Balance of Macrophage Activation by a Complex Coacervate-Based Adhesive Drug Carrier Facilitates Diabetic Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Preparation of Complex Coacervates
2.4. Turbidity Measurements
2.5. Stability Measurements
2.6. Encapsulation Procedure
2.7. Release Kinetics of PAs
2.8. In Vitro Macrophages Culture and Stimulation
2.9. Preparation of Thioglycollate-Elicited Peritoneal Macrophages
2.10. Immunofluorescence
2.11. Quantitative Polymerase Chain Reaction (qPCR)
2.12. Bioinformatic Analyses
2.13. In Vivo Wound Healing in Diabetic Mice
2.14. Wound Immunofluorescence
2.15. Collagen Quantification Using Masson’s Trichrome
2.16. Statistical Analyses
3. Results
3.1. Formation of a Complex Coacervation Base on Och and IP6 Is pH- and Salt-Dependent
3.2. Macroscopic Observation and Stability of ADC
3.3. Drug Encapsulation and In Vitro Drug Release of ADC
3.4. Cytotoxicity and Biocompatibility of ADC and ADC-PA
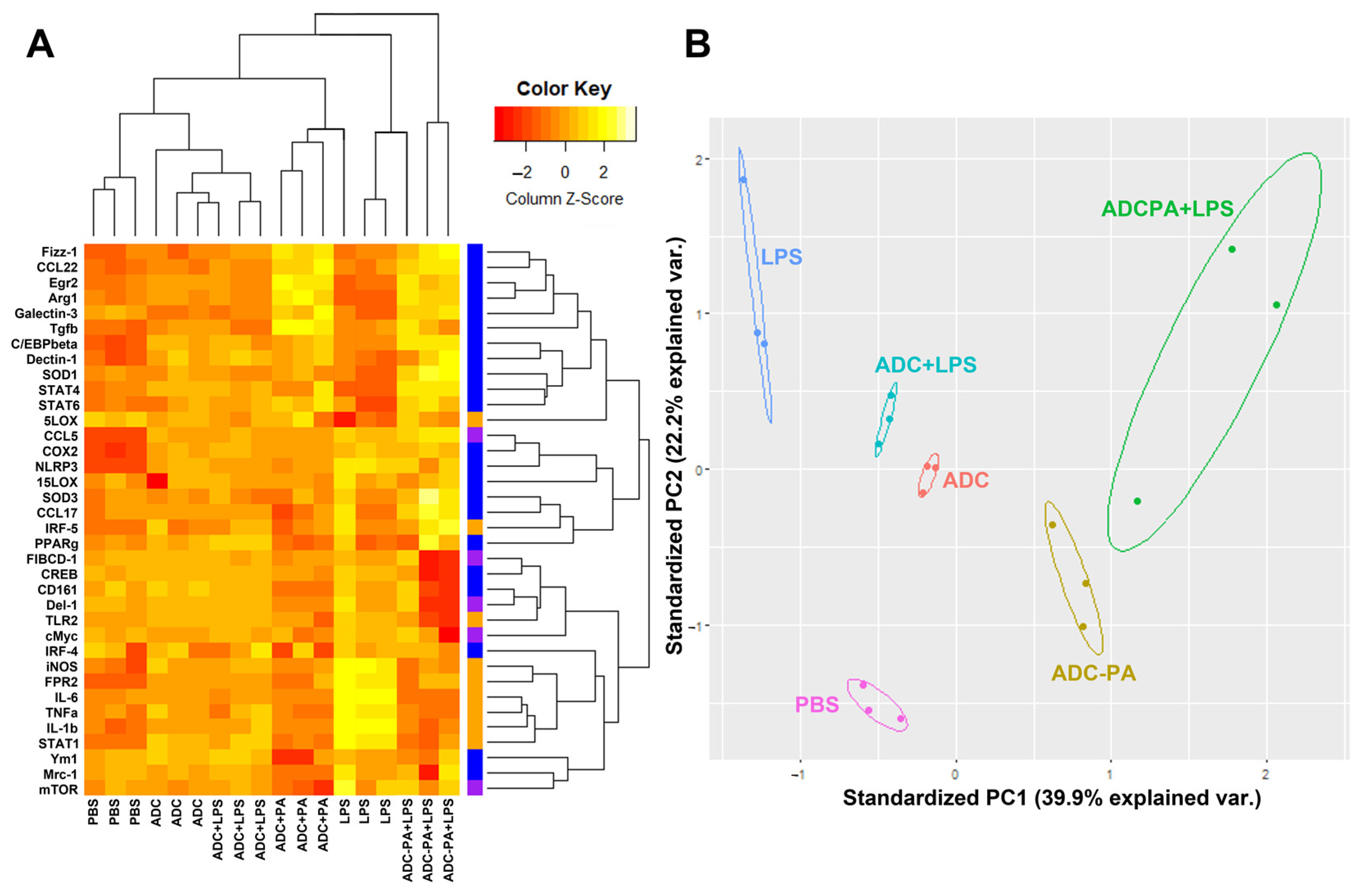
3.5. The Effect of ADC and ADC-PA on the Modulation of LPS-Induced Pro-Inflammatory Responses and Resolution of Inflammation-Associated Gene Expression in Murine Macrophages
3.6. ADC-PA Facilitates Re-Epithelialization and Accelerates the Wound-Healing Process of Diabetic Mice
3.7. Treatment with ADC-PA Leads to a Fine Balance between the Numbers of iNOS+CD206- M1 and iNOS-CD206+ M2 Macrophages in Wound Microenvironments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiber, G.E.; Vileikyte, L.; Boyko, E.J.; del Aguila, M.; Smith, D.G.; Lavery, L.A.; Boulton, A.J. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999, 22, 157–162. [Google Scholar] [CrossRef]
- Gregg, E.W.; Li, Y.; Wang, J.; Rios Burrows, N.; Ali, M.K.; Rolka, D.; Williams, D.E.; Geiss, L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014, 370, 1514–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, J.B.; Garcia del Barco, D.; Cibrian Vera, D.; Savigne, W.; Lopez-Saura, P.; Guillen Nieto, G.; Schultz, G.S. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int. Wound J. 2008, 5, 530–539. [Google Scholar] [CrossRef]
- Faglia, E.; Favales, F.; Morabito, A. New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: A 6.5-Year Follow-Up. Diabetes Care 2001, 24, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiber, G.E.; Raugi, G.J. Preventing Foot ulcers and amputations in diabetes. Lancet 2005, 366, 1676–1677. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Menke, N.B.; Ward, K.R.; Witten, T.M.; Bonchev, D.G.; Diegelmann, R.F. Impaired wound healing. Clin. Dermatol. 2007, 25, 19–25. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Cohen, I.K.; Kaplan, A.M. The role of macrophages in wound repair: A review. Plast. Reconstr. Surg. 1981, 68, 107–113. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Z.; Shi, F.; Zhou, Z.; Sun, F.; Sun, M.-H.; Sun, Q.; Chen, L.; Li, D.; Jiang, C.-Y.; Zhao, R.-Z. M1 Macrophage mediated increased Reactive Oxygen Species (ROS) influence wound healing via the MAPK signaling in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 366, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Paige, J.T.; Kremer, M.; Landry, J.; Hatfield, S.A.; Wathieu, D.; Brug, A.; Lightell, D.J.; Spiller, K.L.; Woods, T.C. Modulation of inflammation in wounds of diabetic patients treated with porcine urinary bladder matrix. Regen. Med. 2019, 14, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.J.; Wang, C.S.; Shao, H. Complex coacervates as a foundation for synthetic underwater adhesives. Adv. Colloid Interface Sci 2011, 167, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.S.; Stewart, R.J. Multipart copolyelectrolyte adhesive of the sandcastle worm, phragmatopoma californica (Fewkes): Catechol oxidase catalyzed curing through peptidyl-DOPA. Biomacromolecules 2013, 14, 1607–1617. [Google Scholar] [CrossRef]
- Wang, C.S.; Stewart, R.J. Localization of the Bioadhesive precursors of the sandcastle worm, phragmatopoma californica (Fewkes). J. Exp. Biol 2012, 215, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Bachus, K.N.; Stewart, R.J. A water-borne adhesive modeled after the sandcastle glue of P. californica. Macromol. Biosci 2009, 9, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Black, K.A.; Priftis, D.; Perry, S.L.; Yip, J.; Byun, W.Y.; Tirrell, M. Protein encapsulation via polypeptide complex coacervation. ACS Macro Lett. 2014, 3, 1088–1091. [Google Scholar] [CrossRef]
- Blocher, W.C.; Perry, S.L. Complex coacervate-based materials for biomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1442. [Google Scholar] [CrossRef]
- Johnson, N.R.; Wang, Y. Coacervate delivery systems for proteins and small molecule drugs. Expert Opin. Drug Deliv. 2014, 11, 1829–1832. [Google Scholar] [CrossRef] [PubMed]
- Versic, R.J. Coacervation for flavor encapsulation. ACS Symp. Ser. 1988, 37, 126–131. [Google Scholar]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. Lwt 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Schmitt, C.; Sanchez, C.; Thomas, F.; Hardy, J. Complex coacervation between β-Lactoglobulin and acacia gum in aqueous medium. Food Hydrocoll. 1999, 13, 483–496. [Google Scholar] [CrossRef]
- Alam, S.S.; Seo, Y.; Lapitsky, Y. Highly sustained release of bactericides from complex coacervates. ACS Appl. Bio Mater. 2020, 3, 8427–8437. [Google Scholar] [CrossRef]
- Park, U.; Lee, M.S.; Jeon, J.; Lee, S.; Hwang, M.P.; Wang, Y.; Yang, H.S.; Kim, K. Coacervate-mediated exogenous growth factor delivery for scarless skin regeneration. Acta Biomater. 2019, 90, 179–191. [Google Scholar] [CrossRef]
- Li, H.; Johnson, N.R.; Usas, A.; Lu, A.; Poddar, M.; Wang, Y.; Huard, J. Sustained release of bone morphogenetic protein 2 via coacervate improves the osteogenic potential of muscle-derived stem cells. Stem Cells Transl. Med. 2013, 2, 667–677. [Google Scholar] [CrossRef]
- Chen, W.C.; Lee, B.G.; Park, D.W.; Kim, K.; Chu, H.; Kim, K.; Huard, J.; Wang, Y. Controlled dual delivery of fibroblast growth factor-2 and interleukin-10 by heparin-based coacervate synergistically enhances ischemic heart repair. Biomaterials 2015, 72, 138–151. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization WHO Traditional Medicine Strategy: 2014–2023; World Health Organization: Geneva, Switzerland, 2013; ISBN 92-4-150609-1.
- Huang, Y. Radioprotective Effect of Grape Seed Proanthocyanidins In Vitro and In Vivo. Oxid. Med. Cell. Longev 2016, 2016, 5706751. [Google Scholar] [CrossRef] [Green Version]
- Houde, V.; Grenier, D.; Chandad, F. Protective effects of grape seed proanthocyanidins against oxidative stress induced by lipopolysaccharides of periodontopathogens. J. Periodontol. 2006, 77, 1371–1379. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, B.; Zhang, H.; Chen, Y.; Fan, C.; Zhang, T.; Qin, T.; Han, J.; Zhang, S.; Chen, X. Inhibition of ROS activity by controlled release of proanthocyanidins from mesoporous silica nanocomposites effectively ameliorates heterotopic ossification in tendon. Chem. Eng. J. 2021, 420, 129415. [Google Scholar] [CrossRef]
- Guo, F.; Hu, Y.; Niu, Q.; Li, Y.; Ding, Y.; Ma, R.; Wang, X.; Li, S.; Xie, J. Grape seed proanthocyanidin extract inhibits human esophageal squamous cancerous cell line ECA109 via the NF-ΚB signaling pathway. Mediat. Inflamm. 2018, 2018, 3403972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Tang, Q.; Huang, H.; Hao, W.; Wei, X. Grape-seed proanthocyanidins inhibit the lipopolysaccharide-induced inflammatory mediator expression in RAW264. 7 macrophages by suppressing MAPK and NF-Κb signal pathways. Environ. Toxicol. Pharmacol. 2016, 41, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-ΚB Pathways in Raw 264.7 Macrophages. Nutr. Res. Pract. 2016, 10, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V. In vitro toxicity assessment of chitosan oligosaccharide coated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Farias, B.S.; Grundmann, D.D.R.; Rizzi, F.Z.; Martins, N.S.S.; Junior, T.R.S.C.; de Almeida Pinto, L.A. Production of Low Molecular Weight Chitosan by Acid and Oxidative Pathways: Effect on Physicochemical Properties. Food Res. Int. 2019, 123, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-K.; Moon, S.-H.; Kim, S.-K. Effect of chitooligosaccharides on calcium bioavailability and bone strength in ovariectomized rats. Life Sci. 2006, 78, 970–976. [Google Scholar] [CrossRef]
- Wee, Y.; Yang, C.; Chen, S.; Yen, Y.; Wang, C. Inositol hexaphosphate modulates the behavior of macrophages through alteration of gene expression involved in pathways of pro-and anti-inflammatory responses, and resolution of inflammation pathways. Food Sci. Nutr. 2021, 9, 3240–3249. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kim, S.; Snyder, S.H. Inositol pyrophosphates as mammalian cell signals. Sci. Signal 2011, 4, re1. [Google Scholar] [CrossRef] [Green Version]
- Omoruyi, F.O.; Stennett, D.; Foster, S.; Dilworth, L. New frontiers for the use of IP6 and inositol combination in treating diabetes mellitus: A review. Molecules 2020, 25, 1720. [Google Scholar] [CrossRef] [Green Version]
- Bartkowiak, A.; Hunkeler, D. Alginate−Oligochitosan microcapsules: A mechanistic study relating membrane and capsule properties to reaction conditions. Chem. Mater. 1999, 11, 2486–2492. [Google Scholar] [CrossRef]
- Puras, G.; Zarate, J.; Aceves, M.; Murua, A.; Díaz, A.; Avilés-Triguero, M.; Fernandez, E.; Pedraz, J. Low molecular weight oligochitosans for non-viral retinal gene therapy. Eur. J. Pharm. Biopharm. 2013, 83, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Delair, T. Colloidal polyelectrolyte complexes of chitosan and dextran sulfate towards versatile nanocarriers of bioactive molecules. Eur. J. Pharm. Biopharm. 2011, 78, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mevold, A.H.; Liu, J.-Y.; Huang, L.-Y.; Liao, H.-L.; Yang, M.-C.; Chan, T.-Y.; Wang, K.-S.; Wang, J.-K.; Wang, Y.-L.; Liu, T.-Y. Core-Shell Structure of gold nanoparticles with inositol hexaphosphate nanohybrids for label-free and rapid detection by SERS nanotechnology. J. Nanomater. 2015, 2015, 857154. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Du, Y.; Yu, X.; Mitsutomi, M.; Aiba, S. Preparation of chitooligosaccharides from chitosan by a complex enzyme. Carbohydr. Res. 1999, 320, 257–260. [Google Scholar] [CrossRef]
- Sing, C.E.; Perry, S.L. Recent Progress in the Science of Complex Coacervation. Soft Matter 2020, 16, 2885–2914. [Google Scholar] [CrossRef] [Green Version]
- Dinicola, S.; Cucina, A.; Antonacci, D.; Bizzarri, M. Anticancer effects of grape seed extract on human cancers: A review. J Carcinog Mutagen S 2014, 8, 390–400. [Google Scholar]
- Ravindranathan, P.; Pasham, D.; Balaji, U.; Cardenas, J.; Gu, J.; Toden, S.; Goel, A. Mechanistic insights into anticancer properties of oligomeric proanthocyanidins from grape seeds in colorectal cancer. Carcinogenesis 2018, 39, 767–777. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Chis, I.C.; Ungureanu, M.I.; Marton, A.; Simedrea, R.; Muresan, A.; Postescu, I.-D.; Decea, N. Antioxidant effects of a grape seed extract in a rat model of diabetes mellitus. Diabetes Vasc. Dis. Res. 2009, 6, 200–204. [Google Scholar] [CrossRef]
- Okudan, N.; Barışkaner, H.; Gökbel, H.; Şahin, A.S.; Belviranlı, M.; Baysal, H. The effect of supplementation of grape seed proanthocyanidin extract on vascular dysfunction in experimental diabetes. J. Med. Food 2011, 14, 1298–1302. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of polyphenols in humans. I. review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Jaggers, G.K.; Oteiza, P.I.; Waterhouse, A.L. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J. Agric. Food Chem. 2013, 61, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Kwik-Uribe, C.; Keen, C.L.; Schroeter, H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am. J. Clin. Nutr. 2012, 95, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Winslow, B.D.; Shao, H.; Stewart, R.J.; Tresco, P.A. Biocompatibility of adhesive complex coacervates modeled after the sandcastle glue of phragmatopoma californica for craniofacial reconstruction. Biomaterials 2010, 31, 9373–9381. [Google Scholar] [CrossRef] [Green Version]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B. Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: A review. Trends Food Sci. Technol. 2018, 71, 121–131. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Trengove, N.J.; Stacey, M.C.; Macauley, S.; Bennett, N.; Gibson, J.; Burslem, F.; Murphy, G.; Schultz, G. Analysis of the acute and chronic wound environments: The role of proteases and their inhibitors. Wound Repair Regen. 1999, 7, 442–452. [Google Scholar] [CrossRef]
- Greener, B.; Hughes, A.; Bannister, N.; Douglass, J. Proteases and PH in chronic wounds. J. Wound Care 2005, 14, 59–61. [Google Scholar] [CrossRef]
- Mao, J.; Chen, L.; Cai, Z.; Qian, S.; Liu, Z.; Zhao, B.; Zhang, Y.; Sun, X.; Cui, W. Advanced biomaterials for regulating polarization of macrophages in wound healing. Adv. Funct. Mater. 2022, 32, 2111003. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial based modulation of macrophage polarization: A review and suggested design principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Dohle, E.; Ramanauskaite, A.; Al-Maawi, S.; Obreja, K.; Magini, R.; Sader, R.; Ghanaati, S.; Schwarz, F. Anti-inflammatory and macrophage polarization effects of cranberry proanthocyanidins (PACs) for periodontal and peri-implant disease therapy. J. Periodontal Res. 2020, 55, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.K.; Chittezhath, M.; Shalova, I.N.; Lim, J.-Y. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012, 53, 11–24. [Google Scholar] [CrossRef]
- Ganesh, G.V.; Ramkumar, K.M. Macrophage mediation in normal and diabetic wound healing responses. Inflamm. Res. 2020, 69, 347–363. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; pros and cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The reactive oxygen species in macrophage polarization: Reflecting its dual role in progression and treatment of human diseases. Oxidative Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [Green Version]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, M.; Okada, Y.; Kitano, A.; Ikeda, K.; Saika, S.; Shinozaki, M. Impaired cutaneous wound healing with excess granulation tissue formation in TNFα-Null mice. Arch. Dermatol. Res. 2009, 301, 531–537. [Google Scholar] [CrossRef]
- Jetten, N. Wound administration of M2-Polarized macrophages does not improve murine cutaneous healing responses. PLoS ONE 2014, 9, 102994. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-S.; Luo, S.-D.; Jia, S.; Wu, W.; Chang, S.-F.; Feng, S.-W.; Yang, C.-H.; Lin, J.-H.; Wee, Y. Balance of Macrophage Activation by a Complex Coacervate-Based Adhesive Drug Carrier Facilitates Diabetic Wound Healing. Antioxidants 2022, 11, 2351. https://doi.org/10.3390/antiox11122351
Wang C-S, Luo S-D, Jia S, Wu W, Chang S-F, Feng S-W, Yang C-H, Lin J-H, Wee Y. Balance of Macrophage Activation by a Complex Coacervate-Based Adhesive Drug Carrier Facilitates Diabetic Wound Healing. Antioxidants. 2022; 11(12):2351. https://doi.org/10.3390/antiox11122351
Chicago/Turabian StyleWang, Ching-Shuen, Shen-Dean Luo, Shihai Jia, Wilfred Wu, Shwu-Fen Chang, Sheng-Wei Feng, Chieh-Hsiang Yang, Jiann-Her Lin, and Yinshen Wee. 2022. "Balance of Macrophage Activation by a Complex Coacervate-Based Adhesive Drug Carrier Facilitates Diabetic Wound Healing" Antioxidants 11, no. 12: 2351. https://doi.org/10.3390/antiox11122351
APA StyleWang, C.-S., Luo, S.-D., Jia, S., Wu, W., Chang, S.-F., Feng, S.-W., Yang, C.-H., Lin, J.-H., & Wee, Y. (2022). Balance of Macrophage Activation by a Complex Coacervate-Based Adhesive Drug Carrier Facilitates Diabetic Wound Healing. Antioxidants, 11(12), 2351. https://doi.org/10.3390/antiox11122351







