Oxidative Regulation of Vascular Cav1.2 Channels Triggers Vascular Dysfunction in Hypertension-Related Disorders
Abstract
1. Introduction
2. Cav1.2 in Vascular Smooth Muscle
2.1. Overview of Cav1.2
2.2. Regulation of Cav1.2
2.2.1. Regulation by Auxiliary Subunits
2.2.2. Regulation by Protein Kinases
2.2.3. Regulation by Small GTPases
2.3. Cav1.2 and Myogenic Tone
3. Roles of Cav1.2 in the Pathogenesis of Hypertension-Related Disorders
3.1. Aberrant Vascular Tone in Hypertension-Related Disorders
3.2. Dysfunction of Vascular Cav1.2 in Hypertension-Related Disorders
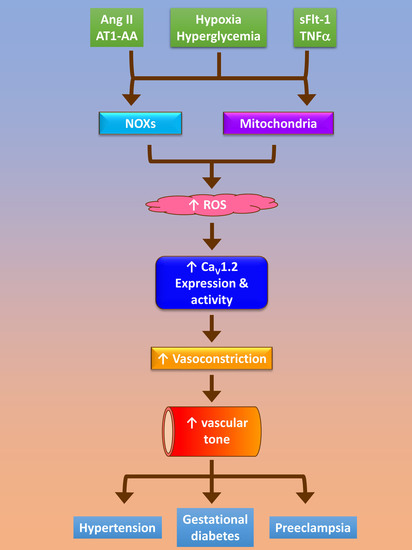
4. Roles of Reactive Oxygen Species (ROS) in the Pathogenesis of Hypertensive Disorders
4.1. Overview of ROS
4.2. Oxidative Stress as a Hallmark in Hypertension-Related Disorders
4.2.1. NOX-Derived ROS in Hypertension-Related Disorders
4.2.2. Mitochondria-Derived ROS in Hypertension-Related Disorders
4.3. A Causative Role of ROS in in Animal Models of Hypertension-Related Disorders
5. Contribution of Dysfunctional Cav1.2 Conferred by ROS to Increased Vascular Tone in Hypertension-Related Disorders
5.1. ROS and Cav1.2 Function/Expression
5.2. ROS and Myogenic Tone
6. Conclusions/Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Woll, K.A.; Van Petegem, F. Calcium-release channels: Structure and function of IP3 receptors and ryanodine receptors. Physiol. Rev. 2022, 102, 209–268. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Syed, A.U.; Prada, M.P.; Nystoriak, M.A.; Santana, L.F.; Nieves-Cintron, M.; Navedo, M.F. Calcium Channels in Vascular Smooth Muscle. Adv. Pharmacol. 2017, 78, 49–87. [Google Scholar] [CrossRef] [PubMed]
- Ottolini, M.; Hong, K.; Sonkusare, S.K. Calcium signals that determine vascular resistance. Wiley Interdiscip Rev. Syst. Biol. Med. 2019, 11, e1448. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Justin, J.; Fayol, A.; Bruno, R.M.; Khettab, H.; Boutouyrie, P. International Guidelines for Hypertension: Resemblance, Divergence and Inconsistencies. J. Clin. Med. 2022, 11, 1975. [Google Scholar] [CrossRef]
- de Boer, I.H.; Bangalore, S.; Benetos, A.; Davis, A.M.; Michos, E.D.; Muntner, P.; Rossing, P.; Zoungas, S.; Bakris, G. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 1273–1284. [Google Scholar] [CrossRef]
- Tsimihodimos, V.; Gonzalez-Villalpando, C.; Meigs, J.B.; Ferrannini, E. Hypertension and Diabetes Mellitus: Coprediction and Time Trajectories. Hypertension 2018, 71, 422–428. [Google Scholar] [CrossRef]
- Shin, D.; Bohra, C.; Kongpakpaisarn, K. Impact of the Discordance Between the American College of Cardiology/American Heart Association and American Diabetes Association Recommendations on Hypertension in Patients With Diabetes Mellitus in the United States. Hypertension 2018, 72, 256–259. [Google Scholar] [CrossRef]
- Whelton, P.K. The elusiveness of population-wide high blood pressure control. Annu. Rev. Public Health 2015, 36, 109–130. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef]
- Rimoldi, S.F.; Scherrer, U.; Messerli, F.H. Secondary arterial hypertension: When, who, and how to screen? Eur. Heart J. 2014, 35, 1245–1254. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Sakran, N.; Graham, Y.; Pintar, T.; Yang, W.; Kassir, R.; Willigendael, E.M.; Singhal, R.; Kooreman, Z.E.; Ramnarain, D.; Mahawar, K.; et al. The many faces of diabetes. Is there a need for re-classification? A narrative review. BMC Endocr. Disord. 2022, 22, 9. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Michael, S.K.; Surks, H.K.; Wang, Y.; Zhu, Y.; Blanton, R.; Jamnongjit, M.; Aronovitz, M.; Baur, W.; Ohtani, K.; Wilkerson, M.K.; et al. High blood pressure arising from a defect in vascular function. Proc. Natl. Acad. Sci. USA 2008, 105, 6702–6707. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; Dechend, R.; Poston, L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension 2004, 44, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Leandro, A.; Azul, L.; Seica, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Montezano, A.C.; Dulak-Lis, M.; Tsiropoulou, S.; Harvey, A.; Briones, A.M.; Touyz, R.M. Oxidative stress and human hypertension: Vascular mechanisms, biomarkers, and novel therapies. Can. J. Cardiol. 2015, 31, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Dolphin, A.C. Voltage-gated calcium channels and their auxiliary subunits: Physiology and pathophysiology and pharmacology. J. Physiol. 2016, 594, 5369–5390. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Kamp, T.J.; Hell, J.W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 2000, 87, 1095–1102. [Google Scholar] [CrossRef]
- Kobrinsky, E.; Tiwari, S.; Maltsev, V.A.; Harry, J.B.; Lakatta, E.; Abernethy, D.R.; Soldatov, N.M. Differential role of the alpha1C subunit tails in regulation of the Cav1.2 channel by membrane potential, beta subunits, and Ca2+ ions. J. Biol. Chem. 2005, 280, 12474–12485. [Google Scholar] [CrossRef]
- Campiglio, M.; Flucher, B.E. The role of auxiliary subunits for the functional diversity of voltage-gated calcium channels. J. Cell. Physiol. 2015, 230, 2019–2031. [Google Scholar] [CrossRef]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef]
- Mikami, A.; Imoto, K.; Tanabe, T.; Niidome, T.; Mori, Y.; Takeshima, H.; Narumiya, S.; Numa, S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature 1989, 340, 230–233. [Google Scholar] [CrossRef]
- Biel, M.; Ruth, P.; Bosse, E.; Hullin, R.; Stuhmer, W.; Flockerzi, V.; Hofmann, F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990, 269, 409–412. [Google Scholar] [CrossRef]
- Murakami, M.; Yamamura, H.; Suzuki, T.; Kang, M.G.; Ohya, S.; Murakami, A.; Miyoshi, I.; Sasano, H.; Muraki, K.; Hano, T.; et al. Modified cardiovascular L-type channels in mice lacking the voltage-dependent Ca2+ channel beta3 subunit. J. Biol. Chem. 2003, 278, 43261–43267. [Google Scholar] [CrossRef]
- Bannister, J.P.; Adebiyi, A.; Zhao, G.; Narayanan, D.; Thomas, C.M.; Feng, J.Y.; Jaggar, J.H. Smooth muscle cell alpha2delta-1 subunits are essential for vasoregulation by Cav1.2 channels. Circ. Res. 2009, 105, 948–955. [Google Scholar] [CrossRef]
- Cox, R.H.; Fromme, S. Expression of Calcium Channel Subunit Variants in Small Mesenteric Arteries of WKY and SHR. Am. J. Hypertens. 2015, 28, 1229–1239. [Google Scholar] [CrossRef]
- Kharade, S.V.; Sonkusare, S.K.; Srivastava, A.K.; Thakali, K.M.; Fletcher, T.W.; Rhee, S.W.; Rusch, N.J. The beta3 subunit contributes to vascular calcium channel upregulation and hypertension in angiotensin II-infused C57BL/6 mice. Hypertension 2013, 61, 137–142. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, M.H.; Zhang, Y.; He, L.L.; Yamada, Y.; Fitzmaurice, A.; Shen, Y.; Zhang, H.; Tong, L.; Yang, J. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature 2004, 429, 675–680. [Google Scholar] [CrossRef]
- Jay, S.D.; Sharp, A.H.; Kahl, S.D.; Vedvick, T.S.; Harpold, M.M.; Campbell, K.P. Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated delta peptides. J. Biol. Chem. 1991, 266, 3287–3293. [Google Scholar] [CrossRef]
- Cheng, X.; Pachuau, J.; Blaskova, E.; Asuncion-Chin, M.; Liu, J.; Dopico, A.M.; Jaggar, J.H. Alternative splicing of Cav1.2 channel exons in smooth muscle cells of resistance-size arteries generates currents with unique electrophysiological properties. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H680–H688. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Liu, J.; Asuncion-Chin, M.; Blaskova, E.; Bannister, J.P.; Dopico, A.M.; Jaggar, J.H. A novel Cav1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J. Biol. Chem. 2007, 282, 29211–29221. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Flockerzi, V.; Kahl, S.; Wegener, J.W. L-type Cav1.2 calcium channels: From in vitro findings to in vivo function. Physiol. Rev. 2014, 94, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liang, M.C.; Soong, T.W. Alternative Splicing of L-type Cav1.2 Calcium Channels: Implications in Cardiovascular Diseases. Genes 2017, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Puri, T.S.; Gerhardstein, B.L.; Zhao, X.L.; Ladner, M.B.; Hosey, M.M. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry 1997, 36, 9605–9615. [Google Scholar] [CrossRef] [PubMed]
- Gerhardstein, B.L.; Puri, T.S.; Chien, A.J.; Hosey, M.M. Identification of the sites phosphorylated by cyclic AMP-dependent protein kinase on the beta 2 subunit of L-type voltage-dependent calcium channels. Biochemistry 1999, 38, 10361–10370. [Google Scholar] [CrossRef]
- Bunemann, M.; Gerhardstein, B.L.; Gao, T.; Hosey, M.M. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the beta(2) subunit. J. Biol. Chem. 1999, 274, 33851–33854. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Zakharov, S.I.; Bellinger, A.M.; Mongillo, M.; Marx, S.O. Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ. Res. 2007, 101, 465–474. [Google Scholar] [CrossRef]
- Li, Y.; Yang, H.; He, T.; Zhang, L.; Liu, C. Post-Translational Modification of Cav1.2 and its Role in Neurodegenerative Diseases. Front. Pharmacol. 2021, 12, 775087. [Google Scholar] [CrossRef]
- Ishikawa, T.; Hume, J.R.; Keef, K.D. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ. Res. 1993, 73, 1128–1137. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, Z.; Sperelakis, N. Cyclic nucleotides regulate the activity of L-type calcium channels in smooth muscle cells from rat portal vein. J. Mol. Cell. Cardiol. 1997, 29, 1411–1421. [Google Scholar] [CrossRef]
- Ruiz-Velasco, V.; Zhong, J.; Hume, J.R.; Keef, K.D. Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circ. Res. 1998, 82, 557–565. [Google Scholar] [CrossRef]
- Keef, K.D.; Hume, J.R.; Zhong, J. Regulation of cardiac and smooth muscle Ca2+ channels (Cav1.2a,b) by protein kinases. Am. J. Physiol. Cell Physiol. 2001, 281, C1743–C1756. [Google Scholar] [CrossRef]
- Fusi, F.; Manetti, F.; Durante, M.; Sgaragli, G.; Saponara, S. The vasodilator papaverine stimulates L-type Ca2+ current in rat tail artery myocytes via a PKA-dependent mechanism. Vasc. Pharmacol. 2016, 76, 53–61. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Nieves-Cintron, M.; Patriarchi, T.; Buonarati, O.R.; Prada, M.P.; Morotti, S.; Grandi, E.; Fernandes, J.D.; Forbush, K.; Hofmann, F.; et al. Ser1928 phosphorylation by PKA stimulates the L-type Ca2+ channel Cav1.2 and vasoconstriction during acute hyperglycemia and diabetes. Sci. Signal. 2017, 10, eaaf9647. [Google Scholar] [CrossRef]
- Fusi, F.; Mugnai, P.; Trezza, A.; Spiga, O.; Sgaragli, G. Fine tuning by protein kinases of Cav1.2 channel current in rat tail artery myocytes. Biochem. Pharmacol. 2020, 182, 114263. [Google Scholar] [CrossRef]
- Syed, A.U.; Reddy, G.R.; Ghosh, D.; Prada, M.P.; Nystoriak, M.A.; Morotti, S.; Grandi, E.; Sirish, P.; Chiamvimonvat, N.; Hell, J.W.; et al. Adenylyl cyclase 5-generated cAMP controls cerebral vascular reactivity during diabetic hyperglycemia. J. Clin. Investig. 2019, 129, 3140–3152. [Google Scholar] [CrossRef]
- Xiong, Z.; Sperelakis, N.; Fenoglio-Preiser, C. Regulation of L-type calcium channels by cyclic nucleotides and phosphorylation in smooth muscle cells from rabbit portal vein. J. Vasc. Res. 1994, 31, 271–279. [Google Scholar] [CrossRef]
- Taguchi, K.; Ueda, M.; Kubo, T. Effects of cAMP and cGMP on L-type calcium channel currents in rat mesenteric artery cells. Jpn. J. Pharmacol. 1997, 74, 179–186. [Google Scholar] [CrossRef]
- Quignard, J.F.; Frapier, J.M.; Harricane, M.C.; Albat, B.; Nargeot, J.; Richard, S. Voltage-gated calcium channel currents in human coronary myocytes. Regulation by cyclic GMP and nitric oxide. J. Clin. Investig. 1997, 99, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhattarai, J.P.; Hwang, P.H.; Han, S.K. Nitric oxide suppresses L-type calcium currents in basilar artery smooth muscle cells in rabbits. Neurol. Res. 2013, 35, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, K.; Groschner, K. Protein kinase-C mediates dual modulation of L-type Ca2+ channels in human vascular smooth muscle. FEBS Lett. 1994, 341, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.; Koh, S.D.; Keef, K.D. Muscarinic M2 receptor stimulation of Cav1.2b requires phosphatidylinositol 3-kinase, protein kinase C, and c-Src. Circ. Res. 2004, 94, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Navedo, M.F.; Amberg, G.C.; Votaw, V.S.; Santana, L.F. Constitutively active L-type Ca2+ channels. Proc. Natl. Acad. Sci. USA 2005, 102, 11112–11117. [Google Scholar] [CrossRef]
- Cobine, C.A.; Callaghan, B.P.; Keef, K.D. Role of L-type calcium channels and PKC in active tone development in rabbit coronary artery. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3079–H3088. [Google Scholar] [CrossRef]
- Nieves-Cintron, M.; Amberg, G.C.; Navedo, M.F.; Molkentin, J.D.; Santana, L.F. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc. Natl. Acad. Sci. USA 2008, 105, 15623–15628. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, J.; Philipson, K.D.; Kotlikoff, M.I.; Blaustein, M.P.; Matteson, D.R. Activation of L-type Ca2+ channels by protein kinase C is reduced in smooth muscle-specific Na+/Ca2+ exchanger knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1484–H1491. [Google Scholar] [CrossRef]
- Weiss, S.; Keren-Raifman, T.; Oz, S.; Ben Mocha, A.; Haase, H.; Dascal, N. Modulation of distinct isoforms of L-type calcium channels by G(q)-coupled receptors in Xenopus oocytes: Antagonistic effects of Gbetagamma and protein kinase C. Channels 2012, 6, 426–437. [Google Scholar] [CrossRef]
- Gulia, J.; Navedo, M.F.; Gui, P.; Chao, J.T.; Mercado, J.L.; Santana, L.F.; Davis, M.J. Regulation of L-type calcium channel sparklet activity by c-Src and PKC-alpha. Am. J. Physiol. Cell Physiol. 2013, 305, C568–C577. [Google Scholar] [CrossRef]
- Navedo, M.F.; Nieves-Cintron, M.; Amberg, G.C.; Yuan, C.; Votaw, V.S.; Lederer, W.J.; McKnight, G.S.; Santana, L.F. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ. Res. 2008, 102, e1–e11. [Google Scholar] [CrossRef]
- Hu, X.Q.; Singh, N.; Mukhopadhyay, D.; Akbarali, H.I. Modulation of voltage-dependent Ca2+ channels in rabbit colonic smooth muscle cells by c-Src and focal adhesion kinase. J. Biol. Chem. 1998, 273, 5337–5342. [Google Scholar] [CrossRef]
- Gui, P.; Chao, J.T.; Wu, X.; Yang, Y.; Davis, G.E.; Davis, M.J. Coordinated regulation of vascular Ca2+ and K+ channels by integrin signaling. Adv. Exp. Med. Biol. 2010, 674, 69–79. [Google Scholar] [CrossRef]
- Wijetunge, S.; Hughes, A.D. pp60c-src increases voltage-operated calcium channel currents in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1995, 217, 1039–1044. [Google Scholar] [CrossRef]
- Wijetunge, S.; Hughes, A.D. Activation of endogenous c-Src or a related tyrosine kinase by intracellular (pY)EEI peptide increases voltage-operated calcium channel currents in rabbit ear artery cells. FEBS Lett. 1996, 399, 63–66. [Google Scholar] [CrossRef]
- Wijetunge, S.; Lymn, J.S.; Hughes, A.D. Effects of protein tyrosine kinase inhibitors on voltage-operated calcium channel currents in vascular smooth muscle cells and pp60(c-src) kinase activity. Br. J. Pharmacol. 2000, 129, 1347–1354. [Google Scholar] [CrossRef]
- Macrez, N.; Mironneau, C.; Carricaburu, V.; Quignard, J.F.; Babich, A.; Czupalla, C.; Nurnberg, B.; Mironneau, J. Phosphoinositide 3-kinase isoforms selectively couple receptors to vascular L-type Ca2+ channels. Circ. Res. 2001, 89, 692–699. [Google Scholar] [CrossRef]
- Pinho, J.F.; Medeiros, M.A.; Capettini, L.S.; Rezende, B.A.; Campos, P.P.; Andrade, S.P.; Cortes, S.F.; Cruz, J.S.; Lemos, V.S. Phosphatidylinositol 3-kinase-delta up-regulates L-type Ca2+ currents and increases vascular contractility in a mouse model of type 1 diabetes. Br. J. Pharmacol. 2010, 161, 1458–1471. [Google Scholar] [CrossRef]
- Le Blanc, C.; Mironneau, C.; Barbot, C.; Henaff, M.; Bondeva, T.; Wetzker, R.; Macrez, N. Regulation of vascular L-type Ca2+ channels by phosphatidylinositol 3,4,5-trisphosphate. Circ. Res. 2004, 95, 300–307. [Google Scholar] [CrossRef]
- Viard, P.; Butcher, A.J.; Halet, G.; Davies, A.; Nurnberg, B.; Heblich, F.; Dolphin, A.C. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat. Neurosci. 2004, 7, 939–946. [Google Scholar] [CrossRef]
- Catalucci, D.; Zhang, D.H.; DeSantiago, J.; Aimond, F.; Barbara, G.; Chemin, J.; Bonci, D.; Picht, E.; Rusconi, F.; Dalton, N.D.; et al. Akt regulates L-type Ca2+ channel activity by modulating Cavalpha1 protein stability. J. Cell Biol. 2009, 184, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, D.; Vecchione, C.; Mascio, G.; Esposito, G.; Cifelli, G.; Martinello, K.; Landolfi, A.; Selvetella, G.; Grieco, P.; Damato, A.; et al. PI3Kgamma inhibition reduces blood pressure by a vasorelaxant Akt/L-type calcium channel mechanism. Cardiovasc. Res. 2012, 93, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Wu, X.; Nurkiewicz, T.R.; Kawasaki, J.; Davis, G.E.; Hill, M.A.; Meininger, G.A. Integrins and mechanotransduction of the vascular myogenic response. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1427–H1433. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Davis, G.E.; Meininger, G.A.; Wilson, E.; Davis, M.J. Regulation of the L-type calcium channel by alpha 5beta 1 integrin requires signaling between focal adhesion proteins. J. Biol. Chem. 2001, 276, 30285–30292. [Google Scholar] [CrossRef] [PubMed]
- Gui, P.; Wu, X.; Ling, S.; Stotz, S.C.; Winkfein, R.J.; Wilson, E.; Davis, G.E.; Braun, A.P.; Zamponi, G.W.; Davis, M.J. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J. Biol. Chem. 2006, 281, 14015–14025. [Google Scholar] [CrossRef]
- Waitkus-Edwards, K.R.; Martinez-Lemus, L.A.; Wu, X.; Trzeciakowski, J.P.; Davis, M.J.; Davis, G.E.; Meininger, G.A. alpha(4)beta(1) Integrin activation of L-type calcium channels in vascular smooth muscle causes arteriole vasoconstriction. Circ. Res. 2002, 90, 473–480. [Google Scholar] [CrossRef]
- Correll, R.N.; Pang, C.; Niedowicz, D.M.; Finlin, B.S.; Andres, D.A. The RGK family of GTP-binding proteins: Regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell. Signal. 2008, 20, 292–300. [Google Scholar] [CrossRef][Green Version]
- Yang, T.; Colecraft, H.M. Regulation of voltage-dependent calcium channels by RGK proteins. Biochim. Biophys. Acta 2013, 1828, 1644–1654. [Google Scholar] [CrossRef][Green Version]
- Katchman, A.; Yang, L.; Zakharov, S.I.; Kushner, J.; Abrams, J.; Chen, B.X.; Liu, G.; Pitt, G.S.; Colecraft, H.M.; Marx, S.O. Proteolytic cleavage and PKA phosphorylation of alpha1C subunit are not required for adrenergic regulation of Cav1.2 in the heart. Proc. Natl. Acad. Sci. USA 2017, 114, 9194–9199. [Google Scholar] [CrossRef]
- Liu, G.; Papa, A.; Katchman, A.N.; Zakharov, S.I.; Roybal, D.; Hennessey, J.A.; Kushner, J.; Yang, L.; Chen, B.X.; Kushnir, A.; et al. Mechanism of adrenergic Cav1.2 stimulation revealed by proximity proteomics. Nature 2020, 577, 695–700. [Google Scholar] [CrossRef]
- Yang, T.; Puckerin, A.; Colecraft, H.M. Distinct RGK GTPases differentially use alpha1- and auxiliary beta-binding-dependent mechanisms to inhibit Cav1.2/Cav2.2 channels. PLoS ONE 2012, 7, e37079. [Google Scholar] [CrossRef]
- Puckerin, A.A.; Chang, D.D.; Shuja, Z.; Choudhury, P.; Scholz, J.; Colecraft, H.M. Engineering selectivity into RGK GTPase inhibition of voltage-dependent calcium channels. Proc. Natl. Acad. Sci. USA 2018, 115, 12051–12056. [Google Scholar] [CrossRef]
- Beguin, P.; Nagashima, K.; Gonoi, T.; Shibasaki, T.; Takahashi, K.; Kashima, Y.; Ozaki, N.; Geering, K.; Iwanaga, T.; Seino, S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature 2001, 411, 701–706. [Google Scholar] [CrossRef]
- Finlin, B.S.; Crump, S.M.; Satin, J.; Andres, D.A. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc. Natl. Acad. Sci. USA 2003, 100, 14469–14474. [Google Scholar] [CrossRef]
- Xu, X.; Marx, S.O.; Colecraft, H.M. Molecular mechanisms, and selective pharmacological rescue, of Rem-inhibited Cav1.2 channels in heart. Circ. Res. 2010, 107, 620–630. [Google Scholar] [CrossRef]
- Bannister, J.P.; Bulley, S.; Leo, M.D.; Kidd, M.W.; Jaggar, J.H. Rab25 influences functional Cav1.2 channel surface expression in arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 2016, 310, C885–C893. [Google Scholar] [CrossRef]
- Davis, M.J.; Hill, M.A. Signaling mechanisms underlying the vascular myogenic response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef]
- Harder, D.R. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ. Res. 1984, 55, 197–202. [Google Scholar] [CrossRef]
- Knot, H.J.; Nelson, M.T. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J. Physiol. 1998, 508 Pt 1, 199–209. [Google Scholar] [CrossRef]
- Kotecha, N.; Hill, M.A. Myogenic contraction in rat skeletal muscle arterioles: Smooth muscle membrane potential and Ca2+ signaling. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1326–H1334. [Google Scholar] [CrossRef][Green Version]
- Welsh, D.G.; Morielli, A.D.; Nelson, M.T.; Brayden, J.E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002, 90, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Waldron, B.J.; Brayden, J.E. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ. Res. 2004, 95, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Naeini, R.; Dedman, A.; Folgering, J.H.; Duprat, F.; Patel, A.; Nilius, B.; Honore, E. TRP channels and mechanosensory transduction: Insights into the arterial myogenic response. Pflügers Arch. 2008, 456, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Choi, S.K.; Lim, M.; Yeon, S.I.; Lee, Y.H. Role of endogenous ENaC and TRP channels in the myogenic response of rat posterior cerebral arteries. PLoS ONE 2013, 8, e84194. [Google Scholar] [CrossRef]
- Nemeth, Z.; Hildebrandt, E.; Ryan, M.J.; Granger, J.P.; Drummond, H.A. Pressure-induced constriction of the middle cerebral artery is abolished in TrpC6 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H42–H50. [Google Scholar] [CrossRef]
- Jackson, W.F. Myogenic Tone in Peripheral Resistance Arteries and Arterioles: The Pressure Is On! Front. Physiol. 2021, 12, 699517. [Google Scholar] [CrossRef]
- Skaik, K.; Shahzad, M. The emerging role of TRPV1 in myogenic tone. J. Physiol. 2022, 600, 2287–2288. [Google Scholar] [CrossRef]
- Nilsson, H.; Jensen, P.E.; Mulvany, M.J. Minor role for direct adrenoceptor-mediated calcium entry in rat mesenteric small arteries. J. Vasc. Res. 1994, 31, 314–321. [Google Scholar] [CrossRef]
- Wesselman, J.P.; VanBavel, E.; Pfaffendorf, M.; Spaan, J.A. Voltage-operated calcium channels are essential for the myogenic responsiveness of cannulated rat mesenteric small arteries. J. Vasc. Res. 1996, 33, 32–41. [Google Scholar] [CrossRef]
- Miller, F.J., Jr.; Dellsperger, K.C.; Gutterman, D.D. Myogenic constriction of human coronary arterioles. Am. J. Physiol. 1997, 273, H257–H264. [Google Scholar] [CrossRef]
- Potocnik, S.J.; Murphy, T.V.; Kotecha, N.; Hill, M.A. Effects of mibefradil and nifedipine on arteriolar myogenic responsiveness and intracellular Ca2+. Br. J. Pharmacol. 2000, 131, 1065–1072. [Google Scholar] [CrossRef]
- Murphy, T.V.; Spurrell, B.E.; Hill, M.A. Mechanisms underlying pervanadate-induced contraction of rat cremaster muscle arterioles. Eur. J. Pharmacol. 2002, 442, 107–114. [Google Scholar] [CrossRef]
- Ahmed, A.; Waters, C.M.; Leffler, C.W.; Jaggar, J.H. Ionic mechanisms mediating the myogenic response in newborn porcine cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2061–H2069. [Google Scholar] [CrossRef][Green Version]
- Abd El-Rahman, R.R.; Harraz, O.F.; Brett, S.E.; Anfinogenova, Y.; Mufti, R.E.; Goldman, D.; Welsh, D.G. Identification of L- and T-type Ca2+ channels in rat cerebral arteries: Role in myogenic tone development. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H58–H71. [Google Scholar] [CrossRef]
- Jackson, W.F.; Boerman, E.M. Voltage-gated Ca2+ channel activity modulates smooth muscle cell calcium waves in hamster cremaster arterioles. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H871–H878. [Google Scholar] [CrossRef]
- Moosmang, S.; Schulla, V.; Welling, A.; Feil, R.; Feil, S.; Wegener, J.W.; Hofmann, F.; Klugbauer, N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J. 2003, 22, 6027–6034. [Google Scholar] [CrossRef]
- Fernandez, J.A.; McGahon, M.K.; McGeown, J.G.; Curtis, T.M. Cav3.1 T-Type Ca2+ Channels Contribute to Myogenic Signaling in Rat Retinal Arterioles. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5125–5132. [Google Scholar] [CrossRef][Green Version]
- Chao, J.T.; Gui, P.; Zamponi, G.W.; Davis, G.E.; Davis, M.J. Spatial association of the Cav1.2 calcium channel with alpha5beta1-integrin. Am. J. Physiol. Cell Physiol. 2011, 300, C477–C489. [Google Scholar] [CrossRef]
- Mederos y Schnitzler, M.; Storch, U.; Meibers, S.; Nurwakagari, P.; Breit, A.; Essin, K.; Gollasch, M.; Gudermann, T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008, 27, 3092–3103. [Google Scholar] [CrossRef]
- Jackson, W.F.; Boerman, E.M. Regional heterogeneity in the mechanisms of myogenic tone in hamster arterioles. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H667–H675. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Ella, S.R.; Davis, M.J.; Braun, A.P. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett. 2010, 584, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Ion channels and the regulation of myogenic tone in peripheral arterioles. Curr. Top. Membr. 2020, 85, 19–58. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Calcium-Dependent Ion Channels and the Regulation of Arteriolar Myogenic Tone. Front. Physiol. 2021, 12, 770450. [Google Scholar] [CrossRef]
- Wallis, S.J.; Firth, J.; Dunn, W.R. Pressure-induced myogenic responses in human isolated cerebral resistance arteries. Stroke 1996, 27, 2287–2290, discussion 2291. [Google Scholar] [CrossRef]
- Falcone, J.C.; Davis, M.J.; Meininger, G.A. Endothelial independence of myogenic response in isolated skeletal muscle arterioles. Am. J. Physiol. 1991, 260, H130–H135. [Google Scholar] [CrossRef]
- Kuo, L.; Chilian, W.M.; Davis, M.J. Coronary arteriolar myogenic response is independent of endothelium. Circ. Res. 1990, 66, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Bagher, P.; Beleznai, T.; Kansui, Y.; Mitchell, R.; Garland, C.J.; Dora, K.A. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc. Natl. Acad. Sci. USA 2012, 109, 18174–18179. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.W.; Sullivan, M.N.; Pritchard, H.A.; Robinson, J.J.; Earley, S. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H2031–H2041. [Google Scholar] [CrossRef]
- Veerareddy, S.; Cooke, C.L.; Baker, P.N.; Davidge, S.T. Vascular adaptations to pregnancy in mice: Effects on myogenic tone. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2226–H2233. [Google Scholar] [CrossRef]
- Carnevale, D.; Facchinello, N.; Iodice, D.; Bizzotto, D.; Perrotta, M.; De Stefani, D.; Pallante, F.; Carnevale, L.; Ricciardi, F.; Cifelli, G.; et al. Loss of EMILIN-1 Enhances Arteriolar Myogenic Tone Through TGF-beta (Transforming Growth Factor-beta)-Dependent Transactivation of EGFR (Epidermal Growth Factor Receptor) and Is Relevant for Hypertension in Mice and Humans. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2484–2497. [Google Scholar] [CrossRef]
- Ren, Y.; D’Ambrosio, M.A.; Liu, R.; Pagano, P.J.; Garvin, J.L.; Carretero, O.A. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1769–H1775. [Google Scholar] [CrossRef]
- Nademi, S.; Lu, C.; Dickhout, J.G. Enhanced Myogenic Constriction in the SHR Preglomerular Vessels Is Mediated by Thromboxane A2 Synthesis. Front. Physiol. 2020, 11, 853. [Google Scholar] [CrossRef]
- Izzard, A.S.; Bund, S.J.; Heagerty, A.M. Myogenic tone in mesenteric arteries from spontaneously hypertensive rats. Am. J. Physiol. 1996, 270, H1–H6. [Google Scholar] [CrossRef]
- Linde, C.I.; Karashima, E.; Raina, H.; Zulian, A.; Wier, W.G.; Hamlyn, J.M.; Ferrari, P.; Blaustein, M.P.; Golovina, V.A. Increased arterial smooth muscle Ca2+ signaling, vasoconstriction, and myogenic reactivity in Milan hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H611–H620. [Google Scholar] [CrossRef]
- Dunn, W.R.; Wallis, S.J.; Gardiner, S.M. Remodelling and enhanced myogenic tone in cerebral resistance arteries isolated from genetically hypertensive Brattleboro rats. J. Vasc. Res. 1998, 35, 18–26. [Google Scholar] [CrossRef]
- Jarajapu, Y.P.; Knot, H.J. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1917–H1922. [Google Scholar] [CrossRef]
- Ahn, D.S.; Choi, S.K.; Kim, Y.H.; Cho, Y.E.; Shin, H.M.; Morgan, K.G.; Lee, Y.H. Enhanced stretch-induced myogenic tone in the basilar artery of spontaneously hypertensive rats. J. Vasc. Res. 2007, 44, 182–191. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Somoza, B.; Conde, M.V.; Fernandez-Alfonso, M.S.; Gonzalez, M.C.; Arribas, S.M. Hypertension increases middle cerebral artery resting tone in spontaneously hypertensive rats: Role of tonic vasoactive factor availability. Clin. Sci. 2008, 114, 651–659. [Google Scholar] [CrossRef]
- Falcone, J.C.; Granger, H.J.; Meininger, G.A. Enhanced myogenic activation in skeletal muscle arterioles from spontaneously hypertensive rats. Am. J. Physiol. 1993, 265, H1847–H1855. [Google Scholar] [CrossRef]
- Shibuya, J.; Ohyanagi, M.; Iwasaki, T. Enhanced myogenic response in resistance small arteries from spontaneously hypertensive rats: Relationship to the voltage-dependent calcium channel. Am. J. Hypertens. 1998, 11, 767–773. [Google Scholar] [CrossRef][Green Version]
- Toth, P.; Csiszar, A.; Tucsek, Z.; Sosnowska, D.; Gautam, T.; Koller, A.; Schwartzman, M.L.; Sonntag, W.E.; Ungvari, Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1698–H1708. [Google Scholar] [CrossRef]
- McCurley, A.; Pires, P.W.; Bender, S.B.; Aronovitz, M.; Zhao, M.J.; Metzger, D.; Chambon, P.; Hill, M.A.; Dorrance, A.M.; Mendelsohn, M.E.; et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat. Med. 2012, 18, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; McCurley, A.; Davel, A.P.; McCarthy, J.; Bender, S.B.; Hong, K.; Yang, Y.; Yoo, J.K.; Aronovitz, M.; Baur, W.E.; et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 2016, 1, e88942. [Google Scholar] [CrossRef] [PubMed]
- Sauve, M.; Hui, S.K.; Dinh, D.D.; Foltz, W.D.; Momen, A.; Nedospasov, S.A.; Offermanns, S.; Husain, M.; Kroetsch, J.T.; Lidington, D.; et al. Tumor Necrosis Factor/Sphingosine-1-Phosphate Signaling Augments Resistance Artery Myogenic Tone in Diabetes. Diabetes 2016, 65, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Bowe, J. Animal models for diabetes: Understanding the pathogenesis and finding new treatments. Biochem. Pharmacol. 2016, 99, 1–10. [Google Scholar] [CrossRef]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental animal models for diabetes and its related complications—A review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef]
- Bunag, R.D.; Tomita, T.; Sasaki, S. Streptozotocin diabetic rats are hypertensive despite reduced hypothalamic responsiveness. Hypertension 1982, 4, 556–565. [Google Scholar] [CrossRef]
- Bagi, Z.; Erdei, N.; Toth, A.; Li, W.; Hintze, T.H.; Koller, A.; Kaley, G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: Enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1610–1616. [Google Scholar] [CrossRef]
- Senador, D.; Kanakamedala, K.; Irigoyen, M.C.; Morris, M.; Elased, K.M. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp. Physiol. 2009, 94, 648–658. [Google Scholar] [CrossRef]
- Bhandari, U.; Kumar, V.; Khanna, N.; Panda, B.P. The effect of high-fat diet-induced obesity on cardiovascular toxicity in Wistar albino rats. Hum. Exp. Toxicol. 2011, 30, 1313–1321. [Google Scholar] [CrossRef]
- Musial, D.C.; da Silva Junior, E.D.; da Silva, R.M.; Miranda-Ferreira, R.; Lima-Landman, M.T.; Jurkiewicz, A.; Garcia, A.G.; Jurkiewicz, N.H. Increase of angiotensin-converting enzyme activity and peripheral sympathetic dysfunction could contribute to hypertension development in streptozotocin-induced diabetic rats. Diab. Vasc. Dis. Res. 2013, 10, 498–504. [Google Scholar] [CrossRef]
- Alameddine, A.; Fajloun, Z.; Bourreau, J.; Gauquelin-Koch, G.; Yuan, M.; Gauguier, D.; Derbre, S.; Ayer, A.; Custaud, M.A.; Navasiolava, N. The cardiovascular effects of salidroside in the Goto-Kakizaki diabetic rat model. J. Physiol. Pharmacol. 2015, 66, 249–257. [Google Scholar]
- Ma, Y.G.; Wang, J.W.; Bai, Y.G.; Liu, M.; Xie, M.J.; Dai, Z.J. Salidroside contributes to reducing blood pressure and alleviating cerebrovascular contractile activity in diabetic Goto-Kakizaki Rats by inhibition of L-type calcium channel in smooth muscle cells. BMC Pharmacol. Toxicol. 2017, 18, 30. [Google Scholar] [CrossRef]
- Wang, Y.W.; Yu, H.R.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Sheen, J.M.; Lin, Y.J.; Chang, K.A.; Chen, C.C.; Tsai, C.C.; et al. Maternal Obesity Related to High Fat Diet Induces Placenta Remodeling and Gut Microbiome Shaping That Are Responsible for Fetal Liver Lipid Dysmetabolism. Front. Nutr. 2021, 8, 736944. [Google Scholar] [CrossRef]
- Carrillo-Sepulveda, M.A.; Maddie, N.; Johnson, C.M.; Burke, C.; Lutz, O.; Yakoub, B.; Kramer, B.; Persand, D. Vascular hyperacetylation is associated with vascular smooth muscle dysfunction in a rat model of non-obese type 2 diabetes. Mol. Med. 2022, 28, 30. [Google Scholar] [CrossRef]
- Choi, S.K.; Kwon, Y.; Byeon, S.; Lee, Y.H. Stimulation of autophagy improves vascular function in the mesenteric arteries of type 2 diabetic mice. Exp. Physiol. 2020, 105, 192–200. [Google Scholar] [CrossRef]
- Zimmermann, P.A.; Knot, H.J.; Stevenson, A.S.; Nelson, M.T. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ. Res. 1997, 81, 996–1004. [Google Scholar] [CrossRef]
- Jarajapu, Y.P.; Guberski, D.L.; Grant, M.B.; Knot, H.J. Myogenic tone and reactivity of cerebral arteries in type II diabetic BBZDR/Wor rat. Eur. J. Pharmacol. 2008, 579, 298–307. [Google Scholar] [CrossRef]
- Ungvari, Z.; Pacher, P.; Kecskemeti, V.; Papp, G.; Szollar, L.; Koller, A. Increased myogenic tone in skeletal muscle arterioles of diabetic rats. Possible role of increased activity of smooth muscle Ca2+ channels and protein kinase C. Cardiovasc. Res. 1999, 43, 1018–1028. [Google Scholar] [CrossRef]
- Ito, I.; Jarajapu, Y.P.; Guberski, D.L.; Grant, M.B.; Knot, H.J. Myogenic tone and reactivity of rat ophthalmic artery in acute exposure to high glucose and in a type II diabetic model. Investig. Ophthalmol. Vis. Sci. 2006, 47, 683–692. [Google Scholar] [CrossRef]
- Prada, M.P.; Syed, A.U.; Buonarati, O.R.; Reddy, G.R.; Nystoriak, M.A.; Ghosh, D.; Simo, S.; Sato, D.; Sasse, K.C.; Ward, S.M.; et al. A Gs-coupled purinergic receptor boosts Ca2+ influx and vascular contractility during diabetic hyperglycemia. Elife 2019, 8, e42214. [Google Scholar] [CrossRef] [PubMed]
- Kublickiene, K.R.; Lindblom, B.; Kruger, K.; Nisell, H. Preeclampsia: Evidence for impaired shear stress-mediated nitric oxide release in uterine circulation. Am. J. Obstet. Gynecol. 2000, 183, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; Kassab, S.E.; Miller, M.T.; Abram, S.R.; Reckelhoff, J.F.; Bennett, W.A.; Granger, J.P. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001, 37, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Fushima, T.; Sekimoto, A.; Minato, T.; Ito, T.; Oe, Y.; Kisu, K.; Sato, E.; Funamoto, K.; Hayase, T.; Kimura, Y.; et al. Reduced Uterine Perfusion Pressure (RUPP) Model of Preeclampsia in Mice. PLoS ONE 2016, 11, e0155426. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.J.; Debrah, J.; Novak, J. Increased myogenic responses of resistance-sized mesenteric arteries after reduced uterine perfusion pressure in pregnant rats. Hypertens. Pregnancy 2011, 30, 45–57. [Google Scholar] [CrossRef]
- Reho, J.J.; Peck, J.; Novak, J.; Ramirez, R.J. Hypertension induced by episodic reductions in uteroplacental blood flow in gravid rat. Hypertens. Pregnancy 2011, 30, 208–220. [Google Scholar] [CrossRef]
- Reho, J.J.; Toot, J.D.; Peck, J.; Novak, J.; Yun, Y.H.; Ramirez, R.J. Increased Myogenic Reactivity of Uterine Arteries from Pregnant Rats with Reduced Uterine Perfusion Pressure. Pregnancy Hypertens. 2012, 2, 106–114. [Google Scholar] [CrossRef]
- Powell, J.S.; Gandley, R.E.; Lackner, E.; Dolinish, A.; Ouyang, Y.; Powers, R.W.; Morelli, A.E.; Hubel, C.A.; Sadovsky, Y. Small extracellular vesicles from plasma of women with preeclampsia increase myogenic tone and decrease endothelium-dependent relaxation of mouse mesenteric arteries. Pregnancy Hypertens. 2022, 28, 66–73. [Google Scholar] [CrossRef]
- Soleymanlou, N.; Jurisica, I.; Nevo, O.; Ietta, F.; Zhang, X.; Zamudio, S.; Post, M.; Caniggia, I. Molecular evidence of placental hypoxia in preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4299–4308. [Google Scholar] [CrossRef]
- Ducsay, C.A.; Goyal, R.; Pearce, W.J.; Wilson, S.; Hu, X.Q.; Zhang, L. Gestational Hypoxia and Developmental Plasticity. Physiol. Rev. 2018, 98, 1241–1334. [Google Scholar] [CrossRef]
- Tong, W.; Giussani, D.A. Preeclampsia link to gestational hypoxia. J. Dev. Orig. Health Dis. 2019, 10, 322–333. [Google Scholar] [CrossRef]
- Grant, I.D.; Giussani, D.A.; Aiken, C.E. Blood pressure and hypertensive disorders of pregnancy at high altitude: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100400. [Google Scholar] [CrossRef]
- Palmer, S.K.; Moore, L.G.; Young, D.; Cregger, B.; Berman, J.C.; Zamudio, S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 m) in Colorado. Am. J. Obstet. Gynecol. 1999, 180, 1161–1168. [Google Scholar] [CrossRef]
- Keyes, L.E.; Armaza, J.F.; Niermeyer, S.; Vargas, E.; Young, D.A.; Moore, L.G. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr. Res. 2003, 54, 20–25. [Google Scholar] [CrossRef]
- Zamudio, S. High-altitude hypoxia and preeclampsia. Front. Biosci. 2007, 12, 2967–2977. [Google Scholar] [CrossRef]
- Hu, X.Q.; Dasgupta, C.; Xiao, D.; Huang, X.; Yang, S.; Zhang, L. MicroRNA-210 Targets Ten-Eleven Translocation Methylcytosine Dioxygenase 1 and Suppresses Pregnancy-Mediated Adaptation of Large Conductance Ca2+-Activated K+ Channel Expression and Function in Ovine Uterine Arteries. Hypertension 2017, 70, 601–612. [Google Scholar] [CrossRef]
- Hu, X.Q.; Song, R.; Romero, M.; Dasgupta, C.; Min, J.; Hatcher, D.; Xiao, D.; Blood, A.; Wilson, S.M.; Zhang, L. Gestational Hypoxia Inhibits Pregnancy-Induced Upregulation of Ca2+ Sparks and Spontaneous Transient Outward Currents in Uterine Arteries Via Heightened Endoplasmic Reticulum/Oxidative Stress. Hypertension 2020, 76, 930–942. [Google Scholar] [CrossRef]
- Tong, W.; Allison, B.J.; Brain, K.L.; Patey, O.V.; Niu, Y.; Botting, K.J.; Ford, S.G.; Garrud, T.A.; Wooding, P.F.B.; Shaw, C.J.; et al. Chronic Hypoxia in Ovine Pregnancy Recapitulates Physiological and Molecular Markers of Preeclampsia in the Mother, Placenta, and Offspring. Hypertension 2022, 79, 1525–1535. [Google Scholar] [CrossRef]
- Chang, K.; Xiao, D.; Huang, X.; Longo, L.D.; Zhang, L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: Role of ERK/PKC pathway. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1840–H1849. [Google Scholar] [CrossRef]
- Hu, X.Q.; Chen, M.; Dasgupta, C.; Xiao, D.; Huang, X.; Yang, S.; Zhang, L. Chronic hypoxia upregulates DNA methyltransferase and represses large conductance Ca2+-activated K+ channel function in ovine uterine arteries. Biol. Reprod. 2017, 96, 424–434. [Google Scholar] [CrossRef]
- Pires, P.W.; Jackson, W.F.; Dorrance, A.M. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H127–H136. [Google Scholar] [CrossRef] [PubMed]
- Tarjus, A.; Belozertseva, E.; Louis, H.; El Moghrabi, S.; Labat, C.; Lacolley, P.; Jaisser, F.; Galmiche, G. Role of smooth muscle cell mineralocorticoid receptor in vascular tone. Pflügers Arch. 2015, 467, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Callera, G.E.; Varanda, W.A.; Bendhack, L.M. Ca2+ influx is increased in 2-kidney, 1-clip hypertensive rat aorta. Hypertension 2001, 38, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Ebeigbe, A.B.; Ezimokhai, M.; Aloamaka, C.P. Responses of arterial smooth muscle from normotensive and pre-eclamptic subjects to the calcium channel agonist, Bay K 8644. Res. Exp. Med. 1987, 187, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Lozinskaya, I.; Cox, R.H. Augmented contributions of voltage-gated Ca2+ channels to contractile responses in spontaneously hypertensive rat mesenteric arteries. Am. J. Hypertens. 1997, 10, 1231–1239. [Google Scholar] [CrossRef]
- Murphy, J.G.; Fleming, J.B.; Cockrell, K.L.; Granger, J.P.; Khalil, R.A. [Ca2+]i signaling in renal arterial smooth muscle cells of pregnant rat is enhanced during inhibition of NOS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R87–R99. [Google Scholar] [CrossRef]
- Pratt, P.F.; Bonnet, S.; Ludwig, L.M.; Bonnet, P.; Rusch, N.J. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension 2002, 40, 214–219. [Google Scholar] [CrossRef]
- Murphy, J.G.; Herrington, J.N.; Granger, J.P.; Khalil, R.A. Enhanced [Ca2+]i in renal arterial smooth muscle cells of pregnant rats with reduced uterine perfusion pressure. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H393–H403. [Google Scholar] [CrossRef]
- White, R.E.; Carrier, G.O. Vascular contraction induced by activation of membrane calcium ion channels is enhanced in streptozotocin-diabetes. J. Pharmacol. Exp. Ther. 1990, 253, 1057–1062. [Google Scholar]
- Chen, W.; Khalil, R.A. Differential [Ca2+]i signaling of vasoconstriction in mesenteric microvessels of normal and reduced uterine perfusion pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1962–R1972. [Google Scholar] [CrossRef]
- Aoki, K.; Asano, M. Effects of Bay K 8644 and nifedipine on femoral arteries of spontaneously hypertensive rats. Br. J. Pharmacol. 1986, 88, 221–230. [Google Scholar] [CrossRef]
- Bannister, J.P.; Bulley, S.; Narayanan, D.; Thomas-Gatewood, C.; Luzny, P.; Pachuau, J.; Jaggar, J.H. Transcriptional upregulation of alpha2delta-1 elevates arterial smooth muscle cell voltage-dependent Ca2+ channel surface expression and cerebrovascular constriction in genetic hypertension. Hypertension 2012, 60, 1006–1015. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Ye, F.; Zhang, L.; Chen, Y.; Zeng, F.; Shi, L. Epigenetic regulation of L-type voltage-gated Ca2+ channels in mesenteric arteries of aging hypertensive rats. Hypertens. Res. 2017, 40, 441–449. [Google Scholar] [CrossRef]
- Cox, R.H.; Lozinskaya, I.M. Augmented calcium currents in mesenteric artery branches of the spontaneously hypertensive rat. Hypertension 1995, 26, 1060–1064. [Google Scholar] [CrossRef]
- Ohya, Y.; Abe, I.; Fujii, K.; Takata, Y.; Fujishima, M. Voltage-dependent Ca2+ channels in resistance arteries from spontaneously hypertensive rats. Circ. Res. 1993, 73, 1090–1099. [Google Scholar] [CrossRef]
- Lozinskaya, I.M.; Cox, R.H. Effects of age on Ca2+ currents in small mesenteric artery myocytes from Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 1997, 29, 1329–1336. [Google Scholar] [CrossRef]
- Simard, J.M.; Li, X.; Tewari, K. Increase in functional Ca2+ channels in cerebral smooth muscle with renal hypertension. Circ. Res. 1998, 82, 1330–1337. [Google Scholar] [CrossRef]
- Takimoto, K.; Li, D.; Nerbonne, J.M.; Levitan, E.S. Distribution, splicing and glucocorticoid-induced expression of cardiac alpha 1C and alpha 1D voltage-gated Ca2+ channel mRNAs. J. Mol. Cell. Cardiol. 1997, 29, 3035–3042. [Google Scholar] [CrossRef]
- Obejero-Paz, C.A.; Lakshmanan, M.; Jones, S.W.; Scarpa, A. Effects of dexamethasone on L-type calcium currents in the A7r5 smooth muscle-derived cell line. FEBS Lett. 1993, 333, 73–77. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, T.; Xu, L.; Huang, C.X. Thyroid hormone increased L-type calcium channel mRNA expression and L-type calcium current of myocytes in rabbits. Biomed. Mater. Eng. 2012, 22, 49–55. [Google Scholar] [CrossRef]
- Wang, J.; Thio, S.S.; Yang, S.S.; Yu, D.; Yu, C.Y.; Wong, Y.P.; Liao, P.; Li, S.; Soong, T.W. Splice variant specific modulation of Cav1.2 calcium channel by galectin-1 regulates arterial constriction. Circ. Res. 2011, 109, 1250–1258. [Google Scholar] [CrossRef]
- Hu, Z.; Li, G.; Wang, J.W.; Chong, S.Y.; Yu, D.; Wang, X.; Soon, J.L.; Liang, M.C.; Wong, Y.P.; Huang, N.; et al. Regulation of Blood Pressure by Targeting Cav1.2-Galectin-1 Protein Interaction. Circulation 2018, 138, 1431–1445. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.G.; Zhang, Y.B.; Bai, Y.G.; Dai, Z.J.; Liang, L.; Liu, M.; Xie, M.J.; Guan, H.T. Berberine alleviates the cerebrovascular contractility in streptozotocin-induced diabetic rats through modulation of intracellular Ca2+ handling in smooth muscle cells. Cardiovasc. Diabetol. 2016, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Wilde, D.W.; Massey, K.D.; Walker, G.K.; Vollmer, A.; Grekin, R.J. High-fat diet elevates blood pressure and cerebrovascular muscle Ca2+ current. Hypertension 2000, 35, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Navedo, M.F.; Takeda, Y.; Nieves-Cintron, M.; Molkentin, J.D.; Santana, L.F. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am. J. Physiol. Cell. Physiol. 2010, 298, C211–C220. [Google Scholar] [CrossRef]
- Youm, J.B.; Park, K.S.; Jang, Y.J.; Leem, C.H. Effects of streptozotocin and unilateral nephrectomy on L-type Ca2+ channels and membrane capacitance in arteriolar smooth muscle cells. Pflugers Arch. 2015, 467, 1689–1697. [Google Scholar] [CrossRef]
- Blidner, A.G.; Rabinovich, G.A. ‘Sweetening’ pregnancy: Galectins at the fetomaternal interface. Am. J. Reprod. Immunol. 2013, 69, 369–382. [Google Scholar] [CrossRef]
- Tirado-Gonzalez, I.; Freitag, N.; Barrientos, G.; Shaikly, V.; Nagaeva, O.; Strand, M.; Kjellberg, L.; Klapp, B.F.; Mincheva-Nilsson, L.; Cohen, M.; et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol. Hum. Reprod. 2013, 19, 43–53. [Google Scholar] [CrossRef]
- Chang, K.; Xiao, D.; Huang, X.; Xue, Z.; Yang, S.; Longo, L.D.; Zhang, L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension 2010, 56, 750–757. [Google Scholar] [CrossRef][Green Version]
- Hu, X.Q.; Xiao, D.; Zhu, R.; Huang, X.; Yang, S.; Wilson, S.; Zhang, L. Pregnancy upregulates large-conductance Ca2+-activated K+ channel activity and attenuates myogenic tone in uterine arteries. Hypertension 2011, 58, 1132–1139. [Google Scholar] [CrossRef]
- Freitag, N.; Tirado-Gonzalez, I.; Barrientos, G.; Herse, F.; Thijssen, V.L.; Weedon-Fekjaer, S.M.; Schulz, H.; Wallukat, G.; Klapp, B.F.; Nevers, T.; et al. Interfering with Gal-1-mediated angiogenesis contributes to the pathogenesis of preeclampsia. Proc. Natl. Acad. Sci. USA 2013, 110, 11451–11456. [Google Scholar] [CrossRef]
- Jin, X.X.; Ying, X.; Dong, M.Y. Galectin-1 expression in the serum and placenta of pregnant women with fetal growth restriction and its significance. BMC Pregnancy Childbirth 2021, 21, 14. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; et al. On the Clinical Pharmacology of Reactive Oxygen Species. Pharmacol. Rev. 2020, 72, 801–828. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Montezano, A.C.; Burger, D.; Ceravolo, G.S.; Yusuf, H.; Montero, M.; Touyz, R.M. Novel Nox homologues in the vasculature: Focusing on Nox4 and Nox5. Clin. Sci. 2011, 120, 131–141. [Google Scholar] [CrossRef]
- Taylor, C.T. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem. J. 2008, 409, 19–26. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Rhee, S.G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Hydrogen peroxide reactivity and specificity in thiol-based cell signalling. Biochem. Soc. Trans. 2020, 48, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Das, U.N. Are free radicals involved in the pathobiology of human essential hypertension? Free Radic. Res. Commun. 1993, 19, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Olivieri, O.; Girelli, D.; Faccini, G.; Zenari, M.L.; Lombardi, S.; Corrocher, R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J. Hypertens. 1998, 16, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Koska, J.; Syrova, D.; Blazicek, P.; Marko, M.; Grna, J.D.; Kvetnansky, R.; Vigas, M. Malondialdehyde, lipofuscin and activity of antioxidant enzymes during physical exercise in patients with essential hypertension. J. Hypertens. 1999, 17, 529–535. [Google Scholar] [CrossRef]
- Redon, J.; Oliva, M.R.; Tormos, C.; Giner, V.; Chaves, J.; Iradi, A.; Saez, G.T. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 2003, 41, 1096–1101. [Google Scholar] [CrossRef]
- Simic, D.V.; Mimic-Oka, J.; Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Opacic, M.; Matic, D.; Ivanovic, B.; Simic, T. Byproducts of oxidative protein damage and antioxidant enzyme activities in plasma of patients with different degrees of essential hypertension. J. Hum. Hypertens. 2006, 20, 149–155. [Google Scholar] [CrossRef]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Bachler, J.P. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin. Sci. 2008, 114, 625–634. [Google Scholar] [CrossRef]
- Montezano, A.C.; Touyz, R.M. Reactive oxygen species, vascular Noxs, and hypertension: Focus on translational and clinical research. Antioxid. Redox Signal. 2014, 20, 164–182. [Google Scholar] [CrossRef]
- Minuz, P.; Patrignani, P.; Gaino, S.; Degan, M.; Menapace, L.; Tommasoli, R.; Seta, F.; Capone, M.L.; Tacconelli, S.; Palatresi, S.; et al. Increased oxidative stress and platelet activation in patients with hypertension and renovascular disease. Circulation 2002, 106, 2800–2805. [Google Scholar] [CrossRef]
- Will, J.C.; Ford, E.S.; Bowman, B.A. Serum vitamin C concentrations and diabetes: Findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 1999, 70, 49–52. [Google Scholar] [CrossRef]
- De Cristofaro, R.; Rocca, B.; Vitacolonna, E.; Falco, A.; Marchesani, P.; Ciabattoni, G.; Landolfi, R.; Patrono, C.; Davi, G. Lipid and protein oxidation contribute to a prothrombotic state in patients with type 2 diabetes mellitus. J. Thromb. Haemost. 2003, 1, 250–256. [Google Scholar] [CrossRef]
- Palanduz, S.; Ademoglu, E.; Gokkusu, C.; Tamer, S. Plasma antioxidants and type 2 diabetes mellitus. Res. Commun. Mol. Pathol. Pharmacol. 2001, 109, 309–318. [Google Scholar]
- Komosinska-Vassev, K.; Olczyk, K.; Olczyk, P.; Winsz-Szczotka, K. Effects of metabolic control and vascular complications on indices of oxidative stress in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2005, 68, 207–216. [Google Scholar] [CrossRef]
- Soliman, G.Z. Blood lipid peroxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med. J. 2008, 49, 129–136. [Google Scholar]
- Calabrese, V.; Cornelius, C.; Leso, V.; Trovato-Salinaro, A.; Ventimiglia, B.; Cavallaro, M.; Scuto, M.; Rizza, S.; Zanoli, L.; Neri, S.; et al. Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim. Biophys. Acta 2012, 1822, 729–736. [Google Scholar] [CrossRef]
- Lutchmansingh, F.K.; Hsu, J.W.; Bennett, F.I.; Badaloo, A.V.; McFarlane-Anderson, N.; Gordon-Strachan, G.M.; Wright-Pascoe, R.A.; Jahoor, F.; Boyne, M.S. Glutathione metabolism in type 2 diabetes and its relationship with microvascular complications and glycemia. PLoS ONE 2018, 13, e0198626. [Google Scholar] [CrossRef]
- Huang, K.; Liang, Y.; Wang, K.; Wu, J.; Luo, H.; Yi, B. Influence of circulating nesfatin-1, GSH and SOD on insulin secretion in the development of T2DM. Front. Public Health 2022, 10, 882686. [Google Scholar] [CrossRef]
- Mikhail, M.S.; Anyaegbunam, A.; Garfinkel, D.; Palan, P.R.; Basu, J.; Romney, S.L. Preeclampsia and antioxidant nutrients: Decreased plasma levels of reduced ascorbic acid, alpha-tocopherol, and beta-carotene in women with preeclampsia. Am. J. Obstet. Gynecol. 1994, 171, 150–157. [Google Scholar] [CrossRef]
- Zusterzeel, P.L.; Mulder, T.P.; Peters, W.H.; Wiseman, S.A.; Steegers, E.A. Plasma protein carbonyls in nonpregnant, healthy pregnant and preeclamptic women. Free Radic. Res. 2000, 33, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Madazli, R.; Benian, A.; Aydin, S.; Uzun, H.; Tolun, N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J. Obstet. Gynaecol. 2002, 22, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Benian, A.; Madazli, R.; Uludag, S.; Uzun, H.; Kaya, S. Plasma malondialdehyde, superoxide dismutase, sE-selectin, fibronectin, endothelin-1 and nitric oxide levels in women with preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Uzun, H.; Benian, A.; Madazli, R.; Topcuoglu, M.A.; Aydin, S.; Albayrak, M. Circulating oxidized low-density lipoprotein and paraoxonase activity in preeclampsia. Gynecol. Obstet. Investig. 2005, 60, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.M.; Pereira, N.R.; Costa, C.A.; Mann, G.E.; Cordeiro, V.S.; de Moura, R.S.; Brunini, T.M.; Mendes-Ribeiro, A.C.; Resende, A.C. L-arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsia. Hypertens. Res. 2013, 36, 783–788. [Google Scholar] [CrossRef]
- Kao, C.K.; Morton, J.S.; Quon, A.L.; Reyes, L.M.; Lopez-Jaramillo, P.; Davidge, S.T. Mechanism of vascular dysfunction due to circulating factors in women with pre-eclampsia. Clin. Sci. 2016, 130, 539–549. [Google Scholar] [CrossRef]
- Babic, G.M.; Markovic, S.D.; Varjacic, M.; Djordjevic, N.Z.; Nikolic, T.; Stojic, I.; Jakovljevic, V. Estradiol decreases blood pressure in association with redox regulation in preeclampsia. Clin. Exp. Hypertens. 2018, 40, 281–286. [Google Scholar] [CrossRef]
- Taravati, A.; Tohidi, F. Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J. Obstet. Gynecol. 2018, 57, 779–790. [Google Scholar] [CrossRef]
- Barden, A.; Beilin, L.J.; Ritchie, J.; Croft, K.D.; Walters, B.N.; Michael, C.A. Plasma and urinary 8-iso-prostane as an indicator of lipid peroxidation in pre-eclampsia and normal pregnancy. Clin. Sci. 1996, 91, 711–718. [Google Scholar] [CrossRef]
- Davi, G.; Ciabattoni, G.; Consoli, A.; Mezzetti, A.; Falco, A.; Santarone, S.; Pennese, E.; Vitacolonna, E.; Bucciarelli, T.; Costantini, F.; et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: Effects of improved metabolic control and vitamin E supplementation. Circulation 1999, 99, 224–229. [Google Scholar] [CrossRef]
- Swei, A.; Lacy, F.; DeLano, F.A.; Schmid-Schonbein, G.W. Oxidative stress in the Dahl hypertensive rat. Hypertension 1997, 30, 1628–1633. [Google Scholar] [CrossRef]
- Newaz, M.A.; Nawal, N.N. Effect of alpha-tocopherol on lipid peroxidation and total antioxidant status in spontaneously hypertensive rats. Am. J. Hypertens. 1998, 11, 1480–1485. [Google Scholar] [CrossRef]
- Schnackenberg, C.G.; Wilcox, C.S. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension 1999, 33, 424–428. [Google Scholar] [CrossRef]
- Zhan, C.D.; Sindhu, R.K.; Pang, J.; Ehdaie, A.; Vaziri, N.D. Superoxide dismutase, catalase and glutathione peroxidase in the spontaneously hypertensive rat kidney: Effect of antioxidant-rich diet. J. Hypertens. 2004, 22, 2025–2033. [Google Scholar] [CrossRef]
- Viel, E.C.; Benkirane, K.; Javeshghani, D.; Touyz, R.M.; Schiffrin, E.L. Xanthine oxidase and mitochondria contribute to vascular superoxide anion generation in DOCA-salt hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H281–H288. [Google Scholar] [CrossRef]
- Lerman, L.O.; Nath, K.A.; Rodriguez-Porcel, M.; Krier, J.D.; Schwartz, R.S.; Napoli, C.; Romero, J.C. Increased oxidative stress in experimental renovascular hypertension. Hypertension 2001, 37, 541–546. [Google Scholar] [CrossRef]
- Welch, W.J.; Mendonca, M.; Aslam, S.; Wilcox, C.S. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K,1C kidney. Hypertension 2003, 41, 692–696. [Google Scholar] [CrossRef]
- Guron, G.S.; Grimberg, E.S.; Basu, S.; Herlitz, H. Acute effects of the superoxide dismutase mimetic tempol on split kidney function in two-kidney one-clip hypertensive rats. J. Hypertens. 2006, 24, 387–394. [Google Scholar] [CrossRef]
- Vural, P.; Kabaca, G.; Firat, R.D.; Degirmencioglu, S. Administration of Selenium Decreases Lipid Peroxidation and Increases Vascular Endothelial Growth Factor in Streptozotocin Induced Diabetes Mellitus. Cell J. 2017, 19, 452–460. [Google Scholar] [CrossRef]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, X.; Liu, D.; Deng, Z.; Hu, W.; Li, Z.; Li, Y. The Hypoglycemic and Renal Protection Properties of Crocin via Oxidative Stress-Regulated NF-kappaB Signaling in db/db Mice. Front. Pharmacol. 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Bin-Jumah, M.N.; Al-Abbasi, F.A.; Nadeem, M.S.; Afzal, M.; Sayyed, N.; Kazmi, I. Fustin Ameliorates Elevated Levels of Leptin, Adiponectin, Serum TNF-alpha, and Intracellular Oxidative Free Radicals in High-Fat Diet and Streptozotocin-Induced Diabetic Rats. ACS Omega 2021, 6, 26098–26107. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.J.; Banek, C.T.; Needham, K.; Gillham, H.; Capoccia, S.; Regal, J.F.; Gilbert, J.S. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension 2013, 61, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.M.; Pinheiro, L.C.; Guimaraes, D.A.; Palei, A.C.; Sertorio, J.T.; Portella, R.L.; Tanus-Santos, J.E. Antihypertensive effects of inducible nitric oxide synthase inhibition in experimental pre-eclampsia. J. Cell. Mol. Med. 2013, 17, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Abuyassin, B.; Ayas, N.; Laher, I. Intermittent hypoxia impairs uterine artery function in pregnant mice. J. Physiol. 2019, 597, 2639–2650. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Kurz, S.; Munzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef]
- Laursen, J.B.; Rajagopalan, S.; Galis, Z.; Tarpey, M.; Freeman, B.A.; Harrison, D.G. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 1997, 95, 588–593. [Google Scholar] [CrossRef]
- Cifuentes, M.E.; Rey, F.E.; Carretero, O.A.; Pagano, P.J. Upregulation of p67(phox) and gp91(phox) in aortas from angiotensin II-infused mice. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2234–H2240. [Google Scholar] [CrossRef]
- Beswick, R.A.; Zhang, H.; Marable, D.; Catravas, J.D.; Hill, W.D.; Webb, R.C. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 2001, 37, 781–786. [Google Scholar] [CrossRef]
- Wu, R.; Millette, E.; Wu, L.; de Champlain, J. Enhanced superoxide anion formation in vascular tissues from spontaneously hypertensive and desoxycorticosterone acetate-salt hypertensive rats. J. Hypertens. 2001, 19, 741–748. [Google Scholar] [CrossRef]
- Landmesser, U.; Cai, H.; Dikalov, S.; McCann, L.; Hwang, J.; Jo, H.; Holland, S.M.; Harrison, D.G. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 2002, 40, 511–515. [Google Scholar] [CrossRef]
- Dantas, A.P.; Franco Mdo, C.; Silva-Antonialli, M.M.; Tostes, R.C.; Fortes, Z.B.; Nigro, D.; Carvalho, M.H. Gender differences in superoxide generation in microvessels of hypertensive rats: Role of NAD(P)H-oxidase. Cardiovasc. Res. 2004, 61, 22–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Griendling, K.K.; Dikalova, A.; Owens, G.K.; Taylor, W.R. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension 2005, 46, 732–737. [Google Scholar] [CrossRef]
- Dikalova, A.; Clempus, R.; Lassegue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; San Martin, A.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 2005, 112, 2668–2676. [Google Scholar] [CrossRef]
- Zhou, X.; Bohlen, H.G.; Miller, S.J.; Unthank, J.L. NAD(P)H oxidase-derived peroxide mediates elevated basal and impaired flow-induced NO production in SHR mesenteric arteries in vivo. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1008–H1016. [Google Scholar] [CrossRef]
- Tanito, M.; Nakamura, H.; Kwon, Y.W.; Teratani, A.; Masutani, H.; Shioji, K.; Kishimoto, C.; Ohira, A.; Horie, R.; Yodoi, J. Enhanced oxidative stress and impaired thioredoxin expression in spontaneously hypertensive rats. Antioxid. Redox Signal. 2004, 6, 89–97. [Google Scholar] [CrossRef]
- Chrissobolis, S.; Drummond, G.R.; Faraci, F.M.; Sobey, C.G. Chronic aldosterone administration causes Nox2-mediated increases in reactive oxygen species production and endothelial dysfunction in the cerebral circulation. J. Hypertens. 2014, 32, 1815–1821. [Google Scholar] [CrossRef]
- Rodrigues, D.; Costa, T.J.; Silva, J.F.; Neto, J.T.O.; Alves, J.V.; Fedoce, A.G.; Costa, R.M.; Tostes, R.C. Aldosterone Negatively Regulates Nrf2 Activity: An Additional Mechanism Contributing to Oxidative Stress and Vascular Dysfunction by Aldosterone. Int. J. Mol. Sci. 2021, 22, 6154. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mussa, S.; Gastaldi, D.; Sadowski, J.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Mechanisms of increased vascular superoxide production in human diabetes mellitus: Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 2002, 105, 1656–1662. [Google Scholar] [CrossRef]
- San Martin, A.; Du, P.; Dikalova, A.; Lassegue, B.; Aleman, M.; Gongora, M.C.; Brown, K.; Joseph, G.; Harrison, D.G.; Taylor, W.R.; et al. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2073–H2082. [Google Scholar] [CrossRef]
- Erdei, N.; Bagi, Z.; Edes, I.; Kaley, G.; Koller, A. H2O2 increases production of constrictor prostaglandins in smooth muscle leading to enhanced arteriolar tone in Type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H649–H656. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chettimada, S.; Ata, H.; Rawat, D.K.; Gulati, S.; Kahn, A.G.; Edwards, J.G.; Gupte, S.A. Contractile protein expression is upregulated by reactive oxygen species in aorta of Goto-Kakizaki rat. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H214–H224. [Google Scholar] [CrossRef]
- Cosentino, F.; Hishikawa, K.; Katusic, Z.S.; Luscher, T.F. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997, 96, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, X.; Gong, M.; Shi, L.; Lincoln, T.; Wang, S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic. Biol. Med. 2007, 42, 852–863. [Google Scholar] [CrossRef]
- Ding, H.; Aljofan, M.; Triggle, C.R. Oxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cells. J. Cell. Physiol. 2007, 212, 682–689. [Google Scholar] [CrossRef]
- Xi, G.; Shen, X.; Maile, L.A.; Wai, C.; Gollahon, K.; Clemmons, D.R. Hyperglycemia enhances IGF-I-stimulated Src activation via increasing Nox4-derived reactive oxygen species in a PKCzeta-dependent manner in vascular smooth muscle cells. Diabetes 2012, 61, 104–113. [Google Scholar] [CrossRef]
- Many, A.; Hubel, C.A.; Fisher, S.J.; Roberts, J.M.; Zhou, Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am. J. Pathol. 2000, 156, 321–331. [Google Scholar] [CrossRef]
- Wang, Y.; Walsh, S.W. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta 2001, 22, 206–212. [Google Scholar] [CrossRef]
- Sikkema, J.M.; van Rijn, B.B.; Franx, A.; Bruinse, H.W.; de Roos, R.; Stroes, E.S.; van Faassen, E.E. Placental superoxide is increased in pre-eclampsia. Placenta 2001, 22, 304–308. [Google Scholar] [CrossRef]
- Hnat, M.D.; Meadows, J.W.; Brockman, D.E.; Pitzer, B.; Lyall, F.; Myatt, L. Heat shock protein-70 and 4-hydroxy-2-nonenal adducts in human placental villous tissue of normotensive, preeclamptic and intrauterine growth restricted pregnancies. Am. J. Obstet. Gynecol. 2005, 193, 836–840. [Google Scholar] [CrossRef]
- Matsubara, K.; Matsubara, Y.; Hyodo, S.; Katayama, T.; Ito, M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J. Obstet. Gynaecol. Res. 2010, 36, 239–247. [Google Scholar] [CrossRef]
- Sedeek, M.; Gilbert, J.S.; LaMarca, B.B.; Sholook, M.; Chandler, D.L.; Wang, Y.; Granger, J.P. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am. J. Hypertens. 2008, 21, 1152–1156. [Google Scholar] [CrossRef]
- Parrish, M.R.; Wallace, K.; Tam Tam, K.B.; Herse, F.; Weimer, A.; Wenzel, K.; Wallukat, G.; Ray, L.F.; Arany, M.; Cockrell, K.; et al. Hypertension in response to AT1-AA: Role of reactive oxygen species in pregnancy-induced hypertension. Am. J. Hypertens. 2011, 24, 835–840. [Google Scholar] [CrossRef]
- Richter, H.G.; Camm, E.J.; Modi, B.N.; Naeem, F.; Cross, C.M.; Cindrova-Davies, T.; Spasic-Boskovic, O.; Dunster, C.; Mudway, I.S.; Kelly, F.J.; et al. Ascorbate prevents placental oxidative stress and enhances birth weight in hypoxic pregnancy in rats. J. Physiol. 2012, 590, 1377–1387. [Google Scholar] [CrossRef]
- Morton, J.S.; Abdalvand, A.; Jiang, Y.; Sawamura, T.; Uwiera, R.R.; Davidge, S.T. Lectin-like oxidized low-density lipoprotein 1 receptor in a reduced uteroplacental perfusion pressure rat model of preeclampsia. Hypertension 2012, 59, 1014–1020. [Google Scholar] [CrossRef]
- Stanley, J.L.; Andersson, I.J.; Hirt, C.J.; Moore, L.; Dilworth, M.R.; Chade, A.R.; Sibley, C.P.; Davidge, S.T.; Baker, P.N. Effect of the anti-oxidant tempol on fetal growth in a mouse model of fetal growth restriction. Biol. Reprod. 2012, 87, 1–8. [Google Scholar] [CrossRef]
- Kusinski, L.C.; Stanley, J.L.; Dilworth, M.R.; Hirt, C.J.; Andersson, I.J.; Renshall, L.J.; Baker, B.C.; Baker, P.N.; Sibley, C.P.; Wareing, M.; et al. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R86–R93. [Google Scholar] [CrossRef]
- Xiao, D.; Hu, X.Q.; Huang, X.; Zhou, J.; Wilson, S.M.; Yang, S.; Zhang, L. Chronic hypoxia during gestation enhances uterine arterial myogenic tone via heightened oxidative stress. PLoS ONE 2013, 8, e73731. [Google Scholar] [CrossRef]
- Rueda-Clausen, C.F.; Stanley, J.L.; Thambiraj, D.F.; Poudel, R.; Davidge, S.T.; Baker, P.N. Effect of prenatal hypoxia in transgenic mouse models of preeclampsia and fetal growth restriction. Reprod. Sci. 2014, 21, 492–502. [Google Scholar] [CrossRef]
- Ganguly, E.; Aljunaidy, M.M.; Kirschenman, R.; Spaans, F.; Morton, J.S.; Phillips, T.E.J.; Case, C.P.; Cooke, C.M.; Davidge, S.T. Sex-Specific Effects of Nanoparticle-Encapsulated MitoQ (nMitoQ) Delivery to the Placenta in a Rat Model of Fetal Hypoxia. Front. Physiol. 2019, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lin, Y.; Chen, L.; Zeng, F.; Zhang, L.; Huang, Y.; Huang, P.; Liao, L.; Yu, Y. Role of DRAM1 in mitophagy contributes to preeclampsia regulation in mice. Mol. Med. Rep. 2020, 22, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.S.; Levasseur, J.; Ganguly, E.; Quon, A.; Kirschenman, R.; Dyck, J.R.B.; Fraser, G.M.; Davidge, S.T. Characterisation of the Selective Reduced Uteroplacental Perfusion (sRUPP) Model of Preeclampsia. Sci. Rep. 2019, 9, 9565. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.A.; Na, H.Y.; Kim, J.E.; Park, S.; Han, K.H.; Kim, Y.J.; Suh, S.H. NADPH oxidase 2-derived superoxide downregulates endothelial KCa3.1 in preeclampsia. Free Radic. Biol. Med. 2013, 57, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Huang, X.; Xiao, D.; Zhang, L. Direct effect of chronic hypoxia in suppressing large conductance Ca2+-activated K+ channel activity in ovine uterine arteries via increasing oxidative stress. J. Physiol. 2016, 594, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Walsh, S.W. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J. Soc. Gynecol. Investig. 1996, 3, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Allahdadi, K.J.; Tostes, R.C.; Webb, R.C. Augmented S-nitrosylation contributes to impaired relaxation in angiotensin II hypertensive mouse aorta: Role of thioredoxin reductase. J. Hypertens. 2011, 29, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chai, Q.; Yu, L.; d’Uscio, L.V.; Katusic, Z.S.; He, T.; Lee, H.C. Reactive oxygen species signaling facilitates FOXO-3a/FBXO-dependent vascular BK channel beta1 subunit degradation in diabetic mice. Diabetes 2012, 61, 1860–1868. [Google Scholar] [CrossRef]
- Holland, O.J.; Cuffe, J.S.M.; Dekker Nitert, M.; Callaway, L.; Kwan Cheung, K.A.; Radenkovic, F.; Perkins, A.V. Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis. 2018, 9, 1150. [Google Scholar] [CrossRef]
- San Jose, G.; Moreno, M.U.; Olivan, S.; Beloqui, O.; Fortuno, A.; Diez, J.; Zalba, G. Functional effect of the p22phox -930A/G polymorphism on p22phox expression and NADPH oxidase activity in hypertension. Hypertension 2004, 44, 163–169. [Google Scholar] [CrossRef]
- Zalba, G.; Beaumont, F.J.; San Jose, G.; Fortuno, A.; Fortuno, M.A.; Etayo, J.C.; Diez, J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 2000, 35, 1055–1061. [Google Scholar] [CrossRef]
- Paravicini, T.M.; Chrissobolis, S.; Drummond, G.R.; Sobey, C.G. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 2004, 35, 584–589. [Google Scholar] [CrossRef]
- Lodi, F.; Cogolludo, A.; Duarte, J.; Moreno, L.; Coviello, A.; Peral De Bruno, M.; Vera, R.; Galisteo, M.; Jimenez, R.; Tamargo, J.; et al. Increased NADPH oxidase activity mediates spontaneous aortic tone in genetically hypertensive rats. Eur. J. Pharmacol. 2006, 544, 97–103. [Google Scholar] [CrossRef]
- Akasaki, T.; Ohya, Y.; Kuroda, J.; Eto, K.; Abe, I.; Sumimoto, H.; Iida, M. Increased expression of gp91phox homologues of NAD(P)H oxidase in the aortic media during chronic hypertension: Involvement of the renin-angiotensin system. Hypertens. Res. 2006, 29, 813–820. [Google Scholar] [CrossRef][Green Version]
- Wind, S.; Beuerlein, K.; Armitage, M.E.; Taye, A.; Kumar, A.H.; Janowitz, D.; Neff, C.; Shah, A.M.; Wingler, K.; Schmidt, H.H. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension 2010, 56, 490–497. [Google Scholar] [CrossRef]
- Camargo, L.L.; Harvey, A.P.; Rios, F.J.; Tsiropoulou, S.; Da Silva, R.N.O.; Cao, Z.; Graham, D.; McMaster, C.; Burchmore, R.J.; Hartley, R.C.; et al. Vascular Nox (NADPH Oxidase) Compartmentalization, Protein Hyperoxidation, and Endoplasmic Reticulum Stress Response in Hypertension. Hypertension 2018, 72, 235–246. [Google Scholar] [CrossRef]
- Beswick, R.A.; Dorrance, A.M.; Leite, R.; Webb, R.C. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 2001, 38, 1107–1111. [Google Scholar] [CrossRef]
- Callera, G.E.; Touyz, R.M.; Tostes, R.C.; Yogi, A.; He, Y.; Malkinson, S.; Schiffrin, E.L. Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension 2005, 45, 773–779. [Google Scholar] [CrossRef]
- Miyata, K.; Rahman, M.; Shokoji, T.; Nagai, Y.; Zhang, G.X.; Sun, G.P.; Kimura, S.; Yukimura, T.; Kiyomoto, H.; Kohno, M.; et al. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J. Am. Soc. Nephrol. 2005, 16, 2906–2912. [Google Scholar] [CrossRef]
- Siuda, D.; Tobias, S.; Rus, A.; Xia, N.; Forstermann, U.; Li, H. Dexamethasone upregulates Nox1 expression in vascular smooth muscle cells. Pharmacology 2014, 94, 13–20. [Google Scholar] [CrossRef]
- Gayen, J.R.; Zhang, K.; RamachandraRao, S.P.; Mahata, M.; Chen, Y.; Kim, H.S.; Naviaux, R.K.; Sharma, K.; Mahata, S.K.; O’Connor, D.T. Role of reactive oxygen species in hyperadrenergic hypertension: Biochemical, physiological, and pharmacological evidence from targeted ablation of the chromogranin a (Chga) gene. Circ. Cardiovasc. Genet. 2010, 3, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Ishizaka, N.; Rajagopalan, S.; Laursen, J.B.; Capers, Q.t.; Taylor, W.R.; Harrison, D.G.; de Leon, H.; Wilcox, J.N.; Griendling, K.K. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res. 1997, 80, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Mollnau, H.; Wendt, M.; Szocs, K.; Lassegue, B.; Schulz, E.; Oelze, M.; Li, H.; Bodenschatz, M.; August, M.; Kleschyov, A.L.; et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 2002, 90, E58–E65. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Neves, M.F.; Amiri, F.; Touyz, R.M.; Schiffrin, E.L. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 2004, 22, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 is involved in angiotensin II-mediated hypertension: A study in Nox1-deficient mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef]
- Wang, D.; Chabrashvili, T.; Borrego, L.; Aslam, S.; Umans, J.G. Angiotensin II infusion alters vascular function in mouse resistance vessels: Roles of O and endothelium. J. Vasc. Res. 2006, 43, 109–119. [Google Scholar] [CrossRef]
- Oelze, M.; Daiber, A.; Brandes, R.P.; Hortmann, M.; Wenzel, P.; Hink, U.; Schulz, E.; Mollnau, H.; von Sandersleben, A.; Kleschyov, A.L.; et al. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension 2006, 48, 677–684. [Google Scholar] [CrossRef]
- Touyz, R.M.; Chen, X.; Tabet, F.; Yao, G.; He, G.; Quinn, M.T.; Pagano, P.J.; Schiffrin, E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: Regulation by angiotensin II. Circ. Res. 2002, 90, 1205–1213. [Google Scholar] [CrossRef]
- Wendt, M.C.; Daiber, A.; Kleschyov, A.L.; Mulsch, A.; Sydow, K.; Schulz, E.; Chen, K.; Keaney, J.F., Jr.; Lassegue, B.; Walter, U.; et al. Differential effects of diabetes on the expression of the gp91phox homologues nox1 and nox4. Free Radic. Biol. Med. 2005, 39, 381–391. [Google Scholar] [CrossRef]
- Ding, H.; Hashem, M.; Triggle, C. Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: Changes in expression of NADPH oxidase subunits and eNOS. Eur. J. Pharmacol. 2007, 561, 121–128. [Google Scholar] [CrossRef]
- Wenzel, P.; Schulz, E.; Oelze, M.; Muller, J.; Schuhmacher, S.; Alhamdani, M.S.; Debrezion, J.; Hortmann, M.; Reifenberg, K.; Fleming, I.; et al. AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic. Biol. Med. 2008, 45, 619–626. [Google Scholar] [CrossRef]
- Rezende, F.; Moll, F.; Walter, M.; Helfinger, V.; Hahner, F.; Janetzko, P.; Ringel, C.; Weigert, A.; Fleming, I.; Weissmann, N.; et al. The NADPH organizers NoxO1 and p47phox are both mediators of diabetes-induced vascular dysfunction in mice. Redox Biol. 2018, 15, 12–21. [Google Scholar] [CrossRef]
- Manea, S.A.; Antonescu, M.L.; Fenyo, I.M.; Raicu, M.; Simionescu, M.; Manea, A. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol. 2018, 16, 332–343. [Google Scholar] [CrossRef]
- Kassan, M.; Choi, S.K.; Galan, M.; Lee, Y.H.; Trebak, M.; Matrougui, K. Enhanced p22phox expression impairs vascular function through p38 and ERK1/2 MAP kinase-dependent mechanisms in type 2 diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H972–H980. [Google Scholar] [CrossRef]
- Bruder-Nascimento, T.; Callera, G.E.; Montezano, A.C.; He, Y.; Antunes, T.T.; Nguyen Dinh Cat, A.; Tostes, R.C.; Touyz, R.M. Vascular injury in diabetic db/db mice is ameliorated by atorvastatin: Role of Rac1/2-sensitive Nox-dependent pathways. Clin. Sci. 2015, 128, 411–423. [Google Scholar] [CrossRef]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriello, A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: The role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef]
- Weidig, P.; McMaster, D.; Bayraktutan, U. High glucose mediates pro-oxidant and antioxidant enzyme activities in coronary endothelial cells. Diabetes Obes. Metab. 2004, 6, 432–441. [Google Scholar] [CrossRef]
- Ulker, S.; McMaster, D.; McKeown, P.P.; Bayraktutan, U. Antioxidant vitamins C and E ameliorate hyperglycaemia-induced oxidative stress in coronary endothelial cells. Diabetes Obes. Metab. 2004, 6, 442–451. [Google Scholar] [CrossRef]
- Cui, X.L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: Involvement in preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef]
- Lim, R.; Acharya, R.; Delpachitra, P.; Hobson, S.; Sobey, C.G.; Drummond, G.R.; Wallace, E.M. Activin and NADPH-oxidase in preeclampsia: Insights from in vitro and murine studies. Am. J. Obstet. Gynecol. 2015, 212, 86.e1–86.e12. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Peracoli, J.C.; Rudge, M.V.; Peracoli, M.T. Tumor necrosis factor-alpha in gestation and puerperium of women with gestational hypertension and pre-eclampsia. Am. J. Reprod. Immunol. 2007, 57, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.H.; Irani, R.A.; Blackwell, S.C.; Ramin, S.M.; Kellems, R.E.; Xia, Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: Correlation with disease severity. Hypertension 2010, 55, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Rieber-Mohn, A.B.; Sugulle, M.; Wallukat, G.; Alnaes-Katjavivi, P.; Leite Storvold, G.; Bolstad, N.; Redman, C.W.; Dechend, R.; Staff, A.C. Auto-antibodies against the angiotensin II type I receptor in women with uteroplacental acute atherosis and preeclampsia at delivery and several years postpartum. J. Reprod. Immunol. 2018, 128, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sankaralingam, S.; Xu, Y.; Sawamura, T.; Davidge, S.T. Increased lectin-like oxidized low-density lipoprotein receptor-1 expression in the maternal vasculature of women with preeclampsia: Role for peroxynitrite. Hypertension 2009, 53, 270–277. [Google Scholar] [CrossRef]
- Tam Tam, K.B.; Lamarca, B.; Arany, M.; Cockrell, K.; Fournier, L.; Murphy, S.; Martin, J.N., Jr.; Granger, J.P. Role of reactive oxygen species during hypertension in response to chronic antiangiogenic factor (sFlt-1) excess in pregnant rats. Am. J. Hypertens. 2011, 24, 110–113. [Google Scholar] [CrossRef][Green Version]
- Brewer, J.; Liu, R.; Lu, Y.; Scott, J.; Wallace, K.; Wallukat, G.; Moseley, J.; Herse, F.; Dechend, R.; Martin, J.N., Jr.; et al. Endothelin-1, oxidative stress, and endogenous angiotensin II: Mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension 2013, 62, 886–892. [Google Scholar] [CrossRef]
- Santana-Garrido, A.; Reyes-Goya, C.; Espinosa-Martin, P.; Sobrevia, L.; Beltran, L.M.; Vazquez, C.M.; Mate, A. Oxidative and Inflammatory Imbalance in Placenta and Kidney of sFlt1-Induced Early-Onset Preeclampsia Rat Model. Antioxidants 2022, 11, 1608. [Google Scholar] [CrossRef]
- Muttukrishna, S.; Knight, P.G.; Groome, N.P.; Redman, C.W.; Ledger, W.L. Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet 1997, 349, 1285–1288. [Google Scholar] [CrossRef]
- Dechend, R.; Viedt, C.; Muller, D.N.; Ugele, B.; Brandes, R.P.; Wallukat, G.; Park, J.K.; Janke, J.; Barta, P.; Theuer, J.; et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 2003, 107, 1632–1639. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.A.; Li, H.Y.; Lee, S.J.; Seok, Y.S.; Kim, T.H.; Han, K.H.; Park, M.H.; Cho, G.J.; Suh, S.H. Altered Redox State Modulates Endothelial KCa2.3 and KCa3.1 Levels in Normal Pregnancy and Preeclampsia. Antioxid. Redox Signal. 2019, 30, 505–519. [Google Scholar] [CrossRef]
- De Keulenaer, G.W.; Alexander, R.W.; Ushio-Fukai, M.; Ishizaka, N.; Griendling, K.K. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem. J. 1998, 329 Pt 3, 653–657. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X. Ox-LDL-induced LOX-1 expression in vascular smooth muscle cells: Role of reactive oxygen species. Fundam. Clin. Pharmacol. 2011, 25, 572–579. [Google Scholar] [CrossRef]
- Chen, X.P.; Xun, K.L.; Wu, Q.; Zhang, T.T.; Shi, J.S.; Du, G.H. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoprotein-induced apoptosis in human umbilical vein endothelial cells: Role of reactive oxygen species. Vascul. Pharmacol. 2007, 47, 1–9. [Google Scholar] [CrossRef]
- Tao, R.; Coleman, M.C.; Pennington, J.D.; Ozden, O.; Park, S.H.; Jiang, H.; Kim, H.S.; Flynn, C.R.; Hill, S.; Hayes McDonald, W.; et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 2010, 40, 893–904. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Billings, F.T.t.; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef]
- De Cavanagh, E.M.; Toblli, J.E.; Ferder, L.; Piotrkowski, B.; Stella, I.; Fraga, C.G.; Inserra, F. Angiotensin II blockade improves mitochondrial function in spontaneously hypertensive rats. Cell. Mol. Biol. 2005, 51, 573–578. [Google Scholar]
- Rodriguez-Iturbe, B.; Sepassi, L.; Quiroz, Y.; Ni, Z.; Wallace, D.C.; Vaziri, N.D. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J. Appl. Physiol. 2007, 102, 255–260. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef]
- Porter, G.A., Jr.; Beutner, G. Cyclophilin D, Somehow a Master Regulator of Mitochondrial Function. Biomolecules 2018, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Itani, H.A.; Dikalova, A.E.; McMaster, W.G.; Nazarewicz, R.R.; Bikineyeva, A.T.; Harrison, D.G.; Dikalov, S.I. Mitochondrial Cyclophilin D in Vascular Oxidative Stress and Hypertension. Hypertension 2016, 67, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Tsai, C.H.; Wu, X.M.; Huang, H.H.; Chen, Z.W.; Lee, B.C.; Chang, Y.Y.; Pan, C.T.; Wu, V.C.; Chou, C.H.; et al. Aldosterone Suppresses Endothelial Mitochondria through Mineralocorticoid Receptor/Mitochondrial Reactive Oxygen Species Pathway. Biomedicines 2022, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassegue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef]
- Kizhakekuttu, T.J.; Wang, J.; Dharmashankar, K.; Ying, R.; Gutterman, D.D.; Vita, J.A.; Widlansky, M.E. Adverse alterations in mitochondrial function contribute to type 2 diabetes mellitus-related endothelial dysfunction in humans. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2531–2539. [Google Scholar] [CrossRef]
- Mackenzie, R.M.; Salt, I.P.; Miller, W.H.; Logan, A.; Ibrahim, H.A.; Degasperi, A.; Dymott, J.A.; Hamilton, C.A.; Murphy, M.P.; Delles, C.; et al. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin. Sci. 2013, 124, 403–411. [Google Scholar] [CrossRef]
- Makino, A.; Scott, B.T.; Dillmann, W.H. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 2010, 53, 1783–1794. [Google Scholar] [CrossRef]
- Cho, Y.E.; Basu, A.; Dai, A.; Heldak, M.; Makino, A. Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am. J. Physiol. Cell. Physiol. 2013, 305, C1033–C1040. [Google Scholar] [CrossRef]
- Keller, A.C.; Knaub, L.A.; McClatchey, P.M.; Connon, C.A.; Bouchard, R.; Miller, M.W.; Geary, K.E.; Walker, L.A.; Klemm, D.J.; Reusch, J.E. Differential Mitochondrial Adaptation in Primary Vascular Smooth Muscle Cells from a Diabetic Rat Model. Oxid. Med. Cell. Longev. 2016, 2016, 8524267. [Google Scholar] [CrossRef]
- Merdzo, I.; Rutkai, I.; Sure, V.N.; McNulty, C.A.; Katakam, P.V.; Busija, D.W. Impaired Mitochondrial Respiration in Large Cerebral Arteries of Rats with Type 2 Diabetes. J. Vasc. Res. 2017, 54, 1–12. [Google Scholar] [CrossRef]
- Merdzo, I.; Rutkai, I.; Sure, V.; Katakam, P.V.G.; Busija, D.W. Effects of prolonged type 2 diabetes on mitochondrial function in cerebral blood vessels. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1086–H1092. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Da Ros, R.; Maier, A.; Zuodar, G.; Ceriello, A. Intermittent high glucose enhances ICAM-1, VCAM-1 and E-selectin expression in human umbilical vein endothelial cells in culture: The distinct role of protein kinase C and mitochondrial superoxide production. Atherosclerosis 2005, 183, 259–267. [Google Scholar] [CrossRef]
- Yu, T.; Sheu, S.S.; Robotham, J.L.; Yoon, Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc. Res. 2008, 79, 341–351. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, X.; Hu, Y.; Li, T.; Gao, Y.; Shi, Y.; Tang, S. Mitochondrial DNA oxidative damage triggering mitochondrial dysfunction and apoptosis in high glucose-induced HRECs. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4203–4209. [Google Scholar] [CrossRef]
- Ungvari, Z.; Labinskyy, N.; Mukhopadhyay, P.; Pinto, J.T.; Bagi, Z.; Ballabh, P.; Zhang, C.; Pacher, P.; Csiszar, A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1876–H1881. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Sun, S.; Sun, Y.; Wang, X. Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: The role of mitochondrial reactive oxygen species. Mol. Cell. Biochem. 2010, 343, 27–35. [Google Scholar] [CrossRef]
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.M.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef]
- Ren, X.; Ren, L.; Wei, Q.; Shao, H.; Chen, L.; Liu, N. Advanced glycation end-products decreases expression of endothelial nitric oxide synthase through oxidative stress in human coronary artery endothelial cells. Cardiovasc. Diabetol. 2017, 16, 52. [Google Scholar] [CrossRef]
- Yoshinaga, A.; Kajihara, N.; Kukidome, D.; Motoshima, H.; Matsumura, T.; Nishikawa, T.; Araki, E. Hypoglycemia Induces Mitochondrial Reactive Oxygen Species Production Through Increased Fatty Acid Oxidation and Promotes Retinal Vascular Permeability in Diabetic Mice. Antioxid. Redox Signal. 2021, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Yung, H.W.; Colleoni, F.; Dommett, E.; Cindrova-Davies, T.; Kingdom, J.; Murray, A.J.; Burton, G.J. Noncanonical mitochondrial unfolded protein response impairs placental oxidative phosphorylation in early-onset preeclampsia. Proc. Natl. Acad. Sci. USA 2019, 116, 18109–18118. [Google Scholar] [CrossRef] [PubMed]
- Vangrieken, P.; Al-Nasiry, S.; Bast, A.; Leermakers, P.A.; Tulen, C.B.M.; Schiffers, P.M.H.; van Schooten, F.J.; Remels, A.H.V. Placental Mitochondrial Abnormalities in Preeclampsia. Reprod. Sci. 2021, 28, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Walsh, S.W. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 1998, 19, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.; Nanri, H.; Ejima, K.; Araki, M.; Fukuda, J.; Yoshimura, K.; Toki, N.; Ikeda, M.; Kashimura, M. Enhancement of mitochondrial oxidative stress and up-regulation of antioxidant protein peroxiredoxin III/SP-22 in the mitochondria of human pre-eclamptic placentae. Placenta 2003, 24, 698–705. [Google Scholar] [CrossRef]
- Vaka, R.; Deer, E.; Cunningham, M.; McMaster, K.M.; Wallace, K.; Cornelius, D.C.; Amaral, L.M.; LaMarca, B. Characterization of Mitochondrial Bioenergetics in Preeclampsia. J. Clin. Med. 2021, 10, 5063. [Google Scholar] [CrossRef]
- Vaka, V.R.; McMaster, K.M.; Cunningham, M.W., Jr.; Ibrahim, T.; Hazlewood, R.; Usry, N.; Cornelius, D.C.; Amaral, L.M.; LaMarca, B. Role of Mitochondrial Dysfunction and Reactive Oxygen Species in Mediating Hypertension in the Reduced Uterine Perfusion Pressure Rat Model of Preeclampsia. Hypertension 2018, 72, 703–711. [Google Scholar] [CrossRef]
- Vaka, V.R.; Cunningham, M.W.; Deer, E.; Franks, M.; Ibrahim, T.; Amaral, L.M.; Usry, N.; Cornelius, D.C.; Dechend, R.; Wallukat, G.; et al. Blockade of endogenous angiotensin II type I receptor agonistic autoantibody activity improves mitochondrial reactive oxygen species and hypertension in a rat model of preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R256–R262. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, P.; Zhu, F.; Liao, J.; Wu, Y.; Hu, M.; Fu, H.; Qiao, J.; Lin, L.; Huang, B.; et al. The Potent Antioxidant MitoQ Protects Against Preeclampsia During Late Gestation but Increases the Risk of Preeclampsia When Administered in Early Pregnancy. Antioxid. Redox Signal. 2021, 34, 118–136. [Google Scholar] [CrossRef]
- Jayaram, A.; Deer, E.; Amaral, L.M.; Campbell, N.; Vaka, V.R.; Cunningham, M.; Ibrahim, T.; Cornelius, D.C.; LaMarca, B.B. The role of tumor necrosis factor in triggering activation of natural killer cell, multi-organ mitochondrial dysfunction and hypertension during pregnancy. Pregnancy Hypertens. 2021, 24, 65–72. [Google Scholar] [CrossRef]
- McCarthy, C.; Kenny, L.C. Therapeutically targeting mitochondrial redox signalling alleviates endothelial dysfunction in preeclampsia. Sci. Rep. 2016, 6, 32683. [Google Scholar] [CrossRef]
- Deer, E.; Vaka, V.R.; McMaster, K.M.; Wallace, K.; Cornelius, D.C.; Amaral, L.M.; Cunningham, M.W.; LaMarca, B. Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: A role for angiotension II type 1 autoantibodies. Am. J. Obstet. Gynecol. MFM 2021, 3, 100275. [Google Scholar] [CrossRef]
- Sanchez-Aranguren, L.C.; Espinosa-Gonzalez, C.T.; Gonzalez-Ortiz, L.M.; Sanabria-Barrera, S.M.; Riano-Medina, C.E.; Nunez, A.F.; Ahmed, A.; Vasquez-Vivar, J.; Lopez, M. Soluble Fms-Like Tyrosine Kinase-1 Alters Cellular Metabolism and Mitochondrial Bioenergetics in Preeclampsia. Front. Physiol. 2018, 9, 83. [Google Scholar] [CrossRef]
- Colleoni, F.; Padmanabhan, N.; Yung, H.W.; Watson, E.D.; Cetin, I.; Tissot van Patot, M.C.; Burton, G.J.; Murray, A.J. Suppression of mitochondrial electron transport chain function in the hypoxic human placenta: A role for miRNA-210 and protein synthesis inhibition. PLoS ONE 2013, 8, e55194. [Google Scholar] [CrossRef]
- Vangrieken, P.; Al-Nasiry, S.; Bast, A.; Leermakers, P.A.; Tulen, C.B.M.; Janssen, G.M.J.; Kaminski, I.; Geomini, I.; Lemmens, T.; Schiffers, P.M.H.; et al. Hypoxia-induced mitochondrial abnormalities in cells of the placenta. PLoS ONE 2021, 16, e0245155. [Google Scholar] [CrossRef]
- Hu, X.Q.; Song, R.; Dasgupta, C.; Romero, M.; Juarez, R.; Hanson, J.; Blood, A.B.; Wilson, S.M.; Zhang, L. MicroRNA-210-mediated mtROS confer hypoxia-induced suppression of STOCs in ovine uterine arteries. Br. J. Pharmacol. 2022, 179, 4640–4654. [Google Scholar] [CrossRef]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef]
- Togliatto, G.; Lombardo, G.; Brizzi, M.F. The Future Challenge of Reactive Oxygen Species (ROS) in Hypertension: From Bench to Bed Side. Int. J. Mol. Sci. 2017, 18, 1988. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vazquez, C.M.; Mate, A.; Sobrevia, L.; Marin, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165354. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhang, L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhang, L. Mitochondrial Dysfunction in the Pathogenesis of Preeclampsia. Curr. Hypertens. Rep. 2022, 24, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Schnackenberg, C.G.; Welch, W.J.; Wilcox, C.S. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: Role of nitric oxide. Hypertension 1998, 32, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Touyz, R.M.; Chen, X.; Schiffrin, E.L. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am. J. Hypertens. 2002, 15, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Onozato, M.; Welch, W.J.; Wilcox, C.S. Blood pressure, blood flow, and oxygenation in the clipped kidney of chronic 2-kidney, 1-clip rats: Effects of tempol and Angiotensin blockade. Hypertension 2010, 55, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Brands, M.W.; Bell, T.D.; Gibson, B. Nitric oxide may prevent hypertension early in diabetes by counteracting renal actions of superoxide. Hypertension 2004, 43, 57–63. [Google Scholar] [CrossRef]
- Onuma, S.; Nakanishi, K. Superoxide dismustase mimetic tempol decreases blood pressure by increasing renal medullary blood flow in hyperinsulinemic-hypertensive rats. Metabolism 2004, 53, 1305–1308. [Google Scholar] [CrossRef]
- Peixoto, E.B.; Pessoa, B.S.; Biswas, S.K.; Lopes de Faria, J.B. Antioxidant SOD mimetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am. J. Nephrol. 2009, 29, 309–318. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Ren, Z.; Gu, J.; Lu, Y.; Wang, X.; Zhang, L. Effects of Diabetic Hyperglycemia on Central Ang-(1-7)-Mas-R-nNOS Pathways in Spontaneously Hypertensive Rats. Cell. Physiol. Biochem. 2016, 40, 1186–1197. [Google Scholar] [CrossRef]
- Hoffmann, D.S.; Weydert, C.J.; Lazartigues, E.; Kutschke, W.J.; Kienzle, M.F.; Leach, J.E.; Sharma, J.A.; Sharma, R.V.; Davisson, R.L. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension 2008, 51, 1058–1065. [Google Scholar] [CrossRef]
- Carlstrom, M.; Brown, R.D.; Sallstrom, J.; Larsson, E.; Zilmer, M.; Zabihi, S.; Eriksson, U.J.; Persson, A.E. SOD1 deficiency causes salt sensitivity and aggravates hypertension in hydronephrosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R82–R92. [Google Scholar] [CrossRef]
- Baumer, A.T.; Kruger, C.A.; Falkenberg, J.; Freyhaus, H.T.; Rosen, R.; Fink, K.; Rosenkranz, S. The NAD(P)H oxidase inhibitor apocynin improves endothelial NO/superoxide balance and lowers effectively blood pressure in spontaneously hypertensive rats: Comparison to calcium channel blockade. Clin. Exp. Hypertens. 2007, 29, 287–299. [Google Scholar] [CrossRef]
- Unger, B.S.; Patil, B.M. Apocynin improves endothelial function and prevents the development of hypertension in fructose fed rat. Indian J. Pharmacol. 2009, 41, 208–212. [Google Scholar] [CrossRef]
- Guimaraes, D.D.; Carvalho, C.C.; Braga, V.A. Scavenging of NADPH oxidase-derived superoxide anions improves depressed baroreflex sensitivity in spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2012, 39, 373–378. [Google Scholar] [CrossRef]
- Wallace, K.; Cornelius, D.C.; Scott, J.; Heath, J.; Moseley, J.; Chatman, K.; LaMarca, B. CD4+ T cells are important mediators of oxidative stress that cause hypertension in response to placental ischemia. Hypertension 2014, 64, 1151–1158. [Google Scholar] [CrossRef]
- Perassa, L.A.; Graton, M.E.; Potje, S.R.; Troiano, J.A.; Lima, M.S.; Vale, G.T.; Pereira, A.A.; Nakamune, A.C.; Sumida, D.H.; Tirapelli, C.R.; et al. Apocynin reduces blood pressure and restores the proper function of vascular endothelium in SHR. Vasc. Pharmacol. 2016, 87, 38–48. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Gongora, M.C.; Harrison, D.G.; Lambeth, J.D.; Dikalov, S.; Griendling, K.K. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H673–H679. [Google Scholar] [CrossRef]
- Youn, J.Y.; Gao, L.; Cai, H. The p47phox- and NADPH oxidase organiser 1 (NOXO1)-dependent activation of NADPH oxidase 1 (NOX1) mediates endothelial nitric oxide synthase (eNOS) uncoupling and endothelial dysfunction in a streptozotocin-induced murine model of diabetes. Diabetologia 2012, 55, 2069–2079. [Google Scholar] [CrossRef]
- Graham, D.; Huynh, N.N.; Hamilton, C.A.; Beattie, E.; Smith, R.A.; Cocheme, H.M.; Murphy, M.P.; Dominiczak, A.F. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 2009, 54, 322–328. [Google Scholar] [CrossRef]
- Dikalova, A.; Mayorov, V.; Xiao, L.; Panov, A.; Amarnath, V.; Zagol-Ikapitte, I.; Vergeade, A.; Ao, M.; Yermalitsky, V.; Nazarewicz, R.R.; et al. Mitochondrial Isolevuglandins Contribute to Vascular Oxidative Stress and Mitochondria-Targeted Scavenger of Isolevuglandins Reduces Mitochondrial Dysfunction and Hypertension. Hypertension 2020, 76, 1980–1991. [Google Scholar] [CrossRef]
- Hool, L.C. The L-type Ca2+ channel as a potential mediator of pathology during alterations in cellular redox state. Heart Lung Circ. 2009, 18, 3–10. [Google Scholar] [CrossRef]
- Scragg, J.L.; Dallas, M.L.; Wilkinson, J.A.; Varadi, G.; Peers, C. Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J. Biol. Chem. 2008, 283, 24412–24419. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, P.; Cserne Szappanos, H.; Ingley, E.; Hool, L. Evidence for redox sensing by a human cardiac calcium channel. Sci. Rep. 2016, 6, 19067. [Google Scholar] [CrossRef] [PubMed]
- Amberg, G.C.; Earley, S.; Glapa, S.A. Local regulation of arterial L-type calcium channels by reactive oxygen species. Circ. Res. 2010, 107, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, N.L.; Amberg, G.C. Hydrogen peroxide mediates oxidant-dependent stimulation of arterial smooth muscle L-type calcium channels. Am. J. Physiol. Cell. Physiol. 2012, 302, C1382–C1393. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, N.L.; Nieves-Cintron, M.; Fresquez, A.M.; Navedo, M.F.; Amberg, G.C. Arterial Smooth Muscle Mitochondria Amplify Hydrogen Peroxide Microdomains Functionally Coupled to L-Type Calcium Channels. Circ. Res. 2015, 117, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Ochi, R.; Dhagia, V.; Lakhkar, A.; Patel, D.; Wolin, M.S.; Gupte, S.A. Rotenone-stimulated superoxide release from mitochondrial complex I acutely augments L-type Ca2+ current in A7r5 aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1118–H1128. [Google Scholar] [CrossRef] [PubMed]
- Chiamvimonvat, N.; O’Rourke, B.; Kamp, T.J.; Kallen, R.G.; Hofmann, F.; Flockerzi, V.; Marban, E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ. Res. 1995, 76, 325–334. [Google Scholar] [CrossRef]
- Fusi, F.; Saponara, S.; Gagov, H.; Sgaragli, G. 2,5-Di-t-butyl-1,4-benzohydroquinone (BHQ) inhibits vascular L-type Ca2+ channel via superoxide anion generation. Br. J. Pharmacol. 2001, 133, 988–996. [Google Scholar] [CrossRef]
- Sotnikova, R. Investigation of the mechanisms underlying H2O2-evoked contraction in the isolated rat aorta. Gen. Pharmacol. 1998, 31, 115–119. [Google Scholar] [CrossRef]
- Yang, Z.W.; Zheng, T.; Wang, J.; Zhang, A.; Altura, B.T.; Altura, B.M. Hydrogen peroxide induces contraction and raises [Ca2+]i in canine cerebral arterial smooth muscle: Participation of cellular signaling pathways. Naunyn-Schmiedeberg Arch. Pharmacol. 1999, 360, 646–653. [Google Scholar] [CrossRef]
- Tabet, F.; Savoia, C.; Schiffrin, E.L.; Touyz, R.M. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2004, 44, 200–208. [Google Scholar] [CrossRef]
- Garcia-Redondo, A.B.; Briones, A.M.; Beltran, A.E.; Alonso, M.J.; Simonsen, U.; Salaices, M. Hypertension increases contractile responses to hydrogen peroxide in resistance arteries through increased thromboxane A2, Ca2+, and superoxide anion levels. J. Pharmacol. Exp. Ther. 2009, 328, 19–27. [Google Scholar] [CrossRef]
- Garcia-Redondo, A.B.; Briones, A.M.; Avendano, M.S.; Hernanz, R.; Alonso, M.J.; Salaices, M. Losartan and tempol treatments normalize the increased response to hydrogen peroxide in resistance arteries from hypertensive rats. J. Hypertens. 2009, 27, 1814–1822. [Google Scholar] [CrossRef]
- Santiago, E.; Contreras, C.; Garcia-Sacristan, A.; Sanchez, A.; Rivera, L.; Climent, B.; Prieto, D. Signaling pathways involved in the H2O2-induced vasoconstriction of rat coronary arteries. Free Radic. Biol. Med. 2013, 60, 136–146. [Google Scholar] [CrossRef]
- Garcia-Redondo, A.B.; Briones, A.M.; Martinez-Revelles, S.; Palao, T.; Vila, L.; Alonso, M.J.; Salaices, M. c-Src, ERK1/2 and Rho kinase mediate hydrogen peroxide-induced vascular contraction in hypertension: Role of TXA2, NAD(P)H oxidase and mitochondria. J. Hypertens. 2015, 33, 77–87. [Google Scholar] [CrossRef]
- Pan, B.X.; Zhao, G.L.; Huang, X.L.; Zhao, K.S. Mobilization of intracellular calcium by peroxynitrite in arteriolar smooth muscle cells from rats. Redox Rep. 2004, 9, 49–55. [Google Scholar] [CrossRef]
- Zimmerman, M.C.; Takapoo, M.; Jagadeesha, D.K.; Stanic, B.; Banfi, B.; Bhalla, R.C.; Miller, F.J., Jr. Activation of NADPH oxidase 1 increases intracellular calcium and migration of smooth muscle cells. Hypertension 2011, 58, 446–453. [Google Scholar] [CrossRef]
- Matoba, T.; Shimokawa, H.; Nakashima, M.; Hirakawa, Y.; Mukai, Y.; Hirano, K.; Kanaide, H.; Takeshita, A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Investig. 2000, 106, 1521–1530. [Google Scholar] [CrossRef]
- Matoba, T.; Shimokawa, H.; Kubota, H.; Morikawa, K.; Fujiki, T.; Kunihiro, I.; Mukai, Y.; Hirakawa, Y.; Takeshita, A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem. Biophys. Res. Commun. 2002, 290, 909–913. [Google Scholar] [CrossRef]
- Matoba, T.; Shimokawa, H.; Morikawa, K.; Kubota, H.; Kunihiro, I.; Urakami-Harasawa, L.; Mukai, Y.; Hirakawa, Y.; Akaike, T.; Takeshita, A. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1224–1230. [Google Scholar] [CrossRef]
- Capettini, L.S.; Cortes, S.F.; Gomes, M.A.; Silva, G.A.; Pesquero, J.L.; Lopes, M.J.; Teixeira, M.M.; Lemos, V.S. Neuronal nitric oxide synthase-derived hydrogen peroxide is a major endothelium-dependent relaxing factor. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2503–H2511. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, T.H.; Inoue, R.; Cribbs, L.; Oike, M.; Ito, Y.; Holstein-Rathlou, N.H.; Jensen, L.J. The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J. Vasc. Res. 2009, 46, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Shaifta, Y.; Snetkov, V.A.; Prieto-Lloret, J.; Knock, G.A.; Smirnov, S.V.; Aaronson, P.I.; Ward, J.P. Sphingosylphosphorylcholine potentiates vasoreactivity and voltage-gated Ca2+ entry via NOX1 and reactive oxygen species. Cardiovasc. Res. 2015, 106, 121–130. [Google Scholar] [CrossRef][Green Version]
- Vogel, P.A.; Yang, X.; Moss, N.G.; Arendshorst, W.J. Superoxide enhances Ca2+ entry through L-type channels in the renal afferent arteriole. Hypertension 2015, 66, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Fernando, V.; Zheng, X.; Walia, Y.; Sharma, V.; Letson, J.; Furuta, S. S-Nitrosylation: An Emerging Paradigm of Redox Signaling. Antioxidants 2019, 8, 404. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, B.; Lim, L.J.Y.; Loh, W.Z.K.; Yu, D.; Tan, B.W.Q.; Liang, M.C.; Huang, Z.; Leo, C.H.; Huang, H.; et al. S-Nitrosylation-Mediated Reduction of Cav1.2 Surface Expression and Open Probability Underlies Attenuated Vasoconstriction Induced by Nitric Oxide. Hypertension 2022, 79, 2854–2866. [Google Scholar] [CrossRef]
- Poteser, M.; Romanin, C.; Schreibmayer, W.; Mayer, B.; Groschner, K. S-nitrosation controls gating and conductance of the alpha 1 subunit of class C L-type Ca2+ channels. J. Biol. Chem. 2001, 276, 14797–14803. [Google Scholar] [CrossRef]
- Schulz, E.; Gori, T.; Munzel, T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Kaludercic, N.; Deshwal, S.; Di Lisa, F. Reactive oxygen species and redox compartmentalization. Front. Physiol. 2014, 5, 285. [Google Scholar] [CrossRef]
- Gopalakrishna, R.; Anderson, W.B. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc. Natl. Acad. Sci. USA 1989, 86, 6758–6762. [Google Scholar] [CrossRef]
- Cosentino-Gomes, D.; Rocco-Machado, N.; Meyer-Fernandes, J.R. Cell signaling through protein kinase C oxidation and activation. Int. J. Mol. Sci. 2012, 13, 10697–10721. [Google Scholar] [CrossRef]
- Chakraborti, S.; Chakraborti, T. Down-regulation of protein kinase C attenuates the oxidant hydrogen peroxide-mediated activation of phospholipase A2 in pulmonary vascular smooth muscle cells. Cell. Signal. 1995, 7, 75–83. [Google Scholar] [CrossRef]
- Li, P.F.; Maasch, C.; Haller, H.; Dietz, R.; von Harsdorf, R. Requirement for protein kinase C in reactive oxygen species-induced apoptosis of vascular smooth muscle cells. Circulation 1999, 100, 967–973. [Google Scholar] [CrossRef]
- Steinberg, S.F. Mechanisms for redox-regulation of protein kinase C. Front. Pharmacol. 2015, 6, 128. [Google Scholar] [CrossRef]
- Sato, H.; Sato, M.; Kanai, H.; Uchiyama, T.; Iso, T.; Ohyama, Y.; Sakamoto, H.; Tamura, J.; Nagai, R.; Kurabayashi, M. Mitochondrial reactive oxygen species and c-Src play a critical role in hypoxic response in vascular smooth muscle cells. Cardiovasc. Res. 2005, 67, 714–722. [Google Scholar] [CrossRef]
- Thakali, K.; Davenport, L.; Fink, G.D.; Watts, S.W. Cyclooxygenase, p38 mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase MAPK, Rho kinase, and Src mediate hydrogen peroxide-induced contraction of rat thoracic aorta and vena cava. J. Pharmacol. Exp. Ther. 2007, 320, 236–243. [Google Scholar] [CrossRef]
- Park, H.J.; Shin, K.C.; Yoou, S.K.; Kang, M.; Kim, J.G.; Sung, D.J.; Yu, W.; Lee, Y.; Kim, S.H.; Bae, Y.M.; et al. Hydrogen peroxide constricts rat arteries by activating Na+-permeable and Ca2+-permeable cation channels. Free Radic. Res. 2019, 53, 94–103. [Google Scholar] [CrossRef]
- Zuhlke, R.D.; Pitt, G.S.; Deisseroth, K.; Tsien, R.W.; Reuter, H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 1999, 399, 159–162. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, J.; Minobe, E.; Shimoara, S.; Hao, L.; Kameyama, M. Regulation of the Cav1.2 cardiac channel by redox via modulation of CaM interaction with the channel. J. Pharmacol. Sci. 2015, 128, 137–143. [Google Scholar] [CrossRef]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef]
- Liu, H.; Colavitti, R.; Rovira, I.I.; Finkel, T. Redox-dependent transcriptional regulation. Circ. Res. 2005, 97, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Pang, L.; Palade, P. Angiotensin II causes endothelial-dependent increase in expression of Cav1.2 protein in cultured arteries. Eur. J. Pharmacol. 2008, 599, 117–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Pang, L.; Palade, P. Angiotensin II upregulates Cav1.2 protein expression in cultured arteries via endothelial H2O2 production. J. Vasc. Res. 2011, 48, 67–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsai, C.T.; Wang, D.L.; Chen, W.P.; Hwang, J.J.; Hsieh, C.S.; Hsu, K.L.; Tseng, C.D.; Lai, L.P.; Tseng, Y.Z.; Chiang, F.T.; et al. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ. Res. 2007, 100, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, L.; Horacek, V.; Vaneckova, I.; Hubacek, J.A.; Oliverio, M.I.; Coffman, T.M.; Navar, L.G. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension 2002, 40, 735–741. [Google Scholar] [CrossRef]
- Cervenka, L.; Vaneckova, I.; Huskova, Z.; Vanourkova, Z.; Erbanova, M.; Thumova, M.; Skaroupkova, P.; Opocensky, M.; Maly, J.; Chabova, V.C.; et al. Pivotal role of angiotensin II receptor subtype 1A in the development of two-kidney, one-clip hypertension: Study in angiotensin II receptor subtype 1A knockout mice. J. Hypertens. 2008, 26, 1379–1389. [Google Scholar] [CrossRef]
- Isidori, A.M.; Graziadio, C.; Paragliola, R.M.; Cozzolino, A.; Ambrogio, A.G.; Colao, A.; Corsello, S.M.; Pivonello, R.; Group, A.B.C.S. The hypertension of Cushing’s syndrome: Controversies in the pathophysiology and focus on cardiovascular complications. J. Hypertens. 2015, 33, 44–60. [Google Scholar] [CrossRef]
- Barbot, M.; Ceccato, F.; Scaroni, C. The Pathophysiology and Treatment of Hypertension in Patients With Cushing’s Syndrome. Front. Endocrinol. 2019, 10, 321. [Google Scholar] [CrossRef]
- Schewe, J.; Seidel, E.; Forslund, S.; Marko, L.; Peters, J.; Muller, D.N.; Fahlke, C.; Stolting, G.; Scholl, U. Elevated aldosterone and blood pressure in a mouse model of familial hyperaldosteronism with ClC-2 mutation. Nat. Commun. 2019, 10, 5155. [Google Scholar] [CrossRef]
- Narayanan, D.; Xi, Q.; Pfeffer, L.M.; Jaggar, J.H. Mitochondria control functional Cav1.2 expression in smooth muscle cells of cerebral arteries. Circ. Res. 2010, 107, 631–641. [Google Scholar] [CrossRef]
- Nowicki, P.T.; Flavahan, S.; Hassanain, H.; Mitra, S.; Holland, S.; Goldschmidt-Clermont, P.J.; Flavahan, N.A. Redox signaling of the arteriolar myogenic response. Circ. Res. 2001, 89, 114–116. [Google Scholar] [CrossRef]
- Cseko, C.; Bagi, Z.; Koller, A. Biphasic effect of hydrogen peroxide on skeletal muscle arteriolar tone via activation of endothelial and smooth muscle signaling pathways. J. Appl. Physiol. 2004, 97, 1130–1137. [Google Scholar] [CrossRef]
- Li, L.; Lai, E.Y.; Wellstein, A.; Welch, W.J.; Wilcox, C.S. Differential effects of superoxide and hydrogen peroxide on myogenic signaling, membrane potential, and contractions of mouse renal afferent arterioles. Am. J. Physiol. Renal. Physiol. 2016, 310, F1197–F1205. [Google Scholar] [CrossRef]
- Wagenfeld, L.; von Domarus, F.; Weiss, S.; Klemm, M.; Richard, G.; Zeitz, O. The effect of reactive oxygen species on the myogenic tone of rat ophthalmic arteries with and without endothelium. Graefe Arch. Clin. Exp. Ophthalmol. 2013, 251, 2339–2344. [Google Scholar] [CrossRef]
- Veerareddy, S.; Cooke, C.L.; Baker, P.N.; Davidge, S.T. Gender differences in myogenic tone in superoxide dismutase knockout mouse: Animal model of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H40–H45. [Google Scholar] [CrossRef][Green Version]
- Ungvari, Z.; Csiszar, A.; Huang, A.; Kaminski, P.M.; Wolin, M.S.; Koller, A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 2003, 108, 1253–1258. [Google Scholar] [CrossRef]
- Lai, E.Y.; Wellstein, A.; Welch, W.J.; Wilcox, C.S. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension 2011, 58, 650–656. [Google Scholar] [CrossRef]
- Lai, E.Y.; Solis, G.; Luo, Z.; Carlstrom, M.; Sandberg, K.; Holland, S.; Wellstein, A.; Welch, W.J.; Wilcox, C.S. p47(phox) is required for afferent arteriolar contractile responses to angiotensin II and perfusion pressure in mice. Hypertension 2012, 59, 415–420. [Google Scholar] [CrossRef]
- Kendrick, D.J.; Mishra, R.C.; John, C.M.; Zhu, H.L.; Braun, A.P. Effects of Pharmacological Inhibitors of NADPH Oxidase on Myogenic Contractility and Evoked Vasoactive Responses in Rat Resistance Arteries. Front. Physiol. 2021, 12, 752366. [Google Scholar] [CrossRef]
- Mironova, G.Y.; Mazumdar, N.; Hashad, A.M.; El-Lakany, M.A.; Welsh, D.G. Defining a role of NADPH oxidase in myogenic tone development. Microcirculation 2022, 29, e12756. [Google Scholar] [CrossRef]
- Gebremedhin, D.; Terashvili, M.; Wickramasekera, N.; Zhang, D.X.; Rau, N.; Miura, H.; Harder, D.R. Redox signaling via oxidative inactivation of PTEN modulates pressure-dependent myogenic tone in rat middle cerebral arteries. PLoS ONE 2013, 8, e68498. [Google Scholar] [CrossRef] [PubMed]
- Frisbee, J.C.; Maier, K.G.; Stepp, D.W. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2160–H2168. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.A.; Sylvester, F.A.; Frisbee, J.C. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R522–R530. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.T.; Goodwill, A.G.; Stanley, S.C.; Frisbee, J.C. Differential impact of dilator stimuli on increased myogenic activation of cerebral and skeletal muscle resistance arterioles in obese zucker rats. Microcirculation 2013, 20, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, G.V.; Sundaresan, N.R.; Gupta, M.P.; White, C. Defective Nrf2-dependent redox signalling contributes to microvascular dysfunction in type 2 diabetes. Cardiovasc. Res. 2013, 100, 143–150. [Google Scholar] [CrossRef]
- Springo, Z.; Tarantini, S.; Toth, P.; Tucsek, Z.; Koller, A.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. Aging Exacerbates Pressure-Induced Mitochondrial Oxidative Stress in Mouse Cerebral Arteries. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1355–1359. [Google Scholar] [CrossRef]
- Spurrell, B.E.; Murphy, T.V.; Hill, M.A. Tyrosine phosphorylation modulates arteriolar tone but is not fundamental to myogenic response. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H373–H382. [Google Scholar] [CrossRef]
- Murphy, T.V.; Spurrell, B.E.; Hill, M.A. Tyrosine phosphorylation following alterations in arteriolar intraluminal pressure and wall tension. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1047–H1056. [Google Scholar] [CrossRef]
- Xiao, D.; Buchholz, J.N.; Zhang, L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: Role of PKC/ERK pathway. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2337–H2343. [Google Scholar] [CrossRef]
- Osol, G.; Laher, I.; Cipolla, M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ. Res. 1991, 68, 359–367. [Google Scholar] [CrossRef]
- Hill, M.A.; Falcone, J.C.; Meininger, G.A. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am. J. Physiol. 1990, 259, H1586–H1594. [Google Scholar] [CrossRef]
- Kizub, I.V.; Pavlova, O.O.; Johnson, C.D.; Soloviev, A.I.; Zholos, A.V. Rho kinase and protein kinase C involvement in vascular smooth muscle myofilament calcium sensitization in arteries from diabetic rats. Br. J. Pharmacol. 2010, 159, 1724–1731. [Google Scholar] [CrossRef]
- Novokhatska, T.; Tishkin, S.; Dosenko, V.; Boldyriev, A.; Ivanova, I.; Strielkov, I.; Soloviev, A. Correction of vascular hypercontractility in spontaneously hypertensive rats using shRNAs-induced delta protein kinase C gene silencing. Eur. J. Pharmacol. 2013, 718, 401–407. [Google Scholar] [CrossRef]
- Csato, V.; Peto, A.; Koller, A.; Edes, I.; Toth, A.; Papp, Z. Hydrogen peroxide elicits constriction of skeletal muscle arterioles by activating the arachidonic acid pathway. PLoS ONE 2014, 9, e103858. [Google Scholar] [CrossRef]
- Shaikh, A. A Practical Approach to Hypertension Management in Diabetes. Diabetes Ther. 2017, 8, 981–989. [Google Scholar] [CrossRef]
- Tocci, G.; Desideri, G.; Roca, E.; Calcullo, C.; Crippa, M.; De Luca, N.; Gaudio, G.V.; Lonati, L.M.; Orselli, L.; Scuteri, A.; et al. How to Improve Effectiveness and Adherence to Antihypertensive Drug Therapy: Central Role of Dihydropyridinic Calcium Channel Blockers in Hypertension. High Blood Press. Cardiovasc. Prev. 2018, 25, 25–34. [Google Scholar] [CrossRef]
- Odigboegwu, O.; Pan, L.J.; Chatterjee, P. Use of Antihypertensive Drugs During Preeclampsia. Front. Cardiovasc. Med. 2018, 5, 50. [Google Scholar] [CrossRef]
- Ojha, U.; Ruddaraju, S.; Sabapathy, N.; Ravindran, V.; Worapongsatitaya, P.; Haq, J.; Mohammed, R.; Patel, V. Current and Emerging Classes of Pharmacological Agents for the Management of Hypertension. Am. J. Cardiovasc. Drugs 2022, 22, 271–285. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drugs Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]



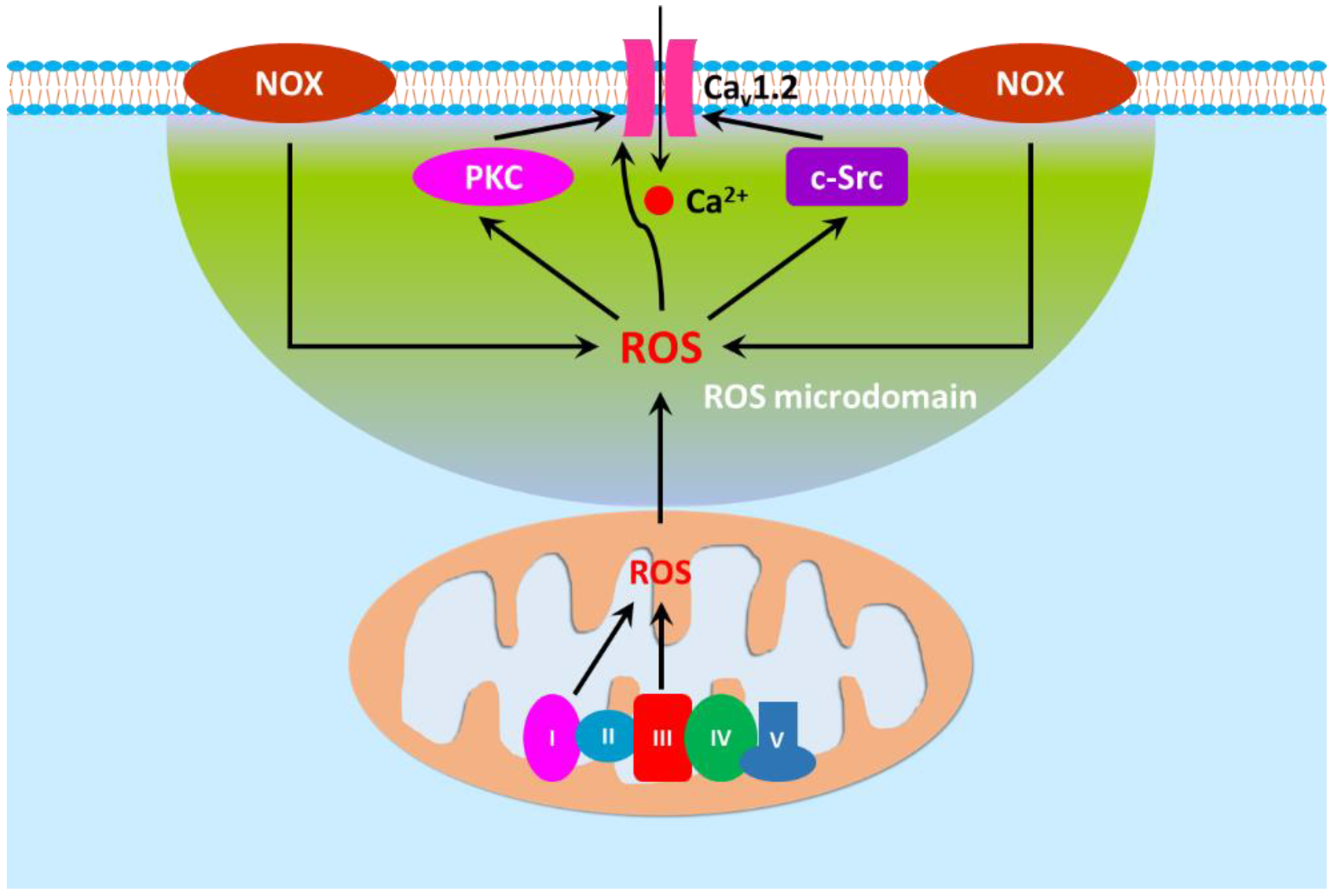
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.-Q.; Zhang, L. Oxidative Regulation of Vascular Cav1.2 Channels Triggers Vascular Dysfunction in Hypertension-Related Disorders. Antioxidants 2022, 11, 2432. https://doi.org/10.3390/antiox11122432
Hu X-Q, Zhang L. Oxidative Regulation of Vascular Cav1.2 Channels Triggers Vascular Dysfunction in Hypertension-Related Disorders. Antioxidants. 2022; 11(12):2432. https://doi.org/10.3390/antiox11122432
Chicago/Turabian StyleHu, Xiang-Qun, and Lubo Zhang. 2022. "Oxidative Regulation of Vascular Cav1.2 Channels Triggers Vascular Dysfunction in Hypertension-Related Disorders" Antioxidants 11, no. 12: 2432. https://doi.org/10.3390/antiox11122432
APA StyleHu, X.-Q., & Zhang, L. (2022). Oxidative Regulation of Vascular Cav1.2 Channels Triggers Vascular Dysfunction in Hypertension-Related Disorders. Antioxidants, 11(12), 2432. https://doi.org/10.3390/antiox11122432