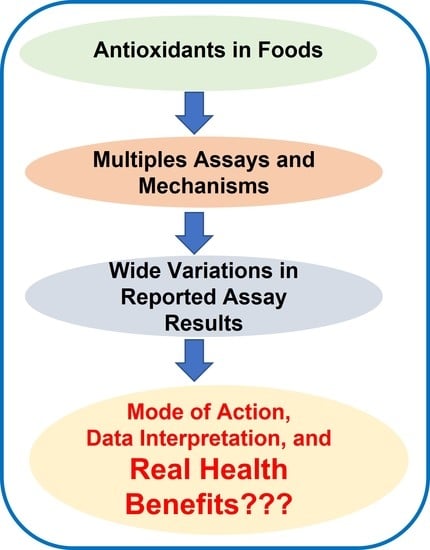
Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays
Abstract
:1. Introduction
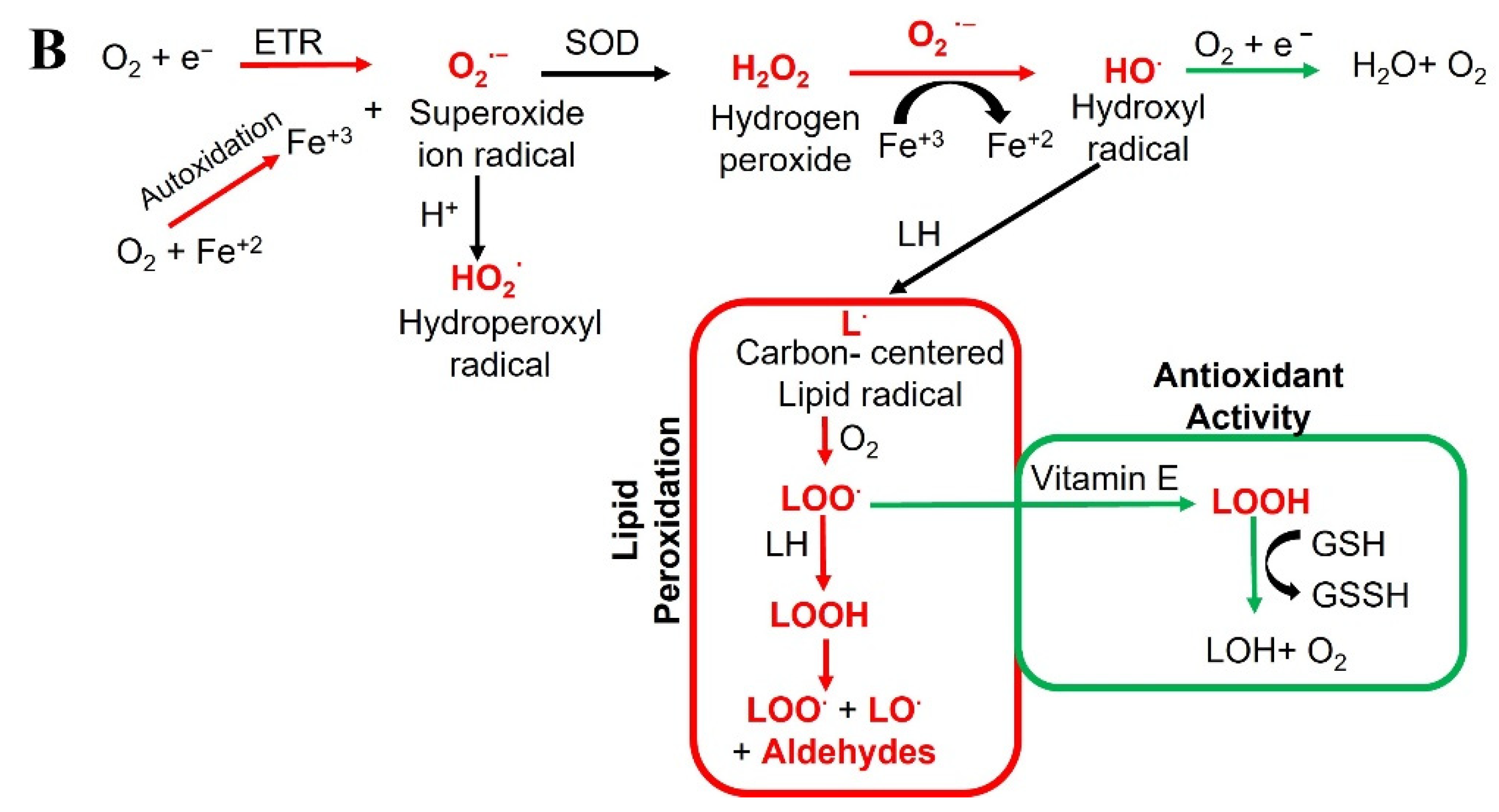
2. Oxidative Stress
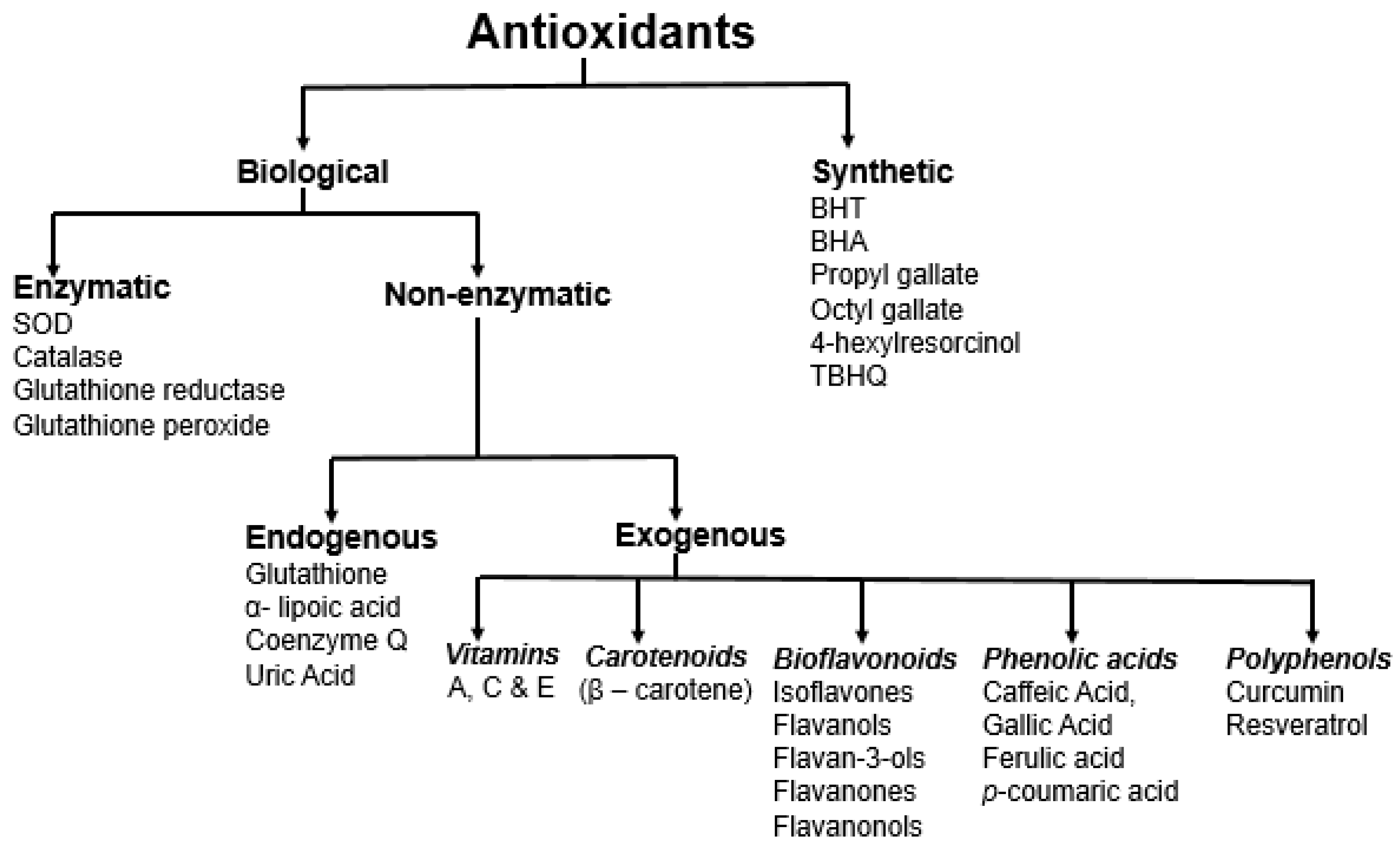
3. Antioxidants
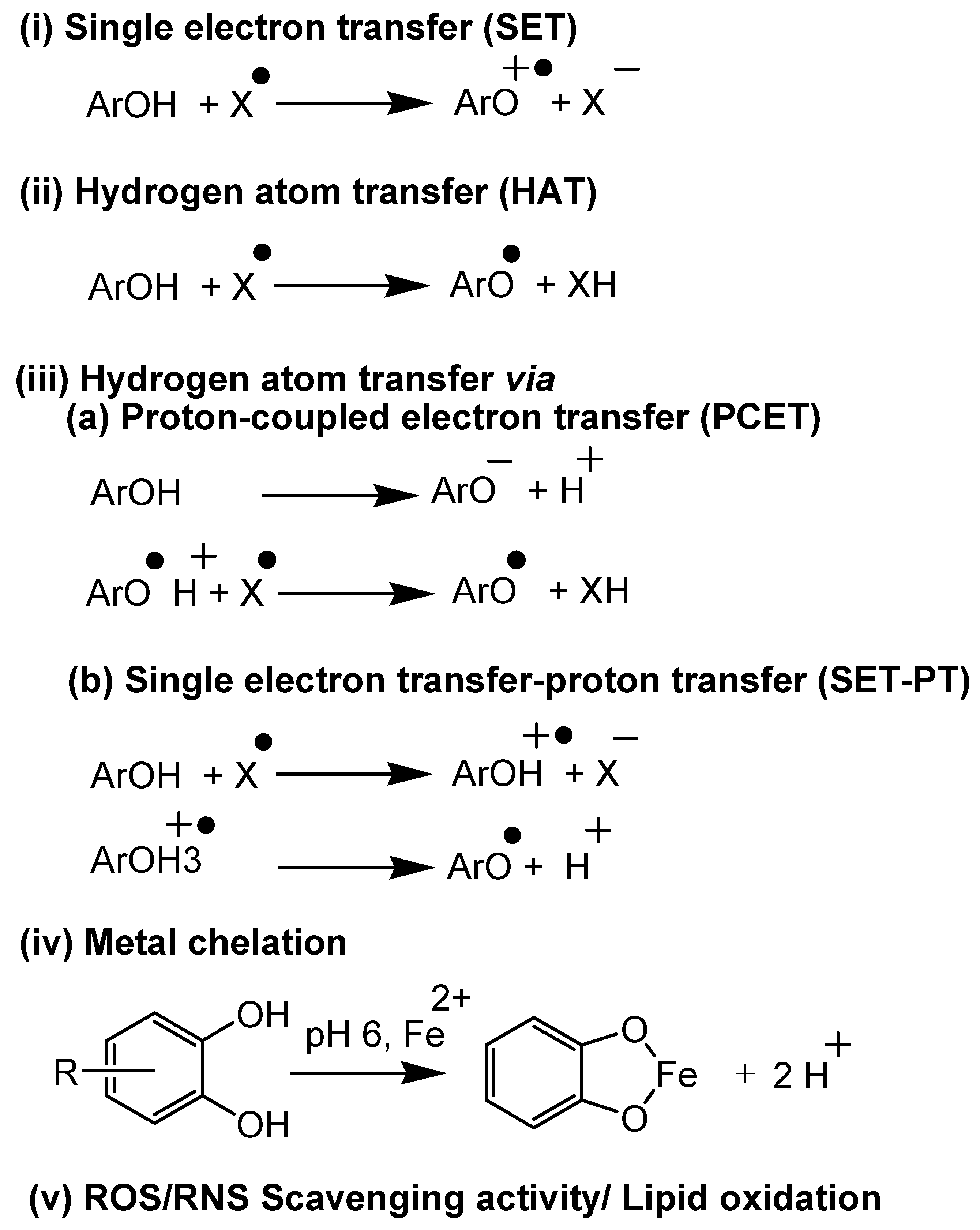
4. Antioxidant Assays
5. Antioxidant Activity of Selected Prominent Foods
5.1. Fruits-Apples and Berries
5.2. Vegetables-Spinach and Olives
5.3. Processed Products-Wine, Coffee, and Tea
5.4. Legumes-Bean, Soybean
5.5. Grains-Corn, Wheat
5.6. Dairy Products-Milk, Yogurt, and Others
6. Strengths and Weaknesses of Antioxidant Assays
6.1. Strengths
6.2. Weaknesses
7. Other Factors Influencing Antioxidant Activity
7.1. Bioaccessibility and Bioavailability of Antioxidants
7.2. Chelation
7.3. In Vivo Assays
7.4. Sample Matrix
7.5. Experimental Parameters
8. Perspectives/Recommendations
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Gökmen, V. Evolution of food antioxidants as a core topic of food science for a century. Food Res. Int. 2018, 105, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Func. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Waterman, K.C.; Adami, R.C.; Alsante, K.M.; Hong, J.; Landis, M.S.; Lombardo, F.; Roberts, C.J. Stabilization of pharmaceuticals to oxidative degradation. Pharm. Dev. Technol. 2002, 7, 1–32. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Wang, X.; Sun, Y.; Zhang, J.; Chen, J.; Shi, Y. An Epigenetic Role of Mitochondria in Cancer. Cells 2022, 11, 2518. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, J.; Beeraka, N.M.; Tang, C.; Babayeva, Y.V.; Sinelnikov, M.Y.; Zhang, X.; Zhang, J.; Liu, J.; Reshetov, I.V.; et al. Advances in the Prevention and Treatment of Obesity-Driven Effects in Breast Cancers. Front. Oncol. 2022, 12, 820968. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics, and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Finley, J.W.; Kong, A.N.; Hintze, K.J.; Jeffery, E.H.; Ji, L.L.; Lei, X.G. Antioxidants in foods: State of the science important to the food industry. J. Agric. Food Chem. 2011, 59, 6837–6846. [Google Scholar] [CrossRef] [PubMed]
- Natural Antioxidants Market Size Worth $ 4.14 Billion By 2022. Available online: grandviewresearch.com (accessed on 4 January 2021).
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amorati, R.; Valgimigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 3. Reactive Oxygen and Nitrogen Species (ROS/RNS) Scavenging Assays, Oxidative Stress Biomarkers, and Chromatographic/Chemometric Assays. J. Agric. Food Chem. 2016, 64, 1046–1070. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Frankel, E.N.; German, J.B. Antioxidants in foods and health: Problems and fallacies in the field. J. Sci. Food Agric. 2006, 86, 1999–2001. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989s–1009s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods. Available online: https://www.researchgate.net/publication/267379608_Oxygen_Radical_Absorbance_Capacity_ORAC_of_selected_foods (accessed on 1 October 2022).
- Food and Drug Administration (FDA); U.S. Department of Health and Human Services; Center for Food Safety and Applied Nutrition. Guidance for Industry, Food Labeling; Nutrient Content Claims; Definition for High Potency and Definition for Antioxidant for Use in Nutrient Content Claims for Dietary Supplements and Conventional Foods; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2008.
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to various food(s)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1489. [Google Scholar]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Halliwell, B. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022 23, 499–515. [CrossRef]
- Carew, J.S.; Huang, P. Mitochondrial defects in cancer. Mol. Cancer 2002, 1, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Ortan, A.; Fierascu, I.C.; Fierascu, I. In vitro and in vivo evaluation of antioxidant properties of wild-growing plants. A short review. Curr. Opin. Food Sci. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Aruoma, O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. 2003, 523, 9–20. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Bektasoglu, B.; Bener, M. Cupric ion reducing antioxidant capacity assay for antioxidants in human serum and for hydroxyl radical scavengers. Methods Mol. Biol. 2010, 594, 215–239. [Google Scholar] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Çelik, S.E. Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Mehdi, M.M.; Rizvi, S.I. N,N-Dimethyl-p-phenylenediamine dihydrochloride-based method for the measurement of plasma oxidative capacity during human aging. Anal. Biochem. 2013, 436, 165–167. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Bohm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006, 95, 200–204. [Google Scholar] [CrossRef]
- Sharma, S.; Vig, A.P. Evaluation of in vitro antioxidant properties of methanol and aqueous extracts of Parkinsonia aculeata L. leaves. Sci. World J. 2013, 2013, 604865. [Google Scholar] [CrossRef] [Green Version]
- Sroka, Z.; Cisowski, W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003, 41, 753–758. [Google Scholar] [CrossRef]
- Gardner, A.M.; Gardner, P.R. Flavohemoglobin detoxifies nitric oxide in aerobic, but not anaerobic, Escherichia coli. Evidence for a novel inducible anaerobic nitric oxide-scavenging activity. J. Biol. Chem. 2002, 277, 8166–8171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarban, S.; Kocyigit, A.; Yazar, M.; Isikan, U.E. Plasma total antioxidant capacity, lipid peroxidation, and erythrocyte antioxidant enzyme activities in patients with rheumatoid arthritis and osteoarthritis. Clin. Biochem. 2005, 38, 981–986. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Rubio, C.P.; Hernandez-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.E.; Kinter, M.T.; Oberley, T.D.; Freeman, M.L.; Frierson, H.F.; Ridnour, L.A.; Tao, Y.; Oberley, L.W.; Spitz, D.R. Enhanced gamma-glutamyl transpeptidase expression and selective loss of CuZn superoxide dismutase in hepatic iron overload. Free Radic. Biol. Med. 1998, 24, 545–555. [Google Scholar] [CrossRef]
- Dadheech, G.; Mishra, S.; Gautam, S.; Sharma, P. Evaluation of antioxidant deficit in schizophrenia. Indian J. Psychiatry 2008, 50, 16–20. [Google Scholar] [PubMed]
- Gungor, N.; Ozyurek, M.; Guclu, K.; Cekic, S.D.; Apak, R. Comparative evaluation of antioxidant capacities of thiol-based antioxidants measured by different in vitro methods. Talanta 2011, 83, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Pinkus, R.; Weiner, L.M.; Daniel, V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappaB, and glutathione S-transferase gene expression. J. Biol. Chem. 1996, 271, 13422–13429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, S.S.; Singh, S.P.; Singhal, P.; Horne, D.; Singhal, J.; Awasthi, S. Antioxidant role of glutathione S-transferases: 4-Hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015, 289, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [Green Version]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (Suppl. 5), 1229–1234. [Google Scholar]
- Nelson, S.K.; Bose, S.K.; Grunwald, G.K.; Myhill, P.; McCord, J.M. The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radic. Biol. Med. 2006, 40, 341–347. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for Measuring Antioxidant Activity and Its Application to Monitoring the Antioxidant Capacity of Wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and Caffeine Content of Green Tea Dietary Supplements and Correlation with Antioxidant Capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (oxygen radical absorbance capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Anese, M.; Nicoli, M.C. Antioxidant Properties of Tea Extracts as Affected by Processing. LWT-Food Sci. Technol. 1998, 31, 694–698. [Google Scholar] [CrossRef]
- Lissi, E.; Salim-Hanna, M.; Pascual, C.; del Castillo, M.D. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic. Biol. Med. 1995, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef] [Green Version]
- Kabouche, A.; Kabouche, Z.; Öztürk, M.; Kolak, U.; Topçu, G. Antioxidant abietane diterpenoids from Salvia barrelieri. Food Chem. 2007, 102, 1281–1287. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Fernando, C.D.; Preethi Soysa, P. Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. MethodsX 2015, 2, 283–291. [Google Scholar] [CrossRef]
- Kunchandy, E.; Rao, M.N.A. Oxygen radical scavenging activity of curcumin. Int. J. Pharm. 1990, 58, 237–240. [Google Scholar] [CrossRef]
- Marcocci, L.; Maguire, J.J.; Droy-Lefaix, M.T.; Packer, L. The Nitric Oxide-Scavenging Properties of Ginkgo Biloba Extract EGb 761. Biochem. Biophys. Res. Commun. 1994, 201, 748–755. [Google Scholar] [CrossRef]
- Kooy, N.W.; Royall, J.A.; Ischiropoulos, H.; Beckman, J.S. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic. Biol. Med. 1994, 16, 149–156. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.-O.; Lee, C.Y. Superoxide Radical Scavenging Activity of the Major Polyphenols in Fresh Plums. J. Agric. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef] [PubMed]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Jagtap, U.B.; Panaskar, S.N.; Bapat, V.A. Evaluation of Antioxidant Capacity and Phenol Content in Jackfruit (Artocarpus heterophyllus Lam.) Fruit Pulp. Plant Foods Hum. Nutr. 2010, 65, 99–104. [Google Scholar] [CrossRef]
- Singal, P.K.; Kapur, N.; Dhillon, K.S.; Beamish, R.E.; Dhalla, N.S. Role of free radicals in catecholamine-induced cardiomyopathy. Can. J. Physiol. Pharmacol. 1982, 60, 1390–1397. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- El-Saadani, M.; Esterbauer, H.; El-Sayed, M.; Goher, M.; Nassar, A.Y.; Jürgens, G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J. Lipid Res. 1989, 30, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Fridovich, I. Superoxide Dismutase: An enzymic function for erythrocuprein (Hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Berker, K.I.; Güçlü, K.; Demirata, B.; Apak, R. A novel antioxidant assay of ferric reducing capacity measurement using ferrozine as the colour forming complexation reagent. Anal. Methods 2010, 2, 1770–1778. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef]
- Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S. Variation in Polyphenolics and Antioxidant Activity of Traditional Apple Cultivars from West Himalaya, Uttarakhand. Hortic. Plant J. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Souid, G.; Timoumi, R.; Le Cerf, D.; Majdoub, H. Partial characterization of the edible Spinacia oleracea polysaccharides: Cytoprotective and antioxidant potentials against Cd induced toxicity in HCT116 and HEK293 cells. Int. J. Biol. Macromol. 2019, 136, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Galla, N.R.; Pamidighantam, P.R.; Karakala, B.; Gurusiddaiah, M.R.; Akula, S. Nutritional, textural and sensory quality of biscuits supplemented with spinach (Spinacia oleracea L.). Int. J. Gastron. Food Sci. 2017, 7, 20–26. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.P.; Ruiz-Medina, A.; Llorent-Martínez, E.J. Phytochemical profile, mineral content, and antioxidant activity of Olea europaea L. cv. Cornezuelo table olives. Influence of in vitro simulated gastrointestinal digestion. Food Chem. 2019, 297, 124933. [Google Scholar] [CrossRef]
- Handa, C.L.; de Lima, F.S.; Guelfi MF, G.; Fernandes, M.d.S.; Georgetti, S.R.; Ida, E.I. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 2019, 271, 274–283. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, Á.; Yáñez, J.A. Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J. Med. Food 2012, 15, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jalao, I.; Sánchez-Moreno, C.; De Ancos, B. Effect of high-pressure processing on flavonoids, hydroxycinnamic acids, dihydrochalcones and antioxidant activity of apple ‘Golden Delicious’ from different geographical origin. Innov. Food Sci. Emer. 2019, 51, 20–31. [Google Scholar] [CrossRef]
- Mihailović, N.R.; Mihailović, V.B.; Kreft, S.; Ćirić, A.R.; Joksović, L.G.; Đurđević, P.T. Analysis of phenolics in the peel and pulp of wild apples (Malus sylvestris (L.) Mill.). J. Food Compos. Anal. 2018, 67, 1–9. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of apple pomace towards extraction of triterpenic acids, antioxidant potential, cytotoxic effects, and inhibition of clinically important enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef]
- Afonso, S.; Ribeiro, C.; Bacelar, E.; Ferreira, H.; Oliveira, I.; Silva, A.P.; Gonçalves, B. Influence of training system on physiological performance, biochemical composition and antioxidant parameters in apple tree (Malus domestica Borkh.). Sci. Hortic. 2017, 225, 394–398. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. In vitro antioxidant activities and polyphenol contents of seven commercially available fruits. Pharmacogn. Res. 2016, 8, 258–264. [Google Scholar]
- Zhao, Y.; Du, S.-k.; Wang, H.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef]
- Stanger, M.C.; Steffens, C.A.; Soethe, C.; Moreira, M.A.; do Amarante CV, T.; Both, V.; Brackmann, A. Phenolic compounds content and antioxidant activity of ‘Galaxy’ apples stored in dynamic controlled atmosphere and ultralow oxygen conditions. Postharvest Biol. Technol. 2018, 144, 70–76. [Google Scholar] [CrossRef]
- Grace, M.H.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M.A. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 277, 336–346. [Google Scholar] [CrossRef]
- Hussain, P.R.; Suradkar, P.; Javaid, S.; Akram, H.; Parvez, S. Influence of postharvest gamma irradiation treatment on the content of bioactive compounds and antioxidant activity of fenugreek (Trigonella foenum–graceum L.) and spinach (Spinacia oleracea L.) leaves. Innov. Food Sci. Emerg. Technol. 2016, 33, 268–281. [Google Scholar] [CrossRef]
- Cheurfa, M.; Abdallah, H.H.; Allem, R.; Noui, A.; Picot-Allain CM, N.; Mahomoodally, F. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food Chem. Toxicol. 2019, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, B.; Maranghi, S.; Paoletti, A.; Marconi, O.; Rosati, A.; Famiani, F.; Benincasa, P. Sprouting olive (Olea europaea L.) seeds as a source of antioxidants from residual whole stones. Sci. Hortic. 2018, 240, 558–560. [Google Scholar] [CrossRef]
- Xu, L.; Yue, Q.; Bian F, e.; Zhai, H.; Yao, Y. Melatonin Treatment Enhances the Polyphenol Content and Antioxidant Capacity of Red Wine. Hortic. Plant J. 2018, 4, 144–150. [Google Scholar] [CrossRef]
- Milat, A.M.; Boban, M.; Teissedre, P.-L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of oxidation and browning of macerated white wine on its antioxidant and direct vasodilatory activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
- Majkić, T.M.; Torović, L.D.; Lesjak, M.M.; Četojević-Simin, D.D.; Beara, I.N. Activity profiling of Serbian and some other European Merlot wines in inflammation and oxidation processes. Food Res. Int. 2019, 121, 151–160. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.H.; Park, J.H.; Jeong, Y.; Ko, K.S. Cellular Antioxidant and Anti-Inflammatory Effects of Coffee Extracts with Different Roasting Levels. J. Med. Food 2017, 20, 626–635. [Google Scholar] [CrossRef]
- Bravo, J.; Monente, C.; Juániz, I.; De Peña, M.P.; Cid, C. Influence of extraction process on antioxidant capacity of spent coffee. Food Res. Int. 2013, 50, 610–616. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Almeida, T.S.D.; Araújo, M.E.M.; Rodríguez, L.G.; Júlio, A.; Mendes, B.G.; Santos, R.M.B.D.; Simões, J.A.M. Influence of preparation procedures on the phenolic content, antioxidant and antidiabetic activities of green and black teas. Braz. J. Pharm. Sci. 2019, 55, e17695. [Google Scholar] [CrossRef]
- Cid-Gallegos, M.S.; Sánchez-Chino, X.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Villa-Treviño, S.; Dávila-Ortíz, G.; Jiménez-Martínez, C. Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model. Nutrients 2020, 12, 2572. [Google Scholar] [CrossRef] [PubMed]
- Peiretti, P.G.; Karamać, M.; Janiak, M.; Longato, E.; Giorgia, M.; Amarowicz, R.; Gai, F. Phenolic Composition and Antioxidant Activities of Soybean (Glycine max (L.) Merr.) Plant during Growth Cycle. Agronomy 2019, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.L.; Yu, Y.P.; Hsieh, J.F.; Kuo, M.I.; Ma, Y.S.; Lu, C.P. Effect of germination on composition profiling and antioxidant activity of the polysaccharide-protein conjugate in black soybean [Glycinemax (L.) Merr.]. Int. J. Biol. Macromol. 2018, 113, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Heshmati, A.; Momtaz, J.; Vahidinia, A. Effect of iron-enrichment on the antioxidant properties of wheat flour and bread. J. Cereal Sci. 2019, 87, 98–102. [Google Scholar] [CrossRef]
- Malunga, L.N.; Izydorczyk, M.; Beta, T. Antiglycemic Effect of Water Extractable Arabinoxylan from Wheat Aleurone and Bran. J. Nutr. Metab. 2017, 2017, 5784759. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aal, E.-S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef]
- Zulueta, A.; Maurizi, A.; Frígola, A.; Esteve, M.J.; Coli, R.; Burini, G. Antioxidant capacity of cow milk, whey and deproteinized milk. Int. Dairy J. 2009, 19, 380–385. [Google Scholar] [CrossRef]
- de Carvalho, M.W.; Arriola, N.D.A.; Pinto, S.S.; Verruck, S.; Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.d.M.C. Stevia-fortified yoghurt: Stability, antioxidant activity and in vitro digestion behaviour. Int. J. Dairy Technol. 2019, 72, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. Phytochemical profiles, antioxidant, and antiproliferative activities of four red-fleshed apple varieties in China. J. Food Sci. 2020, 85, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Zhang, H.-C.; Liu, W.-X.; Li, C.-Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Monomeric Anthocyanins, Percent Polymeric Color, and Antioxidant Capacity of Processed Blackberry Products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; Rodríguez, A.; Stucken, K. Antioxidant, functional properties and health-promoting potential of native South American berries: A review. J. Sci. Food Agric. 2021, 101, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Liović, N.; Bratanić, A.; Zorić, Z.; Pedisić, S.; Režek Jambrak, A.; Krešić, G.; Bilušić, T. The effect of freeze-drying, pasteurisation and high-intensity ultrasound on gastrointestinal stability and antioxidant activity of blueberry phenolics. Int. J. Food Sci. 2021, 56, 1996–2008. [Google Scholar] [CrossRef]
- Dalmau, M.E.; Llabrés, P.J.; Eim, V.S.; Rosselló, C.; Simal, S. Influence of freezing on the bioaccessibility of beetroot (Beta vulgaris) bioactive compounds during in vitro gastric digestion. J. Sci. Food Agric. 2019, 99, 1055–1065. [Google Scholar] [CrossRef]
- Fiorito, S.; Preziuso, F.; Epifano, F.; Scotti, L.; Bucciarelli, T.; Taddeo, V.A.; Genovese, S. Novel biologically active principles from spinach, goji and quinoa. Food Chem. 2019, 276, 262–265. [Google Scholar] [CrossRef]
- Salehi, B.; Tumer, T.B.; Ozleyen, A.; Peron, G.; Dall’Acqua, S.; Rajkovic, J.; Naz, R.; Nosheen, A.; Mudau, F.N.; Labanca, F.; et al. Plants of the genus Spinacia: From bioactive molecules to food and phytopharmacological applications. Trends. Food Sci. Technol. 2019, 88, 260–273. [Google Scholar] [CrossRef]
- Tareq, F.S.; Kotha, R.R.; Ferreira JF, S.; Sandhu, D.; Luthria, D.L. Influence of Moderate to High Salinity on the Phytochemical Profiles of Two Salinity-Tolerant Spinach Genotypes. ACS Food Sci. Technol. 2021, 1, 205–214. [Google Scholar] [CrossRef]
- Kamiloglu, S. Industrial freezing effects on the content and bioaccessibility of spinach (Spinacia oleracea L.) polyphenols. J. Sci. Food Agric. 2020, 100, 4190–4198. [Google Scholar] [CrossRef]
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections between Agriculture and Processing for Health Choices. Antioxidants 2020, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Huertas, E.; Fonolla, J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017, 11, 384–389. [Google Scholar] [CrossRef]
- Pandeya, A.; Rayamajhi, S.; Pokhrel, P.; Giri, B. Evaluation of secondary metabolites, antioxidant activity, and color parameters of Nepali wines. Food Sci. Nutr. 2018, 6, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Tavazzi, I.; Offord, E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem. 2001, 49, 3438–3442. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Nah, J.; Chun, S.; Park, H.; Yang, S.E.; Min, W.K. In vivo antioxidant effect of green tea. Eur. J. Clin. Nutr. 2000, 54, 527–529. [Google Scholar] [CrossRef]
- Benzie, I.; Szeto, Y.T.; Strain, J.J.; Tomlinson, B. Consumption of Green Tea Causes Rapid Increase in Plasma Antioxidant Power in Humans. Nutr. Cancer 1999, 34, 83–87. [Google Scholar] [CrossRef]
- Bahorun, T.; Luximon-Ramma, A.; Neergheen-Bhujun, V.S.; Gunness, T.K.; Googoolye, K.; Auger, C.; Crozier, A.; Aruoma, O.I. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev. Med. 2012, 54, S98–S102. [Google Scholar] [CrossRef]
- Moura-Nunes, N.; Perrone, D.; Farah, A.; Donangelo, C.M. The increase in human plasma antioxidant capacity after acute coffee intake is not associated with endogenous non-enzymatic antioxidant components. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 6), 173–181. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, C.-S.; Sorrells, M.E.; Liu, R.H. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2010, 119, 249–257. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of Antioxidant Properties of Refined and Whole Wheat Flour and Bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov. Food Sci. Emer. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef] [Green Version]
- Alenisan, M.A.; Alqattan, H.H.; Tolbah, L.S.; Shori, A.B. Antioxidant properties of dairy products fortified with natural additives: A review. J. Assoc. Arab. Univ. Basic Appl. Sci. 2017, 24, 101–106. [Google Scholar] [CrossRef]
- Harnly, J. Antioxidant methods. J. Food Compos. Anal. 2017, 64, 145–146. [Google Scholar] [CrossRef]
- Abramovič, H.; Grobin, B.; Poklar Ulrih, N.; Cigić, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Rajesh, J.; Olives, A.I.; Rocchi, D.; Gómez-Carpintero, J.; González, J.F.; Sridharan, V.; Martín, M.A.; Menéndez, J.C. Antioxidants as molecular probes: Structurally novel dihydro-m-terphenyls as turn-on fluorescence chemodosimeters for biologically relevant oxidants. Antioxidants 2020, 9, 605. [Google Scholar] [CrossRef]
- Hermans, N.; Cos, P.; Maes, L.; De Bruyne, T.; Vanden Berghe, D.; Vlietinck, A.J.; Pieters, L. Challenges and pitfalls in antioxidant research. Curr. Med. Chem. 2007, 14, 417–430. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, J.; Ramos, R.; Luís, Â.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Duplancic, D.; Kukoc-Modun, L.; Modun, D.; Radic, N. Simple and Rapid Method for the Determination of Uric Acid-Independent Antioxidant Capacity. Molecules 2011, 16, 7058–7067. [Google Scholar] [CrossRef]
- Lowe, F. Biomarkers of Oxidative Stress, in Systems Biology of Free Radicals and Antioxidants, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health: (Revision 1). EFSA J. 2018, 16, e05136. [Google Scholar]

| Mechanism (Category) | Assay | Technique/Principle | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|
| In vitro | |||||
| Electron transfer (Total Antioxidant Capacity) | CUPRAC (Cupric ion reducing antioxidant capacity method) | In this assay, phenolic groups in the polyphenols are oxidized to quinones, whereas Cu(II) is reduced to Cu(I), which is measured at 450 nm. | (+) Copper reaction rates are faster than that of ferric ions, and it is more specific for antioxidants | (−) it ignores the reaction kinetics | [36] |
| DMPD (N,N-dimethyl-p-phenylene diamine dihydrochloride) method | In the presence TROLOX, reduction of DMPD radical cation by antioxidants, the absorbance at 505 nm is decreased. | (+) easy, cheaper, and reproducible | (−) it ignores the reaction kinetics, and the DMPD radical is a non-physiological radical | [62] | |
| FRAP (Ferric reducing-antioxidant power assay) | Antioxidants at low pH reduce ferric-tripyridyltriazine (FeIII-TPTZ)to FeII form which is measured at 593 nm. | (+) a good representation of electron transfer mechanism (+) the is inexpensive, easy to prepare reagents, reproducibility, and speedy and a straight forward procedure | (−) it ignores the reaction kinetics and non-specific to antioxidants | [51] | |
| Follin-Ciocalteu reducing capacity | In this assay, phenols are oxidized in a basic medium by a mixture of tungstate and molybdate (Folin-Ciocalteu reagent), with the consequent formation of colored molybdenum ions, MoO4+ (750 nm). | (+) easy and reproducible | (−) it ignores the reaction kinetics and non-specific to antioxidants | [14] | |
| Trolox equivalent antioxidant capacity (TEAC) method (ABTS radical cation decolorization assay) | Upon reaction with an antioxidant (Trolox), ABTS (2,2-azo-bis(3-ethylbenz-thiozoline-6-sulfonic acid)) radical cation, which is a blue-green chromophore, reduces and decolorized. This assay uses a diode-array spectrophotometer at 750 nm. | (+) it can screen both hydrophilic and lipophilic antioxidants, easy and reproducible | (−) it ignores the reaction kinetics, and the ABTS is a non-physiological radical (−) The assay is not suitable for the determination of proteins antioxidant activity | [63] | |
| Hydrogen Atom Transfer (Antioxidant Activity) | ORAC (Oxygen radical absorbance capacity) method | AAPH (2,2-azobis-2-aminopropane dihydrochloride) decomposition induces peroxyl radicals, and radical scavengers are used to measure the decrease in fluorescence. AAPH is used as a radical generator and Trolox as the antioxidant control. 485 nm is used as the excitation wavelength, and 520 nm is used as the emission wavelength. | (+) physiologically resemble method, and it takes initiation and propagation into account | (−) lack of consistency and the possible underestimation of antioxidant activity as B-PE can interact with phenolic acids (−) The method has been reported to fail determining both hydrophilic and lipophilic antioxidants | [64] |
| TRAP (Total radical-trapping antioxidant parameter method), both in vivo and in vitro | In this method, the antioxidant potential is assessed by measuring the decay in decoloration. ABAP (2,2′-azo-bis(2-ami-dino-propane)hydrochloride) is a radical initiator that quenches the fluorescence of R-Phycoerythrin (R-PE). | (+) peroxyl radical is a common and physiologically representative radical | (−) detection probe(oxygen) that is not stable and may cause issues in measurements | [65,67,68] | |
| ROS/RNS scavenging activity/lipid oxidation | β-carotene linoleic acid method/conjugated diene assay | The ROS oxidizes linoleic acid, and the resulting products initiate β-carotene oxidation, which leads to discoloration. In the presence of antioxidants, the discoloration will be delayed and measured at 434 nm. | (+) shows a strong correlation with the total phenolics measured by the F-C method. | (−) lack of reproducibility and crude kinetic treatment | [14,68,69] |
| Ferric thiocyanate (FTC) method | During linoleic acid peroxidation, peroxides were formed, which oxidize Fe(II) to Fe(III). The Fe(III) reacts with thiocyanate to form a red color complex, which is measured at 500 nm. | (+) used to measure peroxide amount at the starting phase of peroxidation | (−) lack of specificity | [46,70,71] | |
| Thiobarbituric acid (TBA) method | In this assay, TBA and trichloroacetic acid are mixed with the sample solution, placed in the hot water bath for 10 min, centrifuged in the solution, and supernatant absorbance activity is measured at 552 nm. | (+) used to measure the concentration of free radicals present at the end of peroxide oxidation | (−) Not specific | [14] | |
| Hydrogen peroxide scavenging (H2O2) assay | Antioxidants reduce hydrogen peroxide concentration, which is measured at 230 nm using a spectrophotometer. | (−) most plant and food samples also absorb at this wavelength, which can compromise both the precision and accuracy of the method | [72] | ||
| Hydroxyl radical scavenging activity | In the presence of antioxidant, the degraded product of deoxyribose (TBARS) measured colorimetrically at 532 nm. | (+) Useful for ketone containing antioxidants | (−) Higher concentration of antioxidants required | [73] | |
| Nitric oxide scavenging activity | Under aerobic conditions, nitric oxide reacts with oxygen to form nitrate and nitrite, which can be quantified using Griess reagent, and the absorbance is measured at 546 nm. | (+) relatively simple experimentation and physiologically relevant | (−) detection technique is not easily available and has a long reaction time | [18,74] | |
| Peroxynitrile radical scavenging activity | ONOO.scavenging activity is measured by the oxidation of dihydroxyrhodamine to rhodamine fluorescence spectrophotometer with an excitation wavelength of 485 nm and emission wavelength of 530 nm. | (+) Peroxynitrile is a good oxidizing agent for dihydroxyrhodamine | (−) Under anaerobic conditions, nitric oxide did not oxidize dihydrorhodamine and inhibited spontaneous oxidation of dihydrorhodamine | [75] | |
| Superoxide radical scavenging activity (SRSA/SOD) | This assay is based on the removal rate of superoxide radical (O2−) using antioxidants, which is measured by nitro blue tetrazolium (NBT) at 560 nm. | (+) peroxyl radical is a common and physiologically representative radical | (−) irreproducibility due to the water insolubility issue of diformazan, the end product of NBT reduction | [76,77] | |
| Xanthine oxidase inhibition assay | Xanthine is a substrate in XOD- catalyzed reaction, which yields uric acid as a product. Allopurinol is used as a xanthine oxidase inhibitor, measured at 293 nm. | (+) Possible to get kinetics | (−) Enzyme collection is tricky | [8,78] | |
| ET/HAT_mixed | DPPH scavenging activity | 1-diphenyl-2-picrylhydrazyl (α,α-diphenyl-β-picrylhydrazyl; DPPH) is a stable free radical due to electron delocalization, which prevents its dimerization. DPPH reacts with antioxidants, which diminishes its deep violet color, which is measured at 517 nm (515–518 nm). | (+) easy and reproducible | (−), difficult to get the reaction kinetics, and the DPPH radical is a non-physiological radical | [66] |
| Metal chelation | Ferrous ion chelating activity assay/Ferrozine assay-Fe(II) | TAC assay obtained via reduction of Fe(III) to Fe(II), and formed Fe(II) is determined with ferrozine using spectrophotometric absorbance measurement at 562 nm. | (+) High sensitivity, correlated with structure-activity relationships, higher molar absorptivity, relatively lower interference from foreign ions, wide pH tolerance, complex stability constant, water solubility, and low viscosity | (−) Not correlated with FRAP, DPPH, and TPC | [87,88] |
| Cuprous ion chelating activity/Pyrocatechol violet-Cu(II) | Free Cu(II) that is not complexed with antioxidants is bound to Pyrocatechol, which is assessed at 632 nm. | (+) good repeatability and reproducibility, Cu2+ chelating ability is significantly and positively correlated to DPPH, FRAP, and total phenolic content | [88] | ||
| In vivo | |||||
| Hydrogen atom transfer | Catalase (CAT) | The catalase activity is measured in an erythrocyte lysate as the difference in absorbance (λ240) per unit as the H2O2 maximum absorption wavelength is 240 nm. Catalase activity is used both in vivo and in vitro. | (+) a good representation of physiological conditions | [78] | |
| Electron transfer/reducing power (Total Antioxidant Capacity) | Ferric reducing ability of plasma (FRAP) | This assay is primarily based on the principle that, at low pH, ferric-tripyridyltriazine (FeIII-TPTZ) is reduced to Fe(II). The antioxidant capacity is measured using the increased FeII, which is measured spectrophotometrically at 593 nm. | (+) most simple, rapid, inexpensive tests and very useful for routine analysis, a good representation of electron transfer mechanism | (−) it ignores the reaction kinetics and non-specific to antioxidants | [51,79] |
| γ-glutamyl transpeptidase (GGT) | GGT transfers the γ-glutamyl group from the L-γ-Glutamyl-p-nitroanilide and liberates the chromogen p-nitroanilide (pNA, 418 nm) proportional to the GGT present. | (+) a good representation of physiological conditions | [80] | ||
| Lipid peroxidation inhibition | Glutathione peroxidase (GSHPx) estimation | GSHPx is a seleno-enzyme that catalyzes the reaction of hydroperoxides with GSH to form GSSG and reduction of hydrogen peroxide. | (+) a good representation of physiological conditions | [13,85] | |
| Glutathione reductase (GR) assay | GR catalyzes the reduction of GSSG to GSH. GR activity is determined at 340 nm and 412 nm. One may expect a decrease of activity at 340 nm as a result of the oxidation of NADPH or an increase at 412 nm caused by the reduction of dithiobis (2-nitrobenzoic acid) DTNB. | (+) a good representation of physiological conditions | [13,81] | ||
| Glutathion-S-transferase (GSt) | This assay utilizes 1-Chloro-2,4-dinitrobenzene (CDNB). Potassium phosphate, GSt, and CDNB mixture are incubated at 37 C, pH 6.5 for 5 min, followed by adding substrate. 340 nm absorbance is used for monitoring the assay. | (+) a good representation of physiological conditions | [13] | ||
| LDL assay | The extent of low-density lipoprotein (LDL) oxidation is determined by the amount of lipid peroxides, also by using a thiobarbituric acid reactive substances (TBARS) assay determined at 532 nm. | (+) LDL is a true representation of physiologically | (−) limitations in the isolation of LDL from the blood, and it is difficult to monitor the lag phase | [13,83,84,85] | |
| Lipid peroxidation inhibition | Lipid peroxidation (LPO) assay | Malondialdehyde (MDA) is one of the end products of lipid peroxidation, which is used for the LPO assay measured at 586 nm. | (+) a good representation of physiological conditions | [84,85] | |
| Superoxide dismutase (SOD) method | The SOD assay works based on the absorbance change at 420 nm related to pyrogallol. | (+) a good representation of physiological conditions | [86] | ||
| Samples | Matrix | Assay | Results | Ref. |
|---|---|---|---|---|
| Fruits Apple | Fresh apple | TPC | 6.82 mg GAE/g fw for Benoni cultivars from the location Mukhwa | [89] |
| DPPH | 10.87 mmol AAE/kg fw | |||
| ABTS | 24.57 mmol AAE/kg fw | |||
| FRAP | 24.05 mmol AAE/kg fw | |||
| Fresh apple | TPC | 4.18 ± 0.1 mg GAE/g dw | [96] | |
| DPPH | 22.14 ± 1.2 μmol TE/g dw | |||
| FRAP | 26.98 ± 0.9 μmol TE/g dw | |||
| ABTS | 32.85 ± 1.5 μmol TE/g dw | |||
| Apple peel | TPC | 0.48 g GAE/kg | [102] | |
| DPPH | 121 mol TEAC/kg | |||
| ABTS | 13 mol TEAC/kg | |||
| Wild apples peel and pulp (ultra-sonic extract) | TPC | 8.00 mg GAE/g fw in peel | [97] | |
| 6.64 mg GAE/g fw in pulp | ||||
| DPPH | IC50: 240.00 ± 6.00 μg/mL peel | |||
| IC50: 286.00 ± 7.00 μg/mL pulp | ||||
| ABTS | IC50: 134.00 ± 3.00 μg/mL peel | |||
| IC50: 167.00 ± 4.00 μg/mL pulp | ||||
| Apple pomace | TPC | 3.48 ± 0.12 mg GAE/g apple pomace for MeOH extract | [98] | |
| DPPH | 72.6 ± 1.6% (Inhibition) | |||
| FRAP | 65.8 ± 1.8% (Inhibition) | |||
| ABTS | 84.3 ± 1.6% (Inhibition) | |||
| Apple leaves | TPC | 143.84 ± 37.79 mg GAE/g | [99] | |
| DPPH | 259.68 ± 46.91 μmol TE/g | |||
| ABTS | 625.26 ± 141.31 μmol TE/g | |||
| FRAP | 328.02 ± 130.38 μmol TE/g | |||
| Berries | Blueberry | TPC | 443.60 ± 17.00 mg GAE/g | [100] |
| DPPH | 87.90 ± 0.20% inhibition (100 μg/mL); IC50 1.40 ± 0.10 μg/mL | |||
| ABTS | 23.10 ± 0.60% inhibition (100 μg/mL); IC50 14.00 ± 0.50 μg/mL | |||
| Blackberry | TPC | 269.5 ± 16 mg GAE/g | ||
| DPPH | 77.80 ± 2.00% inhibition (100 μg/mL); IC50 1.30 ± 0.10 μg/mL | |||
| ABTS | 25.30 ± 1.10% inhibition (100 μg/mL); IC50 23.00 ± 5.00 μg/mL | |||
| Black raspberry | TPC | 965.60 ± 2.90 mg GAE/g | ||
| DPPH | 89.03 ± 0.040% inhibition (100 μg/mL); IC50 3.40 ± 0.40 μg/mL | |||
| ABTS | 21.3 ± 1% (per 100 μg/mL); IC50 79.00 ± 18.07 μg/mL | |||
| Red raspberry | TPC | 434.3 ± 6.3 mg GAE g−1 | ||
| DPPH | 87 ± 1.2% inhibition (100 μg/mL); IC50 1.40 ± 0.10 μg/mL | |||
| ABTS | 31.1 ± 0.6% inhibition (100 μg/mL); IC50 15.00 ± 0.90 μg/mL | |||
| Strawberry | TPC | 250.10 ± 17.10 mg GAE/g | ||
| DPPH | 70.20 ± 1.00% inhibition (100 μg/mL); IC50 3.1 ± 0.02 μg/mL | |||
| ABTS | 26.20 ± 0.70% inhibition (100 μg/mL); IC50 9.9 ± 0.40 μg/mL | |||
| Lowbush blueberry | TPC | 24.50 ± 0.69 mg GAE/g | [103] | |
| ABTS | 127.00 ± 5.30 μmol TE/g | |||
| FRAP | 389.00 ± 19.40 μmol FeSO4 equivalent/g | |||
| Vegetables Spinach | Dried, powdered | DPPH | 36.71% inhibition (180 μg sample/mL) | [91] |
| ABTS | 68.34% inhibition (180 μg sample/mL) | |||
| FRAP | 0.14% inhibition (180 μg sample/mL) | |||
| Gamma irradiated (above 0.75 kGy) samples | DPPH | EC50 42–50% inhibition | [104] | |
| FRAP | EC50 0.48–0.7% inhibition | |||
| TPC | 208.9–216.2 mg GAE/g | |||
| Polysaccharides | DPPH | 68.51 ± 0.89% inhibition | [90] | |
| ABTS | 70.12 ± 0.04% inhibition | |||
| FRAP | 1590 ± 53.98 μmol/L at 10 mg/mL BHT and AA | |||
| Olives | Lyophilized table Olive; methanol extract | TPC | 31.52 mg GAE/g | [92] |
| ABTS | 308.68 μmol TE/g | |||
| DPPH | 228.46 μmol TE/g | |||
| Leaves; ethanol extract | DPPH | 69.15 ± 0.06% Inh | [105] | |
| β-carotene bleaching | 54.98 ± 0.03% | |||
| TPC | 82.63 ± 0.02 mg AAE/g extract | |||
| FRAP | 07.53 ± 0.06 mol Fe2+/g extract | |||
| Sprouted olive seeds | TPC | ~4.50 mg GAE/g dw | [106] | |
| ABTS | ~12 μmol TE/g dw | |||
| DPPH | ~11 μmol TE/g dw | |||
| FRAP | ~9 μmol TE/g dw | |||
| Processed food Wine | Red wine | TPC | 317.62 ± 18.75 mg/mL | [107] |
| DPPH | 3.16 ± 0.15 mg GAE/mL | |||
| ABTS | 7.10 ± 0.75 mg TE/mL | |||
| FRAP | 8.20 ± 0.76 mg TE/mL | |||
| Standard white wine | FRAP | 336.70 ± 15.20 μmol TE | [108] | |
| DPPH | 2103.30 ± 115.60 μmol TE | |||
| ABTS | 3037.50 ± 333.30 μmol TE | |||
| ORAC | 4756.70 ± 41.20 μmol TE | |||
| TPC | 305.30 ± 3.40 mg GAE/L | |||
| Merlot wines from Serbia and Spain * Red wine | FRAP | 0.33 ± 0.01 μmol TE/g dry residue | [109] | |
| DPPH | 0.16 ± 0.01 μmol TE/g dry residue | |||
| ABTS | 0.35 ± 0.03 μmol TE/g dry residue | |||
| Coffee | Green coffee- light roasted | DPPH | ~13.00% RSA at 0.5 mg/mL sample | [110] |
| ABTS | ~90.00% RSA at 0.5 mg/mL sample | |||
| Green coffee- medium roasted | DPPH | ~10.00% RSA at 0.5 mg/mL sample | ||
| ABTS | ~90.00% RSA at 0.5 mg/mL sample | |||
| Green coffee- French roasted | DPPH | ~6.50% RSA at 0.5 mg/mL sample | ||
| ABTS | ~90.00% RSA at 0.5 mg/mL sample | |||
| Filtered coffee, water extract | TPC | 13.94 ± 0.2 mg GAE/g dm | [111] | |
| DPPH | 82.40 ± 2.86 μmolTE/g dm | |||
| ABTS | 140.31 ± 2.80 μmolTE/g dm | |||
| Defatted coffee | TPC | 23.43 ± 0.06 mg GAE g−1 dm | ||
| DPPH | 110.33 ± 1.97 μmol TE/g dm | |||
| ABTS | 218.38 ± 0.55 μmol TE/g dm | |||
| Tea | Black Tea (Dianhong Congou) | FRAP | 2670.13 ± 34.02 μmol Fe2+/g dw | [112] |
| TEAC | 994.56 ± 12.64 μmol Trolox/g dw | |||
| TPC | 101.29 ± 1.58 mg GAE/g dw | |||
| Green Tea (Dianqing Tea) | FRAP | 4647.47 ± 57.87 μmol Fe2+/g dw | ||
| TEAC | 2532.41 ± 50.18 μmol Trolox/g dw | |||
| TPC | 252.65 ± 4.74 mg GAE/g dw | |||
| Green Tea leaves | TPC | 0.37 ± 0.02 mg GAE/mL at 90 °C temp | [113] | |
| DPPH | 42.4 ± 2.6% RSA at 90 °C temp | |||
| Green Teabags | TPC | 0.64 ± 0.02 mg GAE/mL at 90 °C temp | ||
| DPPH | 70.3 ± 3.4% RSA at 90 °C temp | |||
| Black Tea leaves | TPC | 0.19 ± 0.00 mg GAE/mL at 90 °C temp | ||
| DPPH | 20.7 ± 1.5% RSA at 90 °C temp | |||
| Black Teabags | TPC | 0.50 ± 0.02 mg GAE/mL at 90 °C temp | ||
| DPPH | 36.0 ± 2.0% RSA at 90 °C temp | |||
| Legumes Beans | Chickpea—60% ethanol extract | TPC | 21.9 ± 2.8 mg GAE/g | [101] |
| TAC | 648 ± 18 (U/g) | |||
| OH scavenging capacity | 66.22 ± 0.09% | |||
| DPPH | ~15% RSA | |||
| Chickpea aqueous extract | TPC | 60.09 ± 4.17 mg GAE/100 g | [114] | |
| ORAC | 52.73± 0.96 mg TE/g dry base | |||
| OH scavenging capacity | 56.36 ± 1.54% | |||
| Soybeans | The aerial part of the soybean | TPC | 42.2 ± 2.23–50.40 ± 1.00 mg CE/g extract 1.40 ± 0.04 to 1.95 ± 0.00 mg CE/g fw for seven growth stages | [115] |
| TEAC | 177.00 ± 11.00–245.00 ± 21.00 μmol TE/g extract 6.26 ± 0.41–8.43 ± 1.28 μmol TE/g fw for seven growth stages | |||
| FRAP | 623.00 ± 3.00–780.00 ± 0.700 μmol Fe2+/g extract 21.4 ± 2.6–28.5 ± 0.7 μmol Fe2+/g fw for seven growth stages | |||
| DPPH | EC50: 0.125–0.22 mg/mL | |||
| Fermented (by M. purpureus) defatted soybean flour | TPC | 2.20 ± 0.03 mg GAE/g | [93] | |
| ABTS | 59.61 ± 6.68 μmol TE/g | |||
| FRAP | 14.26 ± 0.44 μmol TE/g | |||
| DPPH | 0.74 ± 0.02 μmol TE/g | |||
| Water-soluble black soybean polysaccharide from sprouted seeds | TPC | 3.71–6.83 mg GAE/g | [116] | |
| ABTS | IC50: 1.72–3.48 mg/mL | |||
| DPPH | IC50: 4.45–8.00 mg/mL | |||
| Reducing power | IC50: 3.42–5.84 ± 0.12 mg/mL | |||
| Grains Corn | Grounded purple corn extracted with acidified 80:20 methanol: water | TPC | 9.06 ± 0.07 GAE/kg | [94] |
| DPPH | IC50: 66.3 ± 0.80 μg/mL | |||
| ABTS | IC50: 250 ± 0.40 μg/mL | |||
| FRAP | 26.10 ± 0.04 μmol TE/g | |||
| Corn | TPC | ~1230–1410 μg GAE/g dm | [95] | |
| DPPH | 37–45% RSA | |||
| Wheat | Whole fresh flour | TPC | 1556.11 ± 20.42 μg FAE/g | [117] |
| DPPH | 4.68 ± 0.45 μmol TE/g | |||
| FRAP | 42.09 ± 2.82 μmol Fe2+/g | |||
| Wheat aleurone- water extract (WA-f50) | TPC | 26.01 ± 0.40 mg GAE/g | [118] | |
| DPPH | 147.85 ± 8.54 μmol TE/g WEAX | |||
| ABTS | 355.26 ± 0.01 μmol TE/g WEAX | |||
| ORAC | 527.47 ± 13.21 μmol TE/g WEAX | |||
| Wheat bran- water extract (WA-f50) | TPC | 16.78 ± 0.35 mg GAE/g | ||
| DPPH | 106.29± 12.13 μmol TE/g WEAX | |||
| ABTS | 320.40 ± 21.06 μmol TE/g WEAX | |||
| ORAC | 484.91 ± 34.15 μmol TE/g WEAX | |||
| Whole grain flour | DPPH | 3.1 μmol TE/g | [119] | |
| TEAC | 1.3 μmol TE/g | |||
| Peroxyl scavenging capacity | 0.55 mmol TE/g | |||
| Wheat bran | DPPH | 6.7 μmol TE/g | ||
| TEAC | 2.6 μmol TE/g | |||
| ORAC | 1.05 μmol TE/g | |||
| Milk and Dairy products | Milk | ORACFL | Whole milk (UHT): 14,481± 328 μmol TE Deproteinized Milk (UHT): 129 ± 5.9 μmol TE Whole milk (Pasteurized):14,216 ± 1051 μmol TE Deproteinized (Pasteurized): 464 ± 21.4 μmol TE Lowfat milk (UHT): 13,874 ± 312 μmol TE Lowfat Deproteinized Milk (UHT): 35 ± 2.2 μmol TE Lowfat milk (Pasteurized): 13,748 ± 397 μmol TE Deproteinized milk (Pasteurized):610± 16.9 μmol TE | [120] |
| Yoghurt | TPC | Yoghurt; 0.14 ± 0.01 mg GAE/g (control) Yoghurt + 0.25% FSE; 0.43 ± 0.02 mg GAE/g Yoghurt + 0.5% FSE; 0.65 ±0.02 mg GAE/g | [121] | |
| FRAP | Yoghurt; 0.40 ± 0.03 μmol TE/g dw Yoghurt + 0.25% FSE; 2.57 ± 0.09 μmol TE/g dw Yoghurt + 0.5% FSE; 4.19 ± 0.05 μmol TE/g dw | |||
| ABTS | Yoghurt; 0.40 ± 0.04 μmol TE/g dw Yoghurt + 0.25% FSE; 3.63 ± 0.08 μmol TE/g dw Yoghurt + 0.5% FSE; 5.34 ± 0.23 μmol TE/g dw |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. https://doi.org/10.3390/antiox11122388
Kotha RR, Tareq FS, Yildiz E, Luthria DL. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants. 2022; 11(12):2388. https://doi.org/10.3390/antiox11122388
Chicago/Turabian StyleKotha, Raghavendhar R., Fakir Shahidullah Tareq, Elif Yildiz, and Devanand L. Luthria. 2022. "Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays" Antioxidants 11, no. 12: 2388. https://doi.org/10.3390/antiox11122388
APA StyleKotha, R. R., Tareq, F. S., Yildiz, E., & Luthria, D. L. (2022). Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants, 11(12), 2388. https://doi.org/10.3390/antiox11122388