Dimethylthiourea Alleviates Drought Stress by Suppressing Hydrogen Peroxide-Dependent Abscisic Acid-Mediated Oxidative Responses in an Antagonistic Interaction with Salicylic Acid in Brassica napus Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Treatment
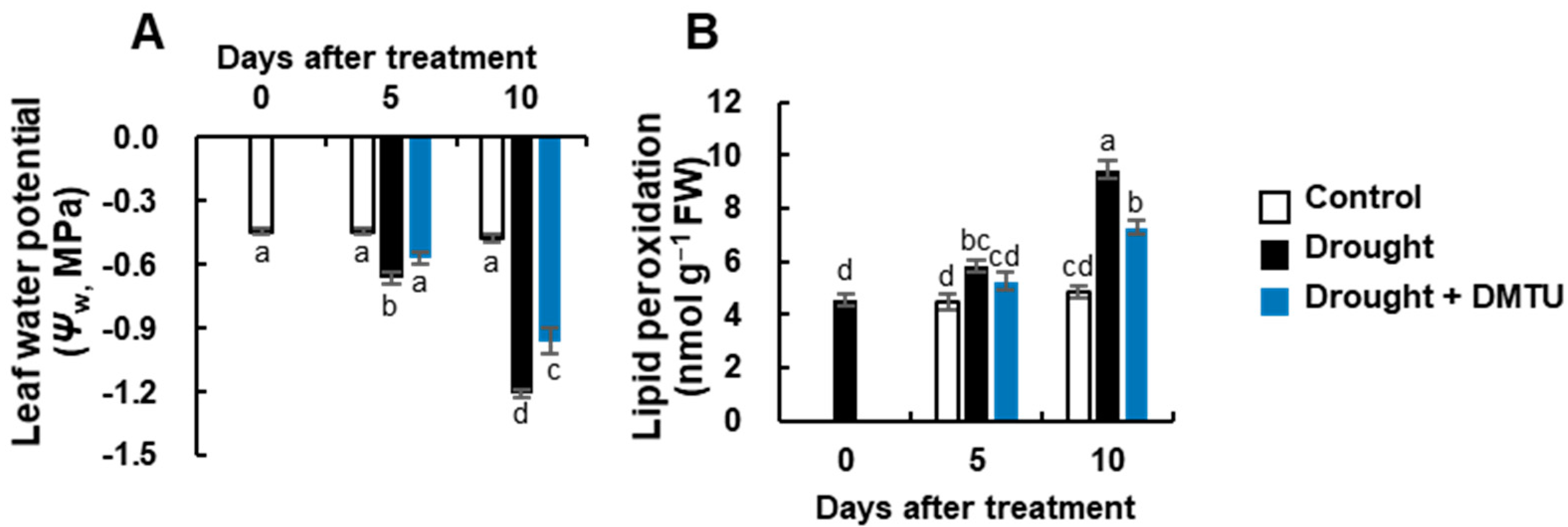
2.2. Measurement of Leaf Water Potential (Ψw)
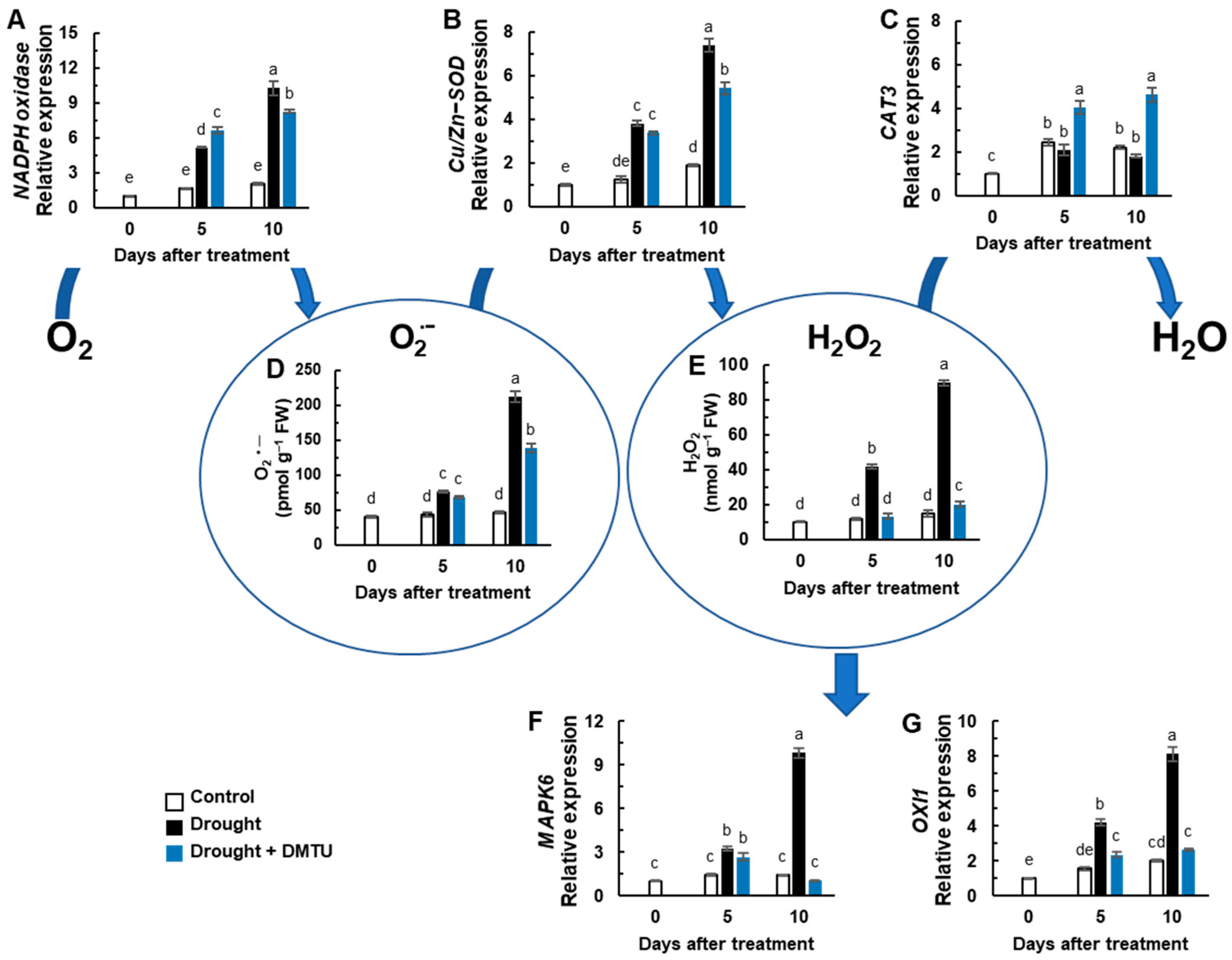
2.3. ROS and MDA Concentrations
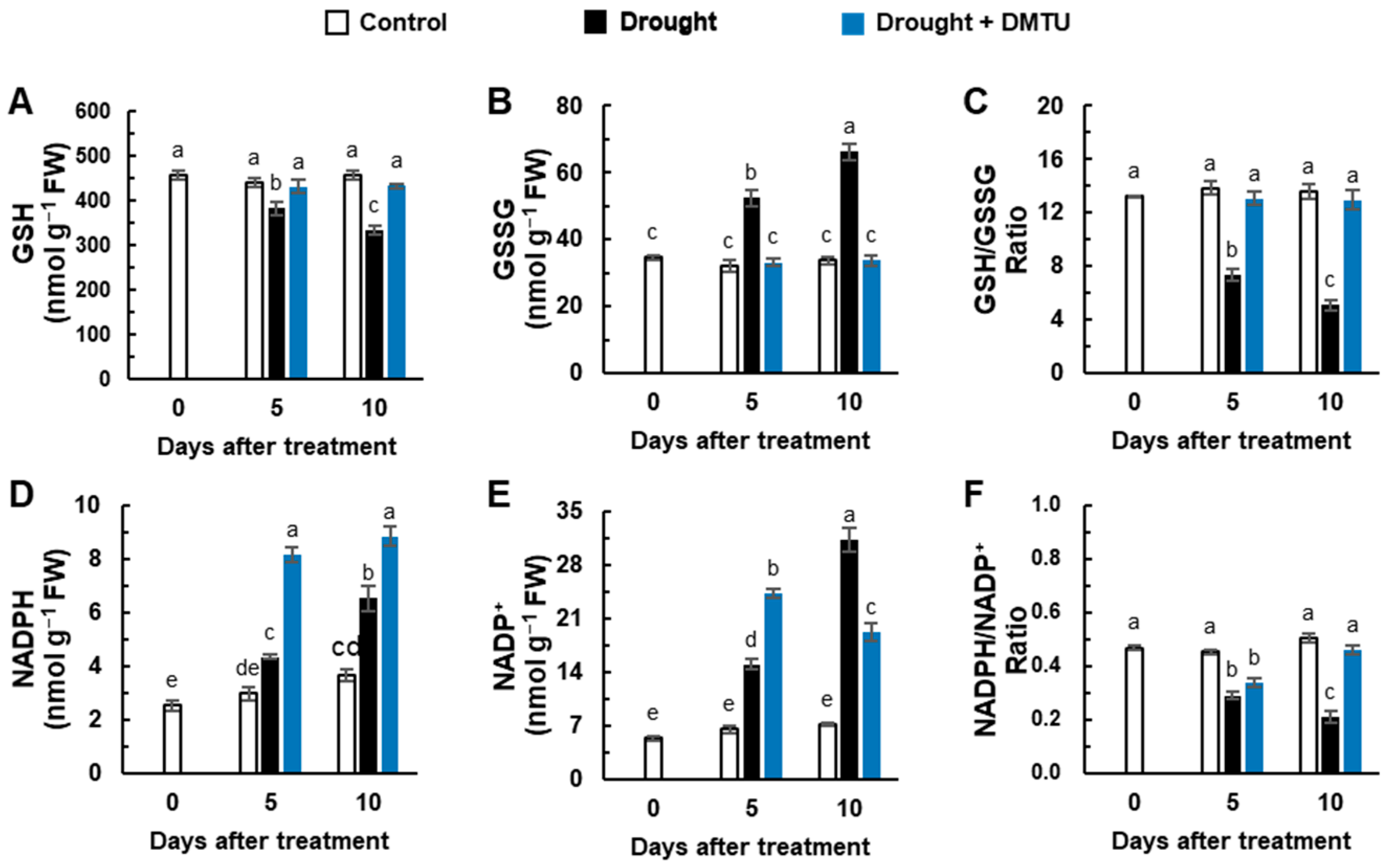
2.4. Redox Status Analysis
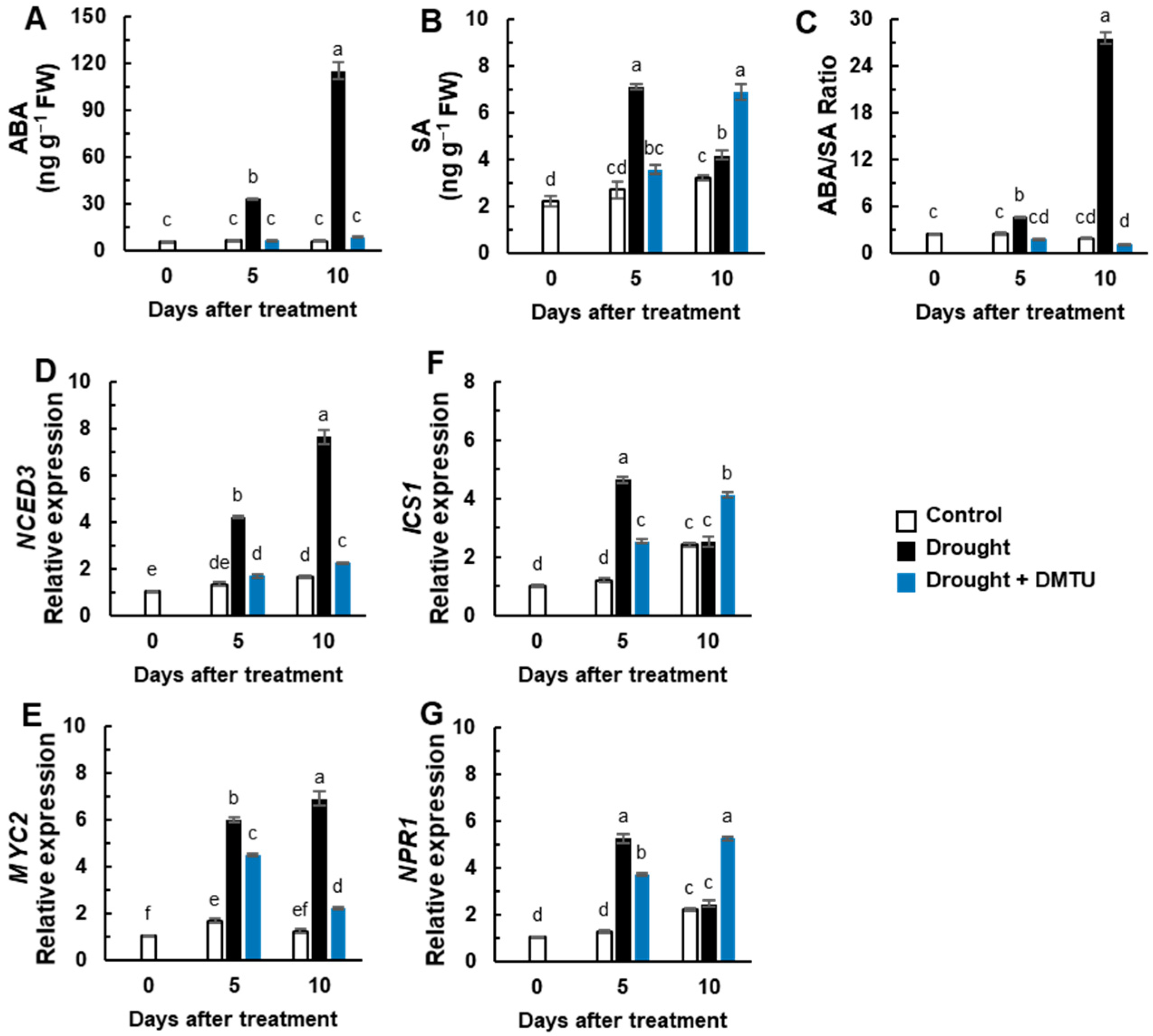
2.5. Phytohormone Analysis
2.6. Isolation of Total RNA and RT-qPCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Leaf Water Potential and Lipid Peroxidation Level
3.2. ROS Status and ROS Signaling Genes Expression
3.3. Endogenous ABA and SA Status, ABA- and SA-Synthesis, and Signaling Genes Expression
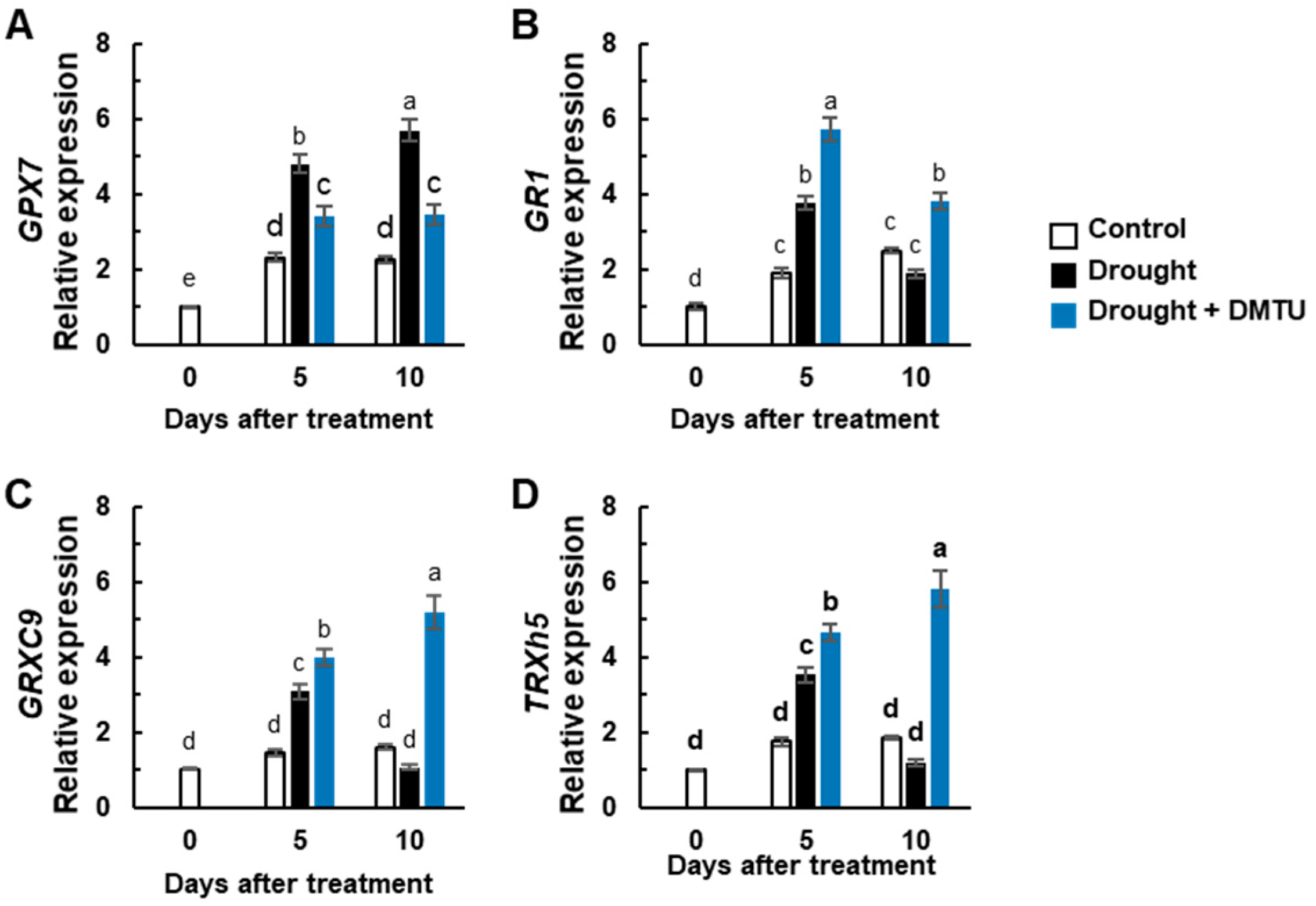
3.4. Redox Changes and the Expression of Redox Signaling Genes
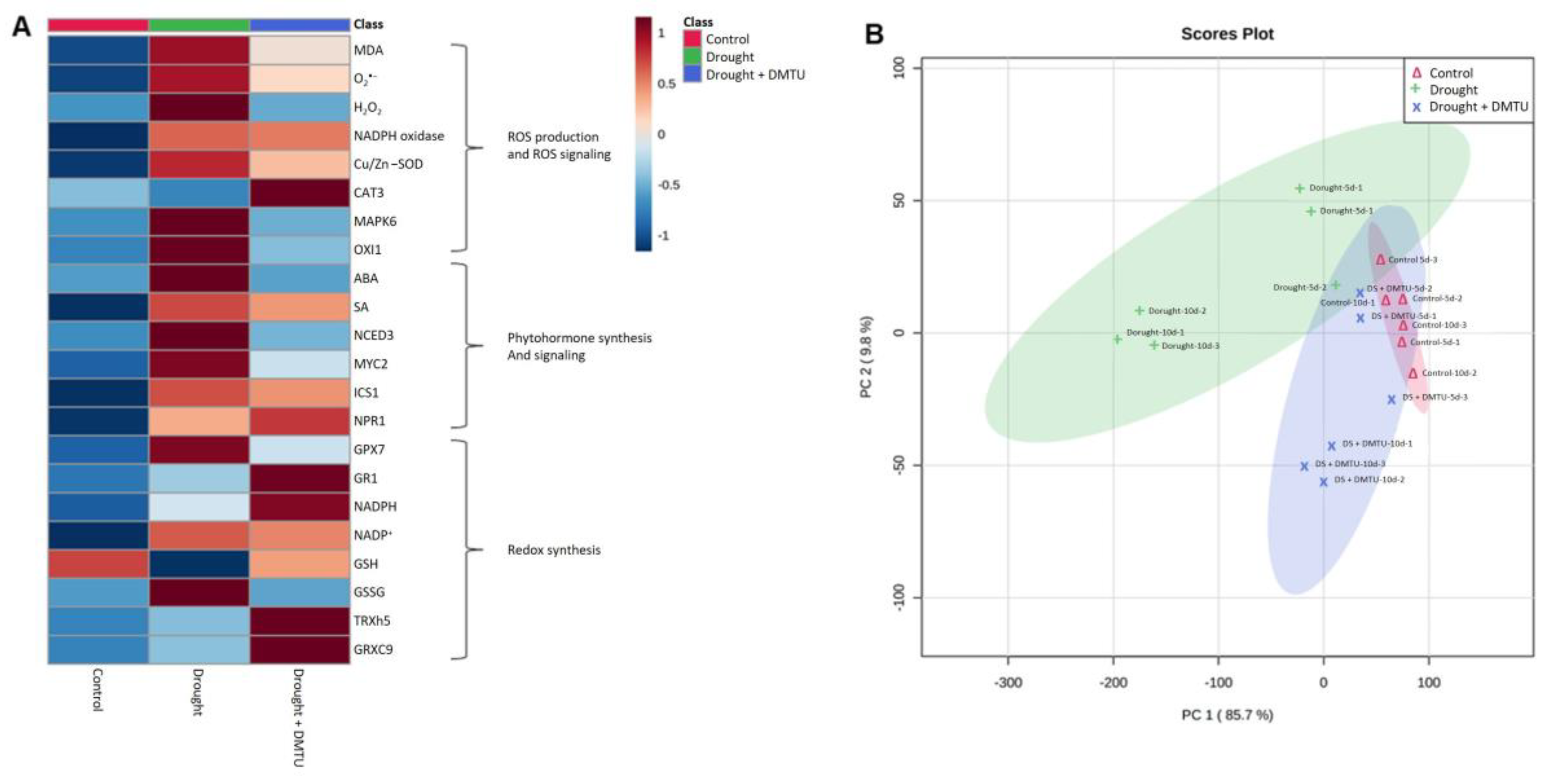
3.5. Heatmap Analysis and PCA of the Targeted ROS System, Redox Signaling, and Hormone Metabolism
3.6. Correlations among Treatment-Responses of Physiological Parameters
4. Discussion
4.1. DMTU-Mediated H2O2-Responsive Antioxidant System and ROS Signaling
4.2. DMTU-Mediated Changes in ROS-Hormonal Interaction
4.3. DMTU-Mediated Hormonal Regulation of NADPH- and GSH-Based Redox
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, B.R.; Zaman, R.; Avice, J.C.; Ourry, A.; Kim, T.H. Sulfur use efficiency is a significant determinant of drought stress tolerance in relation to photosynthetic activity in Brassica napus cultivars. Front. Plant Sci. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Lee, B.R.; La, V.H.; Mamun, M.A.; Bae, D.W.; Kim, T.H. Characterization of salicylic acid- and abscisic acid-mediated photosynthesis, Ca2+ and H2O2 accumulation in two distinct phases of drought stress intensity in Brassica napus. Environ. Exp. Bot. 2021, 186, 104434. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant. Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Redox signaling in plants. Antioxid. Redox Signal. 2013, 18, 2087–2090. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Lee, B.R.; Li, L.S.; Jung, W.J.; Jin, Y.L.; Avice, J.C.; Qurry, A.; Kim, T.H. Water deficit-induced oxidative stress and the activation of antioxidant enzymes in white clover leaves. Biol. Plant. 2009, 53, 505–510. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signaling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Huang, S.; VanAken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress responses in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, N.H.M.B.; Zulfiqar, F.; Raza, A.L.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Singh, J.; Achary, V.M.M.; Reddy, M.K. Redox homeostasis via gene families of ascorbate-glutathione pathway. Front. Plant Sci. 2015, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Rentel, M.C.; Lecourieux, D.; Ouaked, F.; Usher, S.L.; Petersen, L.; Okamoto, H.; Knight, H.; Peck, S.C.; Grierson, C.S.; Hirt, H.; et al. OXI1 kinase is necessary for oxidative burst-mediated signaling in Arabidopsis. Nature 2004, 427, 858–861. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F.V. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.R.; Islam, M.T.; Park, S.H.; Jung, H.I.; Bae, D.W.; Kim, T.H. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Lee, B.R.; La, V.H.; Park, S.H.; Mamun, M.A.; Bae, D.W.; Kim, T.H. H2O2-responsive hormonal status involves oxidative burst signaling and proline metabolism in Rapeseed leaves. Antioxidants 2022, 11, 566. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhou, Y.H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [Green Version]
- Hieno, A.; Naznin, H.A.; Inaba-Hasegawa, K.; Yokogawa, T.; Hayami, N.; Nomoto, M.; Tada, Y.; Yokogawa, T.; Higuchi-Takeuchi, M.; Hanada, K.; et al. Transcriptome analysis and identification of a transcriptional regulatory network in the response to H2O. Plant Physiol. 2019, 180, 1629–1646. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta 2015, 1847, 993–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, X.; Cai, C.; Fan, S.; Wang, L.; Zeng, X. Spatial and temporal calcium signaling and its physiological effects in Moso Bamboo under drought stress. Forests 2019, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.G.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, M.; Zhang, A.; Lu, J. Abscisic acid-induced apoplastic H2O2 accumulation up-regulates the activities of chloroplastic and cytosolic antioxidant enzymes in maize leaves. Planta 2005, 223, 57–68. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, A.; Zhang, J.; Jiang, M. Abscisic acid is a key inducer of hydrogen peroxide production in leaves of maize plants exposed to water stress. J. Exp. Bot. 2006, 47, 1484–1495. [Google Scholar] [CrossRef] [Green Version]
- Verslues, P.E.; Kim, Y.S.; Zhu, J.K. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate:glyoxylate aminotransferase mutant. Plant Mol. Biol. 2007, 64, 205–217. [Google Scholar] [CrossRef]
- Sierla, M.; Waszczak, C.; Vahisalu, T.; Kangasjarvi, J. Reactive oxygen species in the regulation of stomatal movements. Plant Physiol. 2016, 170, 1569–1580. [Google Scholar] [CrossRef] [Green Version]
- Seyfferth, C.; Tsuda, K. Salicylic acid signal transduction: The initiation of biosynthesis, perception and transcription reprogramming. Front. Plant Sci. 2014, 5, 697. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Vasquez, A.; Salinas, P.; Holuique, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef]
- De Agazio, M.; Zacchini, M. Dimethylurea, a hydrogen peroxide trap, partially prevents stress effects and ascorbate peroxidase increase in spermidine-treated maize roots. Plant Cell Environ. 2001, 24, 237–244. [Google Scholar]
- Zong, X.J.; Li, D.P.; Gu, L.K.; Li, D.Q.; Liu, L.X.; Hu, X.L. Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 2009, 229, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Yadu, B.; Chandrakar, V.; Tamboli, R.; Keshavkant, S. Dimethylthiourea antagonizes oxidative responses by up-regulating expressions of pyrroline-5-carboxylate synthetase and antioxidant genes under arsenic stress. Int. J. Environ. Sci. Technol. 2019, 16, 8401–8410. [Google Scholar] [CrossRef]
- Lee, B.R.; Muneer, S.; Park, S.H.; Zhang, Q.; Kim, T.H. Ammonium-induced proline and sucrose accumulation, and their significance in antioxidative activity and osmotic adjustment. Acta Physiol. Planta 2013, 35, 2655–2664. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.R.; Islam, M.T.; Mamun, M.A.; Park, S.H.; Bae, D.W.; Kim, T.H. Characterization of glutamate-mediated hormonal regulatory pathway of the drought responses in relation to proline metabolism in Brassica napus L. Plants 2020, 9, 512. [Google Scholar] [CrossRef]
- Islam, M.T.; Mamun, M.A.; Lee, B.R.; La, V.H.; Jung, W.J.; Bae, D.W.; Kim, T.H. Role of salicylic acid signaling in the biotrophy-necrotrophy transition of Xanthomonas campestris pv. Campestris infection in Brassica napus. Physiol. Mol. Plant Pathol. 2021, 113, 101578. [Google Scholar] [CrossRef]
- Mignolet-Spruyt, L.; Xu, E.; Idanheimo, N.; Hoeberichts, F.A.; Muhlenbock, P.; Brosche, M.; Breusegem, F.V.; Kangasjarvi, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef] [Green Version]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef]
- Jiménez-Quesada, M.J.; Traveso, J.A.; Alche, J. de Diso. NADPH oxidase-dependent superoxide production in plant reproductive tissues. Front. Plant Sci. 2016, 7, 359. [Google Scholar] [CrossRef] [Green Version]
- Asai, S.; Ohta, K.; Yoshioka, H.; Notes, A. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 2008, 20, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zhang, Y.; Jiang, M.-Y. Alternative splicing and differential expression of two transcripts of nicotine adenine dinucleotide phosphate oxidase B gene from Zea mays. J. Integr. Plant Biol. 2009, 51, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Chen, W.H.; Jia, W.; Zhang, J. Mitogen-activated protein kinase kinase 5 (MKK5)-mediated signalling cascade regulates expression of iron superoxide dismutase gene in Arabidopsis under salinity stress. J. Exp. Bot. 2015, 66, 5971–5981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howden, A.J.; Salek, M.; Miguet, L.; Pullen, M.; Thomas, B.; Knight, M.R.; Sweetlove, L.J. The phosphoproteome of Arabidopsis plants lacking the oxidative signal-inducible1 (OXI1) protein kinase. New Phytol. 2011, 190, 49–56. [Google Scholar] [CrossRef]
- Zhang, A.; Jiang, M.; Zhang, J.; Tan, M.; Hu, X. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol. 2006, 141, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Talukda, D. Exogenous thiourea modulates antioxidant defense and glyoxalase systems in lentil genotypes under arsenic stress. J. Plant Stress Physiol. 2016, 2, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Luo, Z.; Dong, H.; Eneji, A.E.; Li, W. H2O2 and ABA signaling are responsible for the increased Na+ efflux and water uptake in Gossypium hirsutum L. roots in the non-saline side under non-uniform root zone salinity. J. Exp. Bot. 2016, 67, 2247–2261. [Google Scholar] [CrossRef] [Green Version]
- La, V.H.; Lee, B.R.; Islam, M.T.; Park, S.H.; Lee, H.; Bae, D.W.; Kim, T.H. Antagonistic shifting from abscisic acid- to salicylic acid-mediated sucrose accumulation contributes to drought tolerance in Brassica napus. Environ. Exp. Bot. 2019, 162, 38–47. [Google Scholar] [CrossRef]
- Anjum, N.A.; Aref, I.M.; Duarte, A.C.; Pereira, E.; Ahamd, I.; Iqbal, M. Glutathione and proline can coordinately make plant withstand the joint attack of metal(loid) and salinity stress. Front. Plant Sci. 2014, 5, 6621. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.R.; Kim, K.Y.; Jung, W.J.; Avice, J.C.; Ourry, A.; Kim, T.H. Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.). J. Exp. Bot. 2007, 58, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.J. The integration of glutathione homeostasis and redox signaling. J. Plant Physiol. 2008, 165, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N.; Malagoli, M.; Wirtz, M.; Hell, R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biol. 2016, 16, 247. [Google Scholar] [CrossRef] [Green Version]
- Herrera, K.; Cahoon, R.E.; Kumaran, S.; Jez, J. Reaction mechanism of glutathione synthetase from Arabidopsis thaliana: Site-directed mutagenesis of active site residues. J. Biol. Chem. 2007, 282, 17157–17165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittova, V.; Tal, M.; Volokita, M.; Guy, M. Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Envrion 2003, 26, 845–856. [Google Scholar] [CrossRef]
- Hou, P.; Wang, F.; Luo, B.; Li, A.; Wang, C.H.; Shabala, L.; Ahmed, H.A.I.; Deng, S.; Zhang, H.; Song, P.; et al. Antioxidant enzymatic activity and osmotic adjustment as components of the drought tolerance mechanism in Carex duriuscula. Plants 2021, 10, 436. [Google Scholar] [CrossRef]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.C.; Chen, J.; Miao, C.; Song, C.P. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Li, Z.; Gao, X.; Chinnusamy, V.; Bressan, R.; Wang, Z.X.; Zhu, J.K.; Wu, J.W.; Liu, D. ROP11 GTPase negatively regulates ABA signaling by protecting ABI1 phosphatase activity from inhibition by the ABA receptor RCAR1/PYL9 in Arabidopsis. J. Integr. Plant Biol. 2012, 54, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Milla, M.A.R.; Maurer, A.; Huete, A.R.; Gustafson, J.P. Glutathione peroxidase genes in Arabidopsis are ubiquitous and regulated by abiotic stresses through diverse signalling pathways. Plant J. 2003, 36, 602–615. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.; Ye, S.; Jiang, L.; Liu, S. Genome-wide identification of glutathione peroxidase (GPX) gene family and their response to abiotic stress in cucumber. 3 Biotech. 2018, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Gansemer, E.R.; McCommis, K.S.; Martino, M.; King-McAlpin, A.Q.; Potthoff, M.J.; Finck, B.N.; Taylor, E.B.; Rutkowski1, D.T. NADPH and Glutathione Redox Link TCA Cycle Activity to Endoplasmic Reticulum Homeostasis. iScience 2020, 23, 101116. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 2013, 18, 2106–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, G.; Queval, G.; Gakiere, B. NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J. Exp. Bot. 2006, 57, 1603–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-R.; La, V.H.; Park, S.-H.; Mamun, M.A.; Bae, D.-W.; Kim, T.-H. Dimethylthiourea Alleviates Drought Stress by Suppressing Hydrogen Peroxide-Dependent Abscisic Acid-Mediated Oxidative Responses in an Antagonistic Interaction with Salicylic Acid in Brassica napus Leaves. Antioxidants 2022, 11, 2283. https://doi.org/10.3390/antiox11112283
Lee B-R, La VH, Park S-H, Mamun MA, Bae D-W, Kim T-H. Dimethylthiourea Alleviates Drought Stress by Suppressing Hydrogen Peroxide-Dependent Abscisic Acid-Mediated Oxidative Responses in an Antagonistic Interaction with Salicylic Acid in Brassica napus Leaves. Antioxidants. 2022; 11(11):2283. https://doi.org/10.3390/antiox11112283
Chicago/Turabian StyleLee, Bok-Rye, Van Hien La, Sang-Hyun Park, Md Al Mamun, Dong-Won Bae, and Tae-Hwan Kim. 2022. "Dimethylthiourea Alleviates Drought Stress by Suppressing Hydrogen Peroxide-Dependent Abscisic Acid-Mediated Oxidative Responses in an Antagonistic Interaction with Salicylic Acid in Brassica napus Leaves" Antioxidants 11, no. 11: 2283. https://doi.org/10.3390/antiox11112283
APA StyleLee, B.-R., La, V. H., Park, S.-H., Mamun, M. A., Bae, D.-W., & Kim, T.-H. (2022). Dimethylthiourea Alleviates Drought Stress by Suppressing Hydrogen Peroxide-Dependent Abscisic Acid-Mediated Oxidative Responses in an Antagonistic Interaction with Salicylic Acid in Brassica napus Leaves. Antioxidants, 11(11), 2283. https://doi.org/10.3390/antiox11112283







