Development of Intestinal Injury and Restoration of Weaned Piglets under Chronic Immune Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Sample Collection
2.3. Analysis of Serum Biochemical Indexes
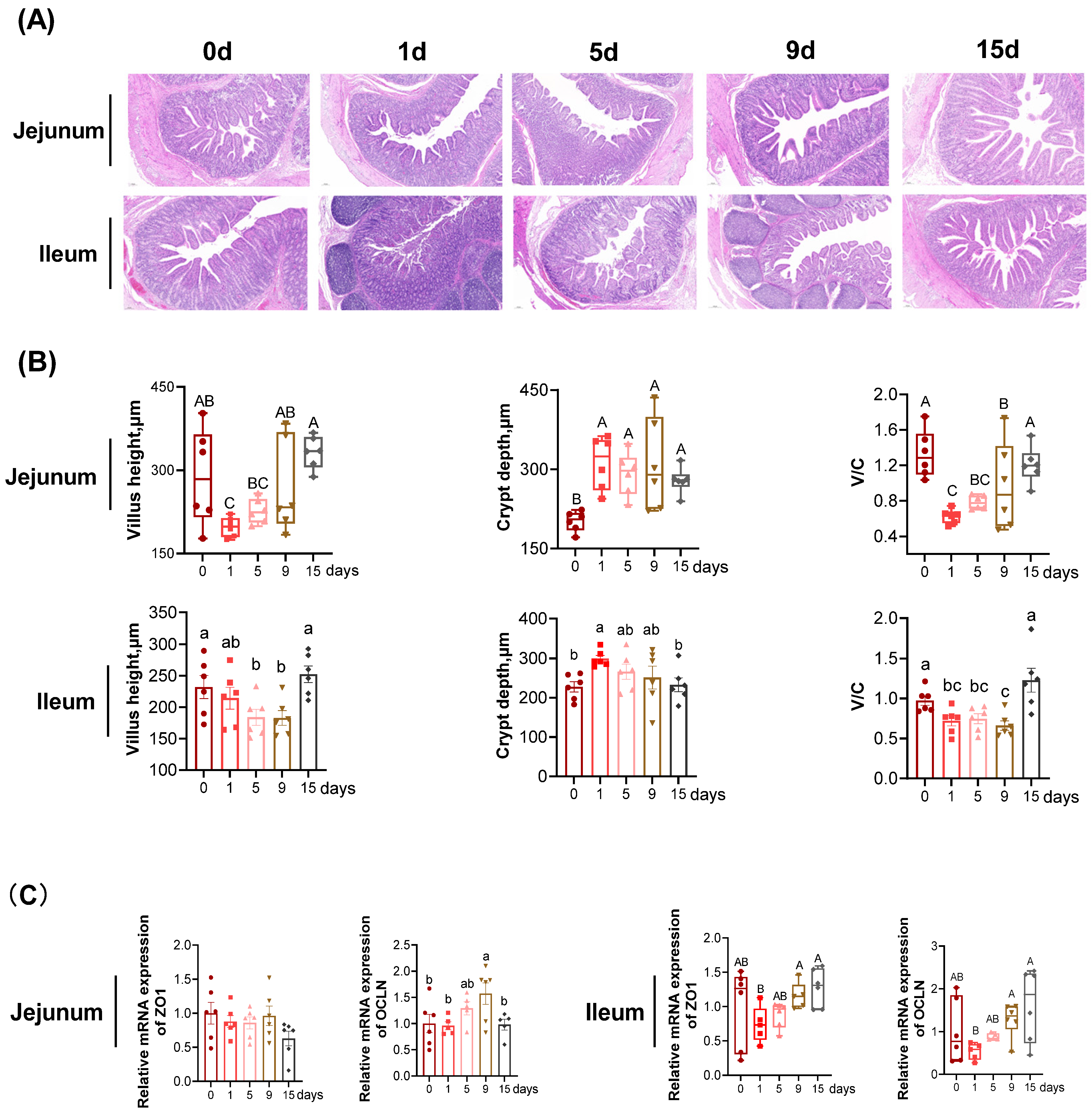
2.4. Intestinal Morphology
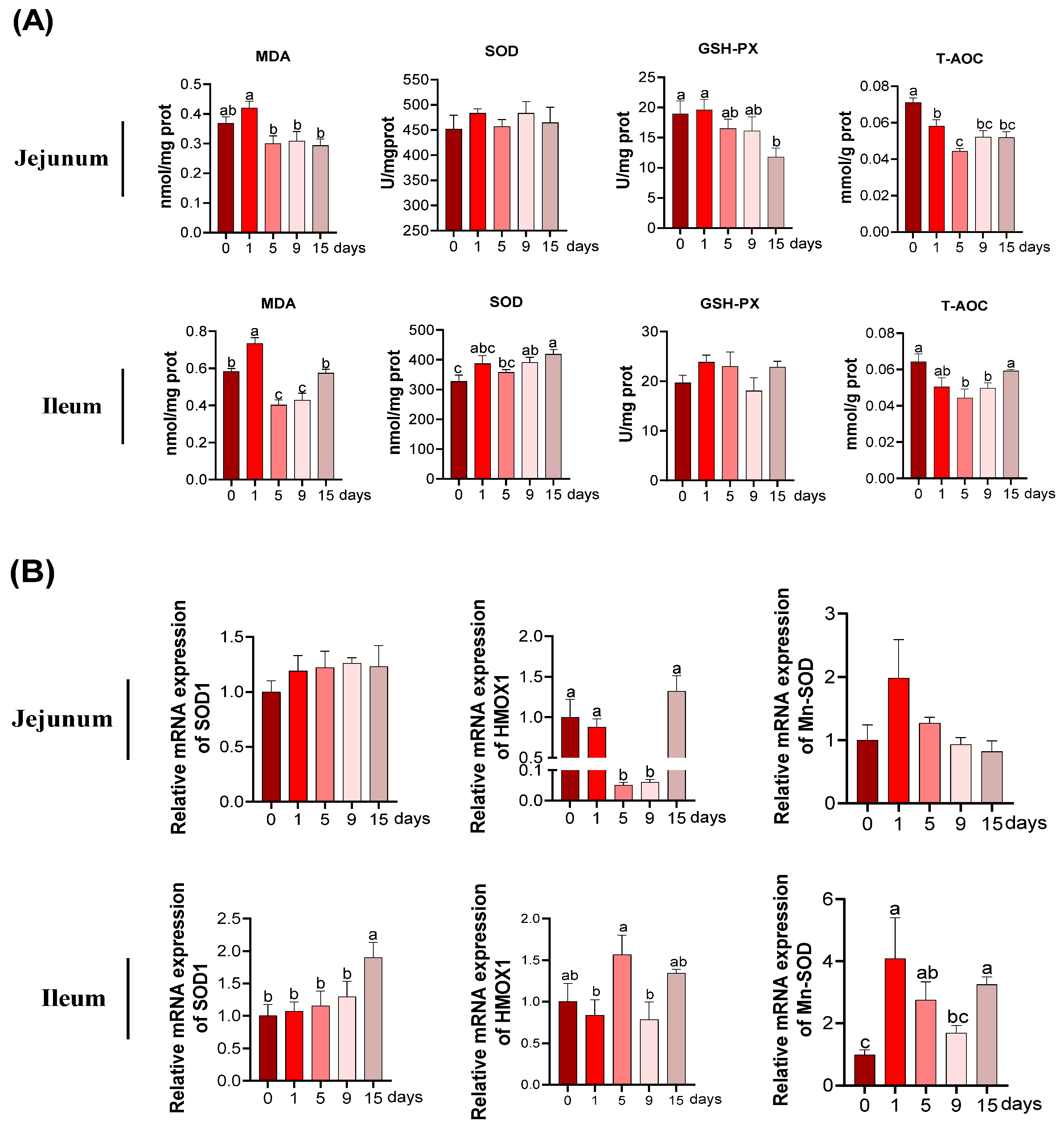
2.5. Redox Parameters Detection
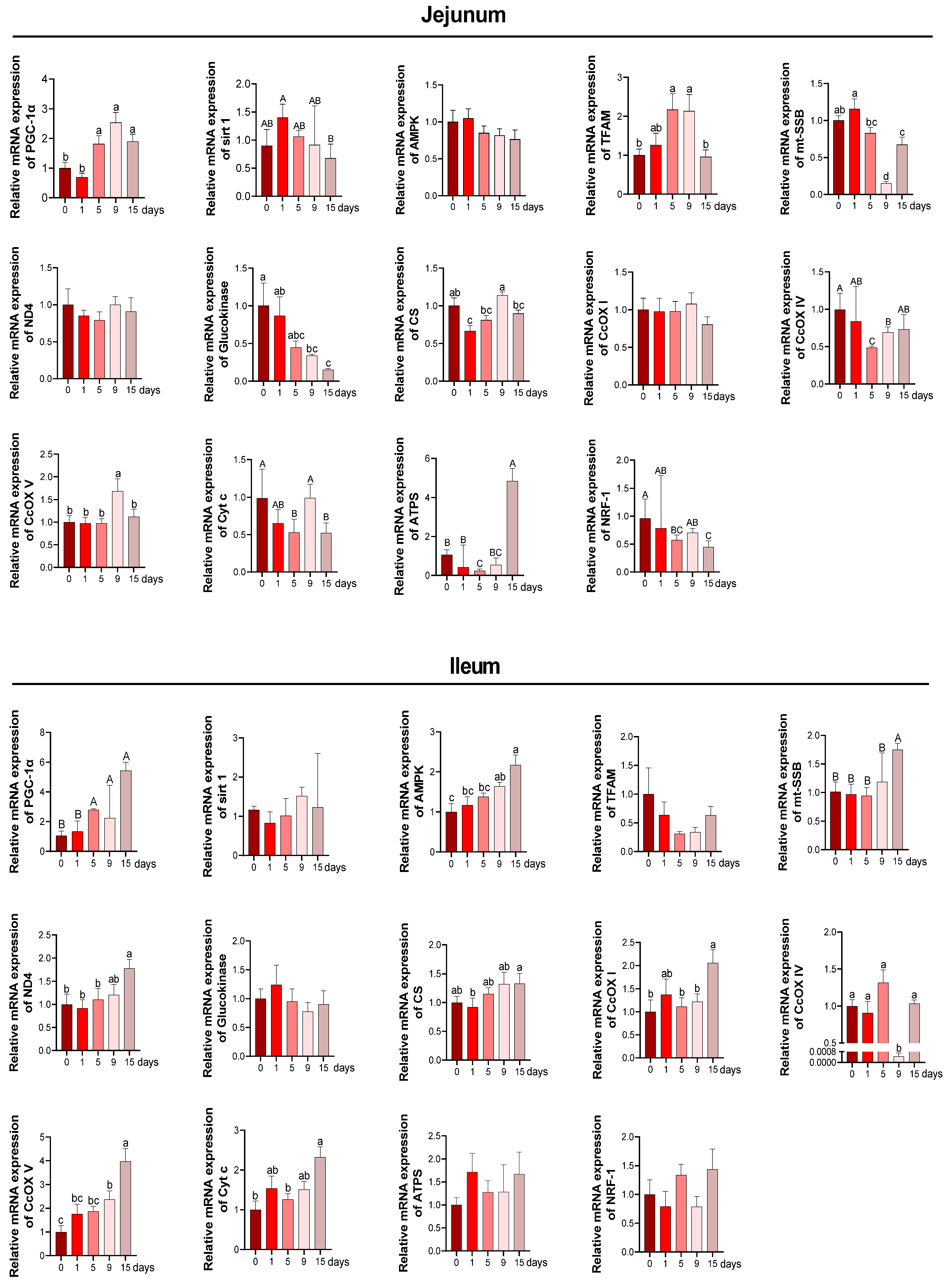
2.6. Quantitative Real-Time PCR
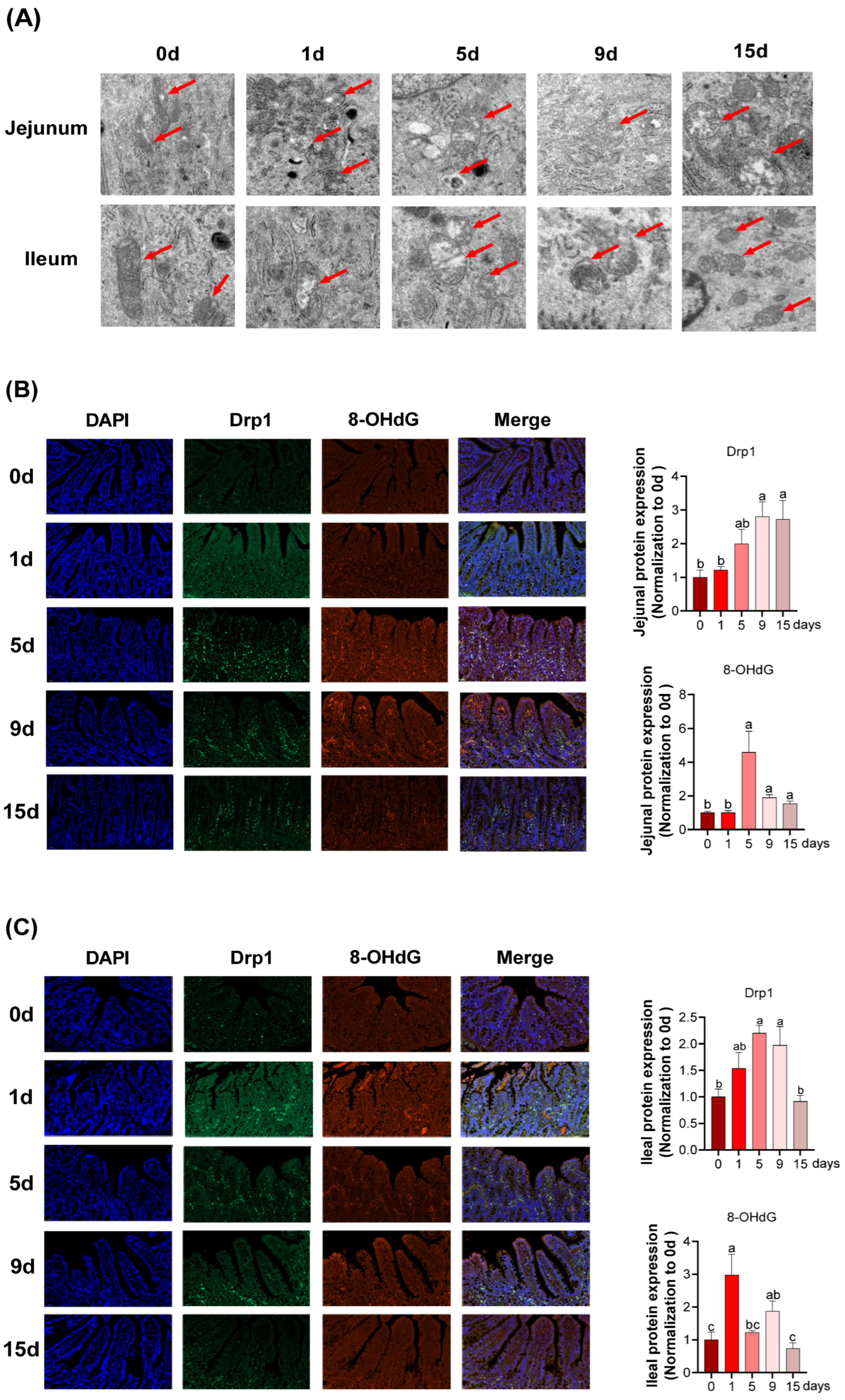
2.7. Immunofluorescence
2.8. Mitochondrial Ultrastructure
2.9. Statistical Analysis
3. Results
3.1. The Intestinal Morphology and Function under LPS-Induced Chronic Immune Stress
3.2. Intestinal Epithelial Inflammation under LPS-Induced Chronic Immune Stress
3.3. The MDA and Anti-Oxidative Parameters under LPS-Induced Chronic Immune Stress
3.4. Intestinal Mitochondria Function and DNA Damage under LPS-Induced Chronic Immune Stress
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabler, N.K.; Spurlock, M.E. Integrating the immune system with the regulation of growth and efficiency. J. Anim. Sci. 2008, 86, E64–E74. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, S.; Liu, Q.; Wang, X.; Shan, T.; Wang, Y. Cathelicidin-WA attenuates LPS-induced inflammation and redox imbalance through activation of AMPK signaling. Free Radic. Biol. Med. 2018, 129, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kai, L.; Zhu, L.; Xu, B.; Chen, N.; Valencak, T.G.; Wang, Y.; Shan, T. Cathelicidin-WA protects against LPS-induced gut damage through enhancing survival and function of intestinal stem cells. Front. Cell Dev. Biol. 2021, 9, 685363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Shi, H.; Wang, X.; Zhu, H.; Pi, D.; Leng, W.; Li, S. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-kappaB and p38 signaling in weaned pigs after LPS challenge. Eur. J. Nutr. 2017, 56, 1433–1443. [Google Scholar] [CrossRef]
- Song, M.; Ye, J.; Zhang, F.; Su, H.; Yang, X.; He, H.; Liu, F.; Zhu, X.; Wang, L.; Gao, P.; et al. Chenodeoxycholic acid (CDCA) protects against the LPS-induced impairment of intestinal epithelial barrier function via FXR-MLCK pathway. J. Agric. Food Chem. 2019, 67, 8868–8874. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Xiao, D.; Tan, B.; Xiao, H.; Wang, J.; Yin, J.; Duan, J.; Huang, R.; Yang, C.; Yin, Y. Chitosan oligosaccharide reduces intestinal inflammation that involves calcium-sensing receptor (CaSR) activation in lipopolysaccharide (LPS)-challenged piglets. J. Agric. Food Chem. 2016, 64, 245–252. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, Q.; Wang, C.; Wu, H.; Jiao, L.; Hong, Q.; Hu, C. LPS challenge increased intestinal permeability, disrupted mitochondrial function and triggered mitophagy of piglets. Innate Immun. 2018, 24, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Song, G.; Shen, H.; Wu, Y.; Zhao, C.; Zhang, Z.; Jiang, Q.; Li, X.; Ma, X.; Tan, B.; et al. Allicin improves intestinal epithelial barrier function and prevents LPS-induced barrier damages of intestinal epithelial cell monolayers. Front. Immunol. 2022, 13, 847861. [Google Scholar] [CrossRef]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef]
- Blachier, F.; Boutry, C.; Bos, C.; Tomé, D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009, 90, 814s–821s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, E.; Moschetta, A.; Haller, D. Mitochondrial function—Gatekeeper of intestinal epithelial cell homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wu, M.M.; Xiao, H.; Ren, W.K.; Duan, J.L.; Yang, G.; Li, T.J.; Yin, Y.L. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 2014, 92, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wu, H.; Wang, C.; Zhang, Q.; Jiao, L.; Lin, F.; Hu, C.H. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets. J. Anim. Sci. 2018, 96, 1795–1805. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Cao, S.; Xiao, H.; Li, X.; Zhu, J.; Gao, J.; Wang, L.; Hu, C. AMPK-PINK1/Parkin Mediated Mitophagy Is Necessary for Alleviating Oxidative Stress-Induced Intestinal Epithelial Barrier Damage and Mitochondrial Energy Metabolism Dysfunction in IPEC-J2. Antioxidants 2021, 10, 2010. [Google Scholar] [CrossRef]
- Novais, A.K.; Deschêne, K.; Martel-Kennes, Y.; Roy, C.; Laforest, J.P.; Lessard, M.; Matte, J.J.; Lapointe, J. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS ONE 2021, 16, e0247188. [Google Scholar] [CrossRef]
- Kim, J.A.; Wei, Y.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Choksi, K.B.; Boylston, W.H.; Rabek, J.P.; Widger, W.R.; Papaconstantinou, J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim. Biophys. Acta 2004, 1688, 95–101. [Google Scholar] [CrossRef]
- Minelli, A.; Bellezza, I.; Conte, C.; Culig, Z. Oxidative stress-related aging: A role for prostate cancer? Biochim. Biophys. Acta 2009, 1795, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Escribano, D.; Campos, P.H.; Gutiérrez, A.M.; Le Floc’h, N.; Cerón, J.J.; Merlot, E. Effect of repeated administration of lipopolysaccharide on inflammatory and stress markers in saliva of growing pigs. Vet. J. 2014, 200, 393–397. [Google Scholar] [CrossRef]
- Rakhshandeh, A.; de Lange, C.F. Evaluation of chronic immune system stimulation models in growing pigs. Animal 2012, 6, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Zheng, C.; Zhong, Y.; Song, B.; Yan, Z.; Kong, X.; Deng, J.; Li, F.; Yin, Y. Beta-hydroxy beta-methyl butyrate decreases muscle protein degradation via increased Akt/FoxO3a signaling and mitochondrial biogenesis in weanling piglets after lipopolysaccharide challenge. Food Funct. 2019, 10, 5152–5165. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Wu, H.; Zhu, H.; Liu, C.; Hou, Y.; Dai, B.; Liu, X.; Liu, Y. Glycine Relieves Intestinal Injury by Maintaining mTOR Signaling and Suppressing AMPK, TLR4, and NOD Signaling in Weaned Piglets after Lipopolysaccharide Challenge. Int. J. Mol. Sci. 2018, 19, 1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academic Press: Washington, DC, USA, 2012. [Google Scholar]
- Wang, C.; Wei, S.; Liu, B.; Wang, F.; Lu, Z.; Jin, M.; Wang, Y. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 2022, 14, 2057779. [Google Scholar] [CrossRef]
- Duan, Y.; Duan, Y.; Li, F.; Li, Y.; Guo, Q.; Ji, Y.; Tan, B.; Li, T.; Yin, Y. Effects of supplementation with branched-chain amino acids to low-protein diets on expression of genes related to lipid metabolism in skeletal muscle of growing pigs. Amino Acids 2016, 48, 2131–2144. [Google Scholar] [CrossRef]
- Qi, M.; Liao, S.; Wang, J.; Deng, Y.; Zha, A.; Shao, Y.; Cui, Z.; Song, T.; Tang, Y.; Tan, B.; et al. MyD88 deficiency ameliorates weight loss caused by intestinal oxidative injury in an autophagy-dependent mechanism. J. Cachexia Sarcopenia Muscle 2022, 13, 677–695. [Google Scholar] [CrossRef]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.; Blikslager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef]
- Talbot, J.; Hahn, P.; Kroehling, L.; Nguyen, H.; Li, D.; Littman, D.R. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 2020, 579, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, S.; Zhang, Q.; Shen, Z.; Feng, J.; Hong, Q.; Lu, J.; Xie, F.; Peng, Y.; Hu, C. Dietary Tributyrin Attenuates Intestinal Inflammation, Enhances Mitochondrial Function, and Induces Mitophagy in Piglets Challenged with Diquat. J. Agric. Food Chem. 2019, 67, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wu, Y.; Jiang, Z.; Zheng, C.; Wang, L.; Yang, X.; Ma, X.; Gao, K.; Hu, Y. Dietary soy isoflavone attenuated growth performance and intestinal barrier functions in weaned piglets challenged with lipopolysaccharide. Int. Immunopharmacol. 2015, 28, 288–294. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Berg, R.D. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995, 3, 149–154. [Google Scholar] [CrossRef]
- Lambert, G.P. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J. Anim. Sci. 2009, 87, E101–E108. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.J.; Zhou, Y.M.; Wu, Y.N.; Zhang, L.L.; Wang, T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet. Immunol. Immunopathol. 2013, 153, 70–76. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Gao, C.; Zhang, K.; Zheng, F.; Kang, J. Evaluation of Fecal Calprotectin, D-Lactic Acid and Bedside Gastrointestinal Ultrasound Image Data for the Prediction of Acute Gastrointestinal Injury in Sepsis Patients. Front. Med. Technol. 2021, 3, 733940. [Google Scholar] [CrossRef]
- Murray, M.J.; Barbose, J.J.; Cobb, C.F. Serum D(-)-lactate levels as a predictor of acute intestinal ischemia in a rat model. J. Surg. Res. 1993, 54, 507–509. [Google Scholar] [CrossRef]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriya, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA. Gastroenterology 2020, 159, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandeh, A.; Dekkers, J.C.; Kerr, B.J.; Weber, T.E.; English, J.; Gabler, N.K. Effect of immune system stimulation and divergent selection for residual feed intake on digestive capacity of the small intestine in growing pigs. J. Anim. Sci. 2012, 90 (Suppl. S4), 233–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montufar-Solis, D.; Garza, T.; Klein, J.R. T-cell activation in the intestinal mucosa. Immunol. Rev. 2007, 215, 189–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chetty, R.; Gatter, K. CD3: Structure, function, and role of immunostaining in clinical practice. J. Pathol. 1994, 173, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Ferenbach, D.; Hughes, J. Macrophages and dendritic cells: What is the difference? Kidney Int. 2008, 74, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [Green Version]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Penberthy, K.K.; Ravichandran, K.S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2016, 269, 44–59. [Google Scholar] [CrossRef] [Green Version]
- Belge, K.U.; Dayyani, F.; Horelt, A.; Siedlar, M.; Frankenberger, M.; Frankenberger, B.; Espevik, T.; Ziegler-Heitbrock, L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J. Immunol. 2002, 168, 3536–3542. [Google Scholar] [CrossRef]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning Is Associated with an Upregulation of Expression of Inflammatory Cytokines in the Intestine of Piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Klaunig, J.E.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharmacol. 2011, 254, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Karihtala, P.; Soini, Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. Apmis 2007, 115, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem. 2011, 11, 341–346. [Google Scholar] [CrossRef]
- Tu, H.K.; Pan, K.F.; Zhang, Y.; Li, W.Q.; Zhang, L.; Ma, J.L.; Li, J.Y.; You, W.C. Manganese superoxide dismutase polymorphism and risk of gastric lesions, and its effects on chemoprevention in a Chinese population. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 1089–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef] [PubMed]
- West, A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology 2017, 391, 54–63. [Google Scholar] [CrossRef]


| Items | Fresh Fish Oil |
|---|---|
| Ingredients, % | Contents, % |
| corn | 30.14 |
| Extruded corn | 30.00 |
| Soybean meal | 9.00 |
| Fish meal | 7.00 |
| Plasma protein powder | 5.00 |
| Whey powder | 9.00 |
| Glucose | 3.00 |
| Soybean oil | 3.80 |
| Limestone | 1.05 |
| Choline chloride | 0.10 |
| Antioxidants | 0.05 |
| Citric acid | 0.50 |
| Salt | 0.10 |
| Vitamin premix a | 0.30 |
| Mineral premix b | 0.15 |
| Lys 98% | 0.45 |
| DL-Met | 0.20 |
| L-Thr | 0.14 |
| L-Trp | 0.02 |
| Total | 100.00 |
| Nutrient content (%) | |
| DE (MJ/kg) | 14.18 |
| CP | 18.61 |
| Ca | 0.80 |
| Total P | 0.56 |
| Available P | 0.38 |
| Lys | 1.37 |
| Met + Cys | 0.75 |
| Thr | 0.81 |
| Trp | 0.22 |
| Gene | Sequence | Size (bp) | GeneBank No. |
|---|---|---|---|
| ZO1 | F:ATCTCGGAAAAGTGCCAGGA R: CCCCTCAGAAACCCATACCA | 172 | XM_003480423.3 |
| OCLN | F:CATGGCTGCCTTCTGCTTCATTGC R:ACCATCACACCCAGGATAGCACTCA | 129 | NM_001163647.2 |
| CLDN1 | F:TATGACCCCATGACCCCAGT R:GCAGCAAAGTAGGGCACCTC | 108 | NM_001244539.1 |
| SOD1 | F: GGTCCTCACTTCAATCCTGAATCC R: CACACCATCTTTGCCAGCAGT | 102 | NM_0011190422 |
| HMOX1 | F: CGCTCCCGAATGAACACTCT R: GCGAGGGTCTCTGGTCCTTA | 148 | NM_001004027.1 |
| Mn-SOD | F: GGACAAATCTGAGCCCTAACG R: CCTTGTTGAAACCGAGCC | 159 | NM_214127 |
| IL-4 | F: GCTGCCCCAGAGAACACGAC R: AGGTTCCTGTCAAGTCCGCTC | 119 | NM_214123.1 |
| TNF-α | F: AACCTCAGATAAGCCCGTCG R: ACCACCAGCTGGTTGTCTTT | 129 | NM_214022.1 |
| NF-κB | F: GACAACATCTCCTTGGCGGG R: TCTGCTCCTGCTGCTTTGAGG | 146 | NM_001048232 |
| PGC-1α | F: CCCGAAACAGTAGCAGAGACAAG R:CTGGGGTCAGAGGAAGAGATAAAG | 111 | NM 213963 |
| SIRT1 | F: TGACTGTGAAGCTGTACGAGGAG R: TGGCTCTATGAAACTGCTCTGG | 143 | EU030283.2 |
| AMPK | F:ACCAGGACCCTTTGGCAGTT R:GAATCAGGTGGGCTTGTTGC | 100 | NM_001167633.1 |
| TFAM | F: GGTCCATCACAGGTAAAGCTGAA R: ATAAGATCGTTTCGCCCAACTTC | 167 | AY923074.1 |
| mt-SSB | F: CTTTGAGGTAGTGCTGTGTCG R: CTCACCCCTGACGATGAAGAC | 143 | AK352341.1 |
| ND4 | F: TTATTGGTGCCGGAGGTACTG R: CCCAGTTTATTCCAGGGTTCTG | 112 | NM 001097468 |
| Glucokinase | F:CTTTTCCCTCCCACACTGCTAT R: GACTCCTCTTCCTGAGACCCTCT | 119 | AK233298.1 |
| CS | F: CCTTTCAGACCCCTACTTGTCCT R: CACATCTTTGCCGACTTCCTTC | 127 | M21197.1 |
| CcOX I | F: ATTATCCTGACGCATACACAGCA R: GCAGATACTTCTCGTTTTGATGC | 127 | AJ950517.1 |
| CcOX IV | F: CCAAGTGGGACTACGACAAGAAC R: CCTGCTCGTTTATTAGCACTGG | 131 | AK233334.1 |
| CcOX V Cyt c | F: ATCTGGAGGTGGTGTTCCTACTG R: GTTGGTGATGGAGGGGACTAAA F: TAGAAAAGGGAGGCAAACACAAG R: GGATTCTCCAGGTACTCCATCAG | 160 154 | AY786556.1 NM 001129970.1 |
| ATPS | F: TGTCCTCCTCCCTATCACACATT R: TAGTGGTTATGACGTTGGCTTGA | 116 | AK230503 |
| NRF-1 | F: GCCAGTGAGATGAAGAGAAACG R: CTACAGCAGGGACCAAAGTTCAC | 166 | AK237171.1 |
| β-actin | F: TCTTTTCCAGCCTTCCTTCTTG R: GAGGTCTTTACGGATGTCAACG | 100 | NM_007393 |
| Items | LPS Treatments | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 d | 1 d | 5 d | 9 d | 15 d | |||
| DAO (mmol/L) | 1.12 ab | 1.86 a | 1.92 a | 1.94 a | 0.74 b | 0.17 | <0.05 |
| LACT (mmol/L) | 6.19 c | 12.49 a | 8.19 b | 8.03 b | 7.43 bc | 0.04 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zheng, C.; Zheng, J.; Duan, G.; Guo, Q.; Zhang, P.; Wan, M.; Duan, Y. Development of Intestinal Injury and Restoration of Weaned Piglets under Chronic Immune Stress. Antioxidants 2022, 11, 2215. https://doi.org/10.3390/antiox11112215
Yu J, Zheng C, Zheng J, Duan G, Guo Q, Zhang P, Wan M, Duan Y. Development of Intestinal Injury and Restoration of Weaned Piglets under Chronic Immune Stress. Antioxidants. 2022; 11(11):2215. https://doi.org/10.3390/antiox11112215
Chicago/Turabian StyleYu, Jiayi, Changbing Zheng, Jie Zheng, Geyan Duan, Qiuping Guo, Peiwen Zhang, Mengliao Wan, and Yehui Duan. 2022. "Development of Intestinal Injury and Restoration of Weaned Piglets under Chronic Immune Stress" Antioxidants 11, no. 11: 2215. https://doi.org/10.3390/antiox11112215
APA StyleYu, J., Zheng, C., Zheng, J., Duan, G., Guo, Q., Zhang, P., Wan, M., & Duan, Y. (2022). Development of Intestinal Injury and Restoration of Weaned Piglets under Chronic Immune Stress. Antioxidants, 11(11), 2215. https://doi.org/10.3390/antiox11112215






