L-Citrulline Supplementation Reduces Blood Pressure and Myocardial Infarct Size under Chronic Intermittent Hypoxia, a Major Feature of Sleep Apnea Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
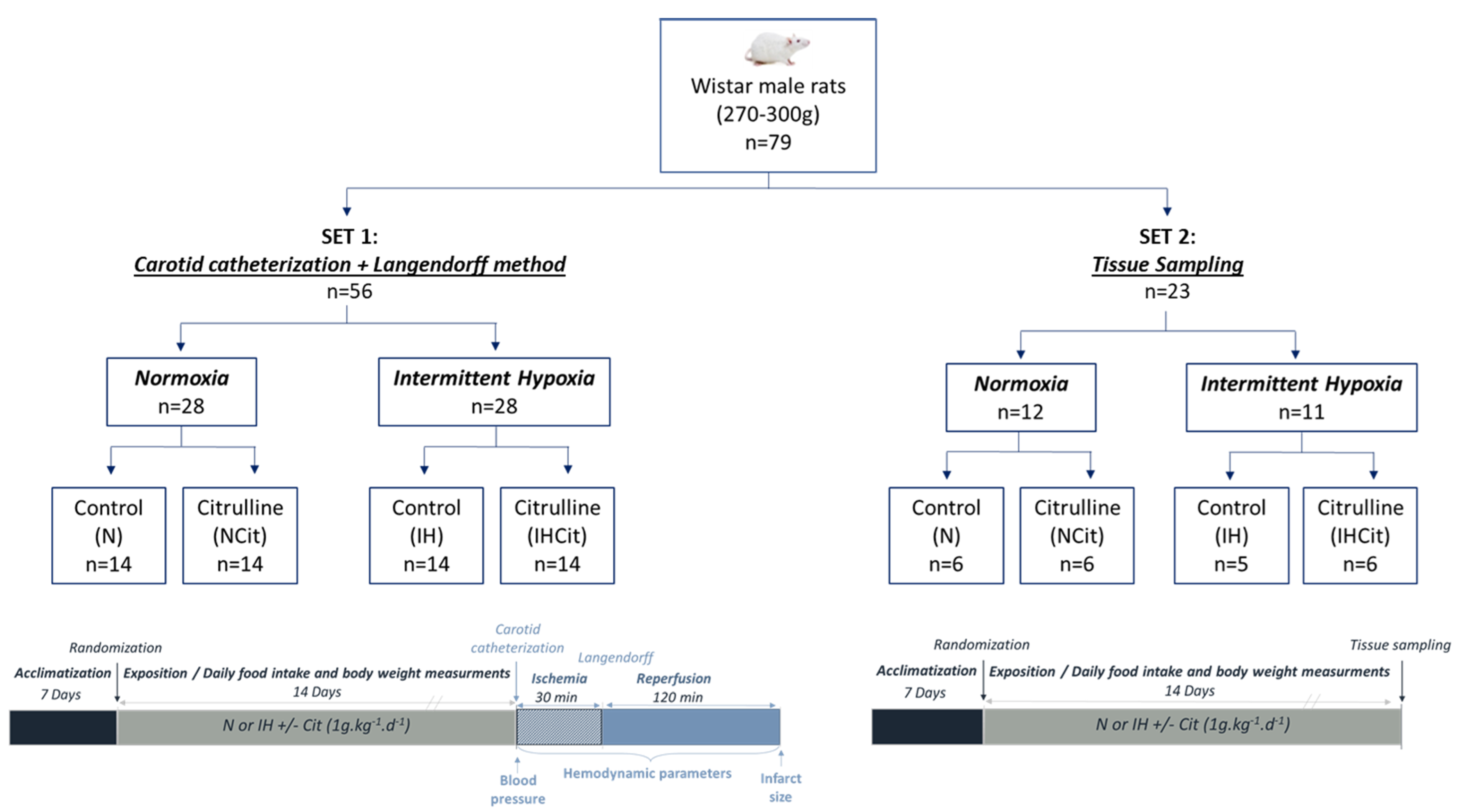
2.2. Animals and Exposition to Normoxia or Intermittent Hypoxia
2.3. Carotid Catheterization, Langendorff, and Ischemia-Reperfusion
2.4. Plasma, Heart, and Aorta Sampling and Extraction
2.5. Oxidative Stress Markers
2.5.1. Dihydroethidium (DHE)
2.5.2. Advanced Oxidation Protein Products (AOPP)
2.5.3. Pro- and Anti-Oxidant Enzyme Activities
2.5.4. Nitrotyrosine (3-NT)
2.5.5. Nitrite/Nitrate
2.6. Statistical Analyses
3. Results
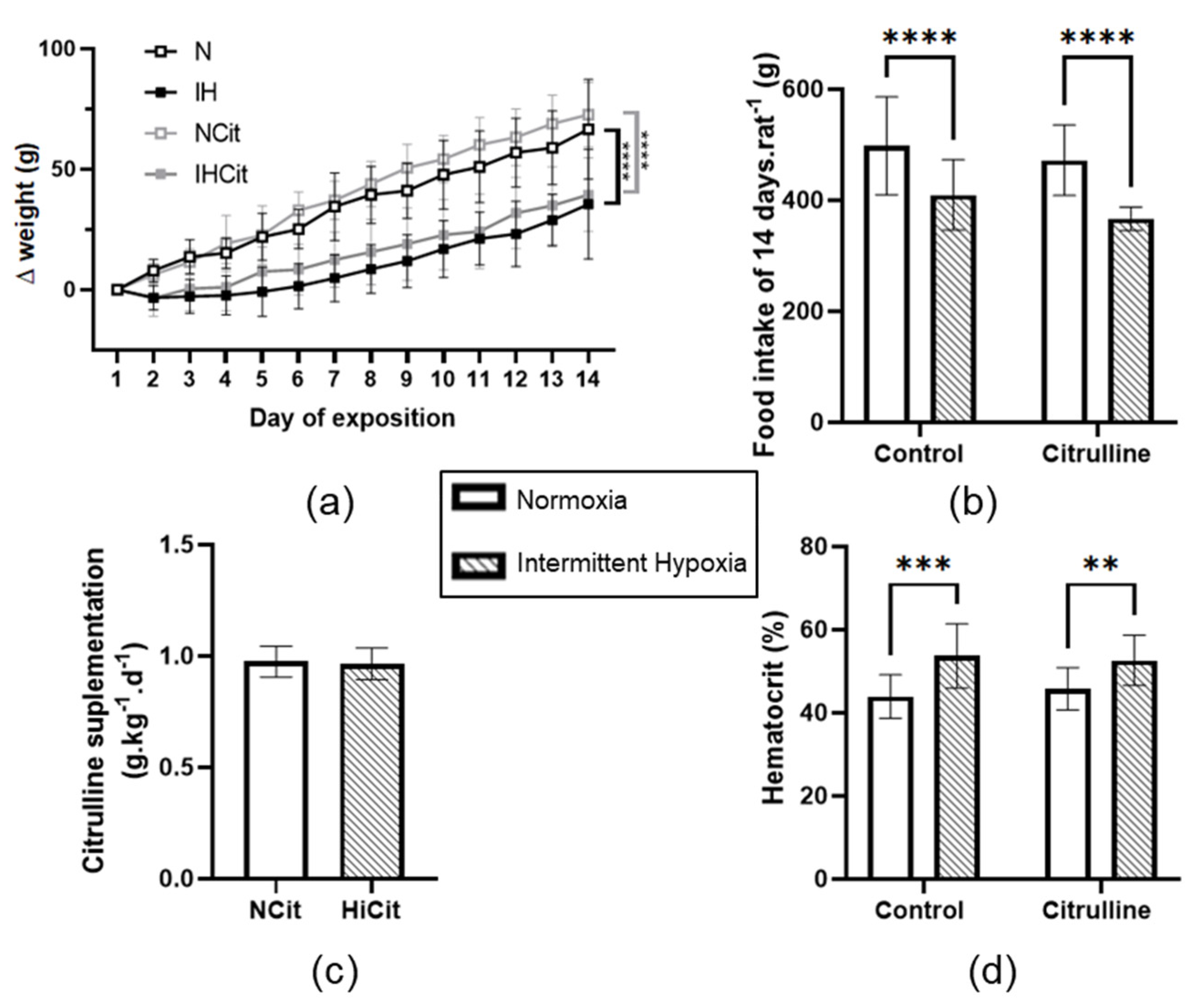
3.1. Citrulline Has No Effect on Body Weight, Food Intake, and Hematocrit
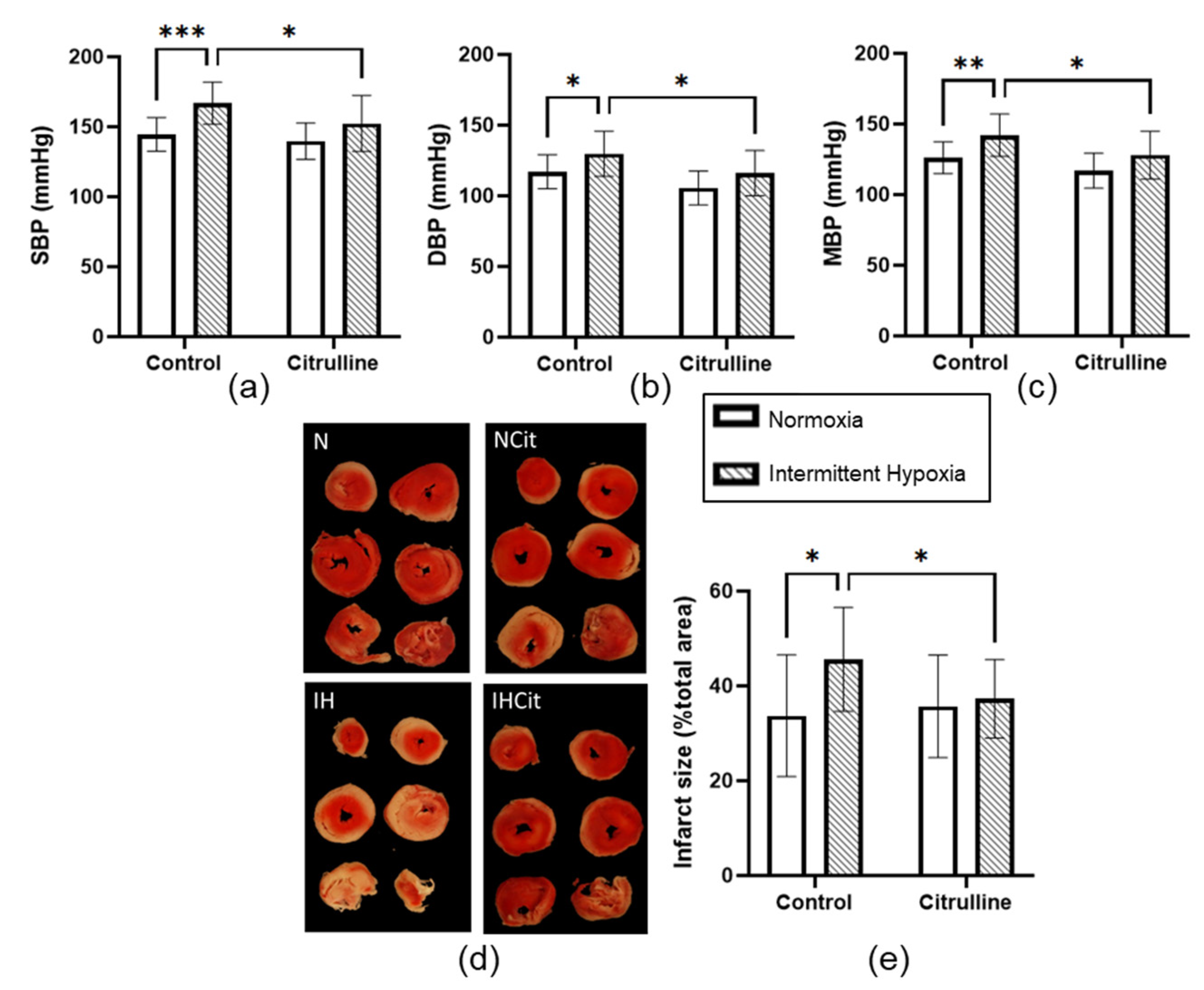
3.2. Citrulline Prevented the IH-Induced Increase in Blood Pressure and Infarct Size
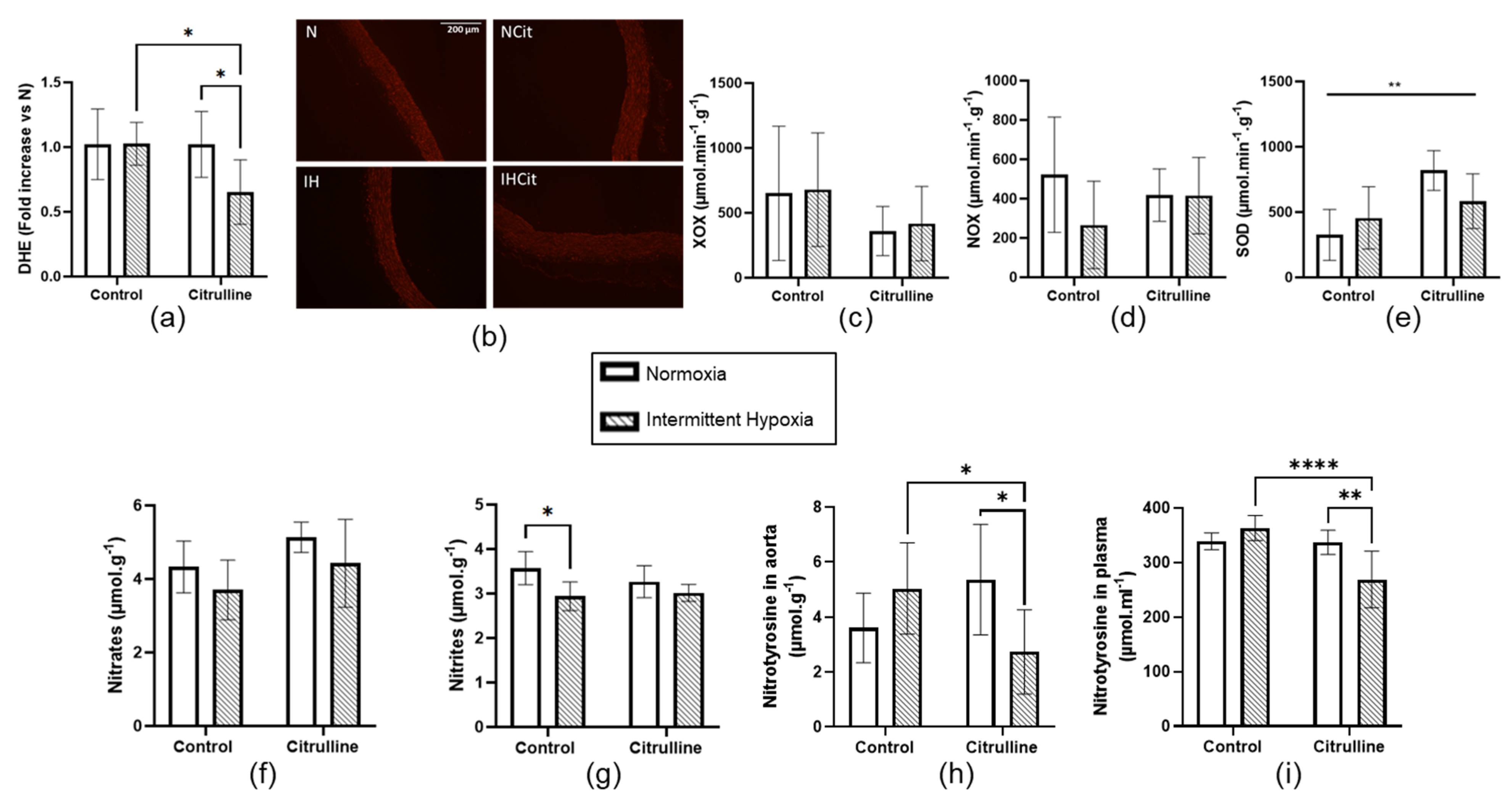
3.3. Citrulline Decreased Superoxide Anion Content and Nitrotyrosine Levels in Aorta
3.4. Citrulline Prevented IH-Induced Increase in Superoxide Anion Content in Heart Tissue
4. Discussion
4.1. Citrulline Did Not Impact the IH-Induced Decrease in Weight Loss and Hematocrit
4.2. Citrulline Significantly Decreased Blood Pressure and Infarct Size under IH
4.3. Oxidative Stress in Aorta and Myocardium
5. Study Limitation
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Normoxia Pair-Fed (Npf) Group
Appendix A.2. Western Blot
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Baillieul, S.; Dekkers, M.; Brill, A.-K.; Schmidt, M.H.; Detante, O.; Pépin, J.-L.; Tamisier, R.; Bassetti, C.L.A. Sleep apnoea and ischaemic stroke: Current knowledge and future directions. Lancet Neurol. 2022, 21, 78–88. [Google Scholar] [CrossRef]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef]
- Buchner, N.J.; Sanner, B.M.; Borgel, J.; Rump, L.C. Continuous Positive Airway Pressure Treatment of Mild to Moderate Obstructive Sleep Apnea Reduces Cardiovascular Risk. Am. J. Respir. Crit. Care Med. 2007, 176, 1274–1280. [Google Scholar] [CrossRef]
- Bratton, D.; Gaisl, T.; Wons, A.M.; Kohler, M. CPAP VS Mandibular Advancement Devices and Blood Pressure in Patients with Obstructive Sleep Apnea. JAMA 2015, 314, 2280–2293. [Google Scholar] [CrossRef] [PubMed]
- Buchner, S.; Satzl, A.; Debl, K.; Hetzenecker, A.; Luchner, A.; Husser, O.; Hamer, O.W.; Poschenrieder, F.; Fellner, C.; Zeman, F.; et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur. Hear. J. 2013, 35, 192–199. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.-L.; Bailly, S.; Rinder, P.; Adler, D.; Benjafield, A.V.; Lavergne, F.; Josseran, A.; Sinel-Boucher, P.; Tamisier, R.; Cistulli, P.A.; et al. Relationship between CPAP Termination and All-Cause Mortality. Chest 2022, 161, 1657–1665. [Google Scholar] [CrossRef]
- Trzepizur, W.; Blanchard, M.; Ganem, T.; Balusson, F.; Feuilloy, M.; Girault, J.-M.; Meslier, N.; Oger, E.; Paris, A.; Pigeanne, T.; et al. Sleep Apnea–Specific Hypoxic Burden, Symptom Subtypes, and Risk of Cardiovascular Events and All-Cause Mortality. Am. J. Respir. Crit. Care Med. 2022, 205, 108–117. [Google Scholar] [CrossRef]
- Pépin, J.-L.; Bailly, S.; Rinder, P.; Adler, D.; Szeftel, D.; Malhotra, A.; Cistulli, P.; Benjafield, A.; Lavergne, F.; Josseran, A.; et al. CPAP Therapy Termination Rates by OSA Phenotype: A French Nationwide Database Analysis. J. Clin. Med. 2021, 10, 936. [Google Scholar] [CrossRef]
- Baillieul, S.; Tamisier, R.; Eckert, D.J.; Pépin, J.-L. Current Knowledge and Perspectives for Pharmacological Treatment in OSA. Arch. Bronconeumol. 2022, 58, 681–684. [Google Scholar] [CrossRef]
- Pépin, J.-L.; Eastwood, P.; Eckert, D.J. Novel avenues to approach non-CPAP therapy and implement comprehensive obstructive sleep apnoea care. Eur. Respir. J. 2021, 59, 2101788. [Google Scholar] [CrossRef]
- Alajmi, M.; Mulgrew, A.; Fox, J.; Davidson, W.; Schulzer, M.; Mak, E.; Ryan, C.; Fleetham, J.; Choi, P.; Ayas, N. Impact of CPAP Therapy on Blood Pressure in Patients with Obstructive Sleep Apnea Hypopnea: A Metaanalysis of Randomized Controlled Trials. Lung 2007, 185, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Gurubhagavatula, I.; Teff, K.; Rader, D.J.; Wadden, T.A.; Townsend, R.; Foster, G.D.; Maislin, G.; Saif, H.; Broderick, P. CPAP, Weight Loss, or Both for Obstructive Sleep Apnea. N. Engl. J. Med. 2014, 370, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.M.; Sharafkhaneh, H.; Hirshkowitz, M.; Elkhatib, R.; Sharafkhaneh, A. Weight and metabolic effects of cpap in obstructive sleep apnea patients with obesity. Respir. Res. 2011, 12, 80–89. [Google Scholar] [CrossRef]
- Mendelson, M.; Bailly, S.; Marillier, M.; Flore, P.; Borel, J.C.; Vivodtzev, I.; Doutreleau, S.; Verges, S.; Tamisier, R.; Pépin, J.L. Obstructive Sleep Apnea Syndrome, Objectively Measured Physical Activity and Exercise Training Interventions: A Systematic Review and Meta-Analysis. Front. Neurol. 2018, 9, 73. [Google Scholar] [CrossRef]
- Dematteis, M.; Godin-Ribuot, D.; Arnaud, C.; Ribuot, C.; Stanke-Labesque, F.; Pépin, J.-L.; Lévy, P. Cardiovas-cular Consequences of Sleep-Disordered Breathing: Contribution of Animal Models to Understanding of the Human Disease. ILAR J. 2009, 50, 262–281. [Google Scholar] [CrossRef]
- Ramond, A.; Godin-Ribuot, D.; Ribuot, C.; Totoson, P.; Koritchneva, I.; Cachot, S.; Levy, P.; Joyeux-Faure, M. Oxidative Stress Mediates Cardiac Infarction Aggravation Induced by Intermittent Hypoxia. Fundam. Clin. Pharmacol. 2013, 27, 252–261. [Google Scholar] [CrossRef]
- Harki, O.; Boete, Q.; Pépin, J.-L.; Arnaud, C.; Belaidi, E.; Faury, G.; Khouri, C.; Briançon-Marjollet, A. Intermittent hypoxia-related alterations in vascular structure and function: A systematic review and meta-analysis of rodent data. Eur. Respir. J. 2021, 59, 2100866. [Google Scholar] [CrossRef]
- Belaidi, E.; Joyeux-Faure, M.; Ribuot, C.; Launois, S.H.; Levy, P.; Godin-Ribuot, D. Major Role for Hypoxia In-ducible Factor-1 and the Endothelin System in Promoting Myocardial Infarction and Hypertension in an An-imal Model of Obstructive Sleep Apnea. J. Am. Coll Cardiol. 2009, 53, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Moulin, S.; Thomas, A.; Arnaud, C.; Arzt, M.; Wagner, S.; Maier, L.S.; Pépin, J.-L.; Godin-Ribuot, D.; Gaucher, J.; Belaidi, E. Cooperation between Hypoxia-Inducible Factor 1α and Activating Transcription Factor 4 in Sleep Apnea–Mediated Myocardial Injury. Can. J. Cardiol. 2020, 36, 936–940. [Google Scholar] [CrossRef]
- Belaidi, E.; Morand, J.; Gras, E.; Pépin, J.L.; Godin-Ribuot, D. Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications. Pharmacol. Ther. 2016, 168, 1–11. [Google Scholar] [CrossRef]
- Semenza, G.L.; Prabhakar, N.R. HIF-1–Dependent Respiratory, Cardiovascular, and Redox Responses to Chronic Intermittent Hypoxia. Antioxid. Redox Signal. 2007, 9, 1391–1396. [Google Scholar] [CrossRef]
- Peng, Y.-J.; Yuan, G.; Ramakrishnan, D.; Sharma, S.; Bosch-Marce, M.; Kumar, G.K.; Semenza, G.L.; Prabhakar, N.R. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J. Physiol. 2006, 577, 705–716. [Google Scholar] [CrossRef]
- Yuan, G.; Khan, S.A.; Luo, W.; Nanduri, J.; Semenza, G.L.; Prabhakar, N.R. Hypoxia-inducible Factor 1 Medi-ates Increased Expression of NADPH Oxidase-2 in Response to Intermittent Hypoxia. J. Cell. Physiol. 2011, 226, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.; Mahmoudi, S.; Hattar, K.; Sibelius, U.; Olschewski, H.; Mayer, K.; Seeger, W.; Grimminger, F. Enhanced Release of Superoxide from Polymorphonuclear Neutrophils in Obstructive Sleep Apnea: Impact of Continuous Positive Airway Pressure Therapy. Am. J. Respir. Crit. Care Med. 2000, 162, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Totoson, P.; Fhayli, W.; Faury, G.; Korichneva, I.; Cachot, S.; Baldazza, M.; Ribuot, C.; Pépin, J.L.; Lévy, P.; Joyeux-Faure, M. Atorvastatin protects against deleterious cardiovascular consequences induced by chronic intermittent hypoxia. Exp. Biol. Med. 2013, 238, 223–232. [Google Scholar] [CrossRef]
- Moulin, S.; Arnaud, C.; Bouyon, S.; Pépin, J.-L.; Godin-Ribuot, D.; Belaidi, E. Curcumin prevents chronic intermittent hypoxia-induced myocardial injury. Ther. Adv. Chronic Dis. 2020, 11, 2040622320922104. [Google Scholar] [CrossRef]
- Farah, C.; Reboul, C. NO Better Way to Protect the Heart during Ischemia–Reperfusion: To Be in the Right Place at the Right Time. Front. Pediatr. 2015, 3, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krause, B.J.; Del Rio, R.; Moya, E.A.; Marquez-Gutierrez, M.; Casanello, P.; Iturriaga, R. Arginase–Endothelial Nitric Oxide Synthase Imbalance Contributes to Endothelial Dysfunction during Chronic Intermittent Hypoxia. J. Hypertens. 2015, 33, 515–524. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L. Cardiovascular Morbidity in Obstructive Sleep Apnea: Oxidative Stress, Inflammation, and Much More. Am. J. Respir. Crit. Care Med. 2008, 177, 369–375. [Google Scholar] [CrossRef]
- Lavie, L.; Hefetz, A.; Luboshitzky, R.; Lavie, P. Plasma Levels of Nitric Oxide and L-Arginine in Sleep Apnea Patients: Effects of nCPAP Treatment. J. Mol. Neurosci. 2003, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ohike, Y.; Kozaki, K.; Iijima, K.; Eto, M.; Kojima, T.; Ohga, E.; Santa, T.; Imai, K.; Hashimoto, M.; Yoshizumi, M. Amelioration of Vascular Endothelial Dysfunction in Obstructive Sleep Apnea Syndrome by Nasal Continuous Positive Airway Pressure Possible Involvement of Nitric Oxide and Asymmetric NG, NG-Dimethylarginine. Circ. J. 2005, 69, 221–226. [Google Scholar] [CrossRef]
- Moinard, C.; Maccario, J.; Walrand, S.; Lasserre, V.; Marc, J.; Boirie, Y.; Cynober, L. Arginine behaviour after arginine or citrulline administration in older subjects. Br. J. Nutr. 2015, 115, 399–404. [Google Scholar] [CrossRef][Green Version]
- Chien, S.-J.; Lin, K.-M.; Kuo, H.-C.; Huang, C.-F.; Lin, Y.-J.; Huang, L.-T.; Tain, Y.-L. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: L-citrulline and nitrate. Transl. Res. 2014, 163, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Juliet, P.A.R.; Matsui-Hirai, H.; Miyazaki, A.; Fukatsu, A.; Funami, J.; Iguchi, A.; Ignarro, L.J. l-citrulline and l-arginine supplementation retards the progression of high-cholesterol-diet-induced atherosclerosis in rabbits. Proc. Natl. Acad. Sci. USA 2005, 102, 13681–13686. [Google Scholar] [CrossRef]
- Heidorn, M.; Frodermann, T.; Böning, A.; Schreckenberg, R.; Schlüter, K.-D. Citrulline Improves Early Post-Ischemic Recovery or Rat Hearts In Vitro by Shifting Arginine Metabolism From Polyamine to Nitric Oxide Formation. Clin. Med. Insights Cardiol. 2018, 12, 1179546818771908. [Google Scholar] [CrossRef] [PubMed]
- Baumgardt, S.L.; Paterson, M.; Leucker, T.M.; Fang, J.; Zhang, D.X.; Bosnjak, Z.J.; Warltier, D.C.; Kersten, J.R.; Ge, Z.-D. Chronic Co-Administration of Sepiapterin and l-Citrulline Ameliorates Diabetic Cardiomyopathy and Myocardial Ischemia/Reperfusion Injury in Obese Type 2 Diabetic Mice. Circ. Hear. Fail. 2016, 9, e002424. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2007, 99, 855–862. [Google Scholar] [CrossRef]
- Figueroa, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kalfon, R. l-Citrulline supplementation attenuates blood pressure, wave reflection and arterial stiffness responses to metaboreflex and cold stress in overweight men. Br. J. Nutr. 2016, 116, 279–285. [Google Scholar] [CrossRef]
- Balderas-Munoz, K.; Castillo-Martínez, L.; Orea-Tejeda, A.; Infante-Vázquez, O.; Utrera-Lagunas, M.; Martínez-Memije, R.; Keirns-Davis, C.; Becerra-Luna, B.; Sánchez-Vidal, G. Improvement of ventricular function in systolic heart failure patients with oral L-citrulline supplementation. Cardiol. J. 2012, 19, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Belaidi, E.; Béguin, P.C.; Lévy, P.; Ribuot, C.; Godin-Ribuot, D. Prevention of HIF-1 activation and iNOS gene targeting by low-dose cadmium results in loss of myocardial hypoxic preconditioning in the rat. Am. J. Physiol. Circ. Physiol. 2008, 294, H901–H908. [Google Scholar] [CrossRef] [PubMed]
- Détrait, M.; Pesse, M.; Calissi, C.; Bouyon, S.; Brocard, J.; Vial, G.; Pépin, J.; Belaidi, E.; Arnaud, C. Short-term intermittent hypoxia induces simultaneous systemic insulin resistance and higher cardiac contractility in lean mice. Physiol. Rep. 2021, 9, e14738. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Oberley, L.W.; Spitz, D.R. Assay of Superoxide Dismutase Activity in Tumor Tissue. In Oxygen Radicals in Biological Systems; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 457–464. [Google Scholar]
- Johansson, L.H.; Borg, L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [CrossRef]
- Ribon-Demars, A.; Jochmans-Lemoine, A.; Ganouna-Cohen, G.; Boreau, A.; Marcouiller, F.; Bairam, A.; Pialoux, V.; Joseph, V. Lung oxidative stress and transcriptional regulations induced by estradiol and intermittent hypoxia. Free Radic. Biol. Med. 2021, 164, 119–129. [Google Scholar] [CrossRef]
- Galiñanes, M.; Matata, B.M. Protein nitration is predominantly mediated by a peroxynitrite-dependent pathway in cultured human leucocytes. Biochem. J. 2002, 367, 467–473. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.L.; Almendros, I.; Wang, Y.; Peris, E.; Qiao, Z.; Gozal, D. Resveratrol Attenuates Intermittent Hypoxia-Induced Macrophage Migration to Visceral White Adipose Tissue and Insulin Resistance in Male Mice. Endocrinology 2015, 156, 437–443. [Google Scholar] [CrossRef]
- Belaidi, E.; Ramond, A.; Joyeux-Faure, M.; Lévy, P.; Ribuot, C.; Godin-Ribuot, D. Contrasting Effects of Intermittent Hypoxia on Myocardial Ischemic Tolerance. In Intermittent Hypoxia: From Molecular Mechanisms to Clinical Applications; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 3–18. [Google Scholar]
- Man, A.W.C.; Zhou, Y.; Lam, U.D.P.; Reifenberg, G.; Werner, A.; Habermeier, A.; Closs, E.I.; Daiber, A.; Münzel, T.; Xia, N.; et al. l-Citrulline ameliorates pathophysiology in a rat model of superimposed preeclampsia. J. Cereb. Blood Flow Metab. 2022, 179, 3007–3023. [Google Scholar] [CrossRef]
- Gaisl, T.; Bratton, D.J.; Kohler, M. The impact of obstructive sleep apnoea on the aorta. Eur. Respir. J. 2015, 46, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, C.; Bouyon, S.; Recoquillon, S.; Brasseur, S.; Lemarié, E.; Briançon-Marjollet, A.; Gonthier, B.; Toral, M.; Faury, G.; Martinez, M.C.; et al. Nonmuscle Myosin Light Chain Kinase: A Key Player in Intermittent Hypoxia-Induced Vascular Alterations. J. Am. Hear. Assoc. 2018, 7, e007893. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Nitta, C.; Henderson, K.; Codianni, S.; Sanchez, L.; Ramiro-Diaz, J.; Howard, T.; Giermakowska, W.; Kanagy, N.; Bosc, L.G. Intermittent hypoxia-induced increases in reactive oxygen species activate NFATc3 increasing endothelin-1 vasoconstrictor reactivity. Vasc. Pharmacol. 2013, 60, 17–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jun, J.; Savransky, V.; Nanayakkara, A.; Bevans, S.; Li, J.; Smith, P.L.; Polotsky, V.Y. Intermittent hypoxia has organ-specific effects on oxidative stress. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1274–R1281. [Google Scholar] [CrossRef]
- Belaidi, E.; Khouri, C.; Harki, O.; Baillieul, S.; Faury, G.; Briançon-Marjollet, A.; Pépin, J.-L.; Arnaud, C. Cardiac consequences of intermittent hypoxia: A matter of dose? A systematic review and meta-analysis in rodents. Eur. Respir. Rev. 2022, 31, 210269. [Google Scholar] [CrossRef]
- Fike, C.D.; Summar, M.; Aschner, J.L. L-citrulline provides a novel strategy for treating chronic pulmonary hypertension in newborn infants. Acta Paediatr. 2014, 103, 1019–1026. [Google Scholar] [CrossRef]
- Huie, R.E.; Padmaja, S. The Reaction of no with Superoxide. Free Radic. Res. Commun. 1993, 18, 195–199. [Google Scholar] [CrossRef]
- Stamler, J.S.; Piantadosi, C.A. O=O NO: It’s CO. J. Clin. Investig. 1996, 97, 2165–2166. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Tan, Y.; Cai, X.; Cai, L.; Cai, J.; Zheng, Y. Deletion of Metallothionein Exacerbates Intermittent Hypoxia-Induced Oxidative and Inflammatory Injury in Aorta. Oxid. Med. Cell. Longev. 2014, 2014, 141053. [Google Scholar] [CrossRef]
- Siques, P.; de Pablo, L.L.; Brito, J.; Arribas, S.M.; Flores, K.; Arriaza, K.; Naveas, N.; González, M.C.; Hoorntje, A.; León-Velarde, F.; et al. Nitric Oxide and Superoxide Anion Balance in Rats Exposed to Chronic and Long Term Intermittent Hypoxia. BioMed Res. Int. 2014, 2014, 610474. [Google Scholar] [CrossRef]
- Badran, M.; Abuyassin, B.; Golbidi, S.; Ayas, N.; Laher, I. Uncoupling of Vascular Nitric Oxide Synthase Caused by Intermittent Hypoxia. Oxid. Med. Cell. Longev. 2016, 2016, 2354870. [Google Scholar] [CrossRef] [PubMed]
- Fike, C.D.; Dikalova, A.; Kaplowitz, M.R.; Cunningham, G.; Summar, M.; Aschner, J.L. Rescue Treatment with L-Citrulline Inhibits Hypoxia-Induced Pulmonary Hypertension in Newborn Pigs. Am. J. Respir. Cell Mol. Biol. 2015, 53, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.J.; Bates, M.L.; Del Rio, R.; Wang, Z.; Dopp, J.M. Oxidative stress augments chemoreflex sensitivity in rats exposed to chronic intermittent hypoxia. Respir. Physiol. Neurobiol. 2016, 234, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Q.; Xu, X.; Zhen, W.-W.; Luo, Y.-L.; Cai, B.; Zhang, S. Protective Effect of Citrulline on the Hearts of Rats with Sepsis Induced by Cecal Ligation and Puncture. BioMed Res. Int. 2018, 2018, 2574501. [Google Scholar] [CrossRef]
- Hassel, C.; Couchet, M.; Jacquemot, N.; Blavignac, C.; Loï, C.; Moinard, C.; Cia, D. Citrulline protects human retinal pigment epithelium from hydrogen peroxide and iron/ascorbate induced damages. J. Cell. Mol. Med. 2022, 26, 2808–2818. [Google Scholar] [CrossRef]
- Goron, A.; Lamarche, F.; Blanchet, S.; Delangle, P.; Schlattner, U.; Fontaine, E.; Moinard, C. Citrulline stimulates muscle protein synthesis, by reallocating ATP consumption to muscle protein synthesis. J. Cachex- Sarcopenia Muscle 2019, 10, 919–928. [Google Scholar] [CrossRef]
- Moinard, C.; Fontaine, E. Direct or indirect regulation of muscle protein synthesis by energy status? Clin. Nutr. 2021, 40, 1893–1896. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Didelija, I.C.; Marini, J.C. Arginase II Plays a Central Role in the Sexual Dimorphism of Arginine Metabolism in C57BL/6 Mice. J. Nutr. 2020, 150, 3133–3140. [Google Scholar] [CrossRef]
- Gonzales, J.U.; Raymond, A.; Ashley, J.; Kim, Y. Doesl-citrulline supplementation improve exercise blood flow in older adults? Exp. Physiol. 2017, 102, 1661–1671. [Google Scholar] [CrossRef]
- Joseph, V.; Pagliardini, S.; Belaidi, E. Editorial: Causes and Consequences of Sleep Apnea: Spotlights on the Roles of Sex and Sex Hormones. Front. Physiol. 2022, 13, 857627. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozcan, B.; Blachot-Minassian, B.; Paradis, S.; Mazière, L.; Chambion-Diaz, M.; Bouyon, S.; Pépin, J.-L.; Pialoux, V.; Arnaud, C.; Moinard, C.; et al. L-Citrulline Supplementation Reduces Blood Pressure and Myocardial Infarct Size under Chronic Intermittent Hypoxia, a Major Feature of Sleep Apnea Syndrome. Antioxidants 2022, 11, 2326. https://doi.org/10.3390/antiox11122326
Ozcan B, Blachot-Minassian B, Paradis S, Mazière L, Chambion-Diaz M, Bouyon S, Pépin J-L, Pialoux V, Arnaud C, Moinard C, et al. L-Citrulline Supplementation Reduces Blood Pressure and Myocardial Infarct Size under Chronic Intermittent Hypoxia, a Major Feature of Sleep Apnea Syndrome. Antioxidants. 2022; 11(12):2326. https://doi.org/10.3390/antiox11122326
Chicago/Turabian StyleOzcan, Bilgehan, Britanny Blachot-Minassian, Stéphanie Paradis, Lucile Mazière, Marie Chambion-Diaz, Sophie Bouyon, Jean-Louis Pépin, Vincent Pialoux, Claire Arnaud, Christophe Moinard, and et al. 2022. "L-Citrulline Supplementation Reduces Blood Pressure and Myocardial Infarct Size under Chronic Intermittent Hypoxia, a Major Feature of Sleep Apnea Syndrome" Antioxidants 11, no. 12: 2326. https://doi.org/10.3390/antiox11122326
APA StyleOzcan, B., Blachot-Minassian, B., Paradis, S., Mazière, L., Chambion-Diaz, M., Bouyon, S., Pépin, J.-L., Pialoux, V., Arnaud, C., Moinard, C., & Belaidi, E. (2022). L-Citrulline Supplementation Reduces Blood Pressure and Myocardial Infarct Size under Chronic Intermittent Hypoxia, a Major Feature of Sleep Apnea Syndrome. Antioxidants, 11(12), 2326. https://doi.org/10.3390/antiox11122326







