Hot-Melt Extrusion Enhances Antioxidant Effects of Mulberry on Probiotics and Pathogenic Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Colloidal Solid Dispersion Systems by Hot-Melt-Extrudate (HME)
2.3. Total Flavonoid Content Analysis of MUL and HME-DDS (Hot-Melt Extrusion Drug Delivery System)
2.4. Total Phenol Content Analysis of HME-DDS
2.5. High Pressure Liquid Chromatography (HPLC) Analysis of Different MUL Formulations
2.6. Measurement of Particle Size, Zeta Size, and Polydispersity Index
2.7. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
2.8. SEM/TEM Instrumentation
2.9. In Vitro Release Study
2.10. Bacterial Strains and Growth Conditions
2.11. Confirmation of Growth Characteristics of Probiotics and Pathogenic Bacteria
2.12. Measurement of Antibacterial Activity against Pathogenic Bacteria of HME-DDS Preparation Extract of Mulberry
2.13. Comparison of Antibacterial Activity of MUL and HME-MUL-F2 with Probiotics
2.14. Proliferative Effect of Probiotics of HME-DDS Preparation Extract of Mulberry
2.15. Effect of Mulberry’s HME-DDS Formulation on pH Change of Probiotics Culture Medium
2.16. ACN Release Characteristics of the Added HME-MUL-F2 Formulation in Terms of the Growth of Probiotic Strains
2.17. Effect of HME-MUL-F2 Formulation on Antibacterial Activity of Probiotics
2.18. Statistical Analysis
3. Results
3.1. Total Flavonoids, Phenol Contents, and Anthocyanin Contents of MUL and HME-DDS
| Total Phenol Contents (mg/g) | Total Flavonoid Contents (mg/g) | ||
|---|---|---|---|
| Raw materials | MUL | 7.19 ± 3.14 e | 3.50 ± 0.20 f |
| MUL-CA | 9.26 ± 1.17 e | 5.29 ± 0.57 de | |
| MUL-F1 | 28.07 ± 3.06 c | 8.19 ± 2.09 b | |
| MUL-F2 | 25.64 ± 0.77 cd | 6.60 ± 0.88 cd | |
| MUL-F3 | 26.99 ± 0.52 cd | 7.75 ± 0.58 bc | |
| Extrusion materials | HME-MUL | 6.82 ± 2.28 e | 3.13 ± 0.09 f |
| HME-MUL-CA | 21.97 ± 0.96 d | 4.95 ± 0.88 e | |
| HME-MUL-F1 | 37.71 ± 7.04 b | 8.33 ± 0.26 b | |
| HME-MUL-F2 | 29.90 ± 0.99 c | 9.88 ± 0.98 a | |
| HME-MUL-F3 | 46.71 ± 0.86 a | 6.84 ± 0.31 bc |
3.2. Characterizations of MUL and MUL-DDS Formulations
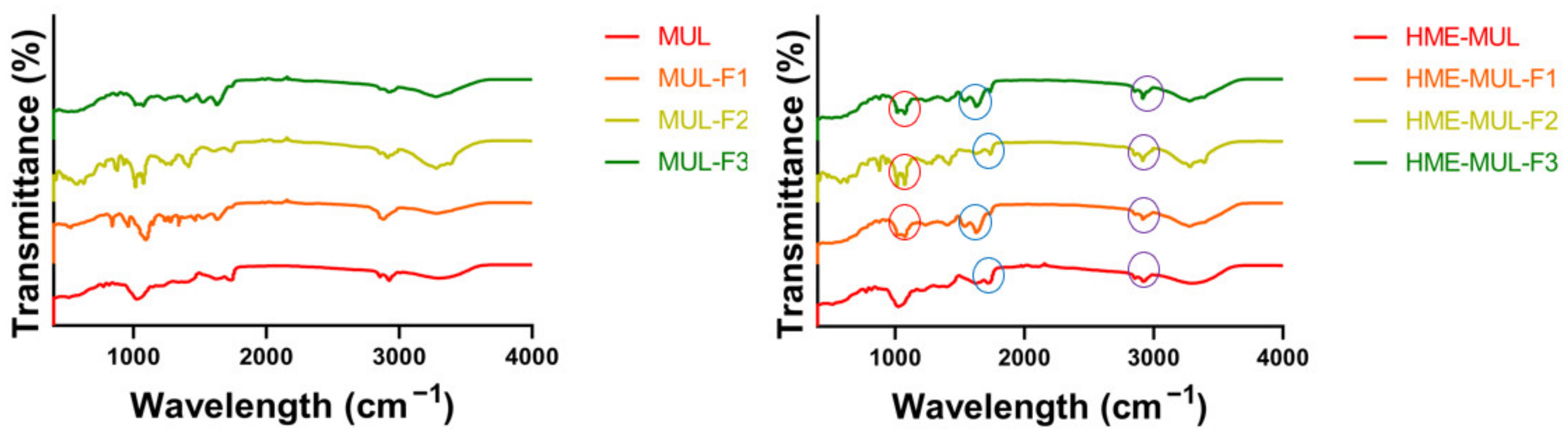
3.3. Structural Change of Compounds MUL and HME-DDS Using FT-IR
3.4. Confirmation of Particle Surface Morphologyof MUL and HME-DDS
3.5. In Vitro Release of non-HME, MUL, and MUL-DDS
3.6. Confirmation of Probiotics and Pathogenic Bacteria Growth Characteristics
3.7. Determination of Antibacterial Activity against Pathogenic Bacteria of HME-DDS Formulation Extract of Mulberry
3.8. Effect of MUL and HME-MUL-F2 on the Antibacterial Ability of Probiotics
3.9. Effects of Sterilized MUL and HME-MUL-F2 on Growth of Probiotics
3.10. Effect of HME-DDS Formulation of Mulberry on pH Change of Probiotics Culture Medium
3.11. Anthocyanin Release Characteristics after HME-DDE Prepation of Mulberry Is Added to Probiotics Strains
3.12. Effect of HME-DDS Formulation of Mulberry on Antibacterial Activity of Probiotics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Buchweitz, M.; Brauch, J.; Carle, R.; Kammerer, D.R. Application of Ferric Anthocyanin Chelates as Natural Blue Food Colorants in Polysaccharide and Gelatin Based Gels. Food Res. Int. 2013, 51, 274–282. [Google Scholar] [CrossRef]
- Kim, I.; Lee, J. Comparison of Different Extraction Solvents and Sonication Times for Characterization of Antioxidant Activity and Polyphenol Composition in Mulberry (Morus Alba L.). Appl. Biol. Chem. 2017, 60, 509–517. [Google Scholar] [CrossRef]
- Kim, H.B. Identification of C3G (Cyanidin-3-Glucoside) from Mulberry Fruits and Quantification with Different Varieties. Korean J. Sericult. Sci. 2003, 45, 90–95. [Google Scholar]
- Bae, S.-H.; Suh, H.-J. Antioxidant Activities of Five Different Mulberry Cultivars in Korea. LWT-Food Sci. Technol. 2007, 40, 955–962. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry Anthocyanin and Its Association with Postprandial Inflammation and Insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef]
- Hu, Y.; Deng, L.; Chen, J.; Zhou, S.; Liu, S.; Fu, Y.; Yang, C.; Liao, Z.; Chen, M. An Analytical Pipeline to Compare and Characterise the Anthocyanin Antioxidant Activities of Purple Sweet Potato Cultivars. Food Chem. 2016, 194, 46–54. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of Anthocyanins and Consequent Effects on the Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as Inflammatory Modulators and the Role of the Gut Microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of Anthocyanins: Gaps in Knowledge, Challenges and Future Research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Bischoff, S.C. “Gut Health”: A New Objective in Medicine? BMC Med. 2011, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K. The Intestinal Microbiota and Its Role in Human Health and Disease. J. Med. Investig. 2016, 63, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natividad, J.M.M.; Verdu, E.F. Modulation of Intestinal Barrier by Intestinal Microbiota: Pathological and Therapeutic Implications. Pharm. Res. 2013, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lee, H.Y.; Nam, S.H.; Baek, J.S. Antifungal Activiti of Angelica gigas with Enhanced Water Solubility of Decursin and Decursinol Angelate by Hot-Melt Extrusion Technology against Candida albocans. Int. J. Transl. Med. 2022, 2, 515–521. [Google Scholar]
- Tan, S.H.; Jiang, L.; Li, W.; Chan, S.H.; Baek, J.S.; Ng, N.K.J.; Sailov, T.; Kharel, S.; Chong, K.K.L.; Loo, S.C.J. Lipid-Polymer Hybrid Nanoparticles Enhances the Potency of Ampicillin against Enterococcus faecalis in a Protozoa Infection Model. ACS Infect. Dis. 2021, 7, 1607–1618. [Google Scholar] [CrossRef]
- Baek, J.S.; Tan, C.H.; Jing, N.K.N.; Yeo, Y.P.; Rice, S.A.; Loo, S.C.J. A Programmable Lipid-Polymer Hybrid Nanoparticle System for Localized, Sustained Antibiotic Delivery to Gram-Positive and Gram-Negative Bacterial Biofilms. Nanoscale Horiz. 2018, 3, 305–311. [Google Scholar] [CrossRef]
- Ishimoto, K.; Miki, S.; Ohno, A.; Nakamura, Y.; Otani, S.; Nakamura, M.; Nakagawa, S. β-Carotene Solid Dispersion Prepared by Hot-Melt Technology Improves Its Solubility in Water. J. Food Sci. Technol. 2019, 56, 3540–3546. [Google Scholar] [CrossRef]
- Aungst, B.J. Optimizing Oral Bioavailability in Drug Discovery: An Overview of Design and Testing Strategies and Formulation Options. J. Pharm. Sci. 2017, 106, 921–929. [Google Scholar] [CrossRef]
- DeBoyace, K.; Wildfong, P.L.D. The Application of Modeling and Prediction to the Formation and Stability of Amorphous Solid Dispersions. J. Pharm. Sci. 2018, 107, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Otani, S.; Miki, S.; Nakamura, Y.; Ishimoto, K.; Ago, Y.; Nakagawa, S. Improved Bioavailability of β-Carotene by Amorphous Solid Dispersion Technology in Rats. J. Nutr. Sci. Vitaminol. 2020, 66, 207–210. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila Aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.-D.; Yu, C.-Y.; Kim, M.-J.; Yun, S.-J.; Lee, S.-J.; Kim, N.-Y.; Chung, I.-M. Comparision of SOD Activity and Phenolic Compound Contents in Various Korean Medicinal Plants. Korean J. Med. Crop Sci. 2004, 12, 191–202. [Google Scholar]
- Vanden Berghe, D.A.; Vlietinck, A.J. Screening methods for antibacterial and antiviral agents from higher plants. In Methods in Plant Biochemistry; Dey, P.M., Harbone, J.D., Eds.; Academic Press: London, UK, 1991; pp. 47–69. [Google Scholar]
- Ishii, K.; Nakamura, S.; Sato, Y. Measurement of dispersion of nanoparticles in a dense suspension by high-sensitivity low-coherence dynamic light scattering. In Proceedings of the International Conference on Optical Particle Characterization (OPC 2014), Tokyo, Japan, 10–14 March 2014; Volume 9232, pp. 94–99. [Google Scholar]
- Chaitanya, K. Molecular Structure, Vibrational Spectroscopic (FT-IR, FR-Raman), UV-Vis spectra, First order Hyperpolarizability, NBO Analysis, HOMO and LUMO analysis, Thermodynamic Properties of Benzophenone 2,4-dicarboxylic acid by ab Initio HF and Density Functional Method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 86, 159–173. [Google Scholar]
- Huseynov, E.; Garibov, A.; Mehdiyeva, R. TEM and SEM Study of Nano SiO2 Particles Exposed to Influence of Neutron Flux. J. Mater. Res. Technol. 2016, 5, 213–218. [Google Scholar] [CrossRef]
- Jin, M.; Jeon, W.J.; Lee, H.; Jung, M.; Kim, H.E.; Won, J.H.; Kim, J.C.; Park, J.H.; Yang, M.J.; Cho, C.W. Preparation and evaluation of Rapid Disintegrating Formulation from Coated Microneedle. Drug Deliv. Transl. Res. 2021, 12, 415–425. [Google Scholar] [CrossRef]
- EN ISO 4833; Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of microorganisms. Colony count technique at 30 °C. International Organization for Standardization (ISO): Geneva, Switzerland, 1993.
- Wang, X.; Huang, M.; Yang, F.; Sun, H.; Zhou, X.; Guo, Y.; Wang, X.; Zhang, M. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef]
- Ti, H.; Zhang, R.; Zhang, M.; Wei, Z.; Chi, J.; Deng, Y.; Zhang, Y. Effect of Extrusion on Phytochemical Profiles in Milled Fractions of Black Rice. Food Chem. 2015, 178, 186–194. [Google Scholar] [CrossRef]
- Chalermchaiwat, P.; Jangchud, K.; Jangchud, A.; Charunuch, C.; Prinyawiwatkul, W. Antioxidant Activity, Free Gamma-Aminobutyric Acid Content, Selected Physical Properties and Consumer Acceptance of Germinated Brown Rice Extrudates as Affected by Extrusion Process. LWT-Food Sci. Technol. 2015, 64, 490–496. [Google Scholar] [CrossRef]
- Obradović, V.; Babić, J.; Šubarić, D.; Ačkar, Đ.; Jozinović, A. Improvement of Nutritional and Functional Properties of Extruded Food Products. J. Food Nutr. Res. 2014, 53, 189–206. [Google Scholar]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-Dependent Studies on the Total Phenolics, Flavonoids, Antioxidant Activities, and Sugar Content in Six Onion Varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Piao, J.; Lee, J.-Y.; Weon, J.B.; Ma, C.J.; Ko, H.-J.; Kim, D.-D.; Kang, W.-S.; Cho, H.-J. Angelica Gigas Nakai and Soluplus-Based Solid Formulations Prepared by Hot-Melting Extrusion: Oral Absorption Enhancing and Memory Ameliorating Effects. PLoS ONE 2015, 10, e0124447. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Bhardwaj, S.P.; Suryanarayanan, R. Controlling the Physical Form of Mannitol in Freeze-Dried Systems. Eur. J. Pharm. Biopharm. 2013, 85, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as Particulate Drug Formulations in Therapy: Rationale for Development and What We Can Expect for the Future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Marks, J.D.; Cromie, W.; Lee, R. Nonionic Surfactant Prevents NMDA-Induced Death in Cultured Hippocampal Neurons. In Proceedings of the Society for Neuroscience Abstracts; Society for Neuroscience: Washington, DC, USA, 1998; Volume 24, p. 462. [Google Scholar]
- Merchant, F.A.; Holmes, W.H.; Capelli-Schellpfeffer, M.; Lee, R.C.; Toner, M. Poloxamer 188 Enhances Functional Recovery of Lethally Heat-Shocked Fibroblasts. J. Surg. Res. 1998, 74, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Taylor, L.S. Evaluation of Amorphous Solid Dispersion Properties Using Thermal Analysis Techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef]
- Hemery, Y.; Chaurand, M.; Holopainen, U.; Lampi, A.-M.; Lehtinen, P.; Piironen, V.; Sadoudi, A.; Rouau, X. Potential of Dry Fractionation of Wheat Bran for the Development of Food Ingredients, Part I: Influence of Ultra-Fine Grinding. J. Cereal Sci. 2011, 53, 1–8. [Google Scholar] [CrossRef]
- Das, S.; Pattanayak, D.; Nayak, A.K.; Yi, D.K.; Nanda, S.S.; Ansari, M.T.; Hasnain, M.S. Alginate–Montmorillonite Composite Systems as Sustained Drug Delivery Carriers. In Alginates in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 187–201. [Google Scholar]
- Ahmed, S. Alginates: Applications in the Biomedical and Food Industries; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 1119487919. [Google Scholar]
- Sime, W.J. Alginates. In Food Gels; Springer: Berlin/Heidelberg, Germany, 1990; pp. 53–78. [Google Scholar]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 9, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Rabinow, B.E. Nanosuspensions in Drug Delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Rana, M.M.; Boateng, J.S.; Mitchell, J.C.; Douroumis, D. Dissolution Enhancement of Poorly Water-Soluble APIs Processed by Hot-Melt Extrusion Using Hydrophilic Polymers. Drug Dev. Ind. Pharm. 2013, 39, 218–227. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nam, S.; Choi, Y.; Kim, M.; Koo, J.S.; Chae, B.-J.; Kang, W.-S.; Cho, H.-J. Fabrication and Characterizations of Hot-Melt Extruded Nanocomposites Based on Zinc Sulfate Monohydrate and Soluplus. Appl. Sci. 2017, 7, 902. [Google Scholar] [CrossRef] [Green Version]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug Nanoparticles: Formulating Poorly Water-Soluble Compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing Agents for Drug Nanocrystals: Effect on Bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiţa, B.; Fuliaş, A.; Bandur, G.; Marian, E.; Tiţa, D. Compatibility Study between Ketoprofen and Pharmaceutical Excipients Used in Solid Dosage Forms. J. Pharm. Biomed. Anal. 2011, 56, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Identificação Espectrométrica de Compostos Orgânicos; Livros Técnicos e Científicos: Rio de Janeiro, Brasil, 2007; p. 490. [Google Scholar]
- Correia, L.P.; Procópio, J.V.V.; de Santana, C.P.; Santos, A.F.O.; de Medeiros Cavalcante, H.M.; Macêdo, R.O. Characterization of Herbal Medicine with Different Particle Sizes Using Pyrolysis GC/MS, SEM, and Thermal Techniques. J. Therm. Anal. Calorim. 2013, 111, 1691–1698. [Google Scholar] [CrossRef]
- Karaman, R. Prodrugs Design Based on Inter-and Intramolecular Chemical Processes. Chem. Biol. Drug Des. 2013, 82, 643–668. [Google Scholar] [CrossRef]
- Kawashima, Y.; Yamamoto, H.; Takeuchi, H.; Hino, T.; Niwa, T. Properties of a Peptide Containing DL-Lactide/Glycolide Copolymer Nanospheres Prepared by Novel Emulsion Solvent Diffusion Methods. Eur. J. Pharm. Biopharm. 1998, 45, 41–48. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, Z.; Nagai, T. Prolonged Hypoglycemic Effect of Insulin-Loaded Polybutylcyanoacrylate Nanoparticles after Pulmonary Administration to Normal Rats. Int. J. Pharm. 2001, 218, 75–80. [Google Scholar] [CrossRef]
- Go, E.J.; Ryu, B.R.; Ryu, S.J.; Kim, B.H.; Lee, H.T.; Kwon, J.W.; Baek, J.S.; Lim, J.D. An Enhanced Water Solubility and Stability of Anthocyanins in Mulberry Processed with Hot Melt Extrusion. Int. J. Mol. Sci. 2021, 22, 12377. [Google Scholar] [CrossRef]
- Censi, S.; Mian, C.; Betterle, C. Insulin Autoimmune Syndrome: From Diagnosis to Clinical Management. Ann. Transl. Med. 2018, 6, 335. [Google Scholar] [CrossRef]
- Jaiswar, D.R.; Pawar, J.N.; Amin, P.D. Hot Melt Extrusion: Continuous Process of Preparation of Sustained Released Matrix Tablet by Using Hydroxypropylcellulose. Am. J. PharmTech Res. 2015, 6, 295–312. [Google Scholar]
- Saulis, G. The Loading of Human Erythrocytes with Small Molecules by Electroporation. Cell. Mol. Biol. Lett. 2005, 10, 23–35. [Google Scholar] [PubMed]
- Tsoneva, I.; Nikolova, B.; Georgieva, M.; Guenova, M.; Tomov, T.; Rols, M.-P.; Berger, M.R. Induction of Apoptosis by Electrotransfer of Positively Charged Proteins as Cytochrome C and Histone H1 into Cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1721, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Cu, Y.; Saltzman, W.M. Controlled Surface Modification with Poly (Ethylene) Glycol Enhances Diffusion of PLGA Nanoparticles in Human Cervical Mucus. Mol. Pharm. 2009, 6, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zuidam, N.J.; Shimoni, E. Overview of Microencapsulates for Use in Food Products or Processes and Methods to Make Them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–29. [Google Scholar]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An Overview of Encapsulation Technologies for Food Applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Lacombe, A.; Tadepalli, S.; Hwang, C.-A.; Wu, V.C.H. Phytochemicals in Lowbush Wild Blueberry Inactivate Escherichia Coli O157: H7 by Damaging Its Cell Membrane. Foodborne Pathog. Dis. 2013, 10, 944–950. [Google Scholar] [CrossRef]
- Vivas, N.; Lonvaud-Funel, A.; Glories, Y. Effect of Phenolic Acids and Anthocyanins on Growth, Viability and Malolactic Activity of a Lactic Acid Bacterium. Food Microbiol. 1997, 14, 291–299. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota in Vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef]
- Keppler, K.; Humpf, H.-U. Metabolism of Anthocyanins and Their Phenolic Degradation Products by the Intestinal Microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and Metabolism of Three Mulberry Anthocyanin Monomers by Rat Gut Microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef]
- Kim, H.J.; White, P.J. In Vitro Fermentation of Oat Flours from Typical and High β-Glucan Oat Lines. J. Agric Food Chem. 2009, 57, 7529–7536. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liu, L.; Sun, Z.; Wang, D.; Mei, L.; Shen, P.; Li, Z.; Tang, S.; Zhang, H.; Zhou, Q.; et al. Fucoidan as a Marine-origin Prebiotic Modulates the Growth and Antibacterial ability of Lactobacillus rhamnosus. Int. J. Biol. Macromol. 2021, 180, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Wang, X.G.; Wang, J.; Chen, Y.J.; Qin, H.L.; Yang, R. Impact of Probiotics Supplement on the gut microbiota in Neonates with Antibiotic Exposure: An Open Label Single-center Randomized Parallel Controlled Study. World J. Pediatr. 2021, 17, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, R.; Ruchdi, M. Antimicrobial Activity of Probiotic Lactobacilli against some Pathogenic Bacteria. Res. Sq. 2022, 28, 1–9. [Google Scholar]
- Coman, M.M.; Mazzotti, L.; Silvi, S.; Orpianesi, C.; Cresci, A.; Verdenelli, M.C. Antimicrobial Activity of SYNBIO Probiotic Formulation in Pathogens isolated from Chronic Ulcerative Lesions: In vitro Studies. J. Appl. Microbiol. 2020, 128, 584–597. [Google Scholar] [CrossRef]













| MUL-HME-F1 | MUL-HME-F2 | MUL-HME-F3 | |
|---|---|---|---|
| Mulberry powder | 50 | 50 | 40 |
| Whey protein isolate | 40 | - | 40 |
| Lecithin | 2.5 | - | 2.5 |
| Ascorbyl palmitate | 2.5 | 5 | 5 |
| Mannitol | 5 | 35 | 5 |
| Sodium alginate | 2.5 | 5 | 5 |
| Poloxamer 188 | 2.5 | 5 | 2.5 |
| Total | 100 | 100 | 100 |
| Anthocyanin Content (mg/g DW) | |||
|---|---|---|---|
| C3G | C3R | ||
| Raw materials | MUL | 43.13 ± 2.63 f | 2.99 ± 1.25 g |
| MUL-CA | 65.07 ± 1.10 f | 18.49 ± 0.89 g | |
| MUL-F1 | 317.39 ± 18.93 c | 136.75 ± 7.97 de | |
| MUL-F2 | 289.22 ± 20.75 d | 166.12 ± 33.38 c | |
| MUL-F3 | 289.13 ± 23.28 d | 126.10 ± 7.09 d | |
| Extrusion materials | HME-MUL | 117.44 ± 1.44 e | 68.91 ± 1.35 f |
| HME-MUL-CA | 591.62 ± 12.65 a | 401.16 ± 13.09 a | |
| HME-MUL-F1 | 402.79 ± 6.78 b | 188.44 ± 3.03 b | |
| HME-MUL-F2 | 325.02 ± 11.12 c | 154.73 ± 11.30 cd | |
| HME-MUL-F3 | 410.76 ± 13.44 b | 164.36 ± 8.46 c | |
| PSA (nm) | PDI (Index) | ZP (mV) | |
|---|---|---|---|
| MUL | 329.67 ± 13.37 | 0.312 ± 0.005 | −25.42 ± 4.87 |
| HME-MUL-F1 | 258.63 ± 21.73 | 0.325 ± 0.004 | −16.76 ± 1.62 |
| HME-MUL-F2 | 152.03 ± 3.19 | 0.297 ± 0.013 | −31.37 ± 0.24 |
| HME-MUL-F3 | 218.20 ± 61.48 | 0.130 ± 0.026 | −23.16 ± 1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, E.J.; Ryu, B.R.; Gim, G.J.; Lee, H.Y.; You, H.S.; Kim, H.B.; Lee, H.T.; Lee, J.Y.; Shim, M.S.; Baek, J.-S.; et al. Hot-Melt Extrusion Enhances Antioxidant Effects of Mulberry on Probiotics and Pathogenic Microorganisms. Antioxidants 2022, 11, 2301. https://doi.org/10.3390/antiox11112301
Go EJ, Ryu BR, Gim GJ, Lee HY, You HS, Kim HB, Lee HT, Lee JY, Shim MS, Baek J-S, et al. Hot-Melt Extrusion Enhances Antioxidant Effects of Mulberry on Probiotics and Pathogenic Microorganisms. Antioxidants. 2022; 11(11):2301. https://doi.org/10.3390/antiox11112301
Chicago/Turabian StyleGo, Eun Ji, Byeong Ryeol Ryu, Gyeong Ju Gim, Ha Yeon Lee, Han Sol You, Hyun Bok Kim, Hyun Tai Lee, Ji Young Lee, Man Sop Shim, Jong-Suep Baek, and et al. 2022. "Hot-Melt Extrusion Enhances Antioxidant Effects of Mulberry on Probiotics and Pathogenic Microorganisms" Antioxidants 11, no. 11: 2301. https://doi.org/10.3390/antiox11112301
APA StyleGo, E. J., Ryu, B. R., Gim, G. J., Lee, H. Y., You, H. S., Kim, H. B., Lee, H. T., Lee, J. Y., Shim, M. S., Baek, J.-S., & Lim, J. D. (2022). Hot-Melt Extrusion Enhances Antioxidant Effects of Mulberry on Probiotics and Pathogenic Microorganisms. Antioxidants, 11(11), 2301. https://doi.org/10.3390/antiox11112301








