Systematic Insight of Resveratrol Activated SIRT1 Interactome through Proximity Labeling Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Plasmid Constructions and Generation of shRNA Expression Cells
2.4. APEX2 Labeling and Preparation of Cell Lysates
2.5. Samples Alkylation, Protein Enrichment, and on Beads Digestion
2.6. LC-MS/MS Analysis
2.7. Cell Viability Assay
2.8. Co-Immunoprecipitation (Co-IP)
2.9. RNA Extraction and Real-Time (RT) PCR
2.10. Intracellular Reactive Oxygen Species (ROS) Determination
2.11. Western Blot Assay
2.12. Statistical Analysis
3. Results
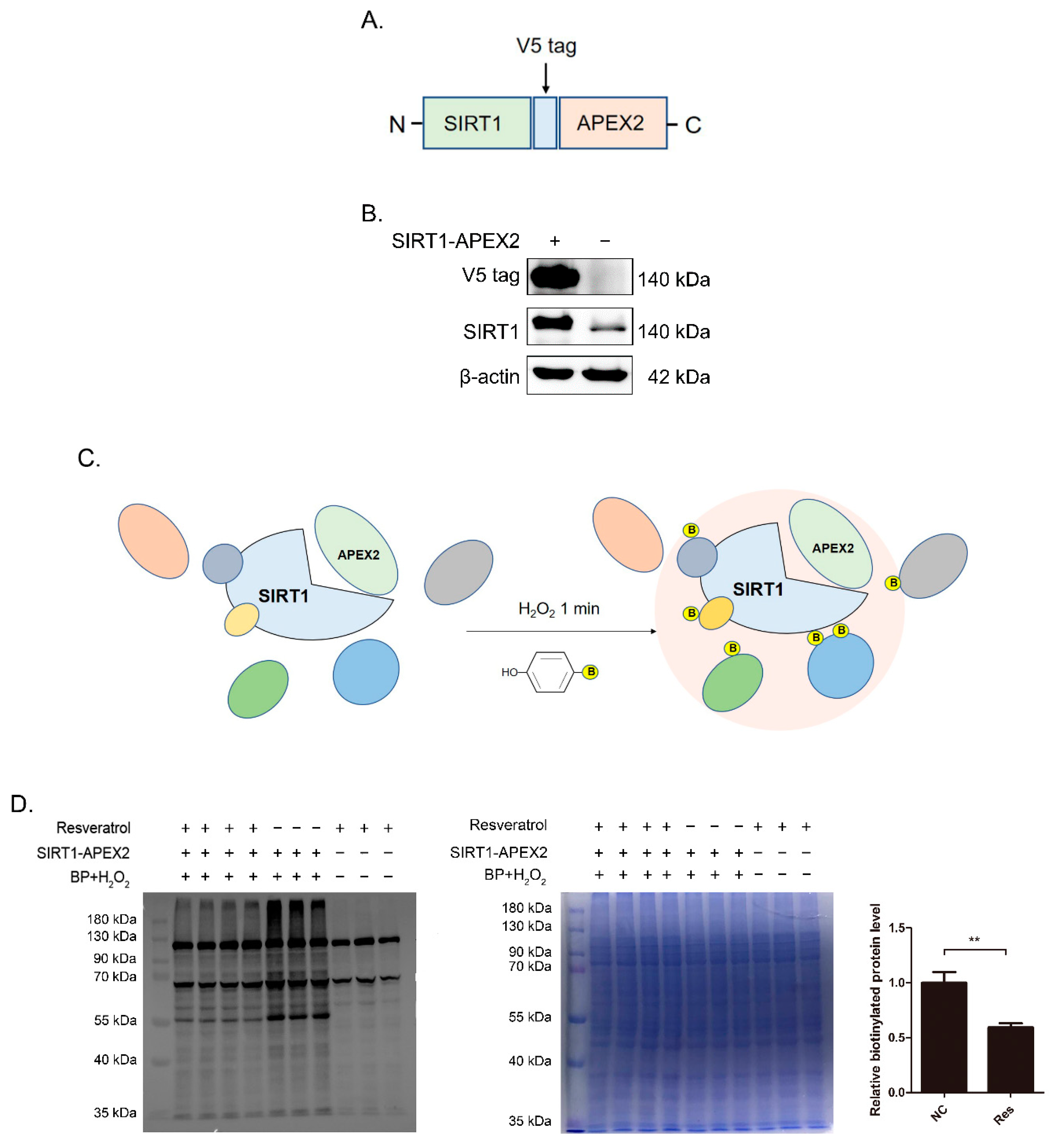
3.1. Construction of SIRT1-APEX2 Plasmid and Identification of Proximity Labeling
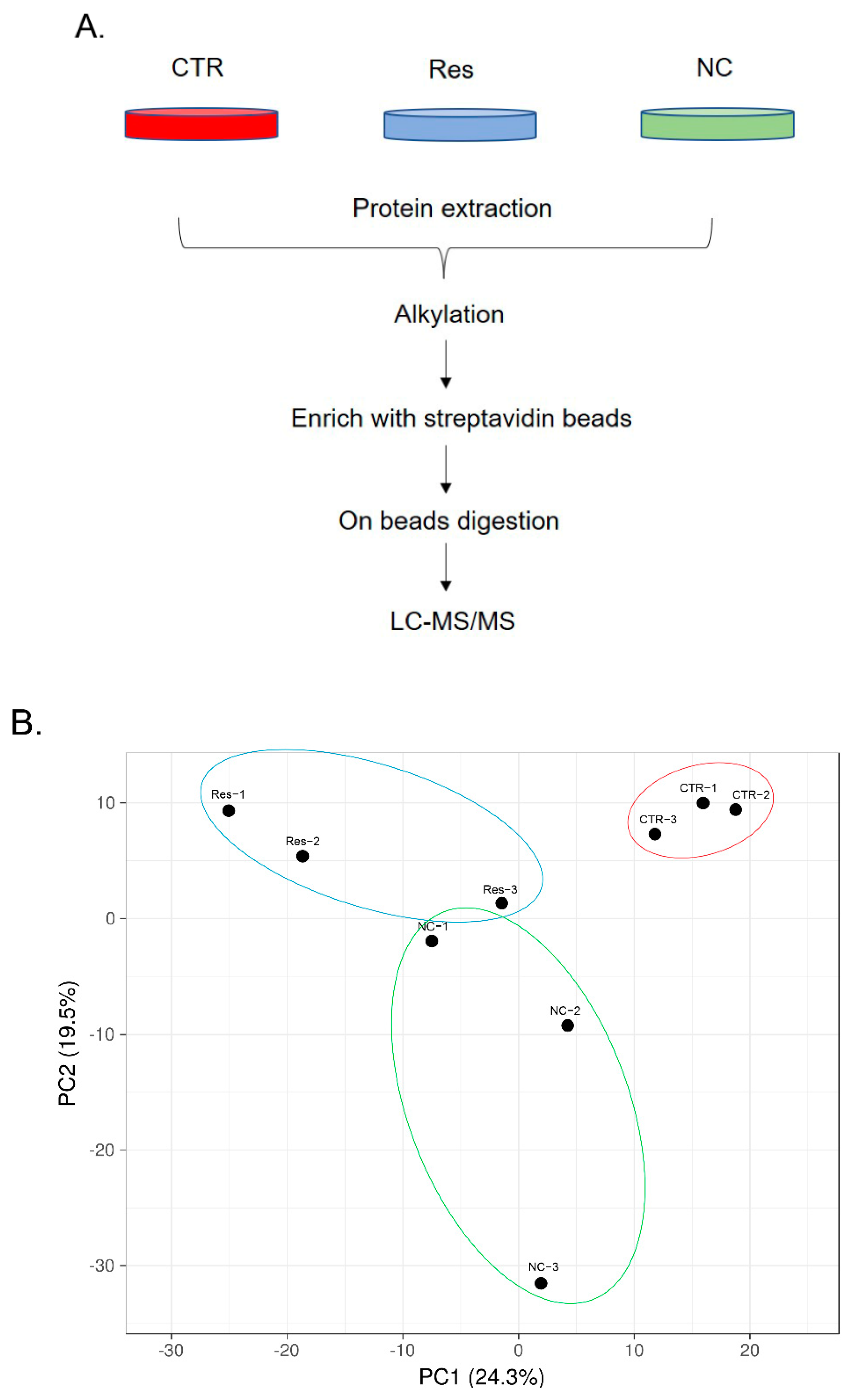
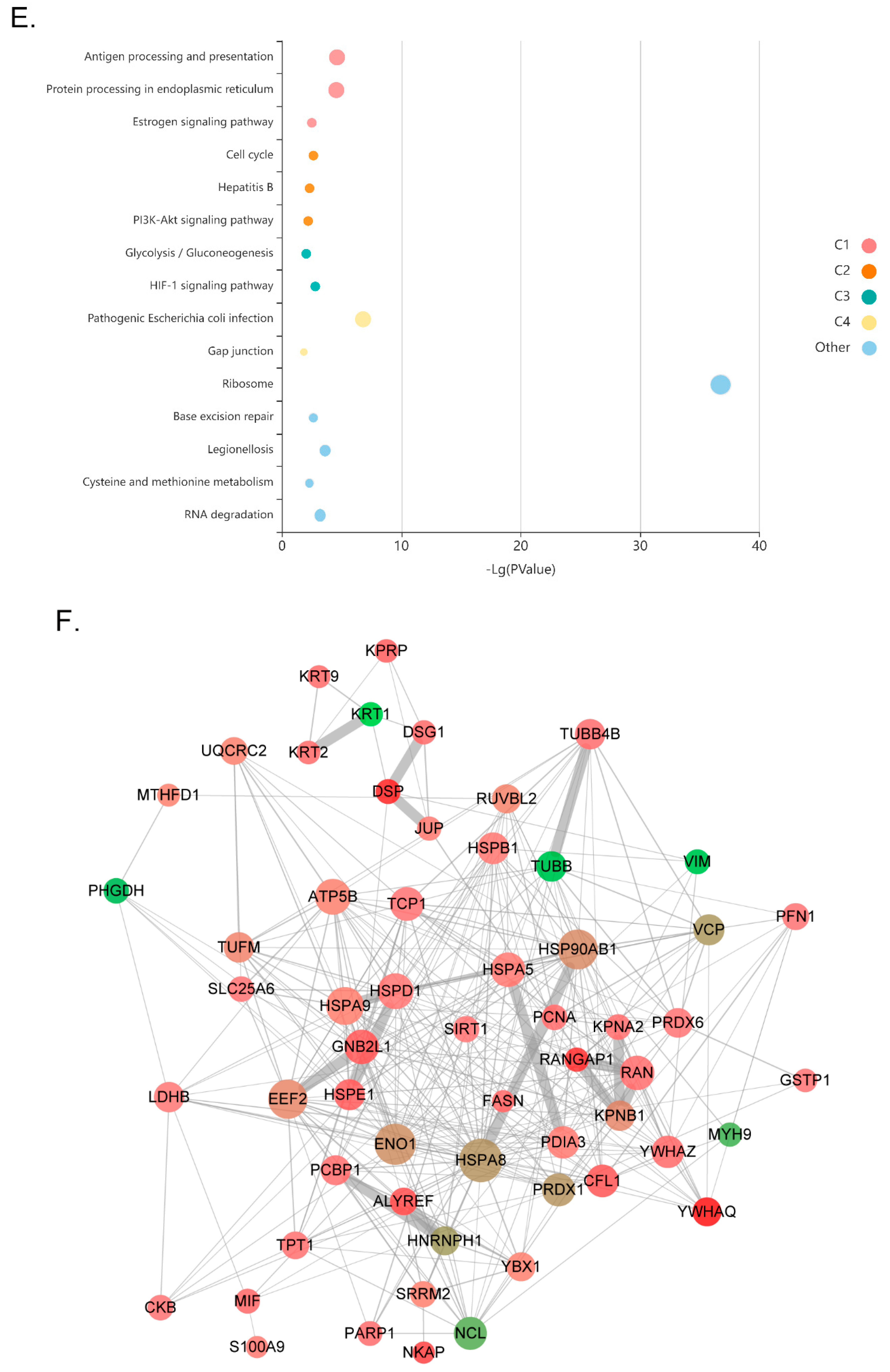
3.2. Dynamic Changes in SIRT1 Interactions in Response to Resveratrol
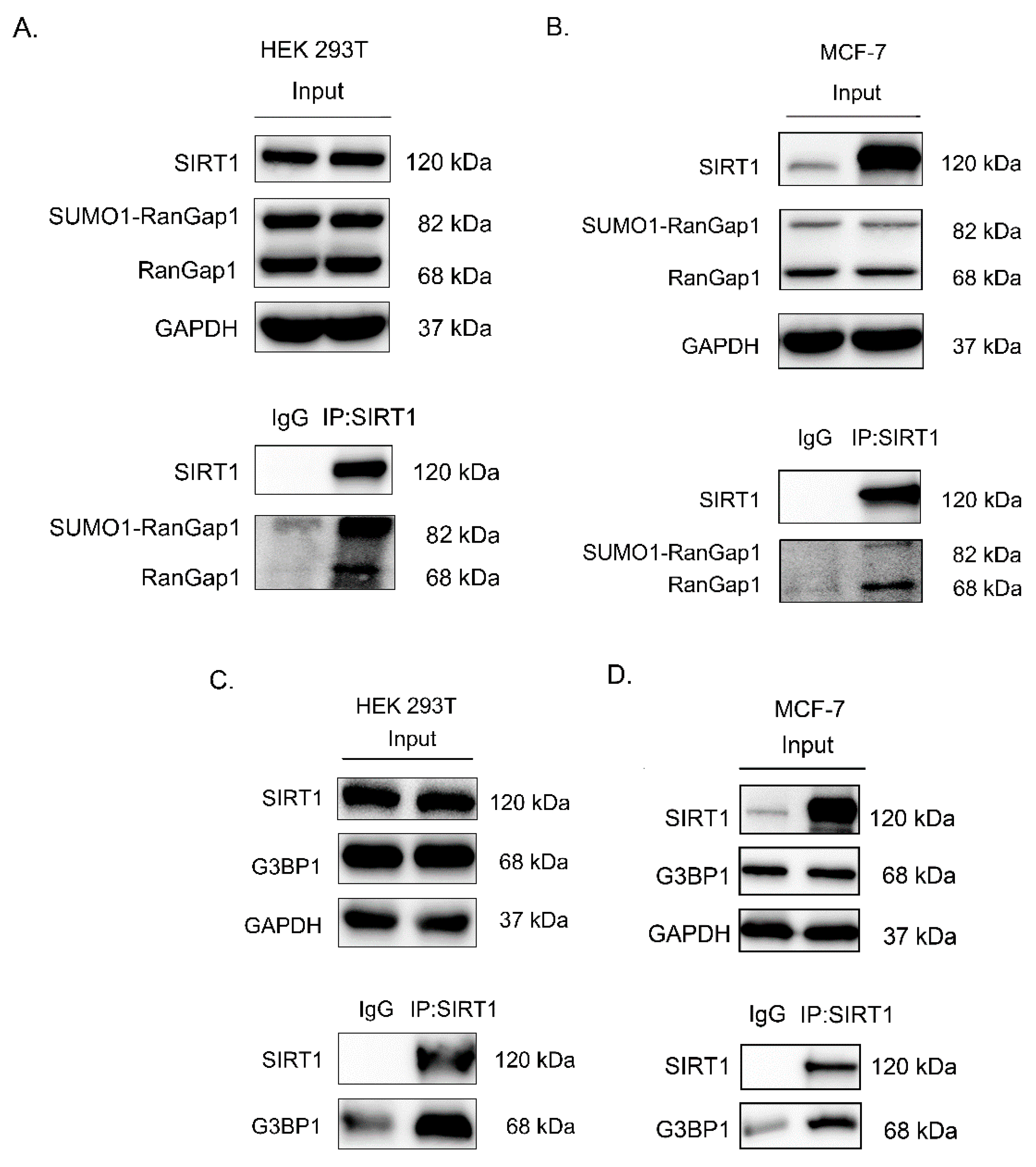
3.3. SIRT1 Interacts with RanGap1 and G3BP1
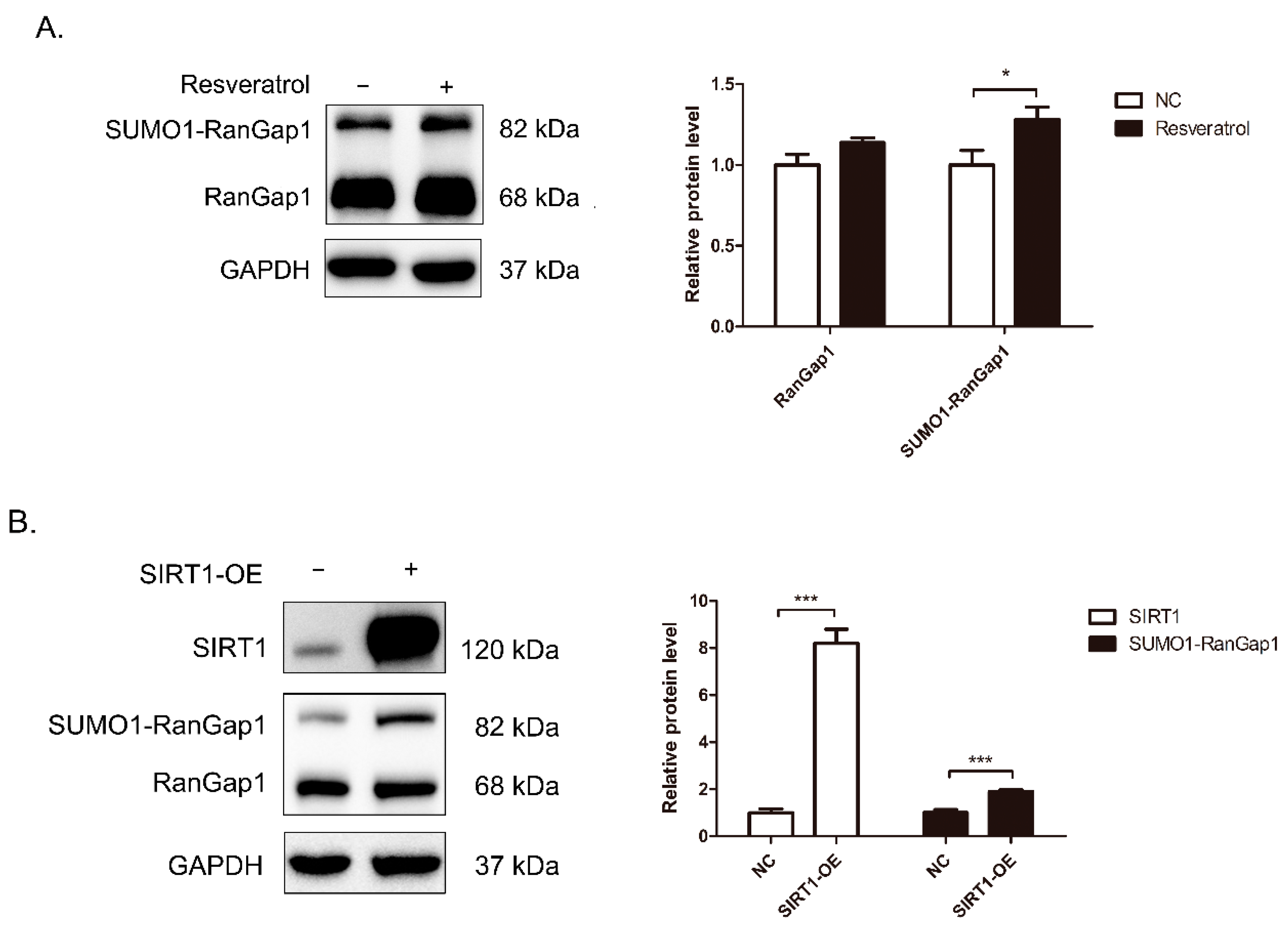
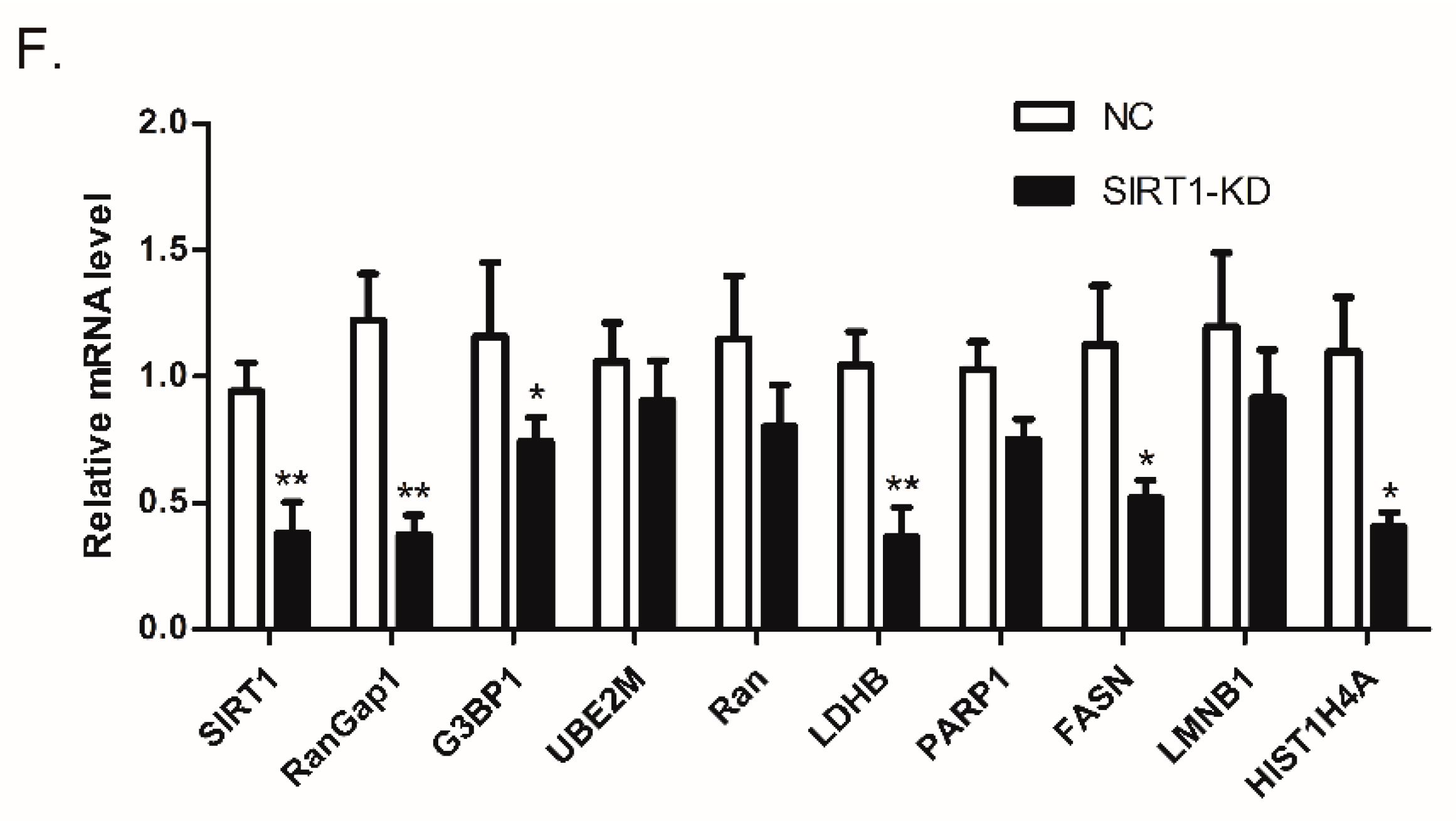
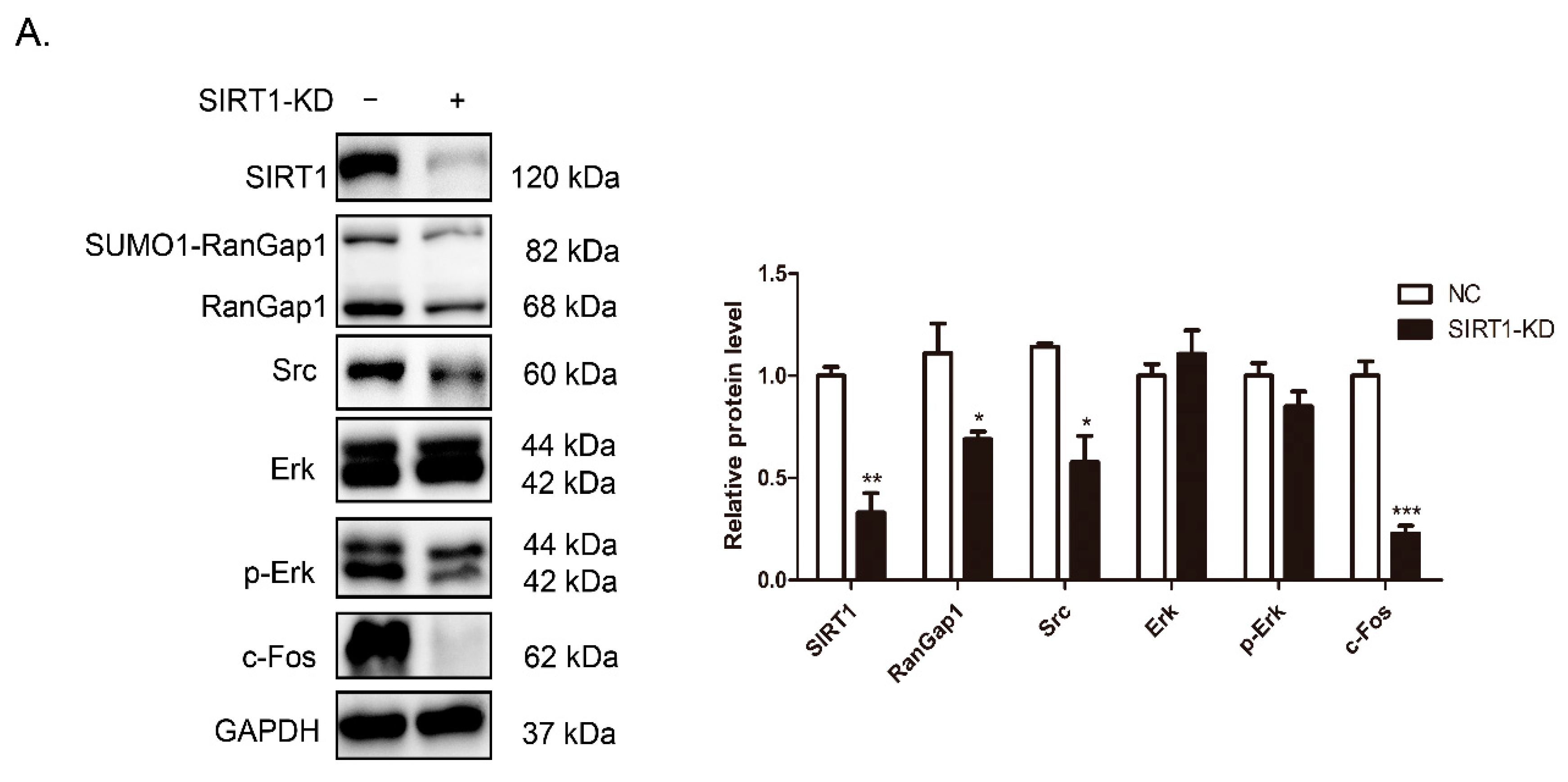
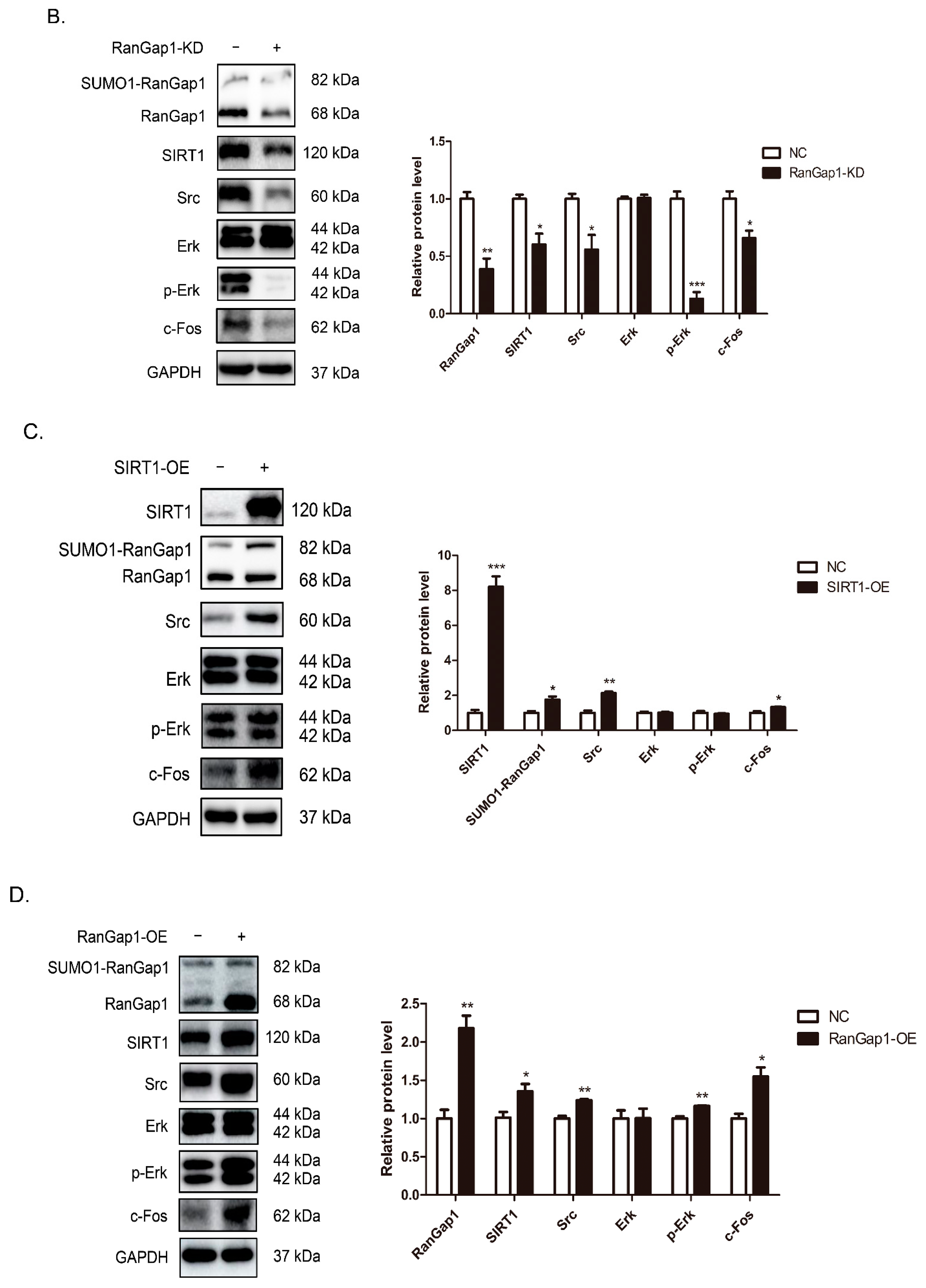
3.4. SIRT1 Regulates RanGap1 Expression
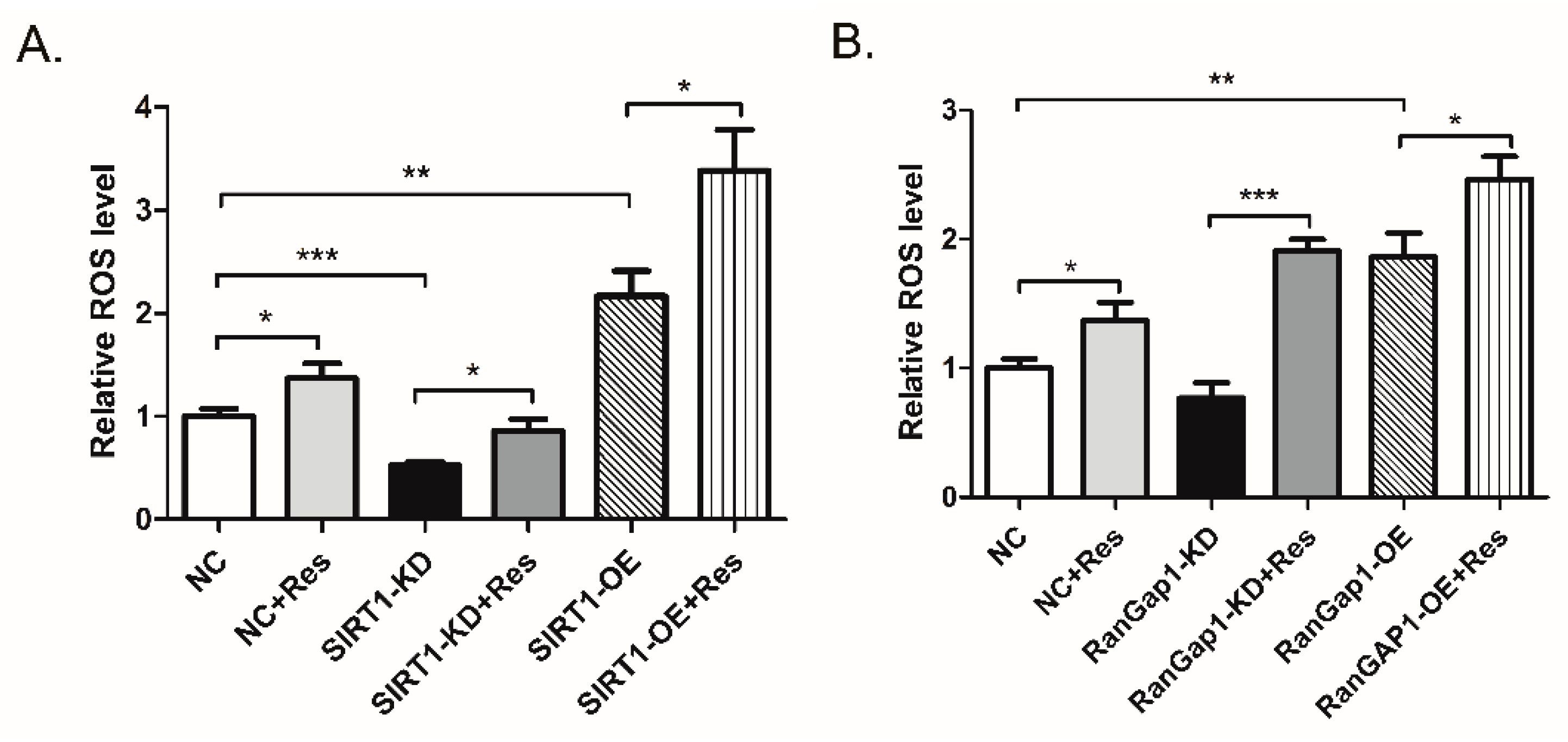
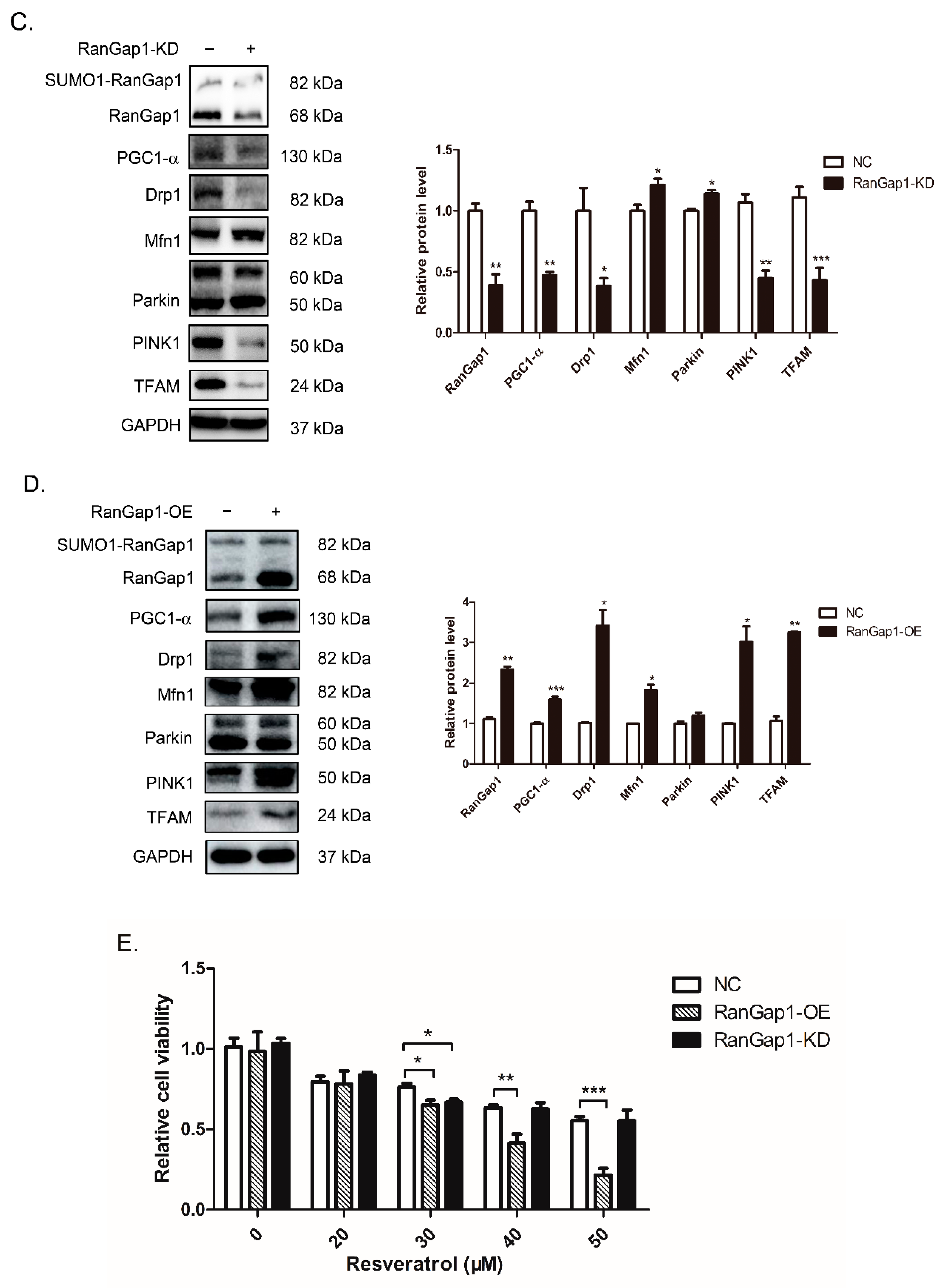
3.5. RanGap1 Affects Antioxidant Capacity and Mitochondrial Functions
3.6. SIRT1 and RanGap1 Participate in the Src/Erk/c-Fos Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gold, D.A.; Sinclair, D.A. Sirtuin evolution at the dawn of animal Life. Mol. Biol. Evol. 2022, 39, 192. [Google Scholar] [CrossRef]
- Behnisch-Cornwell, S.; Grathwol, C.W.; Schulig, L.; Voigt, A.; Baecker, D.; Link, A.; Bednarski, P.J. Correlation analysis of protein expression of 10 HDAC/sirtuin isoenzymes with sensitivities of 23 anticancer drugs in 17 cancer cell lines and potentiation of drug activity by co-treatment with HDAC inhibitors. Cancers 2021, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Jalgaonkar, M.P.; Parmar, U.M.; Kulkarni, Y.A.; Oza, M.J. SIRT1-FOXOs activity regulates diabetic complications. Pharm. Res. 2022, 175, 106014. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rong, F.; Tang, J.; Zhu, C.; Chen, X.; Jia, S.; Wang, Z.; Sun, X.; Deng, H.; Zha, H.; et al. Repression of p53 function by SIRT5-mediated desuccinylation at Lysine 120 in response to DNA damage. Cell Death Differ. 2022, 29, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, S.S.; Moreira, J.B.; Costa, M.; Rodrigues, R.S.; Sebastião, A.M.; Xapelli, S.; Solá, S. The mitochondrial antioxidant Sirtuin3 cooperates with lipid metabolism to safeguard neurogenesis in aging and depression. Cells 2021, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, Y.; Hou, Y.; Zhao, Z.; Liang, N.; Yuan, P.; Yang, T.; Xing, J.; Li, J. Increased mitochondrial fission drives the reprogramming of fatty acid metabolism in hepatocellular carcinoma cells through suppression of Sirtuin 1. Cancer Commun. 2022, 42, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Sun, W.; Sun, H.; Zhang, L. Perfluorooctane sulfonate continual exposure impairs glucose-stimulated insulin secretion via SIRT1-induced upregulation of UCP2 expression. Environ. Pollut. 2021, 278, 116840. [Google Scholar] [CrossRef]
- Myers, M.; Shaik, F.; Shaik, F.; Alway, S.; Mohamed, J. Skeletal muscle gene expression profile in response to caloric restriction and aging: A role for SirT1. Genes 2021, 12, 691. [Google Scholar] [CrossRef] [PubMed]
- Sin, T.K.; Yung, B.Y.; Siu, P.M. Modulation of SIRT1-Foxo1 signaling axis by resveratrol: Implications in skeletal muscle aging and insulin resistance. Cell Physiol. Biochem. 2015, 35, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Jaemin, J.; Kyungmi, J.; Hansoo, L.; Sang-Hoon, K.; Bon-Hong, M.; Kyung-Mi, L.; Myung-Haeng, C.; Gil-Hong, P.; Kee-Ho, L. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp. Mol. Med. 2007, 39, 8–13. [Google Scholar]
- Shinozaki, S.; Chang, K.; Sakai, M.; Shimizu, N.; Yamada, M.; Tanaka, T.; Nakazawa, H.; Ichinose, F.; Yamada, Y.; Ishigami, A.; et al. Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci. Signal. 2014, 7, ra106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Cheng, X.; Wang, D.; Peng, M.; Xue, Z.; Da, Y.; Zhang, N.; Yao, Z.; Li, M.; Xu, A.; et al. Adiponectin promotes pancreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1α signaling. Oncotarget 2014, 5, 4732–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Zhang, M.; Kageyama, S.; Ke, B.; Fujii, T.; Sosa, R.A.; Reed, E.F.; Datta, N.; Zarrinpar, A.; Busuttil, R.W.; et al. Macrophage heme oxygenase-1-SIRT1-p53 axis regulates sterile inflammation in liver ischemia-reperfusion injury. J. Hepatol. 2017, 67, 1232–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herranz, D.; Muñoz-Martin, M.; Cañamero, M.; Mulero, F.; Martinez-Pastor, B.; Fernandez-Capetillo, O.; Serrano, M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Kitada, M.; Koya, D. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes Metab. J. 2013, 37, 315–325. [Google Scholar] [CrossRef]
- Abdelmawgoud, H.; El Awady, R.R. Effect of Sirtuin 1 inhibition on matrix metalloproteinase 2 and Forkhead box O3a expression in breast cancer cells. Genes Dis. 2017, 4, 240–246. [Google Scholar] [CrossRef]
- Lei, M.; Cong, L.; Liu, Z.; Liu, Z.; Ma, Z.; Liu, K.; Li, J.; Deng, Y.; Liu, W.; Xu, B. Resveratrol reduces DRP1-mediated mitochondrial dysfunction via the SIRT1-PGC1alpha signaling pathway in manganese-induced nerve damage in mice. Environ. Toxicol. 2022, 37, 282–298. [Google Scholar] [CrossRef] [PubMed]
- A Fernández-Rodríguez, J.; Almonte-Becerril, M.; Ramil-Gómez, O.; Hermida-Carballo, L.; Viñas-Diz, S.; Vela-Anero, Á.; Concha, Á.; Camacho-Encina, M.; Blanco, F.J.; López-Armada, M.J. Autophagy activation by resveratrol reduces severity of experimental rheumatoid arthritis. Mol. Nutr. Food Res. 2021, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, J.; Wang, J.; Liu, Q. The controversial links among calorie restriction, SIRT1, and resveratrol. Free Radic. Biol. Med. 2011, 51, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gautam, V.K.; Costantini, S.; Paladino, A.; Colonna, G. Interactomic and pharmacological insights on human Sirt-1. Front. Pharmacol. 2012, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.C.; Greco, T.M.; Boonmee, A.; Miteva, Y.; Cristea, I.M. Functional proteomics establishes the interaction of SIRT7 with chromatin remodeling complexes and expands its role in regulation of RNA polymerase I transcription. Mol. Cell Proteom. 2012, 11, 60–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budayeva, H.G.; Cristea, I.M. Human Sirtuin 2 localization, transient interactions, and impact on the proteome point to its role in intracellular trafficking. Mol. Cell Proteom. 2016, 15, 3107–3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, S.S.; Martell, J.D.; Kamer, K.J.; Deerinck, T.J.; Ellisman, M.H.; Mootha, V.K.; Ting, A.Y. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat. Methods 2015, 12, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Honke, K.; Kotani, N. The enzyme-mediated activation of radical source reaction: A new approach to identify partners of a given molecule in membrane microdomains. J. Neurochem. 2011, 116, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.J.; Sizer, I.W. Oxidation of tyramine, tyrosine, and related compounds by peroxidase. J. Biol. Chem. 1959, 234, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Hung, V.; Zou, P.; Rhee, H.-W.; Udeshi, N.D.; Cracan, V.; Svinkina, T.; Carr, S.A.; Mootha, V.K.; Ting, A.Y. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol. Cell 2014, 55, 332–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, H.-W.; Zou, P.; Udeshi, N.D.; Martell, J.D.; Mootha, V.K.; Carr, S.A.; Ting, A.Y. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 2013, 339, 1328–1331. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-L.; Hu, Y.; Udeshi, N.D.; Lau, T.Y.; Wirtz-Peitz, F.; He, L.; Ting, A.Y.; Carr, S.A.; Perrimon, N. Proteomic mapping in live Drosophila tissues using an engineered ascorbate peroxidase. Proc. Natl. Acad. Sci. USA 2015, 112, 12093–12098. [Google Scholar] [CrossRef] [Green Version]
- Hung, V.; Udeshi, N.D.; Lam, S.S.-M.; Loh, K.H.; Cox, K.J.; Pedram, K.; Carr, S.A.; Ting, A.Y. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat. Protoc. 2016, 11, 456–475. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Lesur, A.; Ancheva, L.; Kim, Y.J.; Berchem, G.; van Oostrum, J.; Domon, B. Screening protein isoforms predictive for cancer using immunoaffinity capture and fast LC-MS in PRM mode. Proteom. Clin. Appl. 2015, 9, 695–705. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, H.; Ruzicka, C.; Keire, D.; Ye, H. A general LC-MS/MS method for monitoring potential beta-lactam contamination in drugs and drug-manufacturing surfaces. AAPS J. 2018, 20, 70. [Google Scholar] [CrossRef]
- Ciapetti, G.; Cenni, E.; Pratelli, L.; Pizzoferrato, A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials 1993, 14, 359–364. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Hubbard, B.P.; Gomes, A.P.; Dai, H.; Li, J.; Case, A.W.; Considine, T.; Riera, T.V.; Lee, J.E.; E, S.Y.; Lamming, D.W.; et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 2013, 339, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordbari, S.; Mörchen, B.; Pylaeva, E.; Siakaeva, E.; Spyra, I.; Domnich, M.; Droege, F.; Kanaan, O.; Lang, K.S.; Schadendorf, D.; et al. SIRT1-mediated deacetylation of FOXO3a transcription factor supports pro-angiogenic activity of interferon-deficient tumor-associated neutrophils. Int. J. Cancer 2022, 150, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Furukawa-Hibi, Y.; Chen, C.; Horio, Y.; Isobe, K.; Ikeda, K.; Motoyama, N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int. J. Mol. Med. 2005, 16, 237–243. [Google Scholar] [CrossRef]
- Qiu, D.; Song, S.; Wang, Y.; Bian, Y.; Wu, M.; Wu, H.; Shi, Y.; Duan, H. NAD(P)H: Quinone oxidoreductase 1 attenuates oxidative stress and apoptosis by regulating Sirt1 in diabetic nephropathy. J. Transl. Med. 2022, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yan, H.; An, S.; Shen, M.; Jia, W.; Zhang, R.; Zhao, L.; Huang, G.; Liu, J. SIRT5-mediated deacetylation of LDHB promotes autophagy and tumorigenesis in colorectal cancer. Mol. Oncol. 2019, 13, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Sobuz, S.U.; Sato, Y.; Yoshizawa, T.; Karim, F.; Ono, K.; Sawa, T.; Miyamoto, Y.; Oka, M.; Yamagata, K. SIRT7 regulates the nuclear export of NF-kappaB p65 by deacetylating Ran. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Wang, Z.; Zhang, Z.; Hou, Z.; Han, X.; Yang, F.; Liu, H. Resveratrol regulates Hsp60 in HEK 293T cells during activation of SIRT1 revealed by nascent protein labeling strategy. Food Nutr. Res. 2022, 66, 8224. [Google Scholar] [CrossRef]
- Oi, N.; Yuan, J.; Malakhova, M.; Luo, K.; Li, Y.; Ryu, J.; Zhang, L.; Bode, A.M.; Xu, Z.; Lou, Z.; et al. Resveratrol induces apoptosis by directly targeting Ras-GTPase-activating protein SH3 domain-binding protein 1. Oncogene 2015, 34, 2660–2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Chen, G.F.; Ma, Y.S.; Zhang, H.W.; Zhou, Y.; Liu, G.H.; Chen, D.-Y.; Ping, J.; Liu, Y.-H.; Mou, X.; et al. Hepatic proteomic changes and Sirt1/AMPK signaling activation by oxymatrine treatment in rats with non-alcoholic steatosis. Front. Pharm. 2020, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-H.; Wang, J.-X.; Cai, M.-L.; Shao, R.; Liu, H.; Zhao, W.-L. The roles and mechanisms of G3BP1 in tumour promotion. J. Drug Target. 2019, 27, 300–305. [Google Scholar] [CrossRef]
- Rifaï, K.; Judes, G.; Idrissou, M.; Daures, M.; Bignon, Y.-J.; Penault-Llorca, F.; Bernard-Gallon, D. SIRT1-dependent epigenetic regulation of H3 and H4 histone acetylation in human breast cancer. Oncotarget 2018, 9, 30661–30678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, H.; Li, X.; Ouyang, C.; Ke, H.; Yu, X.; Tan, J.; Chen, J.; Wang, C.; Zhang, L.; Tang, Y.; et al. Salidroside prevents PM2.5-induced BEAS-2B cell apoptosis via SIRT1-dependent regulation of ROS and mitochondrial function. Ecotoxicol. Environ. Saf. 2022, 231, 113170. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Bennett, L.L. Resveratrol enhances the efficacy of sorafenib mediated apoptosis in human breast cancer MCF7 cells through ROS, cell cycle inhibition, caspase 3 and PARP cleavage. Biomed. Pharm. 2016, 84, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xiong, J.; Gao, Y.; Li, J.; Zhu, D.; Shen, X.; Sun, L.; Wang, X. Resveratrol activates PI3K/AKT to reduce myocardial cell apoptosis and mitochondrial oxidative damage caused by myocardial ischemia/reperfusion injury. Acta Histochem. 2021, 123, 151739. [Google Scholar] [CrossRef]
- Huang, Q.; Lou, T.; Lu, J.; Wang, M.; Chen, X.; Xue, L.; Tang, X.; Qi, W.; Zhang, Z.; Su, H.; et al. Major ginsenosides from Panax ginseng promote aerobic cellular respiration and SIRT1-mediated mitochondrial biosynthesis in cardiomyocytes and neurons. J. Ginseng Res. 2022, 46, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.-L.; Chou, H.-C.; Duran, M.; Gruenewald, J.; Waterfield, M.D.; Ridley, A.; Timms, J.F. Major role of epidermal growth factor receptor and Src kinases in promoting oxidative stress-dependent loss of adhesion and apoptosis in epithelial cells. J. Biol. Chem. 2010, 285, 4307–4318. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhao, W.; Yang, J.S.; Cheng, Z.; Luo, H.; Lu, Z.; Tan, M.; Gu, W.; Zhao, Y. Quantitative acetylome analysis reveals the roles of SIRT1 in regulating diverse substrates and cellular pathways. Mol. Cell. Proteom. 2012, 11, 1048. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Feldser, H.G.; Zhang, W.; Zhong, Z.; Loh, C.; Ellis, J. SIRT1 activators promote p65 deacetylation and suppress TNFa stimulated NF-kB activation. FASEB J. 2011, 25, 945. [Google Scholar] [CrossRef]
- Görlich, D.; Panté, N.; Kutay, U.; Aebi, U.; Bischoff, F.R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. Embo J. 1996, 15, 5584–5594. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Schneider, H.; Recino, A.; Richardson, C.; Goldberg, M.; Rudd, C.E. The immune adaptor SLP-76 binds to SUMO-RANGAP1 at nuclear pore complex filaments to regulate nuclear import of transcription factors in T cells. Mol. Cell 2015, 59, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.; Tan, S.-H.; Karpova, T.; McNally, J.G.; Dasso, M. SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J. Cell Biol. 2002, 156, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Shitashige, M.; Satow, R.; Honda, K.; Ono, M.; Hirohashi, S.; Yamada, T. Regulation of Wnt signaling by the nuclear pore complex. Gastroenterology 2008, 134, 1961–1971. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chen, K.-C.; Liu, H.-J.E.; Liu, A.-J.; Wang, K.-L.; Shih, C.-M. MicroRNA-1301-mediated RanGAP1 downregulation induces BCR-ABL nuclear entrapment to enhance imatinib efficacy in chronic myeloid leukemia cells. PLoS ONE 2016, 11, e0156260. [Google Scholar] [CrossRef] [Green Version]
- Weinert, B.T.; Schölz, C.; Wagner, S.A.; Iesmantavicius, V.; Su, D.; Daniel, J.A.; Choudhary, C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013, 4, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Lehman, J.J.; Barger, P.M.; Kovacs, A.; Saffitz, J.E.; Medeiros, D.M.; Kelly, D.P. Peroxisome proliferator–activated receptor γ coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Investig. 2000, 106, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheida, Z.M.; Ahmad, J.; Sakineh, S.B. The effects of resveratrol supplementation in patients with type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease: An umbrella review of meta-analyses of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 1675–1685. [Google Scholar]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol downregulates mir-31 to promote t regulatory cells during prevention of tnbs-induced colitis. Mol. Nutr. Food Res. 2020, 64, 1900633. [Google Scholar] [CrossRef]


| Accession Numbers | Genes | Forward | Reverse |
|---|---|---|---|
| NM_001142498.2 | SIRT1 | TAGCCTTGTCAGATAAGGAAGGA | ACAGCTTCACAGTCAACTTTGT |
| NM_002883.4 | RanGap1 | CACGCCCTCACGGAAGATTC | CTGGGCTATCAGCACGGAG |
| NM_001256799.3 | GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
| NM_198395.2 | G3BP1 | CGGGCGGGAATTTGTGAGA | TCTGTCCGTAGACTGCATCTG |
| NM_003969.4 | UBE2M | GGAAGCCAGTCCTTACGATAAAC | CGTTCTGCTCAAACAGCCG |
| NM_002300.8 | LDHB | CCTCAGATCGTCAAGTACAGTCC | ATCACGCGGTGTTTGGGTAAT |
| NM_006325.5 | RAN | TCTGGCTTGCTAGGAAGCTCA | GCTGGGTCCATGACAACTTCT |
| NM_030752.3 | TCP1 | CCAGCCACGCTATCCAGTC | TCAAGGCAAGCAATTTTTGCAT |
| NM_004104.5 | FASN | AAGGACCTGTCTAGGTTTGATGC | TGGCTTCATAGGTGACTTCCA |
| NM_005573.4 | LMNB1 | GAAAAAGACAACTCTCGTCGCA | GTAAGCACTGATTCCATGTCCA |
| NM_001618.4 | PARP1 | TCTGAGCTTCGGTGGGATGA | TTGGCATACTCTGCTGCAAAG |
| NM_003540.4 | HIST1H4F | AACGCATTTCGGGCCTCATT | GCGCGTAGACAACATCCATTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, T.; Zhang, Z.; Han, X.; Yang, F.; Wang, Z.; Cheng, Y.; Liu, H. Systematic Insight of Resveratrol Activated SIRT1 Interactome through Proximity Labeling Strategy. Antioxidants 2022, 11, 2330. https://doi.org/10.3390/antiox11122330
Su T, Zhang Z, Han X, Yang F, Wang Z, Cheng Y, Liu H. Systematic Insight of Resveratrol Activated SIRT1 Interactome through Proximity Labeling Strategy. Antioxidants. 2022; 11(12):2330. https://doi.org/10.3390/antiox11122330
Chicago/Turabian StyleSu, Tian, Zhengyi Zhang, Xiao Han, Fei Yang, Zhen Wang, Ying Cheng, and Huadong Liu. 2022. "Systematic Insight of Resveratrol Activated SIRT1 Interactome through Proximity Labeling Strategy" Antioxidants 11, no. 12: 2330. https://doi.org/10.3390/antiox11122330





