Aimbire, F. Dual Effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: Action on anti- and pro-inflammatory cytokines. Lasers Surg. Med. 2011, 43, 410–420. [Google Scholar] [CrossRef]
- Hanna, R.; Bensadoun, R.J.; Beken, S.V.; Burton, P.; Carroll, J.; Benedicenti, S. Outpatient Oral Neuropathic Pain Management with Photobiomodulation Therapy: A Prospective Analgesic Pharmacotherapy-Paralleled Feasibility Trial. Antioxidants 2022, 11, 533. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Bensadoun, R.J.; Raber-Durlacher, J.E.; Benedicenti, S. Role of Photobiomodulation Therapy in Neurological Primary Burning Mouth Syndrome. A Systematic Review and Meta-Analysis of Human Randomised Controlled Clinical Trials. Pharmaceutics 2021, 13, 1838. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Bensadoun, R.J.; Benedicenti, S. Role of Photobiomodulation Therapy in Modulating Oxidative Stress in Temporomandibular Disorders. A Systematic Review and Meta-Analysis of Human Randomised Controlled Trials. Antioxidants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Benedicenti, S.; Amaroli, A.; Sălăgean, T.; Pop, I.D.; Todea, D.; Bordea, I.R. Photobiomodulation Therapy in Oral Mucositis and Potentially Malignant Oral Lesions: A Therapy Towards the Future. Cancers 2020, 12, 1949. [Google Scholar] [CrossRef]
- Legouté, F.; Bensadoun, R.J.; Seegers, V.; Pointreau, Y.; Caron, D.; Lang, P.; Prévost, A.; Martin, L.; Schick, U.; Morvant, B.; et al. Low-level laser therapy in treatment of chemoradiotherapy-induced mucositis in head and neck cancer: Results of a randomised, triple blind, multicentre phase III trial. Radiat. Oncol. 2019, 14, 83. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Hanna, R.; Gallus, L.; Benedicenti, A.; Scarfì, S.; Pozzolini, M.; Benedicenti, S. The photobiomodulation effect of higher-fluence 808-nm laser therapy with a flat-top handpiece on the wound healing of the earthworm Dendrobaena veneta: A brief report. Lasers Med. Sci. 2018, 33, 221–225. [Google Scholar] [CrossRef]
- Marashian, S.M.; Hashemian, M.; Pourabdollah, M.; Nasseri, M.; Mahmoudian, S.; Reinhart, F.; Eslaminejad, A. Photobiomodulation Improves Serum Cytokine Response in Mild to Moderate COVID-19: The First Randomized, Double-Blind, Placebo Controlled, Pilot Study. Front. Immunol. 2022, 13, 929837. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, R.; Lei, L.; Liu, H.; Wang, Y.; Wang, Y.; Qian, H.; Dai, T.; Zhang, T.; Lai, Y.; et al. Frontline Science: COVID-19 infection induces readily detectable morphologic and inflammation-related phenotypic changes in peripheral blood monocytes. J. Leukoc. Biol. 2021, 109, 13–22. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- de Castro, M.S.; Miyazawa, M.; Nogueira, E.S.C.; Chavasco, J.K.; Brancaglion, G.A.; Cerdeiram, C.D.; Nogueira, D.A.; Ionta, M.; Hanemann, J.A.C.; Brigagão, M.R.P.L.; et al. Photobiomodulation enhances the Th1 immune response of human monocytes. Lasers Med. Sci. 2022, 37, 135–148. [Google Scholar] [CrossRef]
- Kimizuka, Y.; Katagiri, W.; Locascio, J.J.; Shigeta, A.; Sasaki, Y.; Shibata, M.; Morse, K.; Sîrbulescu, R.F.; Miyatake, M.; Reeves, P.; et al. Brief Exposure of Skin to Near-Infrared Laser Modulates Mast Cell Function and Augments the Immune Response. J. Immunol. 2018, 201, 3587–3603. [Google Scholar] [CrossRef] [Green Version]
- Yokomizo, S.; Katagiri, W.; Maki, Y.; Sano, T.; Inoue, K.; Fukushi, M.; Atochin, D.N.; Kushibiki, T.; Kawana, A.; Kimizuka, Y.; et al. Brief exposure of skin to near-infrared laser augments early vaccine responses. Nanophotonics 2021, 10, 3187–3197. [Google Scholar] [CrossRef]
- Hanna, R.; Dalvi, S.; Amaroli, A.; De Angelis, N.; Benedicenti, S. Effects of photobiomodulation on bone defects grafted with bone substitutes: A systematic review of in vivo animal studies. J. Biophotonics 2021, 14, e202000267. [Google Scholar] [CrossRef]
- Yu, S.; Lan, C.E.; Yu, H.S. Mechanisms of repigmentation induced by photobiomodulation therapy in vitiligo. Exp. Dermatol. 2019, 28, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Arany, P.R. Photobiomodulation Therapy Promotes Expansion of Epithelial Colony Forming Units. Photomed. Laser Surg. 2016, 34, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Oron, U.; Maltz, L.; Tuby, H.; Sorin, V.; Czerniak, A. Enhanced Liver Regeneration Following Acute Hepatectomy by Low-Level Laser Therapy. Photomed. Laser Surg. 2010, 28, 675–678. [Google Scholar] [CrossRef]
- Oron, U. Light therapy and stem cells: A therapeutic intervention of the future? Interv. Cardiol. 2011, 3, 627–629. [Google Scholar] [CrossRef]
- Tomé, R.F.F.; Silva, D.F.B.; dos Santos, C.A.O.; de Vasconcelos, G.; Almeida Rolim, A.K. ILIB (intravascular laser irradiation of blood) as an adjuvant therapy in the treatment of patients with chronic systemic diseases—An integrative literature review. Lasers Med. Sci 2020, 35, 1899–1907. [Google Scholar] [CrossRef]
- Momenzadeh, S.; Abbasi, M.; Ebadifar, A.; Aryani, M.; Bayrami, J.; Nematollahi, F. The intravenous laser blood irradiation in chronic pain and fibromyalgia. J. Lasers Med. Sci. 2015, 6, 6–9. [Google Scholar]
- Kazemikhoo, N.; Kyavar, M.; Razzaghi, Z.; Ansari, F.; Maleki, M.; Ghavidel, A.A.; Gholampour, M.; Ghaffarinejad, M.H. Effects of intravenous and transdermal photobiomodulation on the postoperative complications of coronary artery bypass grafting surgery: A randomized, controlled clinical trial. Lasers Med. Sci. 2021, 36, 1891–1896. [Google Scholar] [CrossRef]
- Chiran, D.A.; Litscher, G.; Weber, M.; Ailioaie, L.M.; Ailioaie, C.; Litscher, D. Intravenous laser blood irradiation increases efficacy of etanercept in selected subtypes of juvenile idiopathic arthritis: An innovative clinical research approach. Evid. Based Complement. Alternat. Med. 2013, 2013, 168134. [Google Scholar] [CrossRef] [Green Version]
- Bakeeva, L.; Manteiffel, V.; Rodichev, E.; Karu, T. Formation of gigantic mitochondria in human blood lymphocytes under the effect of an He-Ne laser. Mol. Biol. Mosk. 1993, 27, 608–617. [Google Scholar]
- Dube, A.; Hausal; Gupta, P.K. Modulation of macrophage structure and function by low level He-Ne irradiation. Photochem. Photobiol. Sci. 2003, 2, 851–855. [Google Scholar] [CrossRef]
- Funk, J.; Kruse, A.; Kirchner, H. Cytokine production after helium-neon laser irradiation in cultures of human peripheral blood mononuclear cells. J. Photochem. Photobiol. 1992, 16, 347–355. [Google Scholar] [CrossRef]
- Luo, G.Y.; Zhao, Y.P.; Liu, T.C.Y.; Liu, S.H. Membranotropic photobiomodulation on red blood cell deformability, Proc. SPIE 6534. In Proceedings of the Fifth International Conference on Photonics and Imaging in Biology and Medicine, Wuhan, China, 1–3 September 2006; p. 653424. [Google Scholar] [CrossRef]
- Hanna, R.; Agas, D.; Benedicenti, S.; Laus, F.; Cuteri, V.; Sabbieti, M.G.; Amaroli, A. A comparative study between the effectiveness of 980nm photobiomodulation, delivered by Gaussian versus flattop profiles on osteoblasts maturation. Front. Endocrinol. 2019, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- de Matos, B.T.L.; Buchaim, D.V.; Pomini, K.T.; Barbalho, S.M.; Guiguer, E.L.; Reis, C.H.B.; Bueno, C.R.S.; Cunha, M.R.D.; Pereira, E.S.B.M.; Buchaim, R.L. Photobiomodulation Therapy as a Possible New Approach in COVID-19: A Systematic Review. Life 2021, 11, 580. [Google Scholar] [CrossRef]
- Walski, T.; Grzeszczuk-Kuć, K.; Gałecka, K.; Trochanowska-Pauk, N.; Bohara, R.; Czerski, A.; Szułdrzyński, K.; Królikowski, W.; Detyna, J.; Komorowska, M. Near-infrared photobiomodulation of blood reversibly inhibits platelet reactivity and reduces hemolysis. Sci. Rep. 2022, 12, 4042. [Google Scholar] [CrossRef]
- Holmes, E.; Barrett, D.W.; Saucedo, C.L.; O’Connor, P.; Liu, H.; Gonzalez-Lima, F. Cognitive Enhancement by Transcranial Photobiomodulation Is Associated With Cerebrovascular Oxygenation of the Prefrontal Cortex. Front. Neurosci. 2019, 13, 1129. [Google Scholar] [CrossRef]
- Murilo, X.; Oliveira, M.X.; Toma, R.L.; Jones, B.J.L.; Cyprien, T.P.; Tier, M.R.; Wallace, C.A.; Renno, A.C.M.; Sabapathy, S.; Laakso, S.E.L. Effects of photobiomodulation therapy (pulsed LASER 904 nm) on muscle oxygenation and performance in exercise-induced skeletal muscle fatigue in young women: A pilot study. In Proceedings of the Mechanisms of Photobiomodulation Therapy XII, San Francisco, CA, USA, 28 January–2 February 2017; p. 100480J. [Google Scholar] [CrossRef] [Green Version]
- Pruitt, T.; Carter, C.; Wang, X.; Wu, A.; Liu, H. Photobiomodulation at Different Wavelengths Boosts Mitochondrial Redox Metabolism and Hemoglobin Oxygenation: Lasers vs. Light-Emitting Diodes In Vivo. Metabolites 2022, 12, 103. [Google Scholar] [CrossRef]
- Linares, S.N.; Beltrame, T.; Ferraresi, C.; Galdino, G.A.M.; Catai, A.M. Photobiomodulation effect on local hemoglobin concentration assessed by near-infrared spectroscopy in humans. Lasers Med. Sci. 2020, 35, 641–649. [Google Scholar] [CrossRef]
- Kitchen, L.C.; Berman, M.; Halper, J.; Chazot, P. Rationale for 1068 nm Photobiomodulation Therapy (PBMT) as a Novel, Non-Invasive Treatment for COVID-19 and Other Coronaviruses: Roles of NO and Hsp70. Int. J. Mol. Sci. 2022, 23, 5221. [Google Scholar] [CrossRef]
- Salehpour, F.; Gholipour-Khalili, S.; Farajdokht, F.; Kamari, F.; Walski, T.; Hamblin, M.R.; DiDuro, J.O.; Cassano, P. Therapeutic potential of intranasal photobiomodulation therapy for neurological and neuropsychiatric disorders: A narrative review. Rev. Neurosci. 2020, 31, 269–286. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Litscher, G.; Weber, M.; Ailioaie, C.; Litscher, D.; Chiran, A.D. Innovations and Challenges by Applying Sublingual Laser Blood Irradiation in Juvenile Idiopathic Arthritis. Int. J. Photoenergy 2014, 2014, 130417. [Google Scholar] [CrossRef] [Green Version]
- Dalvi, S.; Benedicenti, S.; Hanna, R. Is antimicrobial photodynamic therapy an effective treatment modality for aggressive periodontitis? A systematic review of in vivo human randomized controlled clinical trials. Photodiag. Photodyn. Ther. 2021, 34, 102314. [Google Scholar] [CrossRef]
- Dalvi, S.; Benedicenti, S.; Sălăgean, T.; Bordea, I.R.; Hanna, R. Effectiveness of Antimicrobial Photodynamic Therapy in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis of In Vivo Human Randomized Controlled Clinical Trials. Pharmaceutics 2021, 13, 836. [Google Scholar] [CrossRef]
- Dalvi, S.A.; Hanna, R.; Gattani, D.R. Utilisation of antimicrobial photodynamic therapy as an adjunctive tool for open flap debridement in the management of chronic periodontitis: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2019, 25, 440–447. [Google Scholar] [CrossRef]
- Yin, R.; Hamblin, M.R. Antimicrobial Photosensitizers: Drug Discovery Under the Spotlight. Curr. Med. Chem. 2015, 22, 2159–2185. [Google Scholar] [CrossRef]
- Voos, A.C.; Kranz, S.; Tonndorf-Martini, S.; Voelpel, A.; Sigusch, H.; Staudte, H.; Albrecht, V.; Sigusch, B.W. Photodynamic antimicrobial effect of safranine O on an ex vivo periodontal biofilm. Lasers Surg. Med. 2014, 46, 235–243. [Google Scholar] [CrossRef]
- Giannelli, M.; Bani, D. Appropriate laser wavelengths for photodynamic therapy with methylene blue. Lasers Med. Sci. 2018, 33, 1837–1838. [Google Scholar] [CrossRef]
- Schneider, J.E., Jr.; Tabatabaie, T.; Maidt, L.; Smith, R.H.; Nguyen, X.; Pye, Q.; Floyd, R.A. Potential mechanisms of photodynamic inactivation of virus by methylene blue. I. RNA-protein crosslinks and other oxidative lesions in Q beta bacteriophage. Photochem. Photobiol. 1998, 67, 350–357. [Google Scholar]
- Abe, H.; Wagnerm, S.J. Analysis of viral DNA, protein and envelope damage after methylene blue, phthalocyanine derivative or merocyanine 540 photosensitization. Photochem. Photobiol. 1995, 61, 402–409. [Google Scholar] [CrossRef]
- Bachmann, B.K.-H.J.B.; Lambrecht, B.; Mohr, H. Target structures for HIV-1 inactivation by methylene blue and light. J. Med. Virol. 1995, 47, 172–178. [Google Scholar] [CrossRef]
- Pedigo, L.A.; Gibbs, A.J.; Scott, R.J.; Street, C.N. Absence of Bacterial Resistance Following Repeat Exposure to Photodynamic Therapy, Proceedings of the Photodynamic Therapy: Back to the Future; SPIE: Bellingham, WA, USA, 2009; p. 73803H. [Google Scholar]
- Duguay, B.A.; Herod, A.; Pringle, E.S.; Monro, S.M.A.; Hetu, M.; Cameron, C.G.; McFarland, S.A.; McCormick, C. Photodynamic Inactivation of Human Coronaviruses. Viruses 2022, 14, 110. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [Green Version]
- Rustanti, L.; Hobson-Peters, J.; Colmant, A.M.G.; Hall, R.A.; Young, P.R.; Reichenberg, S.; Tolksdorf, F.; Sumian, C.; Gravemann, U.; Seltsam, A.M.; et al. Inactivation of Japanese encephalitis virus in plasma by methylene blue combined with visible light and in platelet concentrates by ultraviolet C light. Transfusion 2020, 60, 2655–2660. [Google Scholar] [CrossRef]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Muller, T.H.; Seltsam, A. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion 2018, 58, 2202–2207. [Google Scholar] [CrossRef] [Green Version]
- Mohr, H.; Knuver-Hopf, J.; Gravemann, U.; Redecker-Klein, A.; Muller, T.H. West Nile virus in plasma is highly sensitive to methylene blue-light treatment. Transfusion 2004, 44, 886–890. [Google Scholar] [CrossRef]
- Papin, J.F.; Floyd, R.A.; Dittmer, D.P. Methylene blue photoinactivation abolishes West Nile virus infectivity in vivo. Antivir. Res. 2005, 68, 84–87. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Jiang, H.; Zhou, J.; Lam, P.; Zhang, L.; et al. Epithelial Cells Lining Salivary Gland Ducts Are Early Target Cells of Severe Acute Respiratory Syndrome Coronavirus Infection in the Upper Respiratory Tracts of Rhesus Macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.; Pérez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903. [Google Scholar] [CrossRef]
- Woelfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Mueller, M.A.; Niemeyer, D.; Jones Kelly, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized cases of coronavirus disease 2019. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Schikora, D.; Hepburn, J.; Plavin, S.R. Reduction of the Viral Load by Non-Invasive Photodynamic Therapy in Early Stages of COVID-19 infection. Am. J. Virol. Dis. 2020, 2, 1–5. [Google Scholar]
- Soria, M.E.; Cortón, M.; Martínez-González, B.; Lobo-Vega, R.; Vázquez-Sirvent, L.; López-Rodríguez, R.; Almoguera, B.; Mahillo, I.; Mínguez, P.; Herrero, A.; et al. High SARS-CoV-2 viral load is associated with a worse clinical outcome of COVID-19 disease. Access Microbiol. 2021, 3, 000259. [Google Scholar] [CrossRef]
- Daniell, H.; Nair, S.K.; Esmaeili, N.; Wakade, G.; Shahid, N.; Ganesan, P.K.; Islam, M.R.; Shepley-McTaggart, A.; Feng, S.; Gary, E.N.; et al. Debulking SARS-CoV-2 in saliva using angiotensin converting enzyme 2 in chewing gum to decrease oral virus transmission and infection. Mol. Ther. 2022, 30, 1966–1978. [Google Scholar] [CrossRef]
- Mroz, P.; Szokalska, A.; Wu, M.X.; Hamblin, M.R. Photodynamic Therapy of Tumors Can Lead to Development of Systemic Antigen-Specific Immune Response. PLoS ONE 2010, 5, e15194. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Tanaka, M.; Vecchio, D.; Garcia-Diaz, M.; Chang, J.; Morimoto, Y.; Hamblin, M.R. Photodynamic Therapy Induces an Immune Response Against a Bacterial Pathogen. Expert Rev. Clin. Immunol. 2012, 8, 479–494. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.H.; Mehran, Y.Z.; Orthaber, A.; Saadat, H.H.; Robert Weber, R.; Wojcik, M. Successful Reduction of SARS-CoV-2 Viral Load by Photodynamic Therapy (PDT) Verified by QPCR—A Novel Approach in Treating Patients in Early Infection Stages. Med. Clin. Res. 2020, 5, 311–325. [Google Scholar]
- Collins, L.M.; Dawes, C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J. Dent. Res. 1987, 66, 1300–1302. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose Response 2009, 7, 58–83. [Google Scholar] [CrossRef]
- Winchester, L.W.; Chou, N.Y. Measurement of sublingual blood velocity as a tool for monitoring sepsis. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 3739–3742. [Google Scholar] [CrossRef] [Green Version]
- Research and Statistics Department Ministry of Tourism Bahamas. 2019. Available online: http://www.tourismtoday.com/sites/default/fles/freequently_requested_statistics_brochure_2019_latest_1.pdf (accessed on 15 August 2022).
- Walter-Sack, I.; Rengelshausen, J.; Oberwittler, H.; Burhenne, J.; Mueller, O.; Meissner, P.; Mikus, G. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur. J. Clin. Pharmacol. 2009, 65, 179–189. [Google Scholar] [CrossRef]
- Grauman Neander, N.; Loner, C.A.; Rotoli, J.M. The Acute Treatment of Methemoglobinemia in Pregnancy. J. Emerg. Med. 2018, 54, 685–689. [Google Scholar] [CrossRef]
- Lobo, C.S.; Rodrigues-Santos, P.; Pereira, D.; Núnez, J.; Trêpa, J.C.D.; Sousa, D.L.; Lourenço, J.V.; Coelho, M.F.; de Almeida, L.P.; da Cunha, J.S.; et al. Photodynamic disinfection of SARS-CoV-2 clinical samples using a methylene blue formulation. Photochem. Photobiol. Sci. 2022, 21, 1101–1109. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Moghaddam, A.B.; Amini, S.; Keramati, M.R.; Zarmehri, A.M.; Alamdari, A.H.; Damsaz, M.; Banpour, H.; Yarahmadi, A.; Koliakos, G. Application of methylene blue -vitamin C -N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur. J. Pharmacol. 2020, 885, 173494. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Hafizi-Lotfabadi, S.; Bagheri-Moghaddam, A.; Safari, H.; Mozdourian, M.; Javidarabshahi, Z.; Peivandi-Yazdi, A.; Ali-Zeraati, A.; Sedaghat, A.; Poursadegh, F.; et al. Methylene Blue for Treatment of Hospitalized COVID-19 Patients: A Randomized, Controlled, Open-Label, Clinical Trial, Phase 2. Rev. Invest. Clin. 2021, 73, 190–198. [Google Scholar] [CrossRef]
- Alamdari, D.H.; Lotfabadi, S.H.; Darban, B.M.; Agheli-Rad, M.; Saadatian, S.; Hashemi, S.H.; Ahmasabadi, F.B.; Morovatdar, N.; Arastoo, M.; Bhushan, B. Methylene Blue for Treatment of Hospitalized COVID-19 Patients, Randomized, Controlled, Open-Label Clinical Trial, Phase 3. Aristotle Biomed. J. 2021, 3, 12–18. [Google Scholar]
- Sigman, S.A.; Mokmeli, S.; Monici, M.; Vetrici, M.A. A 57-Year-Old African American Man with Severe COVID-19 Pneumonia Who Responded to Supportive Photobiomodulation Therapy (PBMT): First Use of PBMT in COVID-19. Am. J. Case Rep. 2020, 21, e926779. [Google Scholar] [CrossRef]
- Williams, R.K.; Raimondo, J.; Cahn, D.; Williams, A.; Schell, D. Whole-organ transdermal photobiomodulation (PBM) of COVID-19: A 50-patient case study. J. Biophotonics 2022, 15, e202100194. [Google Scholar] [CrossRef]
- Aroso, R.T.; Piccirillo, G.; Arnaut, Z.A.; Gonzalez, A.C.S.; Rodrigues, F.M.S.; Pereira, M.M. Photodynamic inactivation of influenza virus as a potential alternative for the control of respiratory tract infection. J. Photochem. Photobiol. 2021, 7, 100043. [Google Scholar]
- Schagen, F.H.; Moor, A.C.; Cheong, S.C.; Cramer, S.J.; van Ormondt, H.; van der Eb, A.J.; Dubbelman, T.M.; Hoeben, R.C. Photodynamic treatment of adenoviral vectors with visible light: An easy and convenient method for viral inactivation. Gene Ther. 1999, 6, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Priyamvada, L.; Burgado, J.; Baker-Wagner, M.; Kitaygorodskyy, A.; Olson, V.; Lingappa, V.R.; Satheshkumar, P.S. New methylene blue derivatives suggest novel anti-orthopoxviral strategies. Antivir. Res. 2021, 191, 105086. [Google Scholar] [CrossRef]



| Actions against SARS-CoV-2 Virus | |
|---|---|
| Reduced Viral Entry |
|
| Reduced Viral Replication |
|
| Photo-Oxidative Viral Inactivation [activation by 660 nm light] |
|
| Reduced Organ Damage | |
| Reduced Cytopathic Effects |
|
| Reduced Hypoxia |
|
| Reduced Hyper-Inflammation |
|
| Broad Spectrum Antimicrobial (Bacterial/Fungal/Other Viruses) | |
| Reduced Secondary Infections |
|
| Type of Device | Semi-Circular Non-Thermal LED Device |
|---|---|
| Light source | Light Emitting Diodes |
| Beam type | Non-Coherent |
| Wavelength | 660 nm |
| Operation emission mode | Continuous mode |
| Number of LEDs | 24 |
| Irradiance per each LED | 5.5m W/cm2 |
| Spot size per each LED | 0.045 cm2 |
| Power output per each LED | 122 mW |
| Total power output | 2928 mW |
| Aperture (surface area of the device) | 18 cm2 |
| Total power density (irradiance) | 163 mW/cm2 |
| Treatment Time | 5 min (300 s) |
| Energy density | 49 J/cm2 |
| Treatment interval | Variable: between 8–24 h (hours) |
| Type of Device | Semi-Circular Non-Thermal LED Device |
|---|---|
| Light source | Light Emitting Diodes |
| Beam type | Non-Coherent |
| Wavelength | 660 nm |
| Operation emission mode | Continuous mode |
| Number of LEDs | 6 |
| Irradiance per each LED | 5.5 mW/cm2 |
| Spot size per LED | 0.045 cm2 |
| Power output per each LED | 122 mW |
| Total power output | 732 mW |
| Device aperture (over sublingual vein) | 9 cm2 |
| Total power density (irradiance) | 81 mW/cm2 |
| Total treatment duration | 5 min (300 s) |
| Estimated blood exposure | 60 s |
| Energy density (blood) | 4.9 J/cm2 |
| Treatment interval | Variable: between 8–24 h (hours) |
| Intervention | August 2020–March 2021 | June/July 2021 (Pilot Study) | LUMINNOVA Upper Respiratory Protocol |
|---|---|---|---|
| Oral MB | Optional: used if symptoms were severe/delayed presentation | Initially 1 mg/kg administered 12-hourly. | Up to 2 mg/kg administered 8-hourly for a total of 8 treatments (unless contraindicated or very mild presentation). |
| Sublingual PBM | Not utilized | Initially utilized for a patient that had not adequately responded to oral MB and in a case where oral MB was contraindicated. | Administered eight treatments every 8 h or until symptoms were resolved |
| Oral/nasal PDT | Total of two treatment sessions 12 h apart. | The number of treatment sessions gradually evolved as we observed the changes in CT values each day. | Treatment course consisted of eight sessions carried out every 8 h. |
| Subject | Post-Acute Sequelae of SARS-CoV-2 | Symptomatic COVID-19 Re-Infection * | Repeat Positive SARS-CoV-2 Test ** |
|---|---|---|---|
| 1 | No PASC | No | No |
| 2 | No PASC | No | No |
| 3 | No PASC | No | No |
| 4 | No PASC | No | No |
| 5 | Significant PASC symptoms | Yes | Yes |
| 6 | No PASC | No | No |
| 7 | No PASC | No | No |
| 8 | Mild PASC symptoms | No | No |
| Case No. (Subject) | Date | Age (yrs) | M/F | Co-Morbidity | Days after Onset | Initial CT Value | Viral Load Reduction Factor * | Days to Negative PCR |
|---|---|---|---|---|---|---|---|---|
| 1 | 26 June 2021 | 61 | F | BMI 31.1 Recurrent Bronchitis | 8 | 16.34/17.19 | 207,000 | 7 |
| 2 | 6 July 2021 | 55 | M | BMI 34 Hypertension Smoker | 5 | 20.17/22.12 | - | Clinically recovered within 12 h (Refused repeat test) |
| 3 | 6 July 2021 | 40 | M | BMI 31.7 | 5 | 18.99/20.43 | 32,768 | 4 |
| 4 | 10 July 2021 | 38 | F | BMI 31.3 | 3 | 11.96/15.97 | 4,194,304 | 4 |
| 5 | 17 July 2021 | 42 | M | BMI 32.5 | 5 | 18.14/19.24 | - | Did not complete treatment protocol. Hospitalized 3 days after discontinuing treatment |
| 6 | 18 July 2021 | 31 | F | BMI 27.6 Pregnant 8 months gestation | 5 | 15.21/16.62 | 450,000 | 7 |
| 7 | 21 July 2021 | 47 | F | BMI 29.2 | 2 | 26.96/29.04 | 128 | 4 |
| 8 | 28 July 2021 | 38 | M | BMI 32.5 | 3 | 6.05/9.41 | 259,000,000 | 8 (Follow up testing was delayed) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hepburn, J.; Williams-Lockhart, S.; Bensadoun, R.J.; Hanna, R. A Novel Approach of Combining Methylene Blue Photodynamic Inactivation, Photobiomodulation and Oral Ingested Methylene Blue in COVID-19 Management: A Pilot Clinical Study with 12-Month Follow-Up. Antioxidants 2022, 11, 2211. https://doi.org/10.3390/antiox11112211
Hepburn J, Williams-Lockhart S, Bensadoun RJ, Hanna R. A Novel Approach of Combining Methylene Blue Photodynamic Inactivation, Photobiomodulation and Oral Ingested Methylene Blue in COVID-19 Management: A Pilot Clinical Study with 12-Month Follow-Up. Antioxidants. 2022; 11(11):2211. https://doi.org/10.3390/antiox11112211
Chicago/Turabian StyleHepburn, Juliette, Susan Williams-Lockhart, René Jean Bensadoun, and Reem Hanna. 2022. "A Novel Approach of Combining Methylene Blue Photodynamic Inactivation, Photobiomodulation and Oral Ingested Methylene Blue in COVID-19 Management: A Pilot Clinical Study with 12-Month Follow-Up" Antioxidants 11, no. 11: 2211. https://doi.org/10.3390/antiox11112211
APA StyleHepburn, J., Williams-Lockhart, S., Bensadoun, R. J., & Hanna, R. (2022). A Novel Approach of Combining Methylene Blue Photodynamic Inactivation, Photobiomodulation and Oral Ingested Methylene Blue in COVID-19 Management: A Pilot Clinical Study with 12-Month Follow-Up. Antioxidants, 11(11), 2211. https://doi.org/10.3390/antiox11112211