Acylated Ghrelin Receptor Agonist HM01 Decreases Lean Body and Muscle Mass, but Unacylated Ghrelin Protects against Redox-Dependent Sarcopenia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. HM01 and Unacylated Ghrelin Delivery
2.3. Assessment of Body Composition
2.4. In Vitro Contractile Properties and Force Generation
2.5. Western Blot
2.6. Statistical Analysis
3. Results
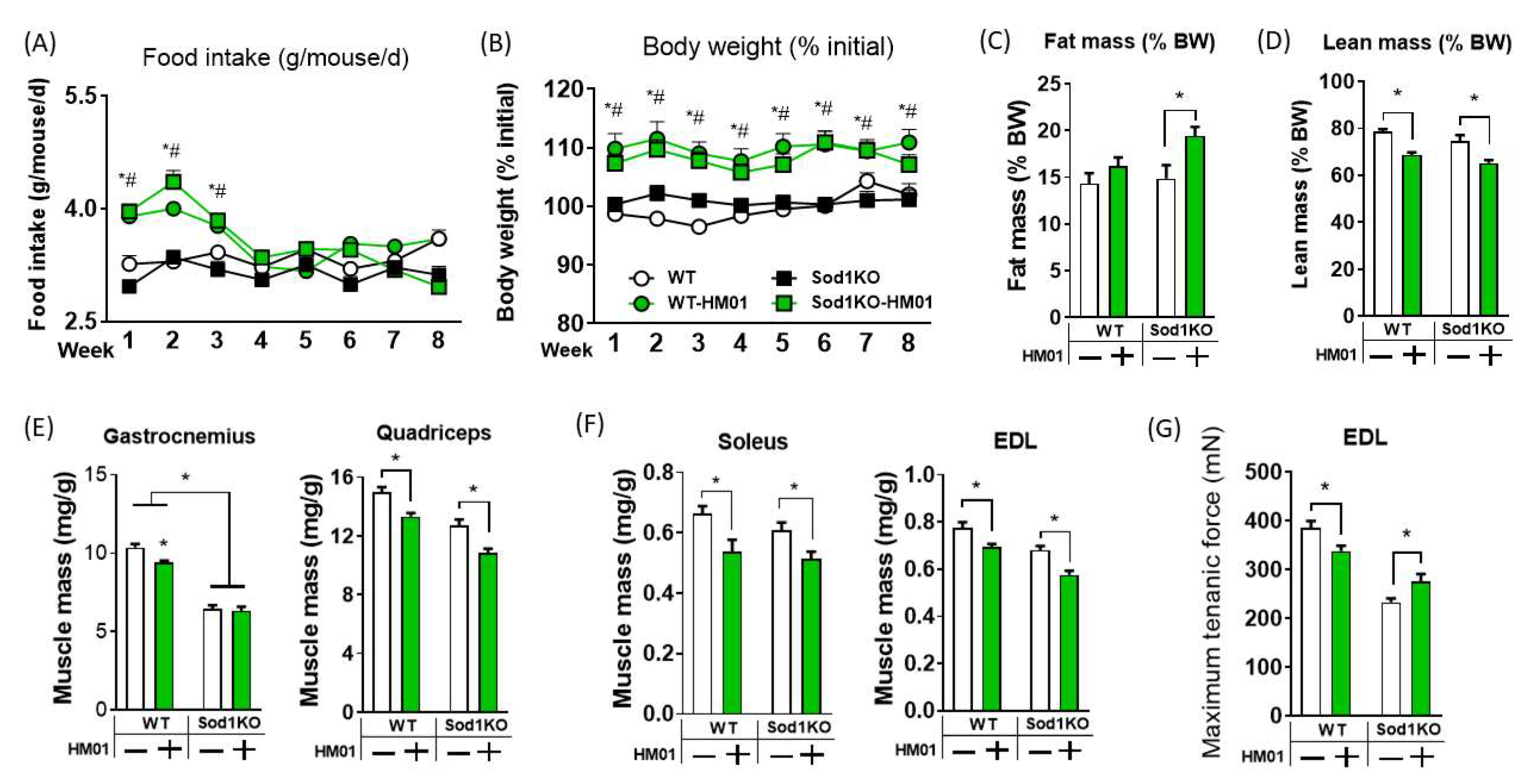
3.1. HM01 Treatment Transiently Increases Food Intake, but Leads to Decreases in Lean Body Mass and Muscle Quantity
3.2. Longer Term Treatment of HM01 Results in Loss of Muscle Mass and Reduced Maximum Force Generation
3.3. GHSR1a Receptor Downstream Pathways and Mechanisms Are Involved in Myogenesis and Protein Synthesis in Muscle
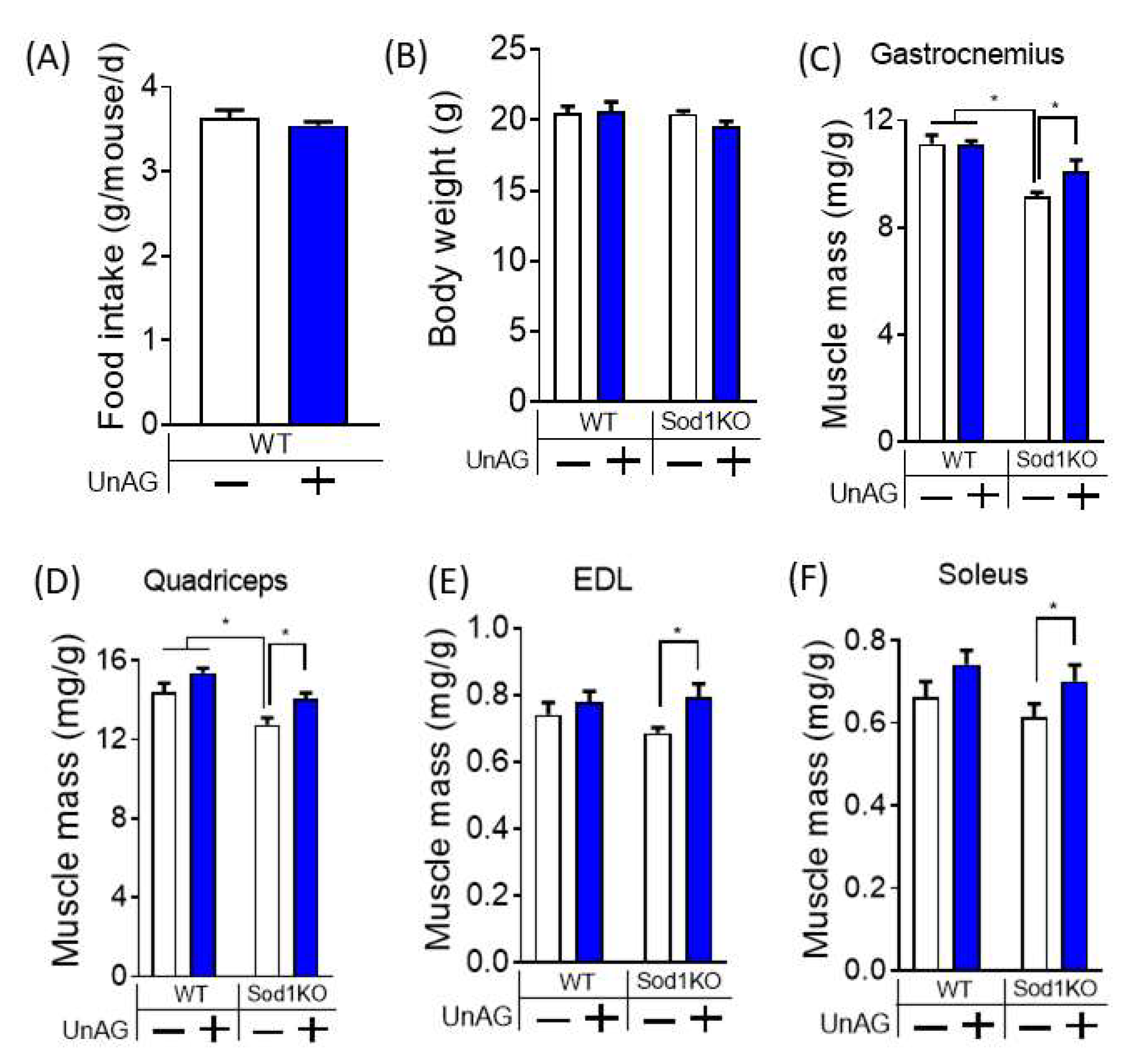
3.4. Unacylated Ghrelin Increases Muscle Mass without Changes in Food Consumption or Body Weights
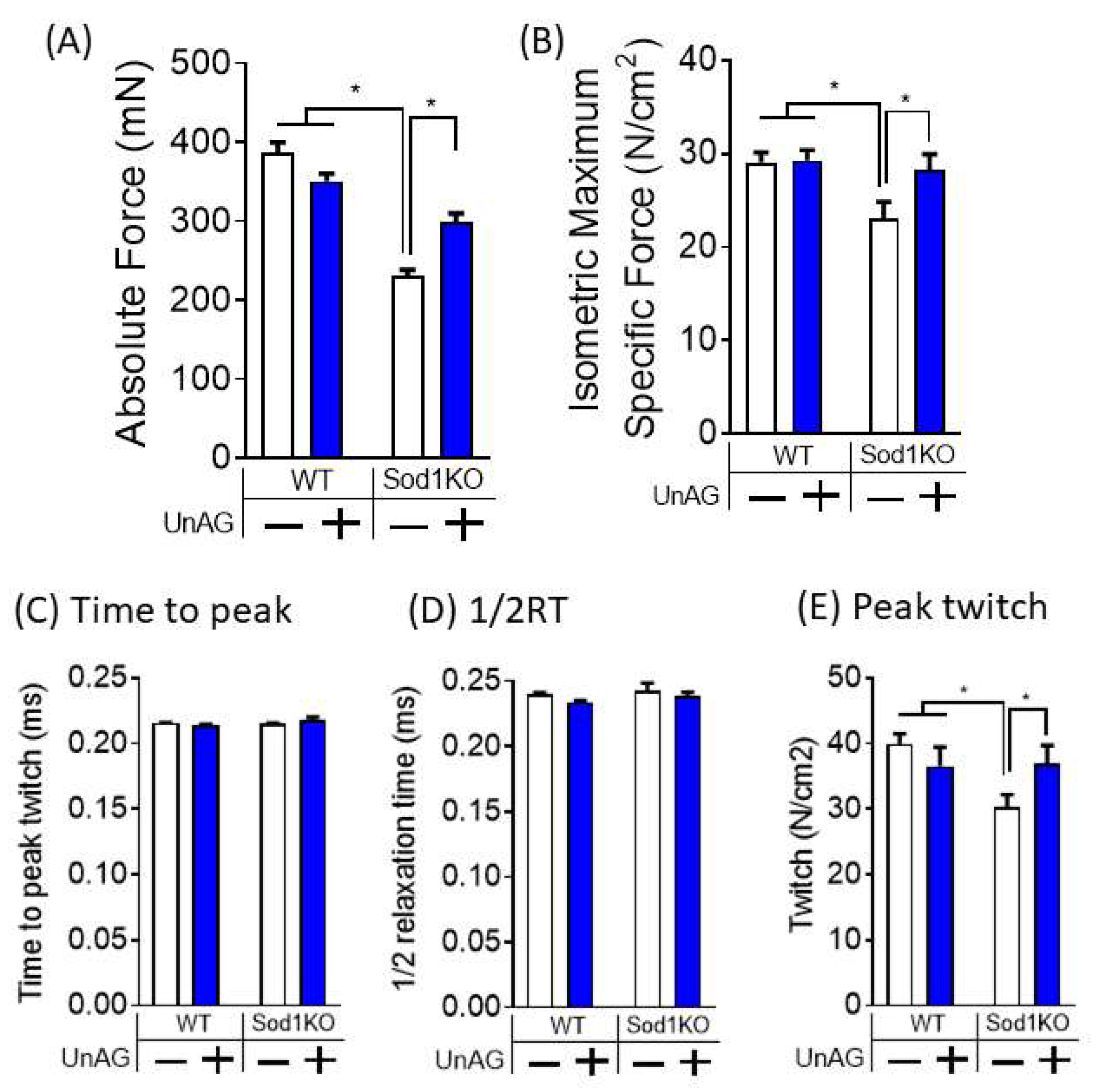
3.5. Unacylated Ghrelin Improves Contractile Properties
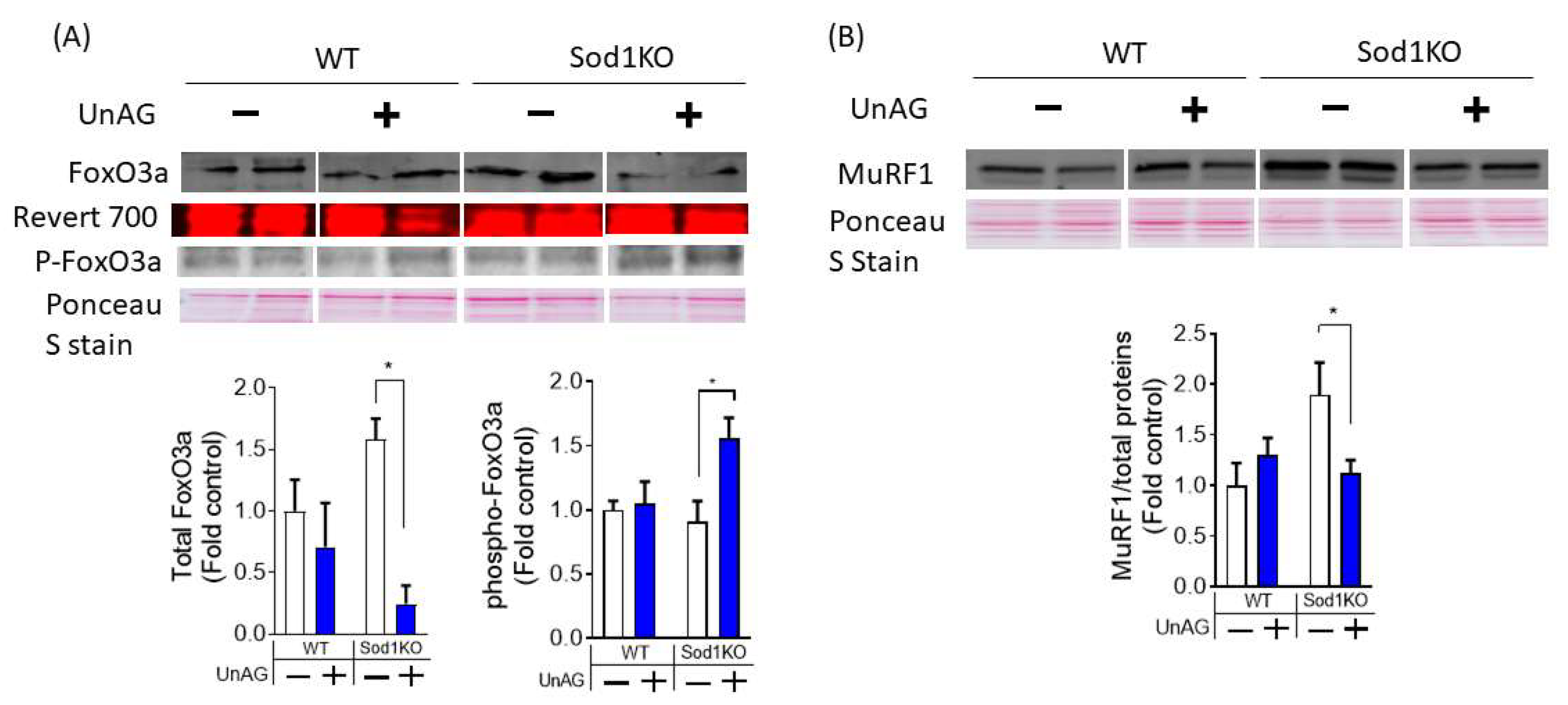
3.6. Downstream Pathways Activated by Unacylated Ghrelin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the Global Challenges of Ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ubaida-Mohien, C.; Lyashkov, A.; Gonzalez-Freire, M.; Tharakan, R.; Shardell, M.; Moaddel, R.; Semba, R.D.; Chia, C.W.; Gorospe, M.; Sen, R.; et al. Discovery Proteomics in Aging Human Skeletal Muscle Finds Change in Spliceosome, Immunity, Proteostasis and Mitochondria. eLife 2019, 8, e49874. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin Is a Growth-Hormone-Releasing Acylated Peptide from Stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.D.; Feighner, S.D.; Cully, D.F.; Arena, J.P.; Liberator, P.A.; Rosenblum, C.I.; Hamelin, M.; Hreniuk, D.L.; Palyha, O.C.; Anderson, J.; et al. A Receptor in Pituitary and Hypothalamus That Functions in Growth Hormone Release. Science 1996, 273, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Guillory, B.; Chen, J.-A.; Patel, S.; Luo, J.; Splenser, A.; Mody, A.; Ding, M.; Baghaie, S.; Anderson, B.; Iankova, B.; et al. Deletion of Ghrelin Prevents Aging-Associated Obesity and Muscle Dysfunction without Affecting Longevity. Aging Cell 2017, 16, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Filigheddu, N.; Gnocchi, V.F.; Coscia, M.; Cappelli, M.; Porporato, P.E.; Taulli, R.; Traini, S.; Baldanzi, G.; Chianale, F.; Cutrupi, S.; et al. Ghrelin and Des-Acyl Ghrelin Promote Differentiation and Fusion of C2C12 Skeletal Muscle Cells. Mol. Biol. Cell 2007, 18, 986–994. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Reano, S.; Ferrara, M.; Angelino, E.; Gnocchi, V.F.; Prodam, F.; Ronchi, G.; Fagoonee, S.; Fornaro, M.; et al. Acylated and Unacylated Ghrelin Impair Skeletal Muscle Atrophy in Mice. J. Clin. Investig. 2013, 123, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Chen, S.; Yang, Y.; Ke, Z. Acylated and Unacylated Ghrelin Inhibit Atrophy in Myotubes Co-Cultured with Colon Carcinoma Cells. Oncotarget 2017, 8, 72872–72885. [Google Scholar] [CrossRef] [Green Version]
- Borner, T.; Loi, L.; Pietra, C.; Giuliano, C.; Lutz, T.A.; Riediger, T. The Ghrelin Receptor Agonist HM01 Mimics the Neuronal Effects of Ghrelin in the Arcuate Nucleus and Attenuates Anorexia-Cachexia Syndrome in Tumor-Bearing Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R89–R96. [Google Scholar] [CrossRef] [Green Version]
- Villars, F.O.; Pietra, C.; Giuliano, C.; Lutz, T.A.; Riediger, T. Oral Treatment with the Ghrelin Receptor Agonist HM01 Attenuates Cachexia in Mice Bearing Colon-26 (C26) Tumors. Int. J. Mol. Sci. 2017, 18, E986. [Google Scholar] [CrossRef]
- Karasawa, H.; Pietra, C.; Giuliano, C.; Garcia-Rubio, S.; Xu, X.; Yakabi, S.; Taché, Y.; Wang, L. New Ghrelin Agonist, HM01 Alleviates Constipation and L-Dopa-Delayed Gastric Emptying in 6-Hydroxydopamine Rat Model of Parkinson’s Disease. Neurogastroenterol. Motil. 2014, 26, 1771–1782. [Google Scholar] [CrossRef] [Green Version]
- Togliatto, G.; Trombetta, A.; Dentelli, P.; Cotogni, P.; Rosso, A.; Tschöp, M.H.; Granata, R.; Ghigo, E.; Brizzi, M.F. Unacylated Ghrelin Promotes Skeletal Muscle Regeneration Following Hindlimb Ischemia via SOD-2-Mediated MiR-221/222 Expression. J. Am. Heart Assoc. 2013, 2, e000376. [Google Scholar] [CrossRef] [Green Version]
- Gortan Cappellari, G.; Semolic, A.; Ruozi, G.; Vinci, P.; Guarnieri, G.; Bortolotti, F.; Barbetta, D.; Zanetti, M.; Giacca, M.; Barazzoni, R. Unacylated Ghrelin Normalizes Skeletal Muscle Oxidative Stress and Prevents Muscle Catabolism by Enhancing Tissue Mitophagy in Experimental Chronic Kidney Disease. FASEB J. 2017, 31, 5159–5171. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, A.; Togliatto, G.; Rolo, A.P.; Teodoro, J.S.; Granata, R.; Ghigo, E.; Columbano, A.; Palmeira, C.M.; Brizzi, M.F. Unacylated Ghrelin Prevents Mitochondrial Dysfunction in a Model of Ischemia/Reperfusion Liver Injury. Cell Death Discov. 2017, 3, 17077. [Google Scholar] [CrossRef] [Green Version]
- Ku, J.M.; Taher, M.; Chin, K.Y.; Barsby, T.; Austin, V.; Wong, C.H.Y.; Andrews, Z.B.; Spencer, S.J.; Miller, A.A. Protective Actions of Des-Acylated Ghrelin on Brain Injury and Blood-Brain Barrier Disruption after Stroke in Mice. Clin. Sci. 2016, 130, 1545–1558. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, B.E.; Irving, D. The Numbers of Limb Motor Neurons in the Human Lumbosacral Cord throughout Life. J. Neurol. Sci. 1977, 34, 213–219. [Google Scholar] [CrossRef]
- Lexell, J.; Downham, D.Y. The Occurrence of Fibre-Type Grouping in Healthy Human Muscle: A Quantitative Study of Cross-Sections of Whole Vastus Lateralis from Men between 15 and 83 Years. Acta Neuropathol. 1991, 81, 377–381. [Google Scholar] [CrossRef]
- Jennekens, F.G.; Tomlinson, B.E.; Walton, J.N. Histochemical Aspects of Five Limb Muscles in Old Age. An Autopsy Study. J. Neurol. Sci. 1971, 14, 259–276. [Google Scholar] [CrossRef]
- Kelly, N.A.; Hammond, K.G.; Stec, M.J.; Bickel, C.S.; Windham, S.T.; Tuggle, S.C.; Bamman, M.M. Quantification and Characterization of Grouped Type I Myofibers in Human Aging. Muscle Nerve 2018, 57, E52–E59. [Google Scholar] [CrossRef]
- Tomonaga, M. Histochemical and Ultrastructural Changes in Senile Human Skeletal Muscle. J. Am. Geriatr. Soc. 1977, 25, 125–131. [Google Scholar] [CrossRef]
- Muller, F.L.; Song, W.; Liu, Y.; Chaudhuri, A.; Pieke-Dahl, S.; Strong, R.; Huang, T.-T.; Epstein, C.J.; Roberts, L.J.; Csete, M.; et al. Absence of CuZn Superoxide Dismutase Leads to Elevated Oxidative Stress and Acceleration of Age-Dependent Skeletal Muscle Atrophy. Free Radic. Biol. Med. 2006, 40, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Lustgarten, M.S.; Liu, Y.; Muller, F.L.; Bhattacharya, A.; Liang, H.; Salmon, A.B.; Brooks, S.V.; Larkin, L.; Hayworth, C.R.; et al. Increased Superoxide in Vivo Accelerates Age-Associated Muscle Atrophy through Mitochondrial Dysfunction and Neuromuscular Junction Degeneration. FASEB J. 2010, 24, 1376–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.C.; Van Remmen, H. Age-Associated Alterations of the Neuromuscular Junction. Exp. Gerontol. 2011, 46, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakellariou, G.K.; Davis, C.S.; Shi, Y.; Ivannikov, M.V.; Zhang, Y.; Vasilaki, A.; Macleod, G.T.; Richardson, A.; Van Remmen, H.; Jackson, M.J.; et al. Neuron-Specific Expression of CuZnSOD Prevents the Loss of Muscle Mass and Function That Occurs in Homozygous CuZnSOD-Knockout Mice. FASEB J. 2014, 28, 1666–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sataranatarajan, K.; Pharaoh, G.; Brown, J.L.; Ranjit, R.; Piekarz, K.M.; Street, K.; Wren, J.D.; Georgescu, C.; Kinter, C.; Kinter, M.; et al. Molecular Changes in Transcription and Metabolic Pathways Underlying Muscle Atrophy in the CuZnSOD Null Mouse Model of Sarcopenia. Geroscience 2020, 42, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Yasunami, M.; Carlson, E.J.; Gillespie, A.M.; Reaume, A.G.; Hoffman, E.K.; Chan, P.H.; Scott, R.W.; Epstein, C.J. Superoxide-Mediated Cytotoxicity in Superoxide Dismutase-Deficient Fetal Fibroblasts. Arch. Biochem. Biophys. 1997, 344, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Ranjit, R.; Kneis, P.; Xu, H.; Piekarz, K.M.; Freeman, W.M.; Kinter, M.; Richardson, A.; Ran, Q.; Brooks, S.V.; et al. Scavenging Mitochondrial Hydrogen Peroxide by Peroxiredoxin 3 Overexpression Attenuates Contractile Dysfunction and Muscle Atrophy in a Murine Model of Accelerated Sarcopenia. Aging Cell 2022, 21, e13569. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ivannikov, M.V.; Walsh, M.E.; Liu, Y.; Zhang, Y.; Jaramillo, C.A.; Macleod, G.T.; Van Remmen, H. The Lack of CuZnSOD Leads to Impaired Neurotransmitter Release, Neuromuscular Junction Destabilization and Reduced Muscle Strength in Mice. PLoS ONE 2014, 9, e100834. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Taylor, C.C.; Sjöström, M. What Is the Cause of the Ageing Atrophy? Total Number, Size and Proportion of Different Fiber Types Studied in Whole Vastus Lateralis Muscle from 15- to 83-Year-Old Men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.S.; Jang, Y.C.; Liu, Y.; Muller, F.L.; Qi, W.; Steinhelper, M.; Brooks, S.V.; Larkin, L.; Shimizu, T.; Shirasawa, T.; et al. Conditional Knockout of Mn-SOD Targeted to Type IIB Skeletal Muscle Fibers Increases Oxidative Stress and Is Sufficient to Alter Aerobic Exercise Capacity. Am. J. Physiol. Cell Physiol. 2009, 297, C1520–C1532. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Ranjit, R.; Premkumar, P.; Pharaoh, G.; Piekarz, K.M.; Matsuzaki, S.; Claflin, D.R.; Riddle, K.; Judge, J.; Bhaskaran, S.; et al. Mitochondrial Oxidative Stress Impairs Contractile Function but Paradoxically Increases Muscle Mass via Fibre Branching. J. Cachexia Sarcopenia Muscle 2019, 10, 411–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Close, R.I. Dynamic Properties of Mammalian Skeletal Muscles. Physiol. Rev. 1972, 52, 129–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Sataranatarajan, K.; Premkumar, P.; Huseman, K.; Van Remmen, H. Restoration of SERCA ATPase Prevents Oxidative Stress-Related Muscle Atrophy and Weakness. Redox Biol. 2019, 20, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Premkumar, P.; Ranjit, R.; Natarajan, K.S.; Ahn, B.; Riddle, K.; Claflin, D.R.; Richardson, A.; Brooks, S.V.; et al. Oxidative Stress-Induced Dysregulation of Excitation-Contraction Coupling Contributes to Muscle Weakness. J. Cachexia Sarcopenia Muscle 2018, 9, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.L.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J.C. The Decline in Skeletal Muscle Mass with Aging Is Mainly Attributed to a Reduction in Type II Muscle Fiber Size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J. Human Aging, Muscle Mass, and Fiber Type Composition. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Delhanty, P.J.D.; van der Eerden, B.C.J.; van der Velde, M.; Gauna, C.; Pols, H.A.P.; Jahr, H.; Chiba, H.; van der Lely, A.J.; van Leeuwen, J.P.T.M. Ghrelin and Unacylated Ghrelin Stimulate Human Osteoblast Growth via Mitogen-Activated Protein Kinase (MAPK)/Phosphoinositide 3-Kinase (PI3K) Pathways in the Absence of GHS-R1a. J. Endocrinol. 2006, 188, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, Y.; Li, E.; Park, S. Ghrelin Protects Spinal Cord Motoneurons against Chronic Glutamate Excitotoxicity by Inhibiting Microglial Activation. Korean J. Physiol. Pharmacol. 2012, 16, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gortan Cappellari, G.; Zanetti, M.; Semolic, A.; Vinci, P.; Ruozi, G.; Falcione, A.; Filigheddu, N.; Guarnieri, G.; Graziani, A.; Giacca, M.; et al. Unacylated Ghrelin Reduces Skeletal Muscle Reactive Oxygen Species Generation and Inflammation and Prevents High-Fat Diet-Induced Hyperglycemia and Whole-Body Insulin Resistance in Rodents. Diabetes 2016, 65, 874–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheriff, S.; Kadeer, N.; Joshi, R.; Friend, L.A.; Howard James, J.; Balasubramaniam, A. Des-Acyl Ghrelin Exhibits pro-Anabolic and Anti-Catabolic Effects on C2C12 Myotubes Exposed to Cytokines and Reduces Burn-Induced Muscle Proteolysis in Rats. Mol. Cell. Endocrinol. 2012, 351, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Vulinović, F.; Grünewald, A.; Unger, M.M.; Möller, J.C.; Klein, C.; Michel, P.P.; Ries, V.; Oertel, W.H.; Alvarez-Fischer, D. Acylated and Unacylated Ghrelin Confer Neuroprotection to Mesencephalic Neurons. Neuroscience 2017, 365, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-S.; Wei, Q.; Wang, H.; Kim, D.M.; Balderas, M.; Wu, G.; Lawler, J.; Safe, S.; Guo, S.; Devaraj, S.; et al. Protective Effects of Ghrelin on Fasting-Induced Muscle Atrophy in Aging Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 621–630. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjit, R.; Van Remmen, H.; Ahn, B. Acylated Ghrelin Receptor Agonist HM01 Decreases Lean Body and Muscle Mass, but Unacylated Ghrelin Protects against Redox-Dependent Sarcopenia. Antioxidants 2022, 11, 2358. https://doi.org/10.3390/antiox11122358
Ranjit R, Van Remmen H, Ahn B. Acylated Ghrelin Receptor Agonist HM01 Decreases Lean Body and Muscle Mass, but Unacylated Ghrelin Protects against Redox-Dependent Sarcopenia. Antioxidants. 2022; 11(12):2358. https://doi.org/10.3390/antiox11122358
Chicago/Turabian StyleRanjit, Rojina, Holly Van Remmen, and Bumsoo Ahn. 2022. "Acylated Ghrelin Receptor Agonist HM01 Decreases Lean Body and Muscle Mass, but Unacylated Ghrelin Protects against Redox-Dependent Sarcopenia" Antioxidants 11, no. 12: 2358. https://doi.org/10.3390/antiox11122358
APA StyleRanjit, R., Van Remmen, H., & Ahn, B. (2022). Acylated Ghrelin Receptor Agonist HM01 Decreases Lean Body and Muscle Mass, but Unacylated Ghrelin Protects against Redox-Dependent Sarcopenia. Antioxidants, 11(12), 2358. https://doi.org/10.3390/antiox11122358






