Evaluation of Selected Oxidant/Antioxidant Parameters in Patients with Relapsing-Remitting Multiple Sclerosis Undergoing Disease-Modifying Therapies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The Determination of AOPP in Serum
2.2.2. The Determination of FRAP in Serum
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leonardi, S.; Maggio, M.G.; Russo, M.; Bramanti, A.; Arcadi, F.A.; Naro, A.; Calabrò, R.S.; De Luca, R. Cognitive Recovery in People with Relapsing/Remitting Multiple Sclerosis: A Randomized Clinical Trial on Virtual Reality-Based Neurorehabilitation. Clin. Neurol. Neurosurg. 2021, 208, 106828. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390. [Google Scholar] [CrossRef]
- Kapica-Topczewska, K.; Brola, W.; Fudala, M.; Tarasiuk, J.; Chorazy, M.; Snarska, K.; Kochanowicz, J.; Kulakowska, A. Prevalence of Multiple Sclerosis in Poland. Mult. Scler. Relat. Disord. 2018, 21, 51–55. [Google Scholar] [CrossRef]
- Brola, W.; Fudala, M.; Flaga, S.; Ryglewicz, D. Need for creating Polish registry of multiple sclerosis patients. Neurol. Neurochir. Pol. 2013, 47, 484–492. [Google Scholar] [CrossRef]
- Katz Sand, I. Classification, Diagnosis, and Differential Diagnosis of Multiple Sclerosis. Curr. Opin. Neurol. 2015, 28, 193–205. [Google Scholar] [CrossRef]
- Huisman, E.; Papadimitropoulou, K.; Jarrett, J.; Bending, M.; Firth, Z.; Allen, F.; Adlard, N. Systematic Literature Review and Network Meta-Analysis in Highly Active Relapsing-Remitting Multiple Sclerosis and Rapidly Evolving Severe Multiple Sclerosis. BMJ Open 2017, 7, e013430. [Google Scholar] [CrossRef]
- Miljković, D.; Spasojević, I. Multiple Sclerosis: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2013, 19, 2286–2334. [Google Scholar] [CrossRef]
- Smith, A.L.; Cohen, J.A.; Hua, L.H. Therapeutic Targets for Multiple Sclerosis: Current Treatment Goals and Future Directions. Neurotherapeutics 2017, 14, 952–960. [Google Scholar] [CrossRef]
- Anlar, O. Treatment of Multiple Sclerosis. CNS Neurol. Disord. Drug Targets 2009, 8, 167–174. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative Stress in Multiple Sclerosis: Central and Peripheral Mode of Action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. The Role of Oxidative Stress in the Pathogenesis of Multiple Sclerosis: The Need for Effective Antioxidant Therapy. J. Neurol. 2004, 251, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Barja, G.; Herrero, A. Oxidative Damage to Mitochondrial DNA Is Inversely Related to Maximum Life Span in the Heart and Brain of Mammals. FASEB J. 2000, 14, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative Damage in Multiple Sclerosis Lesions. Brain J. Neurol. 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed]
- Dewar, D.; Underhill, S.M.; Goldberg, M.P. Oligodendrocytes and Ischemic Brain Injury. J. Cereb. Blood Flow Metab. 2003, 23, 263–274. [Google Scholar] [CrossRef]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.L.M.; Fischer, R. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxid. Med. Cell. Longev. 2020, 2020, 7191080. [Google Scholar] [CrossRef] [PubMed]
- Padureanu, R.; Albu, C.V.; Mititelu, R.R.; Bacanoiu, M.V.; Docea, A.O.; Calina, D.; Padureanu, V.; Olaru, G.; Sandu, R.E.; Malin, R.D.; et al. Oxidative Stress and Inflammation Interdependence in Multiple Sclerosis. J. Clin. Med. 2019, 8, E1815. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Gui, L.-N.; Liu, Y.-Y.; Shi, S.; Cheng, Y. Oxidative Stress Marker Aberrations in Multiple Sclerosis: A Meta-Analysis Study. Front. Neurosci. 2020, 14, 823. [Google Scholar] [CrossRef]
- Adamczyk, B.; Adamczyk-Sowa, M. New Insights into the Role of Oxidative Stress Mechanisms in the Pathophysiology and Treatment of Multiple Sclerosis. Oxid. Med. Cell. Longev. 2016, 2016, 1973834. [Google Scholar] [CrossRef]
- Gironi, M.; Borgiani, B.; Mariani, E.; Cursano, C.; Mendozzi, L.; Cavarretta, R.; Saresella, M.; Clerici, M.; Comi, G.; Rovaris, M.; et al. Oxidative Stress Is Differentially Present in Multiple Sclerosis Courses, Early Evident, and Unrelated to Treatment. J. Immunol. Res. 2014, 2014, 961863. [Google Scholar] [CrossRef]
- Obradovic, D.; Andjelic, T.; Ninkovic, M.; Dejanovic, B.; Kotur-Stevuljevic, J. Superoxide Dismutase (SOD), Advanced Oxidation Protein Products (AOPP), and Disease-Modifying Treatment Are Related to Better Relapse Recovery after Corticosteroid Treatment in Multiple Sclerosis. Neurol. Sci. 2021, 42, 3241–3247. [Google Scholar] [CrossRef]
- Piwowar, A.; Knapik-Kordecka, M.; Warwas, M. AOPP and Its Relations with Selected Markers of Oxidative/Antioxidative System in Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2007, 77, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Espejo, E.; Rodríguez de Fonseca, F.; Gavito, A.L.; Córdoba-Fernández, A.; Chacón, J.; Martín de Pablos, Á. Myeloperoxidase and Advanced Oxidation Protein Products in the Cerebrospinal Fluid in Women and Men with Parkinson’s Disease. Antioxidants 2022, 11, 1088. [Google Scholar] [CrossRef]
- Alderman, C.J.J.; Shah, S.; Foreman, J.C.; Chain, B.M.; Katz, D.R. The Role of Advanced Oxidation Protein Products in Regulation of Dendritic Cell Function. Free Radic. Biol. Med. 2002, 32, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Ouyang, X.; Jiang, Z.; Xie, Z.; Fan, L.; Zhu, D.; Li, L. Advanced Oxidation Protein Products Play Critical Roles in Liver Diseases. Eur. J. Clin. Investig. 2019, 49, e13098. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. On the Evaluation of Disability in Multiple Sclerosis. Neurology 1961, 11, 686–694. [Google Scholar] [CrossRef]
- Gaspari, M.; Roveda, G.; Scandellari, C.; Stecchi, S. An Expert System for the Evaluation of EDSS in Multiple Sclerosis. Artif. Intell. Med. 2002, 25, 187–210. [Google Scholar] [CrossRef]
- Piwowar, A.; Knapik-Kordecka, M.; Warwas, M. Markers of Oxidative Protein Damage in Plasma and Urine of Type 2 Diabetic Patients. Br. J. Biomed. Sci. 2009, 66, 194–199. [Google Scholar] [CrossRef]
- Niepsuj, J.; Franik, G.; Madej, P.; Piwowar, A.; Bizoń, A. Evaluation of Pro/Antioxidant Imbalance in Blood of Women with Polycystic Ovary Syndrome Based on Determination of Oxidized Low-Density Lipoproteins and Ferric Reducing Ability of Plasma Values. Biomedicines 2022, 10, 1564. [Google Scholar] [CrossRef]
- Tobore, T.O. Oxidative/Nitroxidative Stress and Multiple Sclerosis. J. Mol. Neurosci. 2021, 71, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Song, M.-Y.; Song, E.-K.; Kim, E.-K.; Moon, W.S.; Han, M.-K.; Park, J.-W.; Kwon, K.-B.; Park, B.-H. Overexpression of SIRT1 Protects Pancreatic Beta-Cells against Cytokine Toxicity by Suppressing the Nuclear Factor-KappaB Signaling Pathway. Diabetes 2009, 58, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Rajashekaraiah, V. Ferric Reducing Ability of Plasma: A Potential Oxidative Stress Marker in Stored Plasma. Acta Haematol. Pol. 2021, 52, 61–67. [Google Scholar] [CrossRef]
- Cristani, M.; Speciale, A.; Saija, A.; Gangemi, S.; Minciullo, P.L.; Cimino, F. Circulating Advanced Oxidation Protein Products as Oxidative Stress Biomarkers and Progression Mediators in Pathological Conditions Related to Inflammation and Immune Dysregulation. Curr. Med. Chem. 2016, 23, 3862–3882. [Google Scholar] [CrossRef]
- Rodrigues, P.; Bochi, G.V.; Trevisan, G. Advanced Oxidative Protein Products Role in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Mol. Neurobiol. 2021, 58, 5724–5742. [Google Scholar] [CrossRef]
- Adamczyk-Sowa, M.; Galiniak, S.; Żyracka, E.; Grzesik, M.; Naparło, K.; Sowa, P.; Bartosz, G.; Sadowska-Bartosz, I. Oxidative Modification of Blood Serum Proteins in Multiple Sclerosis after Interferon Beta and Melatonin Treatment. Oxid. Med. Cell. Longev. 2017, 2017, 7905148. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Simão, A.N.C.; Oliveira, S.R.; Flauzino, T.; Alfieri, D.F.; de Carvalho Jennings Pereira, W.L.; Kallaur, A.P.; Lozovoy, M.A.B.; Kaimen-Maciel, D.R.; Maes, M.; et al. Antioxidant and Anti-Inflammatory Diagnostic Biomarkers in Multiple Sclerosis: A Machine Learning Study. Mol. Neurobiol. 2020, 57, 2167–2178. [Google Scholar] [CrossRef]
- Ljubisavljevic, S.; Stojanovic, I.; Vojinovic, S.; Stojanov, D.; Stojanovic, S.; Cvetkovic, T.; Savic, D.; Pavlovic, D. The Patients with Clinically Isolated Syndrome and Relapsing Remitting Multiple Sclerosis Show Different Levels of Advanced Protein Oxidation Products and Total Thiol Content in Plasma and CSF. Neurochem. Int. 2013, 62, 988–997. [Google Scholar] [CrossRef]
- Hadžović-Džuvo, A.; Lepara, O.; Valjevac, A.; Avdagić, N.; Hasić, S.; Kiseljaković, E.; Ibragić, S.; Alajbegović, A. Serum Total Antioxidant Capacity in Patients with Multiple Sclerosis. Bosn. J. Basic Med. Sci. 2011, 11, 33. [Google Scholar] [CrossRef]
- Hejazi, E.; Amani, R.; SharafodinZadeh, N.; Cheraghian, B. Comparison of Antioxidant Status and Vitamin D Levels between Multiple Sclerosis Patients and Healthy Matched Subjects. Mult. Scler. Int. 2014, 2014, 539854. [Google Scholar] [CrossRef]
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimer’s Dis. 2021, 82, S109–S126. [Google Scholar] [CrossRef] [PubMed]
- Tselis, A.; Khan, O.; Lisak, R.P. Glatiramer Acetate in the Treatment of Multiple Sclerosis. Neuropsychiatr. Dis. Treat. 2007, 3, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Sanna, A.; Fois, M.L.; Arru, G.; Huang, Y.-M.; Link, H.; Pugliatti, M.; Rosati, G.; Sotgiu, S. Glatiramer Acetate Reduces Lymphocyte Proliferation and Enhances IL-5 and IL-13 Production through Modulation of Monocyte-Derived Dendritic Cells in Multiple Sclerosis. Clin. Exp. Immunol. 2006, 143, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, C.E. Interferon-Beta: Mechanism of Action and Dosing Issues. Neurology 2007, 68, S8–S11. [Google Scholar] [CrossRef]
- Aldabagh, A.A.; Al-Daher, A.G.M.; Abdullah, K.S. The Effect of Interferon-Beta on Oxidative Stress in Patients with Multiple Sclerosis. Ann. Coll. Med. Mosul 2018, 40, 18–23. [Google Scholar] [CrossRef][Green Version]
- Hosseini, A.; Masjedi, A.; Baradaran, B.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Anvari, E.; Jadidi-Niaragh, F. Dimethyl Fumarate: Regulatory Effects on the Immune System in the Treatment of Multiple Sclerosis. J. Cell. Physiol. 2019, 234, 9943–9955. [Google Scholar] [CrossRef]
- Linker, R.A.; Lee, D.-H.; Ryan, S.; van Dam, A.M.; Conrad, R.; Bista, P.; Zeng, W.; Hronowsky, X.; Buko, A.; Chollate, S.; et al. Fumaric Acid Esters Exert Neuroprotective Effects in Neuroinflammation via Activation of the Nrf2 Antioxidant Pathway. Brain J. Neurol. 2011, 134, 678–692. [Google Scholar] [CrossRef]
- Albrecht, P.; Bouchachia, I.; Goebels, N.; Henke, N.; Hofstetter, H.H.; Issberner, A.; Kovacs, Z.; Lewerenz, J.; Lisak, D.; Maher, P.; et al. Effects of Dimethyl Fumarate on Neuroprotection and Immunomodulation. J. Neuroinflamm. 2012, 9, 163. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Patel, K.; Paul, F.; Gold, S.M.; Scheel, M.; Kuchling, J.; Cooper, G.; Asseyer, S.; Chien, C.; Brandt, A.U.; et al. Sex Differences in Brain Atrophy in Multiple Sclerosis. Biol. Sex Differ. 2020, 11, 49. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Gold, S.M. Sex-Related Factors in Multiple Sclerosis Susceptibility and Progression. Nat. Rev. Neurol. 2012, 8, 255–263. [Google Scholar] [CrossRef]
- Tenkorang, M.A.; Snyder, B.; Cunningham, R.L. Sex-Related Differences in Oxidative Stress and Neurodegeneration. Steroids 2018, 133, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijević, M.; Kotur-Stevuljević, J.; Stojić-Vukanić, Z.; Vujnović, I.; Pilipović, I.; Nacka-Aleksić, M.; Leposavić, G. Sex Difference in Oxidative Stress Parameters in Spinal Cord of Rats with Experimental Autoimmune Encephalomyelitis: Relation to Neurological Deficit. Neurochem. Res. 2017, 42, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Stojić-Vukanić, Z.; Kotur-Stevuljević, J.; Nacka-Aleksić, M.; Kosec, D.; Vujnović, I.; Pilipović, I.; Dimitrijević, M.; Leposavić, G. Sex Bias in Pathogenesis of Autoimmune Neuroinflammation: Relevance for Dimethyl Fumarate Immunomodulatory/Anti-Oxidant Action. Mol. Neurobiol. 2018, 55, 3755–3774. [Google Scholar] [CrossRef] [PubMed]
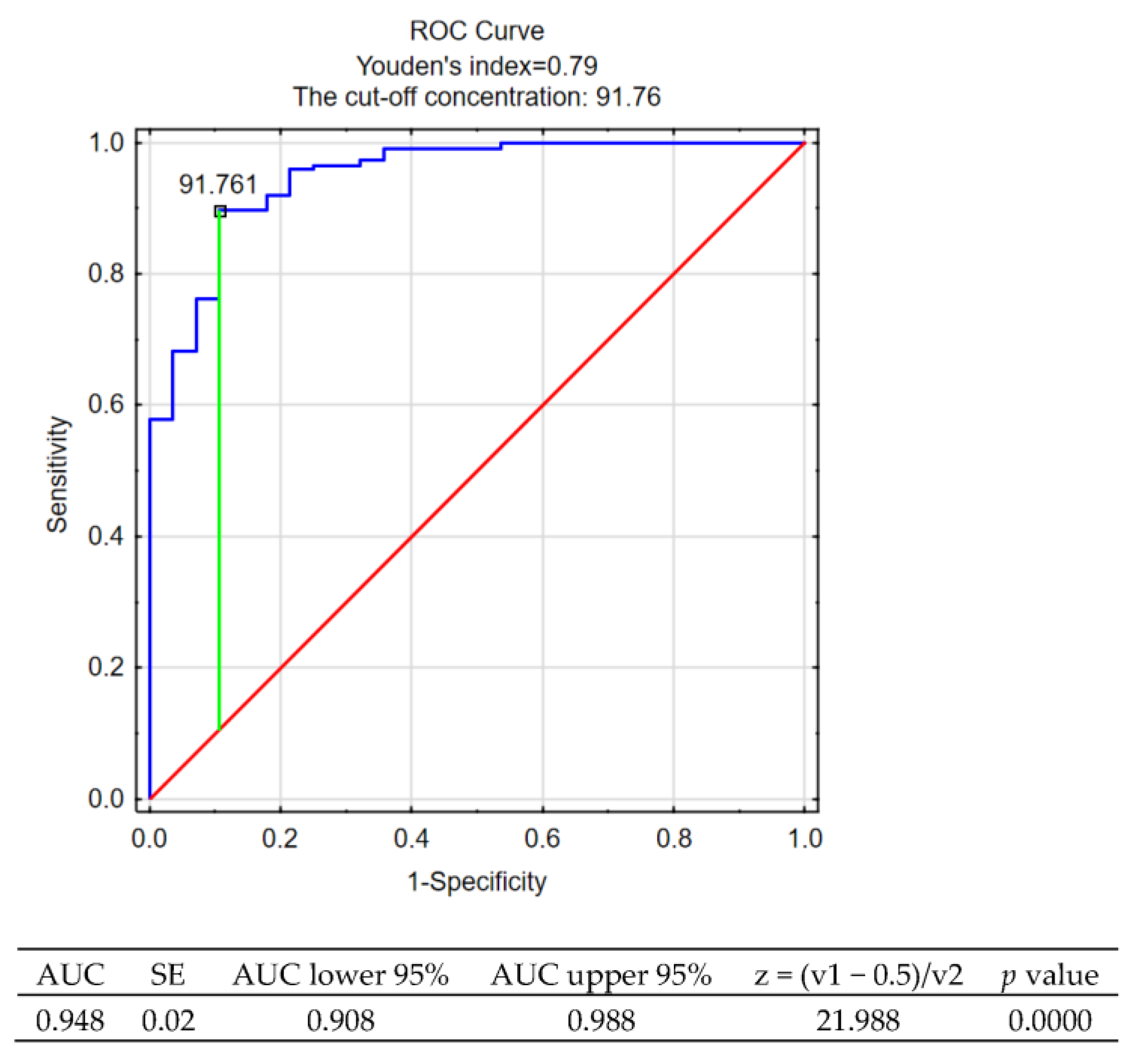
| Variable | Control Group | Patients with RRMS |
|---|---|---|
| Number of subjects | n = 29 | n = 204 |
| Sex [men/women] | 9/20 | 73/131 |
| Age [years] | 41.0 (36.0–41.0) | 43.0 (37.0–51.0) |
| Duration of RRMS [years] | N/A | 12.0 (8.0–15.0) |
| EDSS | N/A | 2.5 (1.5–3.5) |
| FRAP [mM] | 0.9 (0.9–1.1) | 1.0 (0.9–1.1) |
| AOPP [µM] | 70.9 (46.7–79.7) | 141.4 (109.4–201.2) * |
| Variable | Control Group | Patients with RRMS | ||
|---|---|---|---|---|
| Women | Men | Women | Men | |
| Number of subjects | n = 20 | n = 9 | n = 131 | n = 73 |
| Age | 41.0 (33.0–49.0) | 41.0 (36.0–47.5) | 43.0 (37.0–51.0) | 42.5 (35.5–51.0) |
| Disease duration [years] | N/A | N/A | 12.0 (8.0–15.0) | 12.0 (8.5–16.0) |
| EDSS | N/A | N/A | 2.5 (1.5–3.5) | 2.5 (1.5–3.0) |
| FRAP [mM] | 0.9 (0.8–0.9) | 1.1 (1.0–1.1) * | 0.9 (0.8–1.0) | 1.1 (1.0–1.3) * |
| AOPP [µM] | 52.5 (44.0–172.9) | 77.5 (70.6–88.2) ** | 128.6 (102.8–172.9) | 163.8 (128.9–229.5) ** |
| Female Subgroup (n = 131) | ||
|---|---|---|
| Age | NS | NS |
| Disease duration | NS | NS |
| EDSS | NS | r = 0.18; p < 0.041 |
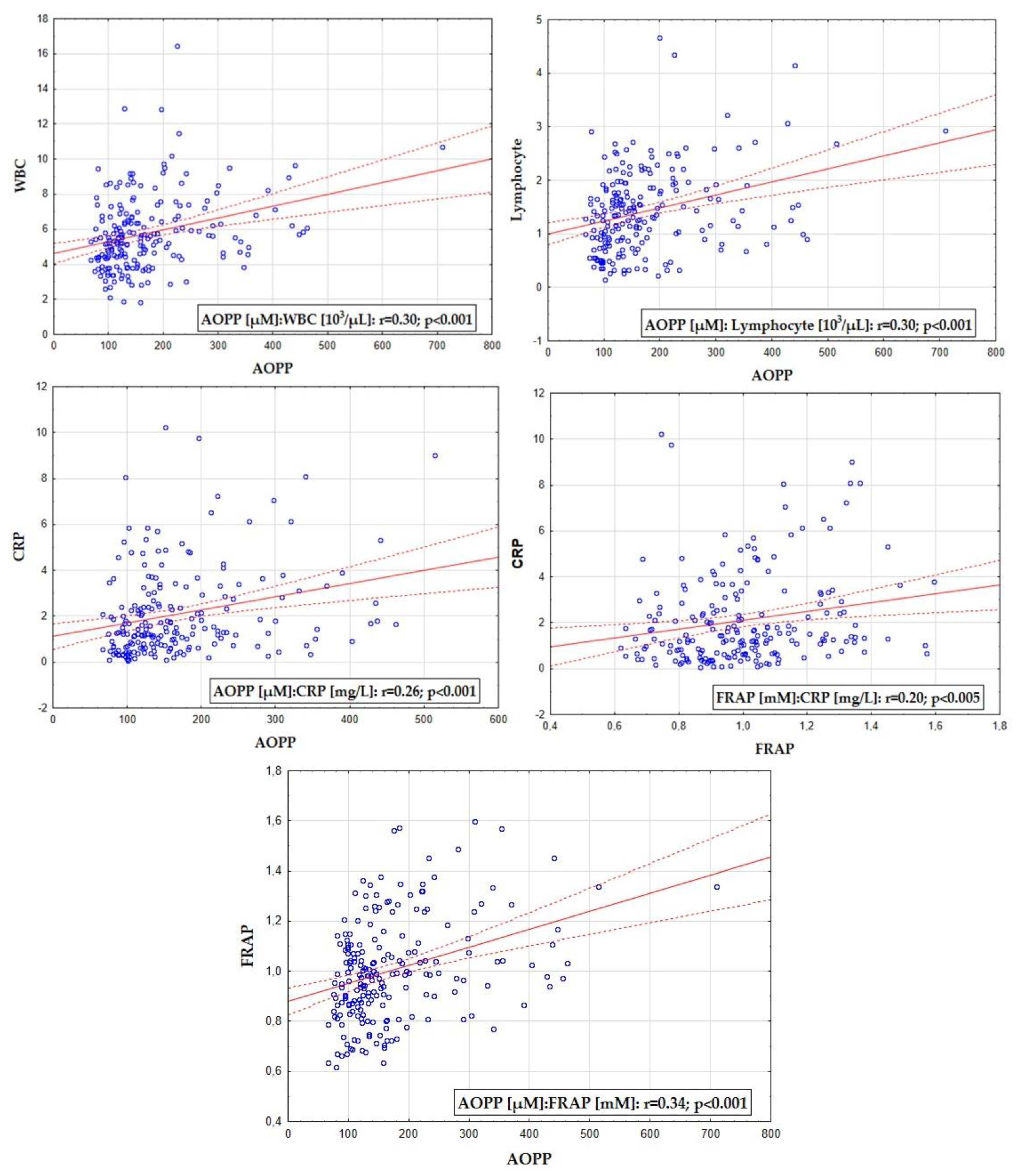
| WBC [103/µL] | NS | r = 0.31; p < 0.002 |
| Lymphocyte [103/µL] | NS | r = 0.28; p < 0.001 |
| CRP [mg/L] | NS | r = 0.31; p < 0.001 |
| AOPP [µM] | r = 0.24; p < 0.001 | - |
| Male Subgroup (n = 73) | ||
| Age | NS | NS |
| Disease duration | NS | r = −0.30; p < 0.028 |
| EDSS | NS | r = −0.27; p < 0.048 |
| WBC [103/µL] | NS | r = 0.29; p < 0.028 |
| Lymphocyte [103/µL] | NS | r = 0.31; p < 0.025 |
| CRP [mg/L] | NS | NS |
| AOPP [µM] | NS | NS |
| Variable | Patients with RRMS | ||||
|---|---|---|---|---|---|
| GA | IFNs | TER | FTY | DMF | |
| Number of subjects | n = 20 | n = 64 | n = 29 | n = 29 | n = 62 |
| Men/Women [number] | 6/14 | 30/34 | 15/14 | 8/21 | 14/48 |
| Age [years] | 44.0 (36.0–53.0) | 43.0 (39.0–51.5) | 46.5 (41.5–57.0) | 42.0 (35.0–48.0) | 41.0 (35.0–47.0) |
| Disease duration [years] | 10.5 (7.5–16.5) | 12.0 (9.0–15.0) | 13.0 (7.0–16.5) | 14.0 (13.0–20.0) | 8.0 (5.0–13.0) * |
| EDSS | 2.0 (1.5–2.8) | 2.0 (1.5–3.0) | 3.0 (2.0–3.8) | 4.0 (3.0–6.0) | 2.0 (1.0–3.0) * |
| WBC [103/µL] | 6.7 (6.1–8.4) | 5.4 (4.4–6.5) | 5.6 (4.6–7.0) | 4.6 (3.8–6.4) | 5.1 (4.3–5.8) * |
| Lymphocytes [103/µL] | 1.9 (1.7–8.4) | 1.5 (1.2–1.9) | 1.5 (1.1–1.8) | 0.5 (0.3–1.5) | 1.0 (0.7–1.5) * |
| CRP [mg/L] | 2.1 (1.1–3.7) | 1.3 (0.7–2.1) | 1.5 (0.9–2.4) | 1.3 (0.6–2.5) | 1.4 (0.7–3.0) |
| FRAP [mM] | 1.0 (0.8–1.1) | 1.0 (0.9–1.1) | 0.9 (0.8–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| AOPP [µM] | 164.1 (122.6–242.1) | 162.8 (120.7–221.9) | 152.5 (112.2–218.2) | 138.3 (109.4–172.9) | 124.02 (97.4–157.8) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizoń, A.; Chojdak-Łukasiewicz, J.; Kołtuniuk, A.; Budrewicz, S.; Pokryszko-Dragan, A.; Piwowar, A. Evaluation of Selected Oxidant/Antioxidant Parameters in Patients with Relapsing-Remitting Multiple Sclerosis Undergoing Disease-Modifying Therapies. Antioxidants 2022, 11, 2416. https://doi.org/10.3390/antiox11122416
Bizoń A, Chojdak-Łukasiewicz J, Kołtuniuk A, Budrewicz S, Pokryszko-Dragan A, Piwowar A. Evaluation of Selected Oxidant/Antioxidant Parameters in Patients with Relapsing-Remitting Multiple Sclerosis Undergoing Disease-Modifying Therapies. Antioxidants. 2022; 11(12):2416. https://doi.org/10.3390/antiox11122416
Chicago/Turabian StyleBizoń, Anna, Justyna Chojdak-Łukasiewicz, Aleksandra Kołtuniuk, Sławomir Budrewicz, Anna Pokryszko-Dragan, and Agnieszka Piwowar. 2022. "Evaluation of Selected Oxidant/Antioxidant Parameters in Patients with Relapsing-Remitting Multiple Sclerosis Undergoing Disease-Modifying Therapies" Antioxidants 11, no. 12: 2416. https://doi.org/10.3390/antiox11122416
APA StyleBizoń, A., Chojdak-Łukasiewicz, J., Kołtuniuk, A., Budrewicz, S., Pokryszko-Dragan, A., & Piwowar, A. (2022). Evaluation of Selected Oxidant/Antioxidant Parameters in Patients with Relapsing-Remitting Multiple Sclerosis Undergoing Disease-Modifying Therapies. Antioxidants, 11(12), 2416. https://doi.org/10.3390/antiox11122416







