Membrane Lipid Reshaping Underlies Oxidative Stress Sensing by the Mitochondrial Proteins UCP1 and ANT1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Reconstitution of UCP1 and ANT1 in Liposomes
2.3. Formation of the Planar Lipid Bilayer Membranes and Measurements of the Membrane Electrical Parameters
2.4. Measurements of the Membrane Elastic Parameters
2.5. Molecular Dynamics Simulations
2.6. Statistics
3. Results
3.1. Impact of DOPE on Membrane Elastic Parameters and SCES
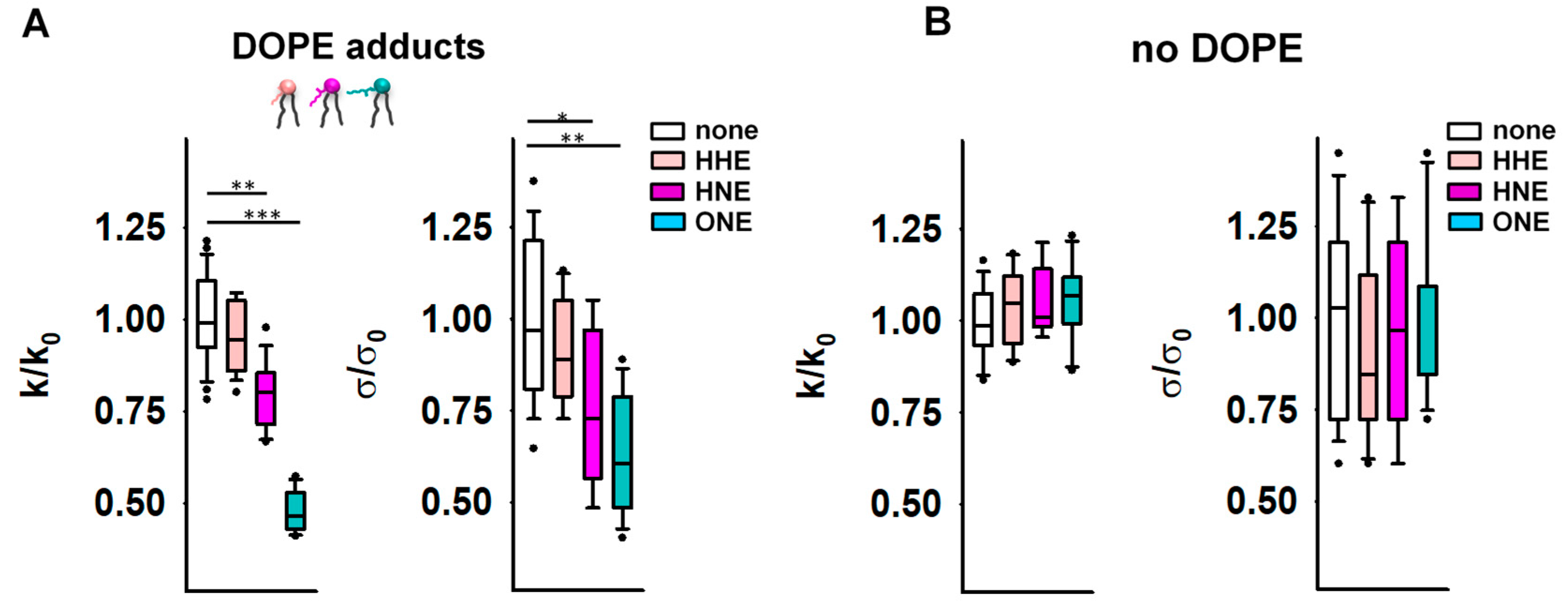
3.2. Reactive Aldehydes Modify the Elastic Properties of the PE-Containing Lipid Bilayer Membranes
3.3. OPC Alters the Elastic Properties of the Lipid Bilayer Membrane
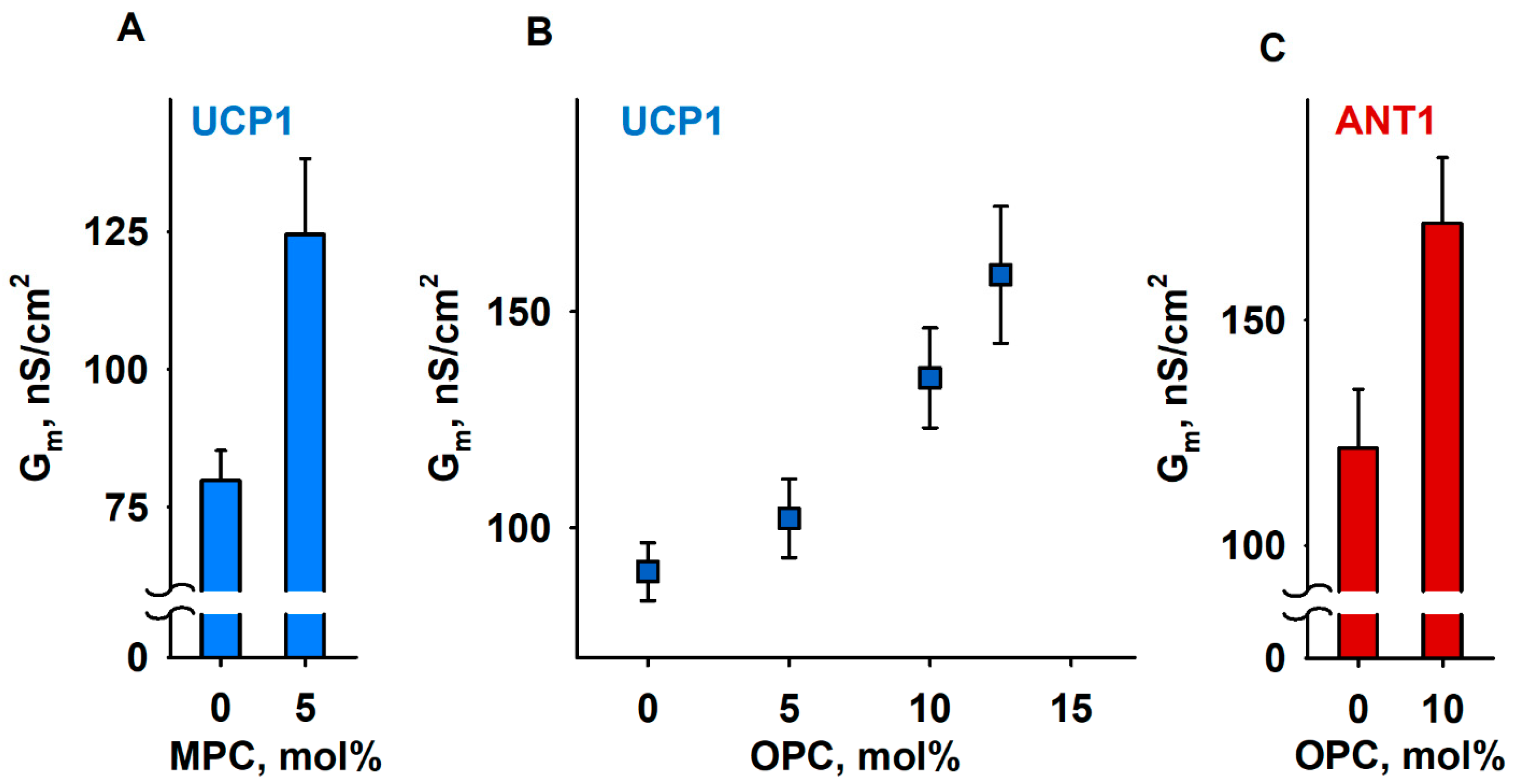
3.4. The IMM Proteins UCP1 and ANT1 Sense Stored Curvature Elastic Stress
3.5. Impact of Lipid Shape on the Lateral Pressure Profiles across the Lipid Bilayer Membrane
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Lamari, F.; Mochel, F.; Sedel, F.; Saudubray, J.M. Disorders of phospholipids, sphingolipids and fatty acids biosynthesis: Toward a new category of inherited metabolic diseases. J. Inherit. Metab. Dis. 2013, 36, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.S.; Park, N.Y.; Cho, D.H. Peroxisome quality control and dysregulated lipid metabolism in neurodegenerative diseases. Exp. Mol. Med. 2020, 52, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Sam, P.N.; Calzada, E.; Acoba, M.G.; Zhao, T.; Watanabe, Y.; Nejatfard, A.; Trinidad, J.C.; Shutt, T.E.; Neal, S.E.; Claypool, S.M. Impaired phosphatidylethanolamine metabolism activates a reversible stress response that detects and resolves mutant mitochondrial precursors. iScience 2021, 24, 102196. [Google Scholar] [CrossRef]
- Eckmann, J.; Eckert, S.H.; Leuner, K.; Muller, W.E.; Eckert, G.P. Mitochondria: Mitochondrial membranes in brain ageing and neurodegeneration. Int. J. Biochem. Cell Biol. 2013, 45, 76–80. [Google Scholar] [CrossRef]
- Kozlov, M.M. Spontaneous and Intrinsic Curvature of Lipid Membranes: Back to the Origins. In Physics of Biological Membranes; Bassereau, P., Sens, P., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 287–309. [Google Scholar]
- Helfrich, W. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Nat. C 1973, 28, 693–703. [Google Scholar] [CrossRef]
- Dymond, M.K. Lipid monolayer spontaneous curvatures: A collection of published values. Chem. Phys. Lipids 2021, 239, 105117. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Vance, J.E.; Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 2013, 1831, 543–554. [Google Scholar] [CrossRef]
- Marsh, D. Lateral pressure profile, spontaneous curvature frustration, and the incorporation and conformation of proteins in membranes. Biophys. J. 2007, 93, 3884–3899. [Google Scholar] [CrossRef]
- Bochicchio, D.; Monticelli, L. The Membrane Bending Modulus in Experiments and Simulations. In Advances in Biomembranes and Lipid Self-Assembly; Iglič, A., Kulkarni, C.V., Rappolt, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 23, pp. 117–143. [Google Scholar]
- van den Brink-van der Laan, E.; Killian, J.A.; de Kruijff, B. Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim. Biophys. Acta 2004, 1666, 275–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsten, M.L.; Baron, R.A.; Seabra, M.C.; Ces, O. Rab1a and Rab5a preferentially bind to binary lipid compositions with higher stored curvature elastic energy. Mol. Membr. Biol. 2013, 30, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; de Kruijff, B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta 1979, 559, 399–420. [Google Scholar] [CrossRef] [PubMed]
- Tuck, S. Extracellular vesicles: Budding regulated by a phosphatidylethanolamine translocase. Curr. Biol. 2011, 21, R988-990. [Google Scholar] [CrossRef] [Green Version]
- Verkleij, A.J.; Leunissen-Bijvelt, J.; de Kruijff, B.; Hope, M.; Cullis, P.R. Non-bilayer structures in membrane fusion. Ciba Found. Symp. 1984, 103, 45–59. [Google Scholar] [CrossRef]
- Siegel, D.P.; Epand, R.M. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: Implications for membrane fusion mechanisms. Biophys. J. 1997, 73, 3089–3111. [Google Scholar] [CrossRef] [Green Version]
- McDonald, C.; Jovanovic, G.; Ces, O.; Buck, M. Membrane Stored Curvature Elastic Stress Modulates Recruitment of Maintenance Proteins PspA and Vipp1. mBio 2015, 6, e01188-15. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, H.; Liu, L.; Sharma, P. Revisiting the curvature-mediated interactions between proteins in biological membranes. Soft Matter 2016, 12, 8907–8918. [Google Scholar] [CrossRef]
- Strandberg, E.; Tiltak, D.; Ehni, S.; Wadhwani, P.; Ulrich, A.S. Lipid shape is a key factor for membrane interactions of amphipathic helical peptides. Biochim. Biophys. Acta 2012, 1818, 1764–1776. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.; Dancourt, J.; Shteyn, V.; Puente, G.; Fong, W.M.; Nag, S.; Bewersdorf, J.; Yamamoto, A.; Antonny, B.; Melia, T.J. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat. Cell Biol. 2014, 16, 415–424. [Google Scholar] [CrossRef]
- Putta, P.; Rankenberg, J.; Korver, R.A.; van Wijk, R.; Munnik, T.; Testerink, C.; Kooijman, E.E. Phosphatidic acid binding proteins display differential binding as a function of membrane curvature stress and chemical properties. Biochim. Biophys. Acta 2016, 1858, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Ronneau, S.; Gonzalez, B.S.; Klutsch, D.; Schaffner-Barbero, C.; Hamoen, L.W. Transmembrane protein sorting driven by membrane curvature. Nat. Commun. 2015, 6, 8728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997, 36, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Mejia, E.M.; Hatch, G.M. Mitochondrial phospholipids: Role in mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef]
- Basu Ball, W.; Neff, J.K.; Gohil, V.M. The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 2018, 592, 1273–1290. [Google Scholar] [CrossRef] [Green Version]
- Bottinger, L.; Horvath, S.E.; Kleinschroth, T.; Hunte, C.; Daum, G.; Pfanner, N.; Becker, T. Phosphatidylethanolamine and cardiolipin differentially affect the stability of mitochondrial respiratory chain supercomplexes. J. Mol. Biol. 2012, 423, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.D.; Basu Ball, W.; Pryce, E.N.; Gohil, V.M. Specific requirements of nonbilayer phospholipids in mitochondrial respiratory chain function and formation. Mol. Biol. Cell 2016, 27, 2161–2171. [Google Scholar] [CrossRef]
- Calzada, E.; Avery, E.; Sam, P.N.; Modak, A.; Wang, C.; McCaffery, J.M.; Han, X.; Alder, N.N.; Claypool, S.M. Phosphatidylethanolamine made in the inner mitochondrial membrane is essential for yeast cytochrome bc1 complex function. Nat. Commun. 2019, 10, 1432. [Google Scholar] [CrossRef] [Green Version]
- Cooke, I.R.; Deserno, M. Coupling between lipid shape and membrane curvature. Biophys. J. 2006, 91, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Bashkirov, P.V.; Chekashkina, K.V.; Akimov, S.A.; Kuzmin, P.I.; Frolov, V.A. Variation of Lipid Membrane Composition Caused by Strong Bending. Biochem. Mosc. Suppl. Ser. A Membr. Cell Biol. 2011, 28, 145–152. [Google Scholar] [CrossRef]
- Beltran-Heredia, E.; Tsai, F.C.; Salinas-Almaguer, S.; Cao, F.J.; Bassereau, P.; Monroy, F. Membrane curvature induces cardiolipin sorting. Commun. Biol. 2019, 2, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias-Wolff, F.; Linden, M.; Lyubartsev, A.P.; Brandt, E.G. Curvature sensing by cardiolipin in simulated buckled membranes. Soft Matter 2019, 15, 792–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, K.M.; Strauss, M.; Daum, B.; Kief, J.H.; Osiewacz, H.D.; Rycovska, A.; Zickermann, V.; Kuhlbrandt, W. Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl. Acad. Sci. USA 2011, 108, 14121–14126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acehan, D.; Malhotra, A.; Xu, Y.; Ren, M.; Stokes, D.L.; Schlame, M. Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria. Biophys. J. 2011, 100, 2184–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanovic, O.; Pashkovskaya, A.A.; Annibal, A.; Vazdar, M.; Burchardt, N.; Sansone, A.; Gille, L.; Fedorova, M.; Ferreri, C.; Pohl, E.E. The molecular mechanism behind reactive aldehyde action on transmembrane translocations of proton and potassium ions. Free Radic. Biol. Med. 2015, 89, 1067–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guichardant, M.; Taibi-Tronche, P.; Fay, L.B.; Lagarde, M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic. Biol. Med. 1998, 25, 1049–1056. [Google Scholar] [CrossRef]
- Bacot, S.; Bernoud-Hubac, N.; Baddas, N.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Lagarde, M.; Guichardant, M. Covalent binding of hydroxy-alkenals 4-HDDE, 4-HHE, and 4-HNE to ethanolamine phospholipid subclasses. J. Lipid Res. 2003, 44, 917–926. [Google Scholar] [CrossRef] [Green Version]
- Vazdar, K.; Vojta, D.; Margetic, D.; Vazdar, M. Reaction Mechanism of Covalent Modification of Phosphatidylethanolamine Lipids by Reactive Aldehydes 4-Hydroxy-2-nonenal and 4-Oxo-2-nonenal. Chem. Res. Toxicol. 2017, 30, 840–850. [Google Scholar] [CrossRef]
- Jovanovic, O.; Skulj, S.; Pohl, E.E.; Vazdar, M. Covalent modification of phosphatidylethanolamine by 4-hydroxy-2-nonenal increases sodium permeability across phospholipid bilayer membranes. Free Radic. Biol. Med. 2019, 143, 433–440. [Google Scholar] [CrossRef]
- Jezek, J.; Jaburek, M.; Zelenka, J.; Jezek, P. Mitochondrial phospholipase A2 activated by reactive oxygen species in heart mitochondria induces mild uncoupling. Physiol. Res. 2010, 59, 737–747. [Google Scholar] [CrossRef]
- Jaburek, M.; Pruchova, P.; Holendova, B.; Galkin, A.; Jezek, P. Antioxidant Synergy of Mitochondrial Phospholipase PNPLA8/iPLA2gamma with Fatty Acid-Conducting SLC25 Gene Family Transporters. Antioxidants 2021, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Bashkirov, P.V.; Kuzmin, P.I.; Vera Lillo, J.; Frolov, V.A. Molecular Shape Solution for Mesoscopic Remodeling of Cellular Membranes. Annu. Rev. Biophys. 2022, 51, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Moldzio, R.; Vazdar, K.; Krewenka, C.; Pohl, E.E. Nutrient deprivation in neuroblastoma cells alters 4-hydroxynonenal-induced stress response. Oncotarget 2017, 8, 8173–8188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macher, G.; Koehler, M.; Rupprecht, A.; Kreiter, J.; Hinterdorfer, P.; Pohl, E.E. Inhibition of mitochondrial UCP1 and UCP3 by purine nucleotides and phosphate. Biochim. Biophys. Acta Biomembr. 2018, 1860, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, J.; Beitz, E.; Pohl, E.E. A Fluorescence-Based Method to Measure ADP/ATP Exchange of Recombinant Adenine Nucleotide Translocase in Liposomes. Biomolecules 2020, 10, 685. [Google Scholar] [CrossRef]
- Beck, V.; Jaburek, M.; Breen, E.P.; Porter, R.K.; Jezek, P.; Pohl, E.E. A new automated technique for the reconstitution of hydrophobic proteins into planar bilayer membranes. Studies of human recombinant uncoupling protein 1. Biochim. Biophys. Acta 2006, 1757, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Rupprecht, A.; Sokolenko, E.A.; Beck, V.; Ninnemann, O.; Jaburek, M.; Trimbuch, T.; Klishin, S.S.; Jezek, P.; Skulachev, V.P.; Pohl, E.E. Role of the transmembrane potential in the membrane proton leak. Biophys. J. 2010, 98, 1503–1511. [Google Scholar] [CrossRef] [Green Version]
- Bashkirov, P.V.; Kuzmin, P.I.; Chekashkina, K.; Arrasate, P.; Vera Lillo, J.; Shnyrova, A.V.; Frolov, V.A. Reconstitution and real-time quantification of membrane remodeling by single proteins and protein complexes. Nat. Protoc. 2020, 15, 2443–2469. [Google Scholar] [CrossRef]
- Frolov, V.A.; Lizunov, V.A.; Dunina-Barkovskaya, A.Y.; Samsonov, A.V.; Zimmerberg, J. Shape bistability of a membrane neck: A toggle switch to control vesicle content release. Proc. Natl. Acad. Sci. USA 2003, 100, 8698–8703. [Google Scholar] [CrossRef] [Green Version]
- Ivchenkov, D.V.; Kuzmin, P.I.; Galimzyanov, T.R.; Shnyrova, A.V.; Bashkirov, P.V.; Frolov, V.A. Nonlinear material and ionic transport through membrane nanotubes. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183677. [Google Scholar] [CrossRef]
- Galimzyanov, T.R.; Bashkirov, P.V.; Blank, P.S.; Zimmerberg, J.; Batishchev, O.V.; Akimov, S.A. Monolayerwise application of linear elasticity theory well describes strongly deformed lipid membranes and the effect of solvent. Soft Matter 2020, 16, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Jaembeck, J.P.; Lyubartsev, A.P. An extension and further validation of an all-atomistic force field for biological membranes. J. Chem. Theory Comput. 2012, 8, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Jaembeck, J.P.; Lyubartsev, A.P. Derivation and systematic validation of a refined all-atom force field for phosphatidylcholine lipids. J. Phys. Chem. B 2012, 116, 3164–3179. [Google Scholar] [CrossRef] [PubMed]
- Jaembeck, J.P.M.; Lyubartsev, A.P. Another piece of the membrane puzzle: Extending slipids further. J. Chem. Theory Comput. 2012, 9, 774–784. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.C.; Kollman, P.A. An Approach to Computing Electrostatic Charges for Molecules. J. Comput. Chem. 1984, 5, 129–145. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A Well-Behaved Electrostatic Potential Based Method Using Charge Restraints for Deriving Atomic Charges-the Resp Model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef] [Green Version]
- Parrinello, M.; Rahman, A. Polymorphic Transitions in Single-Crystals-a New Molecular-Dynamics Method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A linear constraint solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle-an Analytical Version of the Shake and Rattle Algorithm for Rigid Water Models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Vanegas, J.M.; Torres-Sanchez, A.; Arroyo, M. Importance of Force Decomposition for Local Stress Calculations in Biomembrane Molecular Simulations. J. Chem. Theory Comput. 2014, 10, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Torres-Sanchez, A.; Vanegas, J.M.; Arroyo, M. Examining the Mechanical Equilibrium of Microscopic Stresses in Molecular Simulations. Phys. Rev. Lett. 2015, 114, 258102. [Google Scholar] [CrossRef]
- Shi, Z.; Baumgart, T. Membrane tension and peripheral protein density mediate membrane shape transitions. Nat. Commun. 2015, 6, 5974. [Google Scholar] [CrossRef] [Green Version]
- Sorre, B.; Callan-Jones, A.; Manzi, J.; Goud, B.; Prost, J.; Bassereau, P.; Roux, A. Nature of curvature coupling of amphiphysin with membranes depends on its bound density. Proc. Natl. Acad. Sci. USA 2012, 109, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Fuller, N.; Rand, R.P. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys. J. 2001, 81, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Uchida, K. 4-Hydroxy-2-nonenal: A product and mediator of oxidative stress. Prog. Lipid Res. 2003, 42, 318–343. [Google Scholar] [CrossRef]
- Andreyev, A.; Bondareva, T.O.; Dedukhova, V.I.; Mokhova, E.N.; Skulachev, V.P.; Tsofina, L.M.; Volkov, N.I.; Vygodina, T.V. The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria. Eur. J. Biochem. 1989, 182, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, J.; Rupprecht, A.; Skulj, S.; Brkljaca, Z.; Zuna, K.; Knyazev, D.G.; Bardakji, S.; Vazdar, M.; Pohl, E.E. ANT1 Activation and Inhibition Patterns Support the Fatty Acid Cycling Mechanism for Proton Transport. Int. J. Mol. Sci. 2021, 22, 2490. [Google Scholar] [CrossRef] [PubMed]
- Malingriaux, E.A.; Rupprecht, A.; Gille, L.; Jovanovic, O.; Jezek, P.; Jaburek, M.; Pohl, E.E. Fatty acids are key in 4-hydroxy-2-nonenal-mediated activation of uncoupling proteins 1 and 2. PLoS ONE 2013, 8, e77786. [Google Scholar] [CrossRef] [Green Version]
- Garlid, K.D.; Orosz, D.E.; Modriansky, M.; Vassanelli, S.; Jezek, P. On the mechanism of fatty acid-induced proton transport by mitochondrial uncoupling protein. J. Biol. Chem. 1996, 271, 2615–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar] [CrossRef]
- Chen, Y.F.; Tsang, K.Y.; Chang, W.F.; Fan, Z.A. Differential dependencies on [Ca2+] and temperature of the monolayer spontaneous curvatures of DOPE, DOPA and cardiolipin: Effects of modulating the strength of the inter-headgroup repulsion. Soft Matter 2015, 11, 4041–4053. [Google Scholar] [CrossRef]
- Zoni, V.; Khaddaj, R.; Campomanes, P.; Thiam, A.R.; Schneiter, R.; Vanni, S. Pre-existing bilayer stresses modulate triglyceride accumulation in the ER versus lipid droplets. Elife 2021, 10, e62886. [Google Scholar] [CrossRef]
- Renne, M.F.; Bao, X.; Hokken, M.W.; Bierhuizen, A.S.; Hermansson, M.; Sprenger, R.R.; Ewing, T.A.; Ma, X.; Cox, R.C.; Brouwers, J.F.; et al. Molecular species selectivity of lipid transport creates a mitochondrial sink for di-unsaturated phospholipids. EMBO J. 2022, 41, e106837. [Google Scholar] [CrossRef]
- Orsi, M.; Essex, J.W. Physical properties of mixed bilayers containing lamellar and nonlamellar lipids: Insights from coarse-grain molecular dynamics simulations. Faraday Discuss. 2013, 161, 249–272; discussion 273–303, discussion 273–303. [Google Scholar] [CrossRef]
- Kreiter, J.; Brkljača, Z.; Škulj, S.; Bardakji, S.; Vazdar, M.; Pohl, E.E. Mechanism of the ANT-mediated transport of fatty acid anions across the inner mitochondrial membrane. bioRxiv 2022. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovanović, O.; Chekashkina, K.; Škulj, S.; Žuna, K.; Vazdar, M.; Bashkirov, P.V.; Pohl, E.E. Membrane Lipid Reshaping Underlies Oxidative Stress Sensing by the Mitochondrial Proteins UCP1 and ANT1. Antioxidants 2022, 11, 2314. https://doi.org/10.3390/antiox11122314
Jovanović O, Chekashkina K, Škulj S, Žuna K, Vazdar M, Bashkirov PV, Pohl EE. Membrane Lipid Reshaping Underlies Oxidative Stress Sensing by the Mitochondrial Proteins UCP1 and ANT1. Antioxidants. 2022; 11(12):2314. https://doi.org/10.3390/antiox11122314
Chicago/Turabian StyleJovanović, Olga, Ksenia Chekashkina, Sanja Škulj, Kristina Žuna, Mario Vazdar, Pavel V. Bashkirov, and Elena E. Pohl. 2022. "Membrane Lipid Reshaping Underlies Oxidative Stress Sensing by the Mitochondrial Proteins UCP1 and ANT1" Antioxidants 11, no. 12: 2314. https://doi.org/10.3390/antiox11122314
APA StyleJovanović, O., Chekashkina, K., Škulj, S., Žuna, K., Vazdar, M., Bashkirov, P. V., & Pohl, E. E. (2022). Membrane Lipid Reshaping Underlies Oxidative Stress Sensing by the Mitochondrial Proteins UCP1 and ANT1. Antioxidants, 11(12), 2314. https://doi.org/10.3390/antiox11122314






