Oxidative Stress and Intracranial Hypertension after Aneurysmal Subarachnoid Hemorrhage
Abstract
:1. Introduction
2. Characteristics of ICP in aSAH
2.1. Peak of ICP
2.2. Steady State
3. ICP Monitoring in aSAH
4. ICP and Outcome
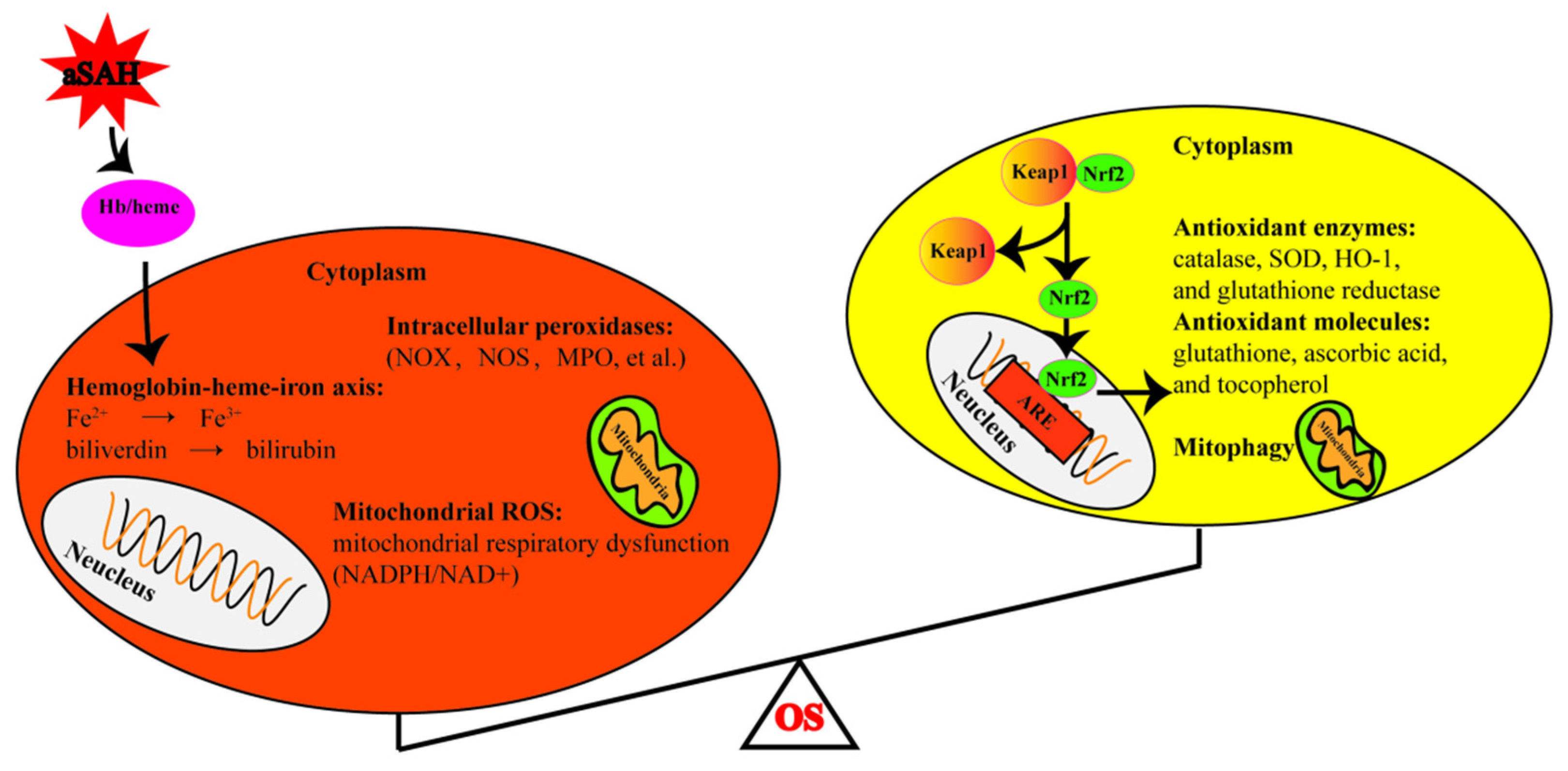
5. Oxidative Stress in aSAH
5.1. Hemoglobin Degradation
5.2. Disrupted Mitochondrial Respiration
5.3. Intracellular Peroxidases Pathways
5.4. Disrupted Antioxidant Systems
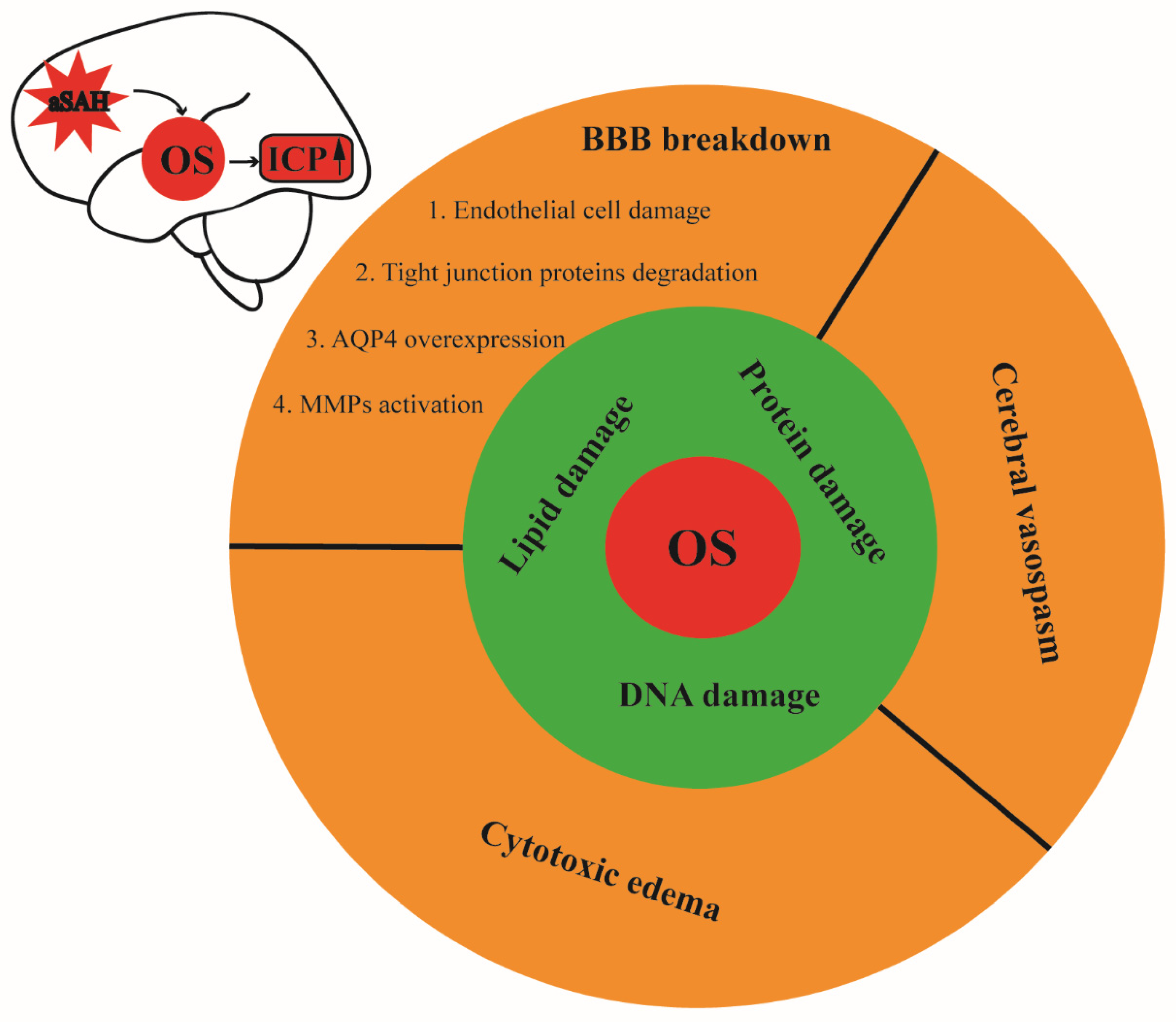
6. Oxidative Stress and Increased ICP
7. Treatment
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Johnston, S.C.; Selvin, S.; Gress, D.R. The Burden, Trends, and Demographics of Mortality from Subarachnoid Hemorrhage. Neurology 1998, 50, 1413–1418. [Google Scholar] [CrossRef]
- le Roux, A.A.; Wallace, M.C. Outcome and Cost of Aneurysmal Subarachnoid Hemorrhage. Neurosurg. Clin. N. Am. 2010, 21, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Sehba, F.A.; Hou, J.; Pluta, R.M.; Zhang, J.H. The Importance of Early Brain Injury after Subarachnoid Hemorrhage. Prog. Neurobiol. 2012, 97, 14–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingelmo Ingelmo, I.; Fabregas Julia, N.; Rama-Maceiras, P.; Hernandez-Palazon, J.; Rubio Romero, R.; Carmona Aurioles, J.; Grupo Ad Hoc de la Seccion de Neurociencia de la Sociedad Espanola de Anestesiologia; Reanimación y Terapéutica del Dolor. Subarachnoid Hemorrhage: Epidemiology, Social Impact and A Multidisciplinary Approach. Rev. Esp. Anestesiol. Reanim. 2010, 57 (Suppl. 2), S4–S15. [Google Scholar] [PubMed]
- van Gijn, J.; Rinkel, G.J. Subarachnoid Haemorrhage: Diagnosis, Causes and Management. Brain 2001, 124, 249–278. [Google Scholar] [CrossRef] [PubMed]
- Heuer, G.G.; Smith, M.J.; Elliott, J.P.; Winn, H.R.; LeRoux, P.D. Relationship between Intracranial Pressure and Other Clinical Variables in Patients with Aneurysmal Subarachnoid Hemorrhage. J. Neurosurg. 2004, 101, 408–416. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Z.; Li, G.; Zhou, L.; Huang, K.; Wu, Z.; Zhan, R.; Shen, J. Inflammation and Oxidative Stress: Potential Targets for Improving Prognosis after Subarachnoid Hemorrhage. Front. Cell. Neurosci. 2021, 15, 739506. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Zhang, J.M. The Updated Role of Oxidative Stress in Subarachnoid Hemorrhage. Curr. Drug Deliv. 2017, 14, 832–842. [Google Scholar] [CrossRef]
- Lin, F.; Li, R.; Tu, W.J.; Chen, Y.; Wang, K.; Chen, X.; Zhao, J. An Update on Antioxidative Stress Therapy Research for Early Brain Injury after Subarachnoid Hemorrhage. Front. Aging Neurosci. 2021, 13, 772036. [Google Scholar] [CrossRef]
- Warren, M.C.; Bump, E.A.; Medeiros, D.; Braunhut, S.J. Oxidative Stress-Induced Apoptosis of Endothelial Cells. Free Radic. Biol. Med. 2000, 29, 537–547. [Google Scholar] [CrossRef]
- Qing, W.G.; Dong, Y.Q.; Ping, T.Q.; Lai, L.G.; Fang, L.D.; Min, H.W.; Xia, L.; Heng, P.Y. Brain Edema after Intracerebral Hemorrhage in Rats: The Role of Iron Overload and Aquaporin 4. J. Neurosurg. 2009, 110, 462–468. [Google Scholar] [CrossRef]
- Fumoto, T.; Naraoka, M.; Katagai, T.; Li, Y.; Shimamura, N.; Ohkuma, H. The Role of Oxidative Stress in Microvascular Disturbances after Experimental Subarachnoid Hemorrhage. Transl. Stroke Res. 2019, 10, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Schenck, H.; Netti, E.; Teernstra, O.; De Ridder, I.; Dings, J.; Niemela, M.; Temel, Y.; Hoogland, G.; Haeren, R. The Role of the Glycocalyx in the Pathophysiology of Subarachnoid Hemorrhage-Induced Delayed Cerebral Ischemia. Front. Cell. Dev. Biol. 2021, 9, 731641. [Google Scholar] [CrossRef]
- Mokri, B. The Monro-Kellie Hypothesis: Applications in CSF Volume Depletion. Neurology 2001, 56, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Nornes, H. The Role of Intracranial Pressure in The Arrest of Hemorrhage in Patients with Ruptured Intracranial Aneurysm. J. Neurosurg. 1973, 39, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keep, R.F.; Andjelkovic, A.V.; Stamatovic, S.M.; Shakui, P.; Ennis, S.R. Ischemia-Induced Endothelial Cell Dysfunction. Acta Neurochir. Suppl. 2005, 95, 399–402. [Google Scholar] [CrossRef]
- Sabri, M.; Lass, E.; Macdonald, R.L. Early Brain Injury: A Common Mechanism in Subarachnoid Hemorrhage and Global Cerebral Ischemia. Stroke Res. Treat. 2013, 2013, 394036. [Google Scholar] [CrossRef] [Green Version]
- Brinker, T.; Seifert, V.; Stolke, D. Acute Changes in The Dynamics of The Cerebrospinal Fluid System during Experimental Subarachnoid Hemorrhage. Neurosurgery 1990, 27, 369–372. [Google Scholar] [CrossRef]
- Grote, E.; Hassler, W. The Critical First Minutes after Subarachnoid Hemorrhage. Neurosurgery 1988, 22, 654–661. [Google Scholar] [CrossRef]
- Furuichi, S.; Endo, S.; Haji, A.; Takeda, R.; Nisijima, M.; Takaku, A. Related Changes in Sympathetic Activity, Cerebral Blood Flow and Intracranial Pressure, and Effect of an Alpha-blocker in Experimental Subarachnoid Haemorrhage. Acta Neurochir. 1999, 141, 415–423, discussion 414–423. [Google Scholar] [CrossRef]
- Westermaier, T.; Jauss, A.; Eriskat, J.; Kunze, E.; Roosen, K. Acute Vasoconstriction: Decrease and Recovery of Cerebral Blood Flow after Various Intensities of Experimental Subarachnoid Hemorrhage in Rats. J. Neurosurg. 2009, 110, 996–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graetz, D.; Nagel, A.; Schlenk, F.; Sakowitz, O.; Vajkoczy, P.; Sarrafzadeh, A. High ICP as Trigger of Proinflammatory IL-6 Cytokine Activation in Aneurysmal Subarachnoid Hemorrhage. Neurol. Res. 2010, 32, 728–735. [Google Scholar] [CrossRef]
- Makino, K.; Osuka, K.; Watanabe, Y.; Usuda, N.; Hara, M.; Aoyama, M.; Takayasu, M.; Wakabayashi, T. Increased ICP Promotes CaMKII-Mediated Phosphorylation of Neuronal NOS at Ser(8)(4)(7) in The Hippocampus Immediately after Subarachnoid Hemorrhage. Brain Res. 2015, 1616, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, T. Early Effects of Experimental Arterial Subarachnoid Haemorrhage on The Cerebral Circulation. Part I: Experimental Subarachnoid Haemorrhage in Cat and Its Pathophysiological Effects. Methods of Regional Cerebral Blood Flow Measurement and Evaluation of Microcirculation. Acta Neurochir. 1984, 72, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Caner, B.; Hou, J.; Altay, O.; Fujii, M.; Zhang, J.H. Transition of Research Focus from Vasospasm to Early Brain Injury after Subarachnoid Hemorrhage. J. Neurochem. 2012, 123 (Suppl. 2), 12–21. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Hishikawa, T.; Nishihiro, S.; Shinji, Y.; Takasugi, Y.; Haruma, J.; Hiramatsu, M.; Kawase, H.; Sato, S.; Mizoue, R.; et al. NADH Fluorescence Imaging and The Histological Impact of Cortical Spreading Depolarization during the Acute Phase of Subarachnoid Hemorrhage in Rats. J. Neurosurg. 2018, 128, 137–143. [Google Scholar] [CrossRef]
- Lu, J.; Chen, F.; Cai, B.; Chen, F.; Kang, D. A Rabbit Model of Aneurysmal Subarachnoid Hemorrhage by Ear Central Artery-Suprasellar Cistern Shunt. J. Clin. Neurosci. 2017, 44, 300–305. [Google Scholar] [CrossRef]
- Friedrich, V.; Bederson, J.B.; Sehba, F.A. Gender Influences the Initial Impact of Subarachnoid Hemorrhage: An Experimental Investigation. PLoS ONE 2013, 8, e80101. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, D.; Lei, J.; Tan, G. Clinical Observation of the Time Course of Raised Intracranial Pressure after Subarachnoid Hemorrhage. Neurol. Sci. 2015, 36, 1203–1210. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Schweizer, T.A. Spontaneous Subarachnoid Haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- Altay, O.; Suzuki, H.; Hasegawa, Y.; Caner, B.; Krafft, P.R.; Fujii, M.; Tang, J.; Zhang, J.H. Isoflurane Attenuates Blood-Brain Barrier Disruption in Ipsilateral Hemisphere after Subarachnoid Hemorrhage in Mice. Stroke 2012, 43, 2513–2516. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.H.; Savarraj, J.P.; Pervez, M.; Jones, W.; Park, J.; Jeon, S.B.; Kwon, S.U.; Chang, T.R.; Lee, K.; Kim, D.H.; et al. The Subarachnoid Hemorrhage Early Brain Edema Score Predicts Delayed Cerebral Ischemia and Clinical Outcomes. Neurosurgery 2018, 83, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Kuyama, H.; Symon, L. An Experimental Study of the Acute Stage of subarachnoid hemorrhage. J. Neurosurg. 1983, 59, 917–924. [Google Scholar] [CrossRef]
- Robba, C.; Graziano, F.; Rebora, P.; Elli, F.; Giussani, C.; Oddo, M.; Meyfroidt, G.; Helbok, R.; Taccone, F.S.; Prisco, L.; et al. Intracranial Pressure Monitoring in Patients with Acute Brain Injury in the Intensive Care Unit (SYNAPSE-ICU): An International, Prospective Observational Cohort Study. Lancet Neurol. 2021, 20, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Zoerle, T.; Lombardo, A.; Colombo, A.; Longhi, L.; Zanier, E.R.; Rampini, P.; Stocchetti, N. Intracranial Pressure after Subarachnoid Hemorrhage. Crit. Care Med. 2015, 43, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossu, G.; Messerer, M.; Stocchetti, N.; Levivier, M.; Daniel, R.T.; Oddo, M. Intracranial Pressure and Outcome in Critically Ill Patients with Aneurysmal Subarachnoid Hemorrhage: A Systematic Review. Minerva Anestesiol. 2016, 82, 684–696. [Google Scholar]
- Magni, F.; Pozzi, M.; Rota, M.; Vargiolu, A.; Citerio, G. High-Resolution Intracranial Pressure Burden and Outcome in Subarachnoid Hemorrhage. Stroke 2015, 46, 2464–2469. [Google Scholar] [CrossRef] [Green Version]
- Carra, G.; Elli, F.; Ianosi, B.; Flechet, M.; Huber, L.; Rass, V.; Depreitere, B.; Guiza, F.; Meyfroidt, G.; Citerio, G.; et al. Association of Dose of Intracranial Hypertension with Outcome in Subarachnoid Hemorrhage. Neurocrit Care. 2021, 34, 722–730. [Google Scholar] [CrossRef]
- Ayer, R.E.; Zhang, J.H. Oxidative Stress in Subarachnoid Haemorrhage: Significance in Acute Brain Injury and Vasospasm. Acta Neurochir. Suppl. 2008, 104, 33–41. [Google Scholar] [CrossRef]
- Sehba, F.A.; Pluta, R.M.; Zhang, J.H. Metamorphosis of Subarachnoid Hemorrhage Research: From Delayed Vasospasm to Early Brain Injury. Mol. Neurobiol. 2011, 43, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, S.L.; Kumar, P.T.; McBride, D.; Zeineddine, H.A.; Leclerc, J.; Choi, H.A.; Dash, P.K.; Grotta, J.; Aronowski, J.; Cardenas, J.C.; et al. Unique Contribution of Haptoglobin and Haptoglobin Genotype in Aneurysmal Subarachnoid Hemorrhage. Front. Physiol. 2018, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Che, M.; Wang, R.; Li, X.; Wang, H.Y.; Zheng, X.F.S. Expanding Roles of Superoxide Dismutases in Cell Regulation and Cancer. Drug Discov Today. 2016, 21, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.H.; Kim, E.M.; Song, J.Y.; Park, J.K.; Um, H.D. Mitochondrial Superoxide dismutase 2 Mediates Gamma-Irradiation-Induced Cancer Cell Invasion. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Papay, F.A.; Levine, H.L.; Schiavone, W.A. Facial Fuzz and Funny Findings. Facial Hair Causing Otalgia and Oropharyngeal pain. Cleve Clin J Med. 1989, 56, 273–276. [Google Scholar] [CrossRef]
- Marzatico, F.; Gaetani, P.; Silvani, V.; Lombardi, D.; Sinforiani, E.; Rodriguez y Baena, R. Experimental Isobaric Subarachnoid Hemorrhage: Regional Mitochondrial Function during the Acute and Late Phase. Surg. Neurol. 1990, 34, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez y Baena, R.; Gaetani, P.; Silvani, V.; Spanu, G.; Marzatico, F. Effect of Nimodipine on Mitochondrial Respiration in Different Rat Brain Areas after Subarachnoid Haemorrhage. Acta Neurochir. Suppl. 1988, 43, 177–181. [Google Scholar] [CrossRef]
- Moro, M.A.; Almeida, A.; Bolanos, J.P.; Lizasoain, I. Mitochondrial Respiratory Chain and Free Radical Generation in stroke. Free Radic. Biol. Med. 2005, 39, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N. Mechanisms of Mitochondrial ROS Production in Assisted Reproduction: The Known, the Unknown, and the Intriguing. Antioxidants 2020, 9, 933. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH Oxidase in Brain Injury and Neurodegenerative Disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Cantu-Medellin, N.; Kelley, E.E. Xanthine Oxidoreductase-Catalyzed Reactive Species Generation: A Process in Critical Need of Reevaluation. Redox Biol. 2013, 1, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Zhang, L.; Hong, J.S.; Zhang, D.; Zhao, J.; Wang, Q. Nicotinamide Adenine Dinucleotide Phosphate Oxidase and Neurodegenerative Diseases: Mechanisms and Therapy. Antioxid. Redox Signal. 2020, 33, 374–393. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wilson, K.; Gu, H.; Wegman-Points, L.; Dooley, S.A.; Pierce, G.L.; Cheng, G.; Pena Silva, R.A.; Heistad, D.D.; Hasan, D. Myeloperoxidase is Increased in Human Cerebral Aneurysms and Increases Formation and Rupture of Cerebral Aneurysms in Mice. Stroke 2015, 46, 1651–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooney, S.J.; Bermudez-Sabogal, S.L.; Byrnes, K.R. Cellular and Temporal Expression of NADPH Oxidase (NOX) Isotypes after Brain Injury. J. Neuroinflammation 2013, 10, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorce, S.; Krause, K.H. NOX Enzymes in the Central Nervous System: From Signaling to Disease. Antioxid. Redox Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Feng, D.; Shen, H.; Tian, X.; Li, H.; Wang, Z.; Chen, G. Involvement of Nox2 and Nox4 NADPH Oxidases in Early Brain Injury after Subarachnoid Hemorrhage. Free Radic. Res. 2017, 51, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Suh, Y.S.; Lee, M.S.; Kim, K.Y.; Lee, J.H.; Lee, H.S.; Hong, K.W.; Kim, C.D. Vascular NAD(P)H Oxidase Triggers Delayed Cerebral Vasospasm after Subarachnoid Hemorrhage in Rats. Stroke 2002, 33, 2687–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.K.; Pennathur, S.; Perier, C.; Tieu, K.; Teismann, P.; Wu, D.C.; Jackson-Lewis, V.; Vila, M.; Vonsattel, J.P.; Heinecke, J.W.; et al. Ablation of The Inflammatory Enzyme Myeloperoxidase Mitigates Features of Parkinson’s Disease in Mice. J. Neurosci. 2005, 25, 6594–6600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, P.S.; Mendez, A.J.; Jacob, J.S.; Crowley, J.R.; Growdon, W.; Hyman, B.T.; Heinecke, J.W. Neuronal Expression of Myeloperoxidase is Increased in Alzheimer’s Disease. J. Neurochem. 2004, 90, 724–733. [Google Scholar] [CrossRef]
- Nagra, R.M.; Becher, B.; Tourtellotte, W.W.; Antel, J.P.; Gold, D.; Paladino, T.; Smith, R.A.; Nelson, J.R.; Reynolds, W.F. Immunohistochemical and Genetic Evidence of Myeloperoxidase Involvement in Multiple Sclerosis. J. Neuroimmunol. 1997, 78, 97–107. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Mou, R.T.; Feng, D.X.; Wang, Z.; Chen, G. The Role of Nitric Oxide in stroke. Med. Gas Res. 2017, 7, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Khey, K.M.W.; Huard, A.; Mahmoud, S.H. Inflammatory Pathways Following Subarachnoid Hemorrhage. Cell. Mol. Neurobiol. 2020, 40, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Lenz, I.J.; Plesnila, N.; Terpolilli, N.A. Role of Endothelial Nitric Oxide Synthase for Early Brain Injury after Subarachnoid Hemorrhage in Mice. J. Cereb. Blood Flow Metab. 2021, 41, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Sehba, F.A.; Bederson, J.B. Nitric Oxide in Early Brain Injury after Subarachnoid Hemorrhage. Acta Neurochir. Suppl. 2011, 110, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, G.; Zhu, W.W.; Zhou, D. Activation of Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) in the Basilar Artery after Subarachnoid Hemorrhage in Rats. Ann. Clin. Lab. Sci. 2010, 40, 233–239. [Google Scholar] [PubMed]
- Zolnourian, A.; Galea, I.; Bulters, D. Neuroprotective Role of the Nrf2 Pathway in Subarachnoid Haemorrhage and Its Therapeutic Potential. Oxidative Med. Cell. Longev. 2019, 2019, 6218239. [Google Scholar] [CrossRef]
- Antognelli, C.; Trapani, E.; Delle Monache, S.; Perrelli, A.; Daga, M.; Pizzimenti, S.; Barrera, G.; Cassoni, P.; Angelucci, A.; Trabalzini, L.; et al. KRIT1 Loss-of-Function Induces A Chronic Nrf2-Mediated Adaptive Homeostasis That Sensitizes Cells to Oxidative Stress: Implication for Cerebral Cavernous Malformation disease. Free Radic. Biol. Med. 2018, 115, 202–218. [Google Scholar] [CrossRef]
- Harada, N.; Kanayama, M.; Maruyama, A.; Yoshida, A.; Tazumi, K.; Hosoya, T.; Mimura, J.; Toki, T.; Maher, J.M.; Yamamoto, M.; et al. Nrf2 Regulates Ferroportin 1-Mediated Iron Efflux and Counteracts Lipopolysaccharide-Induced Ferroportin 1 mRNA Suppression in Macrophages. Arch. Biochem. Biophys. 2011, 508, 101–109. [Google Scholar] [CrossRef]
- Chen, M.; Regan, R.F. Time Course of Increased Heme Oxygenase Activity and Expression after Experimental Intracerebral Hemorrhage: Correlation with Oxidative Injury. J. Neurochem. 2007, 103, 2015–2021. [Google Scholar] [CrossRef]
- Morris, C.M.; Candy, J.M.; Edwardson, J.A.; Bloxham, C.A.; Smith, A. Evidence For the Localization of Haemopexin Immunoreactivity in Neurones in the Human Brain. Neurosci. Lett. 1993, 149, 141–144. [Google Scholar] [CrossRef]
- Zhao, X.; Song, S.; Sun, G.; Strong, R.; Zhang, J.; Grotta, J.C.; Aronowski, J. Neuroprotective Role of Haptoglobin after Intracerebral Hemorrhage. J. Neurosci. 2009, 29, 15819–15827. [Google Scholar] [CrossRef] [Green Version]
- Jeyapaul, J.; Jaiswal, A.K. Nrf2 and c-Jun Regulation of Antioxidant Response Element (ARE)-Mediated Expression and Induction of Gamma-Glutamylcysteine Synthetase Heavy Subunit Gene. Biochem. Pharmacol. 2000, 59, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.Q.; Li, X.F.; Lei, X.H.; Wang, Z.Y.; Ma, D.Y.; Wang, Y.N.; Ye, W. c-Jun N-terminal Kinase Inhibition Attenuates Early Brain Injury Induced Neuronal Apoptosis via Decreasing p53 Phosphorylation and Mitochondrial Apoptotic Pathway Activation in Subarachnoid Hemorrhage Rats. Mol. Med. Rep. 2019, 19, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Yatsushige, H.; Ostrowski, R.P.; Tsubokawa, T.; Colohan, A.; Zhang, J.H. Role of c-Jun N-Terminal Kinase in Early Brain Injury after Subarachnoid Hemorrhage. J. Neurosci. Res. 2007, 85, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Chiang, S.; Kalinowski, D.S.; Bae, D.H.; Sahni, S.; Richardson, D.R. The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxidative Med. Cell. Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Wu, P.; Budbazar, E.; Zhu, Q.; Sun, C.; Mo, J.; Peng, J.; Gospodarev, V.; Tang, J.; Shi, H.; et al. Mitophagy Reduces Oxidative Stress Via Keap1 (Kelch-Like Epichlorohydrin-Associated Protein 1)/Nrf2 (Nuclear Factor-E2-Related Factor 2)/PHB2 (Prohibitin 2) Pathway after Subarachnoid Hemorrhage in Rats. Stroke 2019, 50, 978–988. [Google Scholar] [CrossRef]
- Link, T.E.; Murakami, K.; Beem-Miller, M.; Tranmer, B.I.; Wellman, G.C. Oxyhemoglobin-Induced Expression of R-type Ca2+ Channels in Cerebral Arteries. Stroke 2008, 39, 2122–2128. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, M.; Morielli, A.D.; Zvarova, K.; Tranmer, B.I.; Penar, P.L.; Wellman, G.C. Oxyhemoglobin-Induced Suppression of Voltage-Dependent K+ Channels in Cerebral Arteries by Enhanced Tyrosine Kinase Activity. Circ. Res. 2006, 99, 1252–1260. [Google Scholar] [CrossRef] [Green Version]
- Sabri, M.; Ai, J.; Knight, B.; Tariq, A.; Jeon, H.; Shang, X.; Marsden, P.A.; Loch Macdonald, R. Uncoupling of Endothelial Nitric Oxide Synthase after Experimental Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2011, 31, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Rangel-Castilla, L.; Gopinath, S.; Robertson, C.S. Management of Intracranial Hypertension. Neurol. Clin. 2008, 26, 521–541. [Google Scholar] [CrossRef]
- Mak, C.H.; Lu, Y.Y.; Wong, G.K. Review and Recommendations on Management of Refractory Raised Intracranial Pressure in Aneurysmal Subarachnoid Hemorrhage. Vasc. Health Risk Manag. 2013, 9, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, N.M.; Wang, J.Z.; Pasarikovski, C.R.; Guha, D.; Al-Mufti, F.; Mamdani, M.; Saposnik, G.; Schweizer, T.A.; Macdonald, R.L. Management of Raised Intracranial Pressure in Aneurysmal Subarachnoid Hemorrhage: Time for a consensus? Neurosurg. Focus 2017, 43, E13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, T.; Juvela, S.; Unterberg, A.; Jung, C.; Forsting, M.; Rinkel, G.; European Stroke, O. European Stroke Organization Guidelines for the Management of Intracranial Aneurysms and Subarachnoid Haemorrhage. Cerebrovasc Dis. 2013, 35, 93–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/american Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, W. Evaluation of the Effectiveness of Lumbar Punctures in Aneurysmal Subarachnoid Hemorrhage Patient with External Ventricular Drainage. World Neurosurg. 2021, 151, e1–e9. [Google Scholar] [CrossRef]
- Konovalov, A.; Shekhtman, O.; Pilipenko, Y.; Okishev, D.; Ershova, O.; Oshorov, A.; Abramyan, A.; Kurzakova, I.; Eliava, S. External Ventricular Drainage in Patients with Acute Aneurysmal Subarachnoid Hemorrhage after Microsurgical Clipping: Our 2006–2018 Experience and a Literature Review. Cureus 2021, 13, e12951. [Google Scholar] [CrossRef]
- Duan, F.; Wang, G.; Ma, X.; Zhao, Y.; Xu, X.; Dong, F. A Controlled Study of Continuous Lumbar Drainage of Fluid and Lumbar Puncture Drainage for Aneurysmal SAH after Intracranial Aneurysm Clipping. J. Healthcare Eng. 2021, 2021, 2827493. [Google Scholar] [CrossRef]
- Wang, A.Y.; Hsieh, P.C.; Chen, C.C.; Chin, S.C.; Wu, Y.M.; Chen, C.T.; Chang, C.H.; Wu, T.E. Effect of Intracranial Pressure Control on Improvement of Cerebral Perfusion after Acute Subarachnoid Hemorrhage: A Comparative Angiography Study Based on Temporal Changes of Intracranial Pressure and Systemic Pressure. World Neurosurg. 2018, 120, e290–e296. [Google Scholar] [CrossRef]
- van Lieshout, J.H.; Pumplun, I.; Fischer, I.; Kamp, M.A.; Cornelius, J.F.; Steiger, H.J.; Boogaarts, H.D.; Petridis, A.K.; Beseoglu, K. Volume of Cerebrospinal Fluid Drainage as a Predictor for Pretreatment Aneurysmal Rebleeding. J. Neurosurg. 2018, 128, 1778–1784. [Google Scholar] [CrossRef]
- Olson, D.M.; Zomorodi, M.; Britz, G.W.; Zomorodi, A.R.; Amato, A.; Graffagnino, C. Continuous Cerebral Spinal Fluid Drainage Associated with Complications in Patients Admitted with Subarachnoid Hemorrhage. J. Neurosurg. 2013, 119, 974–980. [Google Scholar] [CrossRef]
- Cagnazzo, F.; Chalard, K.; Lefevre, P.H.; Garnier, O.; Derraz, I.; Dargazanli, C.; Gascou, G.; Riquelme, C.; Bonafe, A.; Perrini, P.; et al. Optimal Intracranial Pressure in Patients with Aneurysmal Subarachnoid Hemorrhage Treated with Coiling and Requiring External Ventricular Drainage. Neurosurg. Rev. 2021, 44, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Kubota, A.; Murai, Y.; Morita, A. A Case of Suspected Low-Pressure Hydrocephalus Caused by Spinal Drainage Following Subarachnoid Hemorrhage. J. Nippon. Med. Sch. 2021, 89, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Linsler, S.; Schmidtke, M.; Steudel, W.I.; Kiefer, M.; Oertel, J. Automated Intracranial Pressure-Controlled Cerebrospinal Fluid External Drainage with LiquoGuard. Acta Neurochir. 2013, 155, 1589–1594, discussion 1585–1594. [Google Scholar] [CrossRef]
- Al-Tamimi, Y.Z.; Bhargava, D.; Feltbower, R.G.; Hall, G.; Goddard, A.J.; Quinn, A.C.; Ross, S.A. Lumbar Drainage of Cerebrospinal Fluid after Aneurysmal Subarachnoid Hemorrhage: A Prospective, Randomized, Controlled trial (LUMAS). Stroke 2012, 43, 677–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Xin, D.Q.; Hu, Q.; Wang, L.X.; Qiu, J.; Yuan, H.T.; Chu, X.L.; Liu, D.X.; Li, G.; Wang, Z. Neuroprotective Mechanism of L-cysteine after Subarachnoid Hemorrhage. Neural Regen. Res. 2020, 15, 1920–1930. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.S.; Wang, H.D.; Zhang, X.; Yu, Q.; Li, W.; Zhou, M.L.; Wang, X.L. Astaxanthin Activates Nuclear Factor Erythroid-related Factor 2 and the Antioxidant Responsive Element (Nrf2-ARE) Pathway in the Brain after Subarachnoid Hemorrhage in Rats and Attenuates Early Brain Injury. Mar. Drugs 2014, 12, 6125–6141. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, J.; Wang, Z.; You, W.; Wu, L.; Ji, C.; Chen, G. Dimethylfumarate Alleviates Early Brain Injury and Secondary Cognitive Deficits after Experimental Subarachnoid Hemorrhage via Activation of Keap1-Nrf2-ARE System. J. Neurosurg. 2015, 123, 915–923. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Guo, S.; Wang, J.; Shen, Y.; Zhang, J.; Wu, Q. Nrf2/HO-1 Mediates the Neuroprotective Effect of Mangiferin on Early Brain Injury after Subarachnoid Hemorrhage by Attenuating Mitochondria-Related Apoptosis and Neuroinflammation. Sci. Rep. 2017, 7, 11883. [Google Scholar] [CrossRef]
- Song, S.; Chen, Y.; Han, F.; Dong, M.; Xiang, X.; Sui, J.; Li, Y.; Yang, H.; Liu, J. Aloperine Activates the Nrf2-ARE Pathway When Ameliorating Early Brain Injury in A Subarachnoid Hemorrhage Model. Exp. Ther. Med. 2018, 15, 3847–3855. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Lu, Y.; Wan, J.; Dai, H.; Zhou, X.; Lv, S.; Chen, X.; Zhang, X.; Hang, C.; et al. Cerebroprotection by Salvianolic Acid B after Experimental Subarachnoid Hemorrhage Occurs via Nrf2- and SIRT1-Dependent Pathways. Free Radic. Biol. Med. 2018, 124, 504–516. [Google Scholar] [CrossRef]
- Wang, T.; Xu, L.; Gao, L.; Zhao, L.; Liu, X.H.; Chang, Y.Y.; Liu, Y.L. Paeoniflorin Attenuates Early Brain Injury through Reducing Oxidative Sress and Neuronal Apoptosis after Subarachnoid Hemorrhage in Rats. Metab. Brain Dis. 2020, 35, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, C.; Li, X.; Liang, G. Oleanolic Acid Reduces Oxidative Stress and Neuronal Apoptosis after Experimental Subarachnoid Hemorrhage by Regulating Nrf2/HO-1 Pathway. Drug Dev Res. 2022, 83, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhang, W.; Zou, C.; Han, S.; Tian, Q.; Wang, J.; He, P.; Guo, Y.; Li, M. Andrographolide Attenuates Blood-Brain Barrier Disruption, Neuronal Apoptosis, and Oxidative Stress Through Activation of Nrf2/HO-1 Signaling Pathway in Subarachnoid Hemorrhage. Neurotox Res. 2022, 40, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Y.; Wang, Y.H.; Hang, C.H. The Mfn1-betaIIPKC Interaction Regulates Mitochondrial Dysfunction via Sirt3 Following Experimental Subarachnoid Hemorrhage. Transl. Stroke Res. 2022, 13, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, Y.; Zhu, S.; Wang, C.; Dai, J.; Zhang, G.; Zheng, B.; Xu, S.; Wang, L.; Zhang, T.; et al. Mdivi-1 Alleviates Early Brain Injury after Experimental Subarachnoid Hemorrhage in Rats, Possibly via Inhibition of Drp1-Activated Mitochondrial Fission and Oxidative Stress. Neurochem. Res. 2017, 42, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Zhou, J.; Li, G.; Chen, W.; Zhong, W.; Chen, Z. SS31 Attenuates Oxidative Stress and Neuronal Apoptosis in Early Brain Injury following Subarachnoid Hemorrhage Possibly by the Mitochondrial Pathway. Neurosci. Lett. 2020, 717, 134654. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, P.; Zhang, J.H.; Li, Y.; Xu, S.; Wang, C.; Wang, L.; Zhang, G.; Dai, J.; Zhu, S.; et al. Docosahexaenoic Acid Alleviates Oxidative Stress-Based Apoptosis via Improving Mitochondrial Dynamics in Early Brain Injury after Subarachnoid Hemorrhage. Cell. Mol. Neurobiol. 2018, 38, 1413–1423. [Google Scholar] [CrossRef]
- Fan, L.F.; He, P.Y.; Peng, Y.C.; Du, Q.H.; Ma, Y.J.; Jin, J.X.; Xu, H.Z.; Li, J.R.; Wang, Z.J.; Cao, S.L.; et al. Mdivi-1 Ameliorates Early Brain Injury after Subarachnoid Hemorrhage via the Suppression of Inflammation-Related Blood-Brain Barrier Disruption and Endoplasmic Reticulum Stress-Based Apoptosis. Free Radic. Biol. Med. 2017, 112, 336–349. [Google Scholar] [CrossRef]
- Zhang, X.S.; Lu, Y.; Tao, T.; Wang, H.; Liu, G.J.; Liu, X.Z.; Liu, C.; Xia, D.Y.; Hang, C.H.; Li, W. Fucoxanthin Mitigates Subarachnoid Hemorrhage-Induced Oxidative Damage via Sirtuin 1-Dependent Pathway. Mol. Neurobiol. 2020, 57, 5286–5298. [Google Scholar] [CrossRef]
- Liu, H.; Guo, W.; Guo, H.; Zhao, L.; Yue, L.; Li, X.; Feng, D.; Luo, J.; Wu, X.; Cui, W.; et al. Bakuchiol Attenuates Oxidative Stress and Neuron Damage by Regulating Trx1/TXNIP and the Phosphorylation of AMPK after Subarachnoid Hemorrhage in Mice. Front. Pharmacol. 2020, 11, 712. [Google Scholar] [CrossRef]
- Zhuang, K.; Zuo, Y.C.; Sherchan, P.; Wang, J.K.; Yan, X.X.; Liu, F. Hydrogen Inhalation Attenuates Oxidative Stress Related Endothelial Cells Injury after Subarachnoid Hemorrhage in Rats. Front. Neurosci. 2019, 13, 1441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, T.; Li, Y.; Guo, Y.; Liu, B.; Tian, Y.; Wu, P.; Shi, H. Metformin Attenuates Early Brain Injury after Subarachnoid Hemorrhage in Rats via AMPK-Dependent Mitophagy. Exp. Neurol. 2022, 353, 114055. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, T.; Gao, L.; Zheng, J.; Yan, J.; Zhang, J.; Shao, A. Apelin-13/APJ System Attenuates Early Brain Injury via Suppression of Endoplasmic Reticulum Stress-Associated TXNIP/NLRP3 Inflammasome Activation and Oxidative Stress in A AMPK-Dependent Manner after Subarachnoid Hemorrhage in Rats. J. Neuroinflammation 2019, 16, 247. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Su, J.; Liu, X.; Zhao, Y.; Wang, C.; Li, X. Naringin Alleviates Early Brain Injury after Experimental Subarachnoid Hemorrhage by Reducing Oxidative Stress and Inhibiting Apoptosis. Brain Res. Bull. 2017, 133, 42–50. [Google Scholar] [CrossRef]
- Fu, P.; Hu, Q. 3,4-Dihydroxyphenylethanol Alleviates Early Brain Injury by Modulating Oxidative Stress and Akt and Nuclear Factor-KappaB Pathways in A Rat Model of Subarachnoid Hemorrhage. Exp. Ther. Med. 2016, 11, 1999–2004. [Google Scholar] [CrossRef]
| Anti-OS | Pathway | Medicine | Possible Effects of ICP | Effects of OS |
|---|---|---|---|---|
| Upregulate anti-OS system | Keap1-Nrf2-ARE | Andrographolide [103] | attenuate neuronal apoptosis, BBB disruption, and brain edema | |
| Oleanolic acid [102] | reduce brain edema, BBB disruption, and neuronal apoptosis | increase the levels of superoxide dismutase, catalase, and GSH-Px | ||
| Paeoniflorin [101] | attenuate brain water content, Evans blue extravasation, and neuronal apoptosis | decrease ROS, MDA, 3-nitrotyrosine, and 8-OHDG levels; increase SOD, GSH-Px, and CAT activity | ||
| Salvianolic acid A and B [100] | reduce brain edema and neuronal apoptosis | suppress ROS; decrease lipid peroxidation; and increase GSH-Px, GSH, and SOD activities | ||
| Aloperine [99] | ameliorate brain edema and cellular apoptosis | decrease MDA and increase GST | ||
| Mangiferin [98] | ameliorate brain edema and cellular apoptosis | decrease MDA; increase SOD, CAT, and GSH | ||
| Dimethylfumarate [97] | attenuate brain edema and BBB impairment | decrease MDA; increase SOD, NADPH NQO1, and GST-a1 activities | ||
| Astaxanthin [96] | attenuate brain edema, BBB disruption, and cellular apoptosis | decrease MDA; increase NQO1 and GST-a1 activities | ||
| L-cysteine [95] | decrease brain water content | reduce ROS content and decrease endoplasmic reticulum stress | ||
| Reduce ROS | Mitochondrial pathway | Docosahexaenoic acid [107] | ameliorate mitochondrial dysfunction, reduce brain edema, and attenuate OxyHb-induced neuronal death | attenuate MDA levels and SOD stress |
| SS31 [106] | ameliorate mitochondrial dysfunction, brain edema, and Evans blue dye extravasation; decrease neuronal apoptosis | reduce MDA levels and restore the activities of GSH-Px and SOD | ||
| Mdivi-1 [108] (a selective Drp1 inhibitor), dynamin-related protein-1 (Drp1, a dominator of mitochondrial fission) | ameliorate BBB disruption and brain edema, decrease the expression of MMP-9, and prevent the degradation of tight-junction proteins | reduce ROS levels | ||
| Mdivi-1 [105] | attenuate the release of cytochrome C from mitochondria, inhibit excessive mitochondrial fission, restore the ultra-structure of mitochondria, alleviate brain edema and BBB permeability, and attenuate apoptotic cell death | reduce levels of MDA, 3-NT, and 8-OHdG; improve SOD activity | ||
| Mfn1-βIIPKC [104] | attenuate the OxyHb-induced neuronal injury and apoptosis; reduce brain edema | enhance the activities of its downstream mitochondrial antioxidant enzymes | ||
| Fucoxanthin [109] | improve mitochondrial morphology, ameliorate neural apoptosis, and reduce brain edema | decrease intracellular MDA, nitrotyrosine, and 8-OHDG production and increase endogenous antioxidant systems (including GSH-Px, GSH, SOD, and catalase) | ||
| Bakuchiol [110] | alleviate BBB disruption (decrease EB extravasation; increase claudin-5, occludin, and zonula occludens-1; and decrease matrix metalloproteinase-9) and brain edema; inhibit cellular apoptosis by regulating the protein levels of Bcl-2, Bax, and cleaved caspase-3 | attenuate oxidative stress by reducing reactive oxygen species, MDA, 3-NT, 8-OHDG, gp91 phox, and 4-HNE; increase the activities of SOD and GSH-Px | ||
| Hydrogen [111] | reduce the expression of apoptotic makers in the vessels, brain edema, microthrombi formation, and vasospasm | decrease MDA concentration, 8-OHDG-positive cells, and the expression of 4-HNE and HO-1; increase SOD2 | ||
| Metformin [112] | attenuate brain edema and disrupt BBB permeability | alleviate OS | ||
| Other pathways | ER stress | Apelin-13 [113] | attenuate brain edema and preserve BBB integrity (Evans blue staining) | reduce MPO and ROS |
| MAPK | Naringin [114] | ameliorate brain edema and BBB integrity | decrease MDA; increase the activities of CAT, GSH-Px enzymes, and the GSH/GSSG ratio | |
| Akt and NF-κB pathways | 3,4-Dihydroxyphenylethanol [115] | induce a reduction in the brain water content and decrease BBB permeability | decrease MDA; augment the activities of SOD, CAT, and GSH-PX | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, G.; Eser, P.; Mo, J. Oxidative Stress and Intracranial Hypertension after Aneurysmal Subarachnoid Hemorrhage. Antioxidants 2022, 11, 2423. https://doi.org/10.3390/antiox11122423
Hao G, Eser P, Mo J. Oxidative Stress and Intracranial Hypertension after Aneurysmal Subarachnoid Hemorrhage. Antioxidants. 2022; 11(12):2423. https://doi.org/10.3390/antiox11122423
Chicago/Turabian StyleHao, Guangshan, Pinar Eser, and Jun Mo. 2022. "Oxidative Stress and Intracranial Hypertension after Aneurysmal Subarachnoid Hemorrhage" Antioxidants 11, no. 12: 2423. https://doi.org/10.3390/antiox11122423
APA StyleHao, G., Eser, P., & Mo, J. (2022). Oxidative Stress and Intracranial Hypertension after Aneurysmal Subarachnoid Hemorrhage. Antioxidants, 11(12), 2423. https://doi.org/10.3390/antiox11122423







