Size-Controllable Prussian Blue Nanoparticles Using Pluronic Series for Improved Antioxidant Activity and Anti-Inflammatory Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PB/Plu NPs
2.3. Characterization of PB/Plu NPs
2.4. Stability of PB/Plu NPs
2.5. In Situ Antioxidant Activity of PB/Plu NPs
2.6. In Vitro Cytotoxicity
2.7. In Vitro Antioxidant Activity
2.8. In Vitro Anti-Inflammatory Activity
2.9. In Vitro Wound-Healing Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of PB/Plu NPs
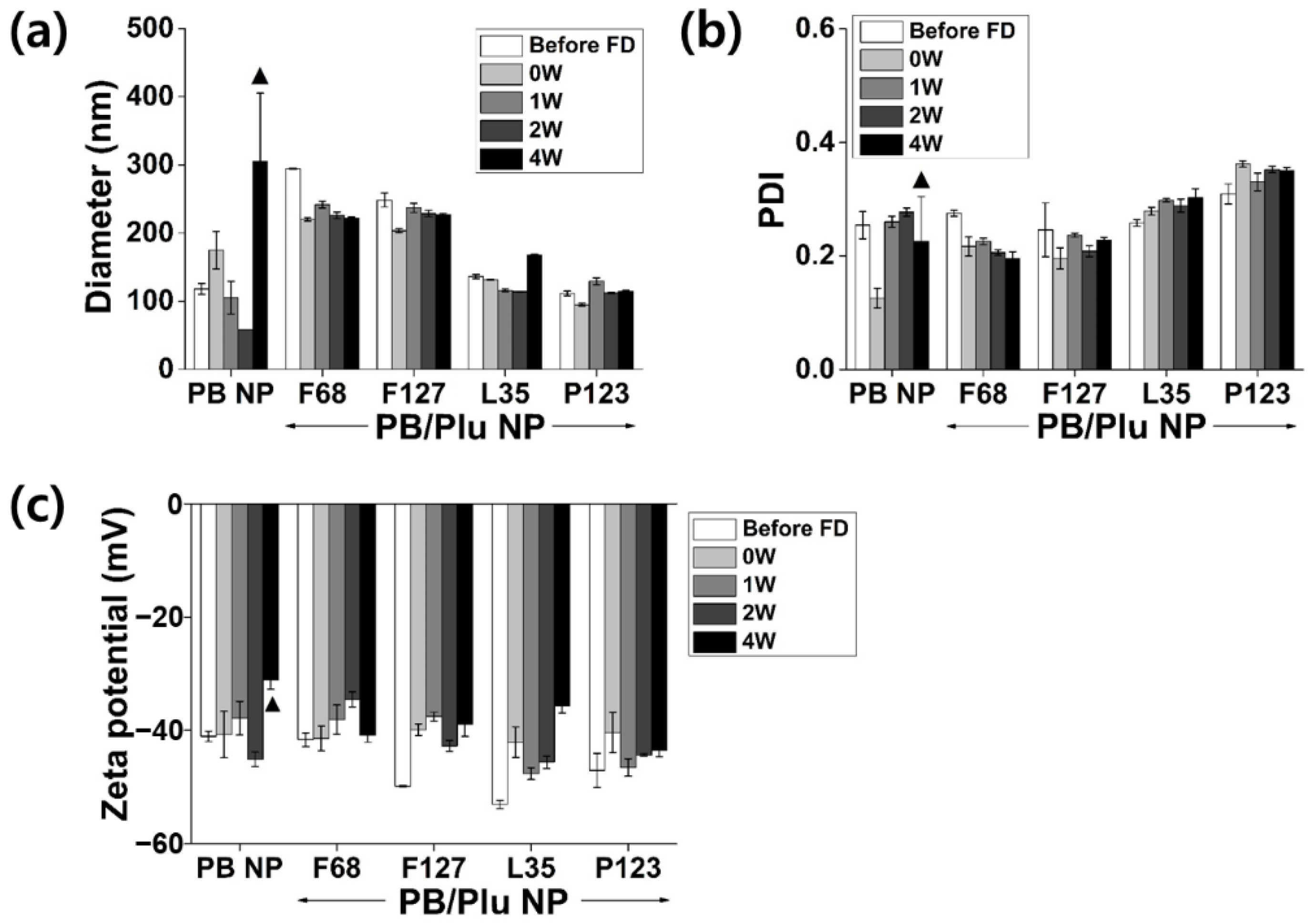
3.2. Stability of PB/Plu NPs
3.3. In Situ Antioxidant Activity of PB/Plu NPs
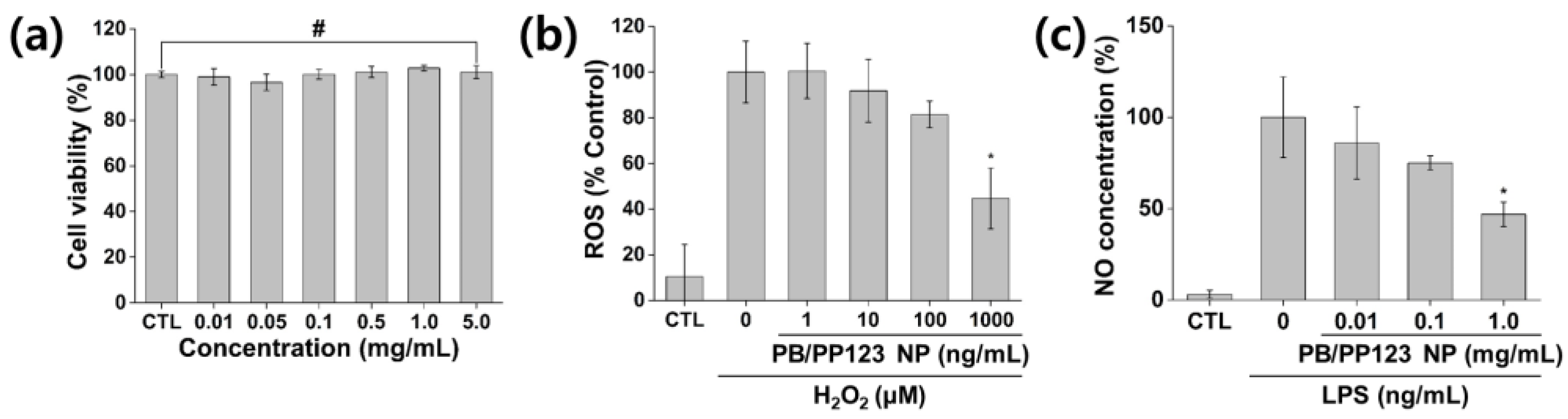
3.4. In Vitro Cytotoxicity and Antioxidant and Anti-Inflammatory Activities
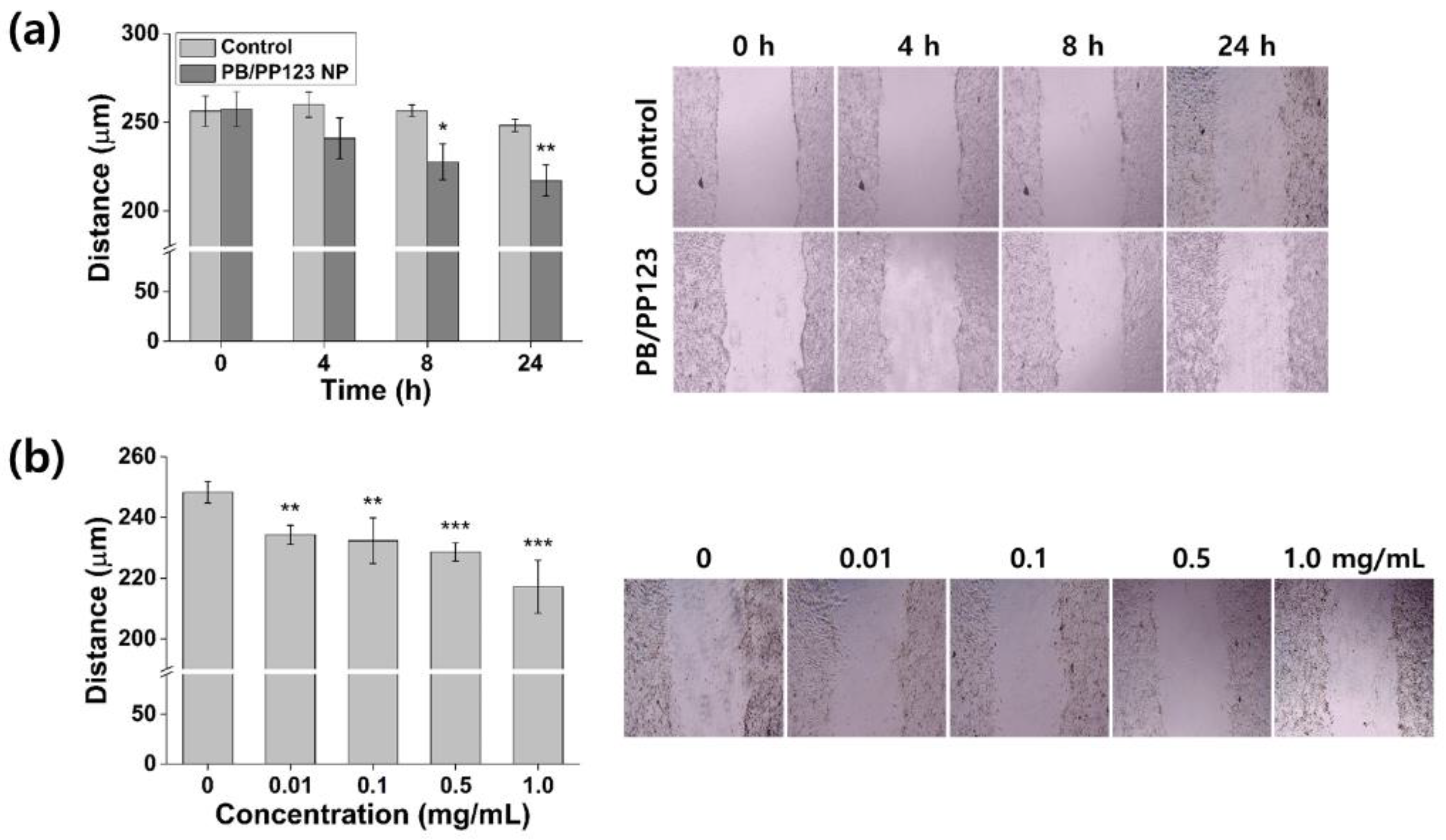
3.5. In Vitro Wound-Healing Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bandyopadhyay, U.; Das, D.; Banerjee, R.K. Reactive oxygen species: Oxidative damage and pathogenesis. Curr. Sci. 1999, 77, 658–666. [Google Scholar]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, 14S–22S. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitta, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Free radicals and other reactive species in disease. eLS 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Okayama, Y. Oxidative stress in allergic and inflammatory skin diseases. Curr. Drug Target Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Cassano, N.; Meo, M.D.; Scoppio, B.M.; Loviglio, M.C.; Vecchio, S.D.; Vena, G.A. Does oxidative stress play a role in the pathogenesis of urticarias? Eur. J. Inflamm. 2005, 3, 5–10. [Google Scholar] [CrossRef]
- Elswaifi, S.F.; Palmieri, J.R.; Hockey, K.S.; Rzigalinski, B.A. Antioxidant nanoparticles for control of infectious disease. Infect. Disord. Drug Targets 2009, 9, 445–452. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Liu, C.; Zhang, Y.; Ren, J.; Qu, X. Selenium-based nanozyme as biomimetic antioxidant machinery. Chem. Eur. J. 2018, 24, 10224–10230. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, S.; Yin, J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef]
- Farah, A.; Billing, C.; Dikio, C.W.; Dibofori-Orji, A.N.; Oyedeji, O.O.; Wankasi, D.; Mtunzi, F.M.; Dikio, E.D. Synthesis of Prussian blue and its electrochemical detection of hydrogen peroxide based on cetyltrimethylammonium bromide (CTAB) modified glassy carbon electrode. Int. J. Electrochem. Sci. 2013, 8, 21132–212146. [Google Scholar]
- Zhao, J.; Cai, X.; Gao, W.; Zhang, L.; Zou, D.; Zheng, Y.; Li, Z.; Chen, H. Prussian blue nanozyme with multienzyme activity reduces colitis in mice. ACS Appl. Mater. Interfaces 2018, 10, 26108–26117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tu, M.; Gao, W.; Cai, X.; Song, F.; Chen, Z.; Zhang, Q.; Wang, J.; Jin, C.; Shi, J.; et al. Hollow Prussian blue nanozymes drive neuroprotection against ischemic stroke via attenuating oxidative stress, counteracting inflammation, and suppressing cell apoptosis. Nano Lett. 2019, 19, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Jeon, J.; Lee, M.S.; Yang, H.S.; Tae, G. Antioxidant and anti-inflammatory activities of Prussian blue nanozyme promotes full-thickness skin wound healing. Mater. Sci. Eng. C. 2021, 119, 111596. [Google Scholar] [CrossRef]
- Sanz, I.M.C.; Franco, M.D.L.; Castro, B.; Hidalgo, P.L.P. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Luo, Z.; Qi, B.; Sun, Y.; Chen, Y.; Lin, J.; Qin, H.; Wang, N.; Shi, R.; Shang, X.; Chen, S.; et al. Engineering bioactive M2 macrophage-polarized, anti-inflammatory, miRNA-based liposomes for functional muscle repair: From exosomal mechanisms to biomaterials. Small 2022, 18, 2201957. [Google Scholar] [CrossRef] [PubMed]
- Ludi, A. Prussian blue, an inorganic evergreen. J. Chem. Educ. 1981, 58, 1013. [Google Scholar] [CrossRef]
- Chen, L.C.; Huang, Y.H.; Ho, K.C. A complementary electrochromic system based on Prussian blue and indium hexacyanoferrate. J. Solid State Electrochem. 2002, 7, 6–10. [Google Scholar] [CrossRef]
- Tung, T.S.; Ho, K.C. Cycling and at-rest stabilities of a complementary electrochromic device containing poly(3,4-ethylenedioxythiophene) and Prussian blue. Sol. Energy Mater. Sol. Cells 2006, 90, 521–537. [Google Scholar] [CrossRef]
- Omura, A.; Kurihara, Y.; Ishizaki, M.; Kurihara, M.; Moritomo, Y. Oxidization process of Fe-Ni mixed Prussian blue analogue investigated by valence-differential spectroscopy. Jpn. J. Appl. Phys. 2011, 50, 032401. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, J.; Shi, C.; Chen, H. Multilayer membranes via layer-by-layer deposition of organic polymer protected Prussian blue nanoparticles and glucose oxidase for glucose biosensing. Langmuir 2005, 21, 9630–9634. [Google Scholar] [CrossRef]
- Fu, G.; Sanjay, S.T.; Li, X. Cost-effective and sensitive colorimetric immunosensing using an iron oxide-to Prussian blue nanoparticle conversion strategy. Analyst 2016, 141, 3883–3889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itaya, K.; Uchida, I.; Neff, V.D. Electrochemistry of polynuclear transition metal cyanides: Prussian blue and its analogues. Acc. Chem. Res. 1986, 19, 162–168. [Google Scholar] [CrossRef]
- Patra, C.R. Prussian blue nanoparticles and their analogues for application to cancer theranostics. Nanomedicine 2016, 11, 569–572. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, Y.; Cai, X.; Gao, W.; Tang, X.; Chen, Y.; Chen, J.; Chen, L.; Tian, Q.; Yang, S.; et al. Large-scale synthesis of monodisperse Prussian blue nanoparticles for cancer theranostics via an “in situ modification” strategy. Int. J. Nanomed. 2018, 14, 271–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uemura, T.; Kitagawa, S. Prussian blue nanoparticles protected by poly(vinylpyrrolidone). J. Am Chem. Soc. 2003, 125, 7814–7815. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Liang, X.L.; Ma, F.; Jing, L.J.; Lin, L.; Yang, Y.B.; Feng, S.S.; Fu, G.L.; Yue, X.L.; Dai, Z.F. Chitosan stabilized Prussian blue nanoparticles for photothermally enhanced gene delivery. Colloids Surf. B Biointerfaces 2014, 123, 629–638. [Google Scholar] [CrossRef]
- Cheng, L.; Gong, H.; Zhu, W.; Liu, J.; Wang, X.; Liu, G.; Liu, Z. PEGylated Prussian blue nanocubes as a theranostic agent for simultaneous cancer imaging and photothermal therapy. Biomaterials 2014, 35, 9844–9852. [Google Scholar] [CrossRef]
- Sobczyński, J.; Kristensen, S.; Berg, K. The influence of Pluronics nanovehicles on dark cytotoxicity, photocytotoxicity, and localization of four model photosensitizers in cancer cells. Photochem. Photobiol. Sci. 2014, 13, 8–22. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef] [Green Version]
- Rahme, K.; Oberdisse, J.; Schweins, R.; Gaillard, C.; Marty, J.D.; Mingotaud, C.; Gauffre, F. Pluronics-stabilized gold nanoparticles: Investigation of the structure of the polymer-particle hybrid. ChemPhysChem 2008, 9, 2230–2236. [Google Scholar] [CrossRef]
- Abdullin, T.I.; Bondar, O.V.; Shtyrlin, Y.G.; Kahraman, M.; Culha, M. Pluronic® block copolymer-mediated interactions of organic compounds with noble metal nanoparticles for SERS analysis. Langmuir 2010, 26, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lee, J.S.; Sung, D.; Lee, J.H.; Moh, S.H.; Lim, J.; Choi, W.I. Synergistic antioxidant activity of size controllable chitosan-templated Prussian blue nanoparticle. Nanomedicine 2019, 14, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound-healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Vipin, A.K.; Fugetsu, B.; Sakata, I.; Isogai, A.; Endo, M.; Li, M.; Dresselhaus, M.S. Cellulose nanofiber backboned Prussian blue nanoparticles as powerful adsorbents for the selective elimination of radioactive cesium. Sci. Rep. 2016, 15, 37009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, W.I.; Kim, Y.H.; Tae, G.; Lee, S.Y.; Kim, K.; Kwon, I.C. In-vivo tumor targeting of Pluronic-based nano-carriers. J. Control. Release 2010, 147, 109–117. [Google Scholar] [CrossRef]
- Shiba, F.; Mameuda, U.; Tatejima, S.; Okawa, Y. Synthesis of uniform Prussian blue nanoparticles by a polyol process using a polyethylene glycol aqueous solution. RSC Adv. 2019, 9, 34589–34594. [Google Scholar] [CrossRef] [Green Version]
- Uemura, T.; Ohba, M.; Kitagawa, S. Size and surface effects of Prussian blue nanoparticles protected by organic polymers. Inorg. Chem. 2004, 43, 7339–7345. [Google Scholar] [CrossRef]
- Barick, K.C.; Ekta; Gawali, S.L.; Sarkar, A.; Kunwar, A.; Priyadarsini, K.I.; Hassan, P.A. Pluronic stabilized Fe3O4 magnetic nanoparticles for intracellular delivery of curcumin. RSC Adv. 2016, 6, 98674–98681. [Google Scholar] [CrossRef]
- Ge, C.; Li, P.; Lai, J.; Qiu, P. In situ synthesis and characterization of Prussian blue nanocubes on graphene oxide and its application for H2O2 reduction. Indian J. Chem. 2018, 57A, 26–33. [Google Scholar]
- Raval, A.; Pillai, S.A.; Bahadur, A.; Bahadur, P. Systematic characterization of Pluronic® micelles and their application for solubilization and in vitro release of some hydrophobic anticancer drugs. J. Mol. Liq. 2017, 230, 473–481. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur. Polym. J. 2016, 79, 140–149. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.H.; Li, J.; Tang, H.Q. Antioxidant activity of polyaniline nanofibers. Chin. Chem. Lett. 2007, 18, 1005–1008. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, N.; Zhang, Y. Prussian blue nanoparticles possess potential anti-inflammatory properties via scavenging reactive oxygen species. Inflamm. Cell Signal. 2017, 4, e1342. [Google Scholar]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Shalini, V.; Bhaskar, S.; Kumar, K.S.; Mohanlal, S.; Jayalekshmy, A.; Helen, A. Molecular mechanisms of anti-inflammatory action of the flavonoid, tricin from Njavara rice (Oryza sativa L.) in human peripheral blood mononuclear cells: Possible role in the inflammatory signaling. Int. Immunopharmacol. 2012, 14, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Chen, D.Q.; Jin, M.H.; Jin, Y.H.; Li, J.; Shen, G.N.; Li, W.L.; Gong, Y.X.; Mao, Y.Y.; Xie, D.P.; et al. Anti-inflammatory effect of hispidin on LPS induced macrophage inflammation through MAPK and JAK1/STAT3 signaling pathways. Appl. Biol. Chem. 2020, 63, 21. [Google Scholar] [CrossRef]
- Steiling, H.; Munz, B.; Werner, S.; Brauchle, M. Different types of ROS-scavenging enzymes are expressed during cutaneous wound repair. Exp. Cell Res. 1999, 247, 484–494. [Google Scholar] [CrossRef]
- Mohammad, G.; Mishra, V.K.; Pandey, H.P. Antioxidant properties of some nanoparticle may enhance wound healing in T2DM patient. Dig. J. Nanomater. Biostruct. 2008, 3, 159–162. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.; Lee, J.S.; Sung, D.; Yang, S.; Choi, W.I. Size-Controllable Prussian Blue Nanoparticles Using Pluronic Series for Improved Antioxidant Activity and Anti-Inflammatory Efficacy. Antioxidants 2022, 11, 2392. https://doi.org/10.3390/antiox11122392
Oh H, Lee JS, Sung D, Yang S, Choi WI. Size-Controllable Prussian Blue Nanoparticles Using Pluronic Series for Improved Antioxidant Activity and Anti-Inflammatory Efficacy. Antioxidants. 2022; 11(12):2392. https://doi.org/10.3390/antiox11122392
Chicago/Turabian StyleOh, Hyeryeon, Jin Sil Lee, Daekyung Sung, Siyoung Yang, and Won Il Choi. 2022. "Size-Controllable Prussian Blue Nanoparticles Using Pluronic Series for Improved Antioxidant Activity and Anti-Inflammatory Efficacy" Antioxidants 11, no. 12: 2392. https://doi.org/10.3390/antiox11122392





