Hatched Eggshell Membrane Can Be a Novel Source of Antioxidant Hydrolysates to Protect against H2O2-Induced Oxidative Stress in Human Chondrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Scanning Electron Microscopy
2.3. The Chemical Characterization of ESM
2.4. Amino Acid Analysis
2.5. Preparation of Eggshell Membrane Protein Hydrolysates
2.6. Degree of Hydrolysis (DH)
2.7. Determination of Molecular Weight (MW) Distribution
2.8. Nano-LC-ESI-MS/MS Analysis
2.9. Determinati on of Chemical Antioxidative Activity
2.9.1. DPPH Radical Scavenging Activity Assay
2.9.2. Fe2+-Chelating Activity Assay
2.9.3. Reducing Power Assay
2.10. Membrane Ultrafiltration
2.11. Effects of HEMH-I on H2O2-Induced SW1353 Cells
2.11.1. Cell Culture
2.11.2. Cytotoxicity Analysis of HEMH-I and H2O2 on SW1353 Cells
2.11.3. Cytoprotective Effect of HEMH-I on H2O2-Damaged SW1353 Cells
2.11.4. Determination of Intracellular ROS
2.11.5. Western Blotting Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Structure and Chemical Composition of ESM from Two Sources of Chicken Eggs
3.1.1. Electron Microscopic Scanning of ESM
3.1.2. Proximate Composition of ESM
3.1.3. Amino Acid Composition of ESM
3.2. Enzymatic Hydrolysis of ESM
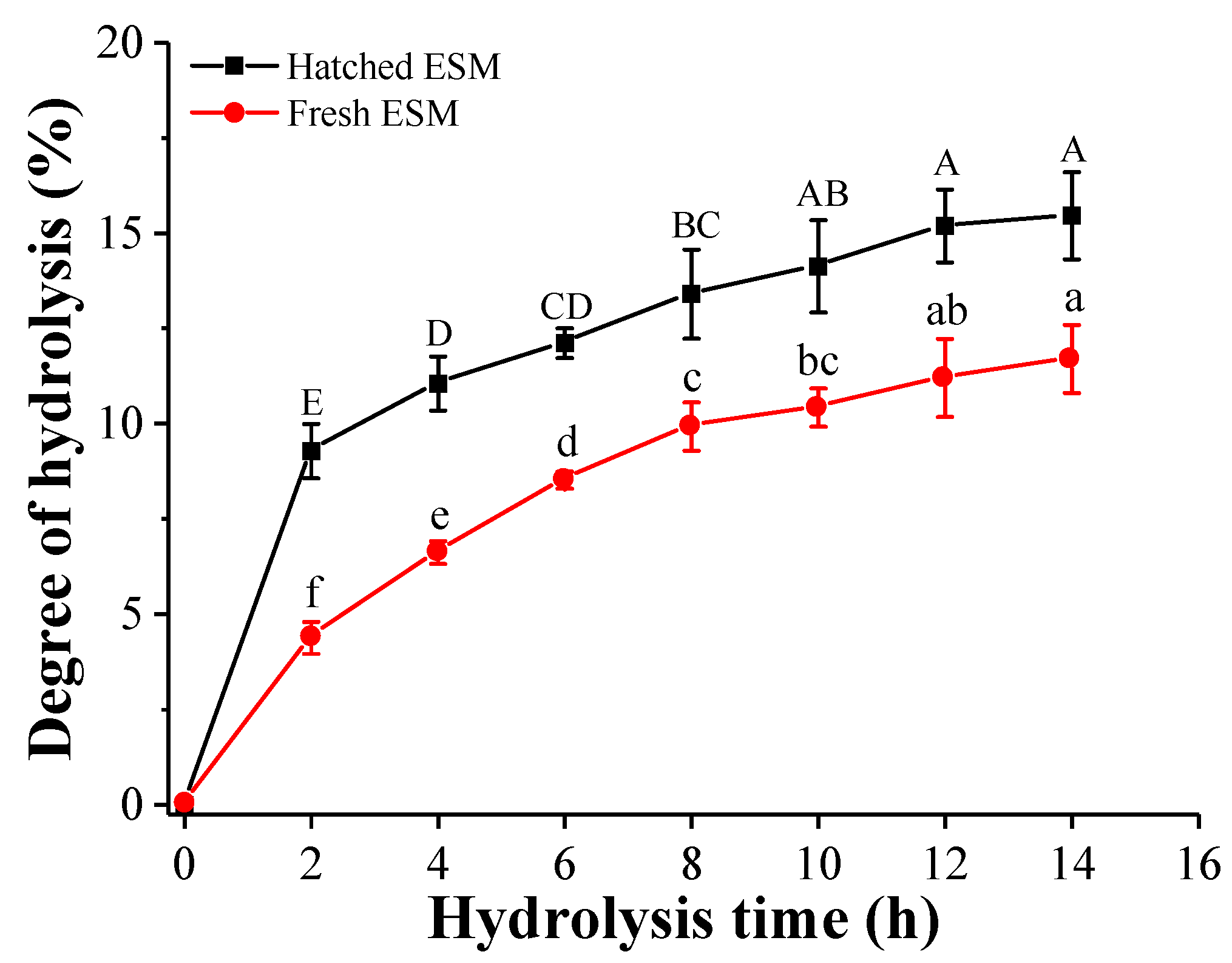
3.2.1. Degree of Hydrolysis (DH)
3.2.2. Molecular Weight Distributions of ESM Hydrolysates
3.2.3. Amino Acid Composition of HEMH and FEMH Fractions
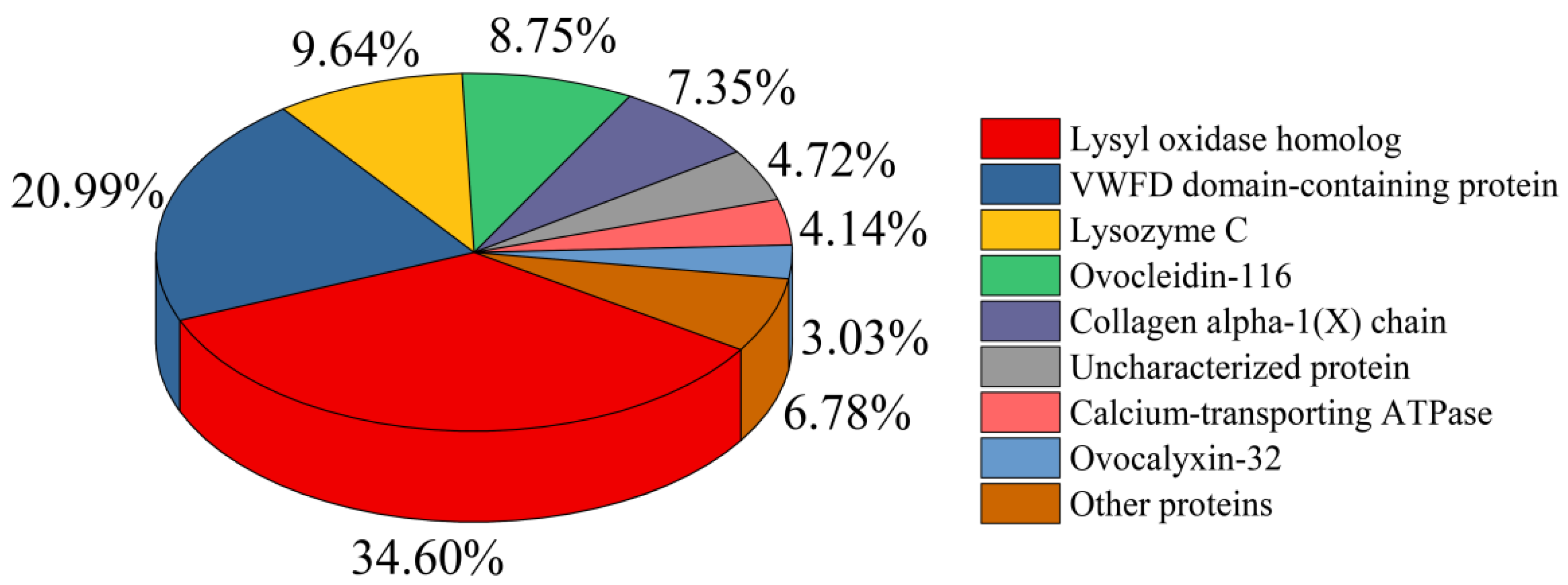
3.2.4. Nano-LC-ESI-MS/MS Analysis of HEMH
3.3. The Antioxidation Activity of HEMH and Ultrafiltration Fractions
3.4. Cytoprotective Effect of HEMH-I on H2O2-Induced SW1353 Human Chondrocytes
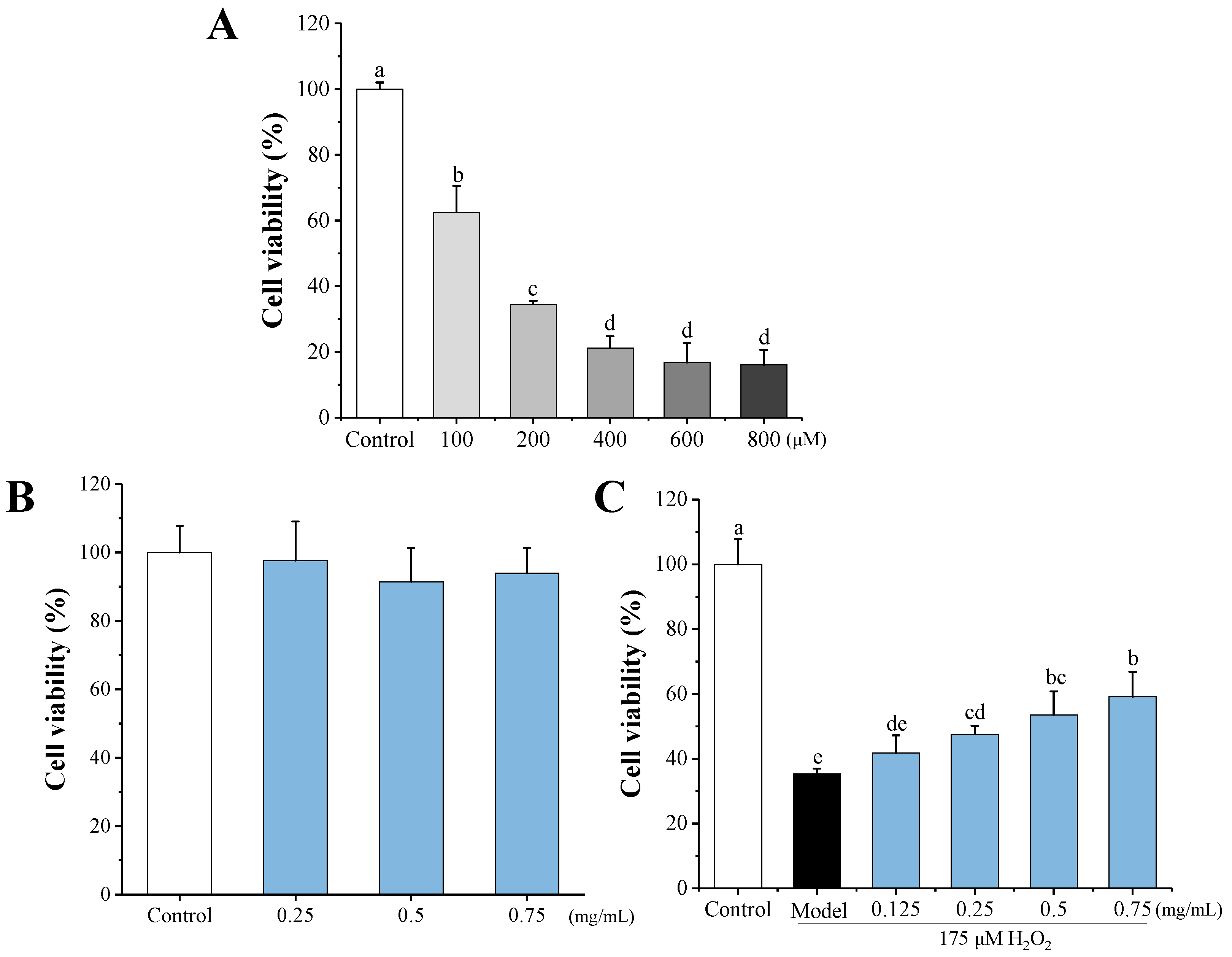
3.4.1. Effects of HEMH-I on Cell Viability of H2O2-Induced SW1353 Human Chondrocytes
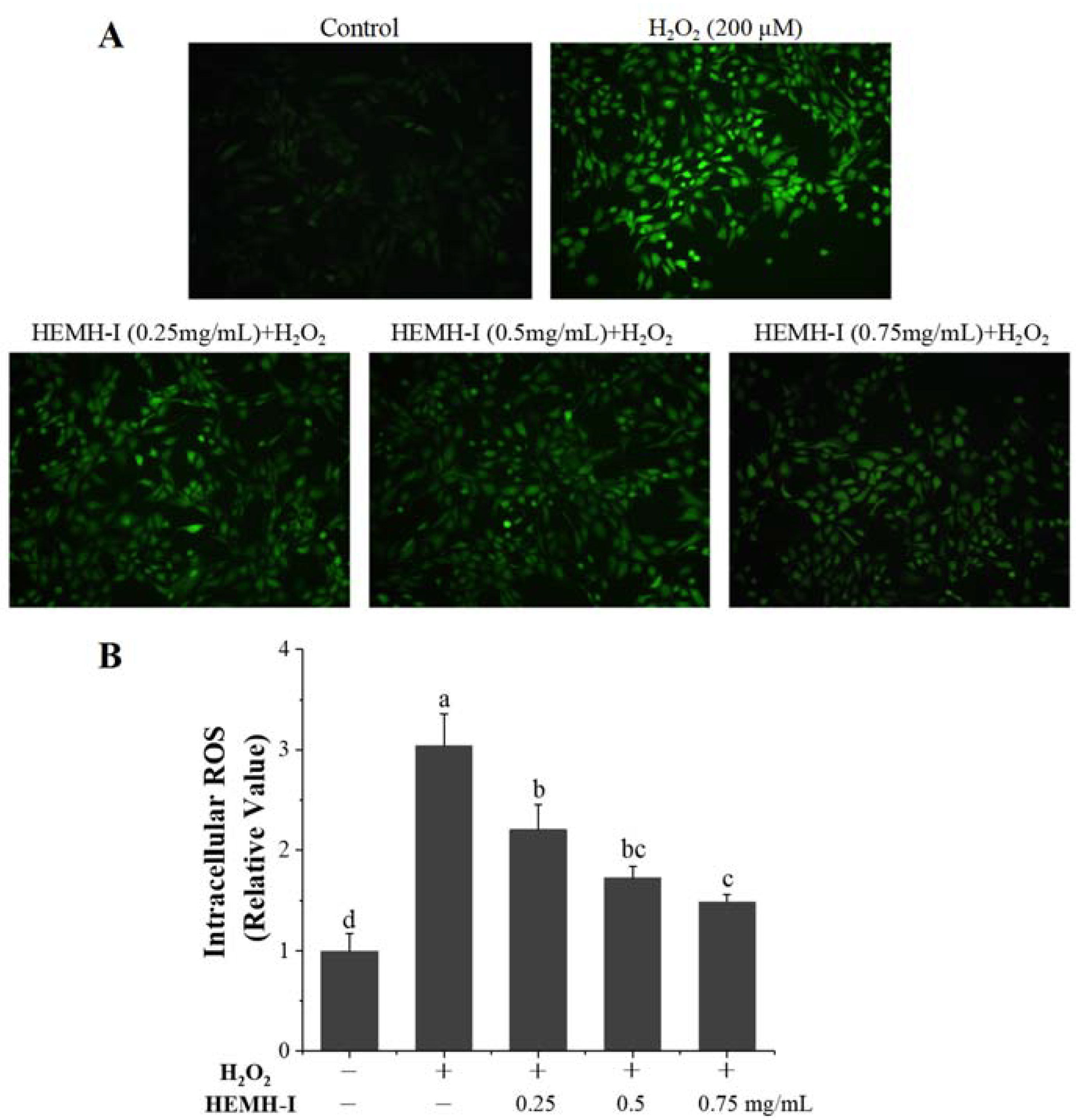
3.4.2. Effects of HEMH-I on ROS Levels in H2O2-Induced SW1353 Human Chondrocytes
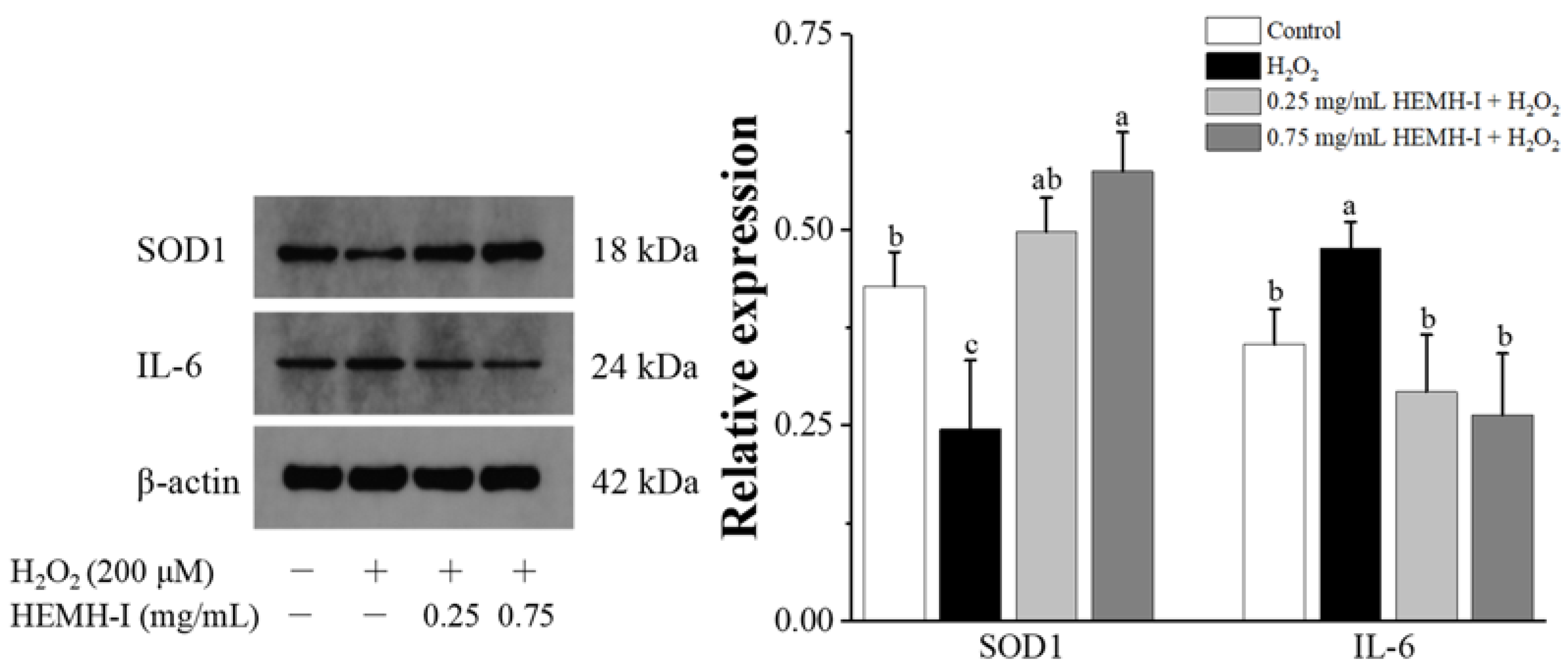
3.4.3. Effects of HEMH-I on Antioxidant Enzymes and Inflammatory Factor Expression in SW1353 Human Chondrocytes
3.4.4. Effect of HEMH-I on the Expression of Collagen II, MMP3, and MMP13 in SW1353 Human Chondrocytes
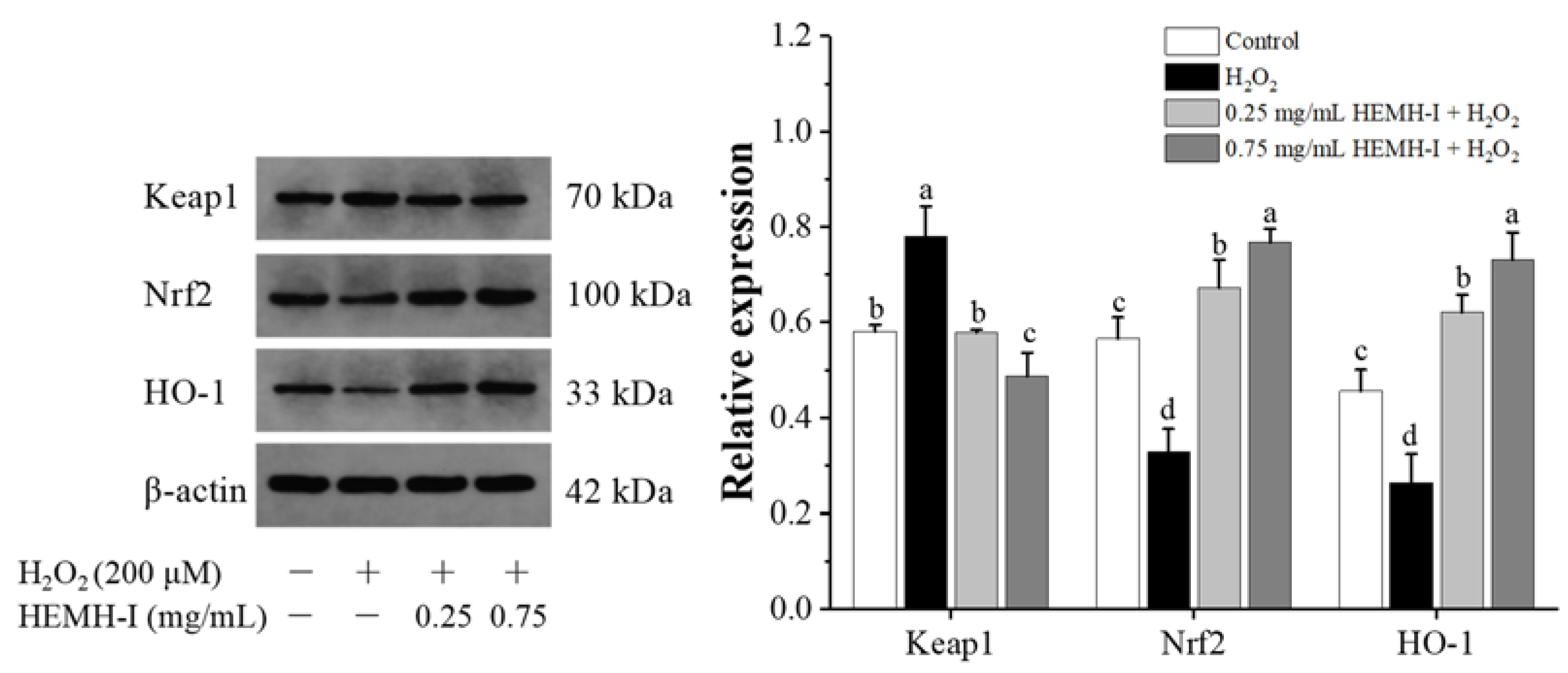
3.4.5. Effect of HEMH-I on the Activation of Keap1/Nrf2/HO-1 Pathway of SW1353 Human Chondrocytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Dong, Y.; Bao, Z.; Zhang, S.; Lin, S.; Sun, N. Advances in the activity evaluation and cellular regulation pathways of food-derived antioxidant peptides. Trends Food Sci. Technol. 2022, 122, 171–186. [Google Scholar] [CrossRef]
- Kim, E.; Lee, H.; Jeong, G. Cudratricusxanthone O Inhibits HO-Induced Cell Damage by Activating Nrf2/HO-1 Pathway in Human Chondrocytes. Antioxidants 2020, 9, 788. [Google Scholar] [CrossRef]
- Zhuang, C.; Wang, Y.; Zhang, Y.; Xu, N. Oxidative stress in osteoarthritis and antioxidant effect of polysaccharide from angelica sinensis. Int. J. Biol. Macromol. 2018, 115, 281–286. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, M.L.; Fernández-Romero, A.M.; Rabasco, A.M. Towards the antioxidant therapy in Osteoarthritis: Contribution of nanotechnology. J. Drug Deliv. Sci. Technol. 2017, 42, 94–106. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef]
- Poulet, B.; Beier, F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Res. Ther. 2016, 18, 32. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322. [Google Scholar] [CrossRef]
- Yang, J.; Sun-waterhouse, D.; Xiao, Y.; He, W.; Su, G. Osteoarthritis-alleviating effects in papain-induced model rats of chicken cartilage hydrolysate and its peptide fractions. Int. J. Food Sci. Technol. 2019, 54, 2711–2717. [Google Scholar] [CrossRef]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2015, 95, 702–707. [Google Scholar] [CrossRef]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmad, N.; Abdullah; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Xiao, N.; Huang, X.; He, W.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Zhao, Y.; Tu, Y. A review on recent advances of egg byproducts: Preparation, functional properties, biological activities and food applications. Food Res. Int. 2021, 147, 110563. [Google Scholar] [CrossRef] [PubMed]
- Kulshreshtha, G.; Diep, T.; Hudson, H.A.; Hincke, M.T. High value applications and current commercial market for eggshell membranes and derived bioactives. Food Chem. 2022, 382, 132270. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Production and characterization of functional properties of protein hydrolysates from egg shell membranes by lactic acid bacteria fermentation. J. Food Sci. Technol-Mysore 2017, 54, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.C.; Zhao, J.Y.; Ahn, D.U.; Jin, Y.G.; Huang, X. Separation and Identification of Highly Efficient Antioxidant Peptides from Eggshell Membrane. Antioxidants 2019, 8, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Zhou, Y.H.; Ma, M.H.; Cai, Z.X.; Li, T. Chemiluminescence Evaluation of Antioxidant Activity and Prevention of DNA Damage Effect of Peptides Isolated from Soluble Eggshell Membrane Protein Hydrolysate. J. Agric. Food Chem. 2010, 58, 12137–12142. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Peptides derived from eggshell membrane improve antioxidant enzyme activity and glutathione synthesis against oxidative damage in Caco-2 cells. J. Funct. Foods 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Glatz, P.; Miao, Z.; Rodda, B. Handling and Treatment of Poultry Hatchery Waste: A Review. Sustainability 2011, 3, 216–237. [Google Scholar] [CrossRef] [Green Version]
- King’Ori, A.M. A Review of the uses of poultry eggshells and shell membranes. Int. J. Poult. Sci. 2011, 10, 908–912. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Sun, Q.; Munagapati, V.S.; Saratale, G.D.; Park, J.; Kim, D.S. The use of eggshell membrane for the treatment of dye-containing wastewater: Batch, kinetics and reusability studies. Chemosphere 2021, 281, 130777. [Google Scholar] [CrossRef]
- Cordeiro, C.M.M.; Hincke, M.T. Quantitative proteomics analysis of eggshell membrane proteins during chick embryonic development. J. Proteom. 2016, 130, 11–25. [Google Scholar] [CrossRef]
- Rath, N.C.; Liyanage, R.; Makkar, S.K.; Lay, J.O. Protein profiles of hatchery egg shell membrane. Proteome Sci. 2017, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.E.; Suso, H.P.; Hincke, M.T. In-depth comparative analysis of the chicken eggshell membrane proteome. J. Proteom. 2017, 155, 49–62. [Google Scholar] [CrossRef]
- Zhu, L.; Xiong, H.; Huang, X.; Guyonnet, V.; Ma, M.; Chen, X.; Zheng, Y.; Wang, L.; Hu, G. Identification and molecular mechanisms of novel antioxidant peptides from two sources of eggshell membrane hydrolysates showing cytoprotection against oxidative stress: A combined in silico and in vitro study. Food Res. Int. 2022, 157, 111266. [Google Scholar] [CrossRef]
- Sykiotis, G.P. Keap1/Nrf2 Signaling Pathway. Antioxidants 2021, 10, 828. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Jia, Y.; Wang, Y.; Li, R.; Li, S.; Zhang, M.; He, C.; Chen, H. The structural characteristic of acidic-hydrolyzed corn silk polysaccharides and its protection on the HO-injured intestinal epithelial cells. Food Chem. 2021, 356, 129691. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, Y.; Wu, P.; Wang, Y.; Kwaku Golly, M.; Ma, H. The necessity of walnut proteolysis based on evaluation after in vitro simulated digestion: ACE inhibition and DPPH radical-scavenging activities. Food Chem. 2020, 311, 125960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and Characterization of Antioxidant Peptides from Alcalase-Hydrolyzed Soybean (Glycine max L.) Hydrolysate and Their Cytoprotective Effects in Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Skonberg, D.I.; Myracle, A.D. Anti-Hyperglycemic Effects of Green Crab Hydrolysates Derived by Commercially Available Enzymes. Foods 2020, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, M.; Zhang, C.; Huang, F. The protective effects of dehydrocostus lactone against TNF-α-induced degeneration of extracellular matrix (ECM) in SW1353 cells. Aging 2020, 12, 17137–17149. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Anal, A.K. Optimization of extraction of functional protein hydrolysates from chicken egg shell membrane (ESM) by ultrasonic assisted extraction (UAE) and enzymatic hydrolysis. LWT-Food Sci. Technol. 2016, 69, 295–302. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Handa, C.L.; Xu, J. Effects of ultrasound pre-treatment on the structure of β-conglycinin and glycinin and the antioxidant activity of their hydrolysates. Food Chem. 2017, 218, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, K.S.; Lee, D.; Kim, D.; Lim, K.T.; Lee, K.-H.; Seonwoo, H.; Kim, J. Eggshell membrane: Review and impact on engineering. Biosyst. Eng. 2016, 151, 446–463. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, K.; Li, D.; Guyonnet, V.; Hincke, M.T.; Mine, Y. Avian Eggshell Membrane as a Novel Biomaterial: A Review. Foods 2021, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Rose-Martel, M.; Smiley, S.; Hincke, M.T. Novel identification of matrix proteins involved in calcitic biomineralization. J. Proteom. 2015, 116, 81–96. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Liu, S.; Tang, Y.; Mo, B.; Liao, H. New zonal structure and transition of the membrane to mammillae in the eggshell of chicken Gallus domesticus. J. Struct. Biol. 2018, 203, 162–169. [Google Scholar] [CrossRef]
- Chien, Y.C.; Hincke, M.T.; Vali, H.; McKee, M.D. Ultrastructural matrix-mineral relationships in avian eggshell, and effects of osteopontin on calcite growth in vitro. J. Struct. Biol. 2008, 163, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Lomholt, J.P. The development of the oxygen permeability of the avian egg shell and its membranes during incubation. J. Exp. Zool. 1976, 198, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, A.B.; Marie, P.; Nys, Y.; Hincke, M.T.; Gautron, J. Amorphous calcium carbonate controls avian eggshell mineralization: A new paradigm for understanding rapid eggshell calcification. J. Struct. Biol. 2015, 190, 291–303. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Suso, H.P.; Maqbool, A.; Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mater. Sci. Eng. C 2019, 95, 192–203. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef]
- Liu, C.M.; Wang, F.; Zhong, J.Z.; Yang, X.; Ru-Yan, D.; Zhong, Y.J. Functional properties and amino acid composition of cashew nut protein. Sci. Technol. Food Ind. 2016, 37, 88–92. [Google Scholar] [CrossRef]
- Kaweewong, K.; Garnjanagoonchorn, W.; Jirapakkul, W.; Roytrakul, S. Solubilization and identification of hen eggshell membrane proteins during different times of chicken embryo development using the proteomic approach. Protein J. 2013, 32, 297–308. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.B.; Wang, S.Y.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Zamorano-Apodaca, J.C.; Garcia-Sifuentes, C.O.; Carvajal-Millan, E.; Vallejo-Galland, B.; Scheuren-Acevedo, S.M.; Lugo-Sanchez, M.E. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chem. 2020, 331, 127350. [Google Scholar] [CrossRef]
- Mune, M.A.; Minka, S.R.; Henle, T. Investigation on antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activity of Bambara bean protein hydrolysates. Food Chem. 2018, 250, 162–169. [Google Scholar] [CrossRef]
- Piotrowicz, I.B.B.; Garces-Rimon, M.; Moreno-Fernandez, S.; Aleixandre, A.; Salas-Mellado, M.; Miguel-Castro, M. Antioxidant, Angiotensin-Converting Enzyme Inhibitory Properties and Blood-Pressure-Lowering Effect of Rice Bran Protein Hydrolysates. Foods 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Habinshuti, I.; Mu, T.H.; Zhang, M. Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein. Ultrason Sonochem. 2020, 69, 105262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qu, J.; Thakur, K.; Zhang, J.G.; Mocan, A.; Wei, Z.J. Purification and identification of an antioxidative peptide from peony (Paeonia suffruticosa Andr.) seed dreg. Food Chem. 2019, 285, 266–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.; Dai, Z. Antioxidant and Cryoprotective Effects of Bone Hydrolysates from Bighead Carp (Aristichthys nobilis) in Freeze-Thawed Fish Fillets. Foods 2021, 10, 1409. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant activity of enzymatic hydrolysates from eggshell membrane proteins and its protective capacity in human intestinal epithelial Caco-2 cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Sierra, L.; Fan, H.; Zapata, J.; Wu, J. Antioxidant peptides derived from hydrolysates of red tilapia (Oreochromis sp.) scale. LWT 2021, 146, 111631. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.; Stevens, J. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shen, Y.; Li, Y. Antioxidant Activities of Sorghum Kafirin Alcalase Hydrolysates and Membrane/Gel Filtrated Fractions. Antioxidants 2019, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrasco-Castilla, J.; Hernandez-Alvarez, A.J.; Jimenez-Martinez, C.; Jacinto-Hernandez, C.; Alaiz, M.; Giron-Calle, J.; Vioque, J.; Davila-Ortiz, G. Antioxidant and metal chelating activities of peptide fractions from phaseolin and bean protein hydrolysates. Food Chem. 2012, 135, 1789–1795. [Google Scholar] [CrossRef]
- Falade, E.O.; Mu, T.-H.; Zhang, M. Improvement of ultrasound microwave-assisted enzymatic production and high hydrostatic pressure on emulsifying, rheological and interfacial characteristics of sweet potato protein hydrolysates. Food Hydrocolloid 2021, 117, 106684. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Dong, X.; Zhang, Y.; Zhou, X.; Huang, M. Purification and identification of antioxidant peptides from duck plasma proteins. Food Chem. 2020, 319, 126534. [Google Scholar] [CrossRef]
- Pang, K.L.; Chow, Y.Y.; Leong, L.M.; Law, J.X.; Ghafar, N.A.; Soelaiman, I.N.; Chin, K.Y. Establishing SW1353 Chondrocytes as a Cellular Model of Chondrolysis. Life 2021, 11, 272. [Google Scholar] [CrossRef]
- Kim, S.; Na, J.Y.; Song, K.B.; Choi, D.S.; Kim, J.H.; Kwon, Y.B.; Kwon, J. Protective Effect of Ginsenoside Rb1 on Hydrogen Peroxide-induced Oxidative Stress in Rat Articular Chondrocytes. J. Ginseng Res. 2012, 36, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, Y.P.; Moses, J.C.; Bhardwaj, N.; Mandal, B.B. Overcoming the Dependence on Animal Models for Osteoarthritis Therapeutics—The Promises and Prospects of In Vitro Models. Adv. Healthc. Mater. 2021, 10, 2100961. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Xiang, X.W.; Du, M.; Zhang, L.F.; Cheng, N.X.; Liu, X.L.; Zheng, B.; Wen, Z.S. Protective effect of polysaccharides of sea cucumber Acaudina leucoprocta on hydrogen peroxide-induced oxidative injury in RAW264.7 cells. Int. J. Biol. Macromol. 2019, 139, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, L.; Liu, Y.; Huang, C.; Xia, W.; Zhou, H.; Zhou, Z.; Zhou, X. Luteolin Protects Chondrocytes from H2O2-Induced Oxidative Injury and Attenuates Osteoarthritis Progression by Activating AMPK-Nrf2 Signaling. Oxid. Med. Cell Longev. 2022, 2022, 5635797. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Milaras, C.; Lepetsos, P.; Dafou, D.; Potoupnis, M.; Tsiridis, E. Association of Matrix Metalloproteinase (MMP) Gene Polymorphisms with Knee Osteoarthritis: A Review of the Literature. Cureus 2021, 13, e18607. [Google Scholar] [CrossRef] [PubMed]
- Baek, A.; Jung, S.H.; Pyo, S.; Kim, S.Y.; Cho, S.R. 3’-sialyllactose protects SW1353 chondrocytic cells from interleukin-1β-induced oxidative stress and inflammation. Front. Pharmacol. 2020, 12, 609817. [Google Scholar] [CrossRef]
- Yang, J.; Song, X.; Feng, Y.; Liu, N.; Fu, Z.; Wu, J.; Li, T.; Chen, H.; Chen, J.; Chen, C.; et al. Natural ingredients-derived antioxidants attenuate H2O2-induced oxidative stress and have chondroprotective effects on human osteoarthritic chondrocytes via Keap1/Nrf2 pathway. Free Radic. Biol. Med. 2020, 152, 854–864. [Google Scholar] [CrossRef]
- Boyenle, I.D.; Divine, U.C.; Adeyemi, R.; Ayinde, K.S.; Olaoba, O.T.; Apu, C.; Du, L.; Lu, Q.; Yin, X.; Adelusi, T.I. Direct Keap1-kelch inhibitors as potential drug candidates for oxidative stress-orchestrated diseases: A review on In silico perspective. Pharmacol. Res. 2021, 167, 105577. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Hong, H.; Wang, N.; Chen, J.; Lu, S.; Zhang, H.; Zhang, X.; Bei, C. Xanthohumol suppresses inflammation in chondrocytes and ameliorates osteoarthritis in mice. Biomed. Pharmacother. 2021, 137, 111238. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]



| Raw Material | Protein Content | Ash Content | Ca Content | Saccharide |
|---|---|---|---|---|
| Hatched ESM | 92.98 ± 1.22 b | 3.62 ± 0.12 a | 0.66 ± 0.01 a | 2.1 ± 0.24 a |
| Fresh ESM | 96.00 ± 1.38 a | 2.96 ± 0.14 b | 0.45 ± 0.01 b | 1.81 ± 0.12 b |
| Amino Acid | Hatched ESM | Fresh ESM |
|---|---|---|
| Asp | 7.69 ± 0.19 | 7.68 ± 0.13 |
| Thr | 4.74 ± 0.09 b | 4.97 ± 0.02 a |
| Ser | 5.08 ± 0.08 | 5.22 ± 0.02 |
| Glu | 10.29 ± 0.23 | 10.21 ± 0.14 |
| Gly | 5.57 ± 0.13 | 5.35 ± 0.13 |
| Ala | 3.22 ± 0.06 a | 2.61 ± 0.12 b |
| * Cys | 7.34 ± 0.12 b | 9.56 ± 0.44 a |
| Val | 3.45 ± 0.06 | 3.65 ± 0.12 |
| Met | 2.55 ± 0.07 a | 2.34 ± 0.03 b |
| Ile | 2.77 ± 0.06 a | 2.62 ± 0.04 b |
| Leu | 4.39 ± 0.19 a | 3.61 ± 0.06 b |
| Tyr | 1.44 ± 0.03 a | 1.16 ± 0.02 b |
| Phe | 1.73 ± 0.03 a | 1.32 ± 0.04 b |
| His | 2.95 ± 0.05 b | 3.18 ± 0.04 a |
| Lys | 3.16 ± 0.07 a | 2.47 ± 0.03 b |
| Arg | 5.6 ± 0.12 | 5.67 ± 0.01 |
| * Pro | 6.27 ± 0.09 b | 6.64 ± 0.08 a |
| TAA | 74.57 ± 1.6 | 73.48 ± 0.99 |
| EAA | 22.8 ± 0.58 | 21.34 ± 0.81 |
| EAA/TAA | 30.57 ± 0.12 | 29.04 ± 0.71 |
| DPPH Radical Scavenging Activity (μmol TE/g) | Fe2+ Chelating Activity (%) | Fe3+ Reducing Power (A700) | |
|---|---|---|---|
| FEMH | 51.93 ± 2.47 d | 56.51 ± 1.17 e | 0.10 ± 0.01 f |
| HEMH | 153.51 ± 12.63 a | 80.11 ± 0.30 b | 0.67 ± 0.005 b |
| HEMH-I | 156.5 ± 8.97 a | 83.26 ± 1.95 a | 0.864 ± 0.003 a |
| HEMH-II | 111.94 ± 9.85 b | 80.88 ± 0.13 b | 0.655 ± 0.007 c |
| HEMH-III | 116.89 ± 8.6 b, c | 70.87 ± 0.36 c | 0.51 ± 0.002 d |
| HEMH-IV | 98.45 ± 10.91 c | 61.21 ± 0.24 d | 0.47 ± 0.000 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Ma, M.; Ahn, D.U.; Guyonnet, V.; Wang, L.; Zheng, Y.; He, Q.; Xiong, H.; Huang, X. Hatched Eggshell Membrane Can Be a Novel Source of Antioxidant Hydrolysates to Protect against H2O2-Induced Oxidative Stress in Human Chondrocytes. Antioxidants 2022, 11, 2428. https://doi.org/10.3390/antiox11122428
Zhu L, Ma M, Ahn DU, Guyonnet V, Wang L, Zheng Y, He Q, Xiong H, Huang X. Hatched Eggshell Membrane Can Be a Novel Source of Antioxidant Hydrolysates to Protect against H2O2-Induced Oxidative Stress in Human Chondrocytes. Antioxidants. 2022; 11(12):2428. https://doi.org/10.3390/antiox11122428
Chicago/Turabian StyleZhu, Lingjiao, Meihu Ma, Dong Uk Ahn, Vincent Guyonnet, Limei Wang, Yuting Zheng, Qin He, Hanguo Xiong, and Xi Huang. 2022. "Hatched Eggshell Membrane Can Be a Novel Source of Antioxidant Hydrolysates to Protect against H2O2-Induced Oxidative Stress in Human Chondrocytes" Antioxidants 11, no. 12: 2428. https://doi.org/10.3390/antiox11122428
APA StyleZhu, L., Ma, M., Ahn, D. U., Guyonnet, V., Wang, L., Zheng, Y., He, Q., Xiong, H., & Huang, X. (2022). Hatched Eggshell Membrane Can Be a Novel Source of Antioxidant Hydrolysates to Protect against H2O2-Induced Oxidative Stress in Human Chondrocytes. Antioxidants, 11(12), 2428. https://doi.org/10.3390/antiox11122428








