Impact of Maternal and Offspring Dietary Zn Supplementation on Growth Performance and Antioxidant and Immune Function of Offspring Broilers
Abstract
:1. Introduction
2. Materials and Methods
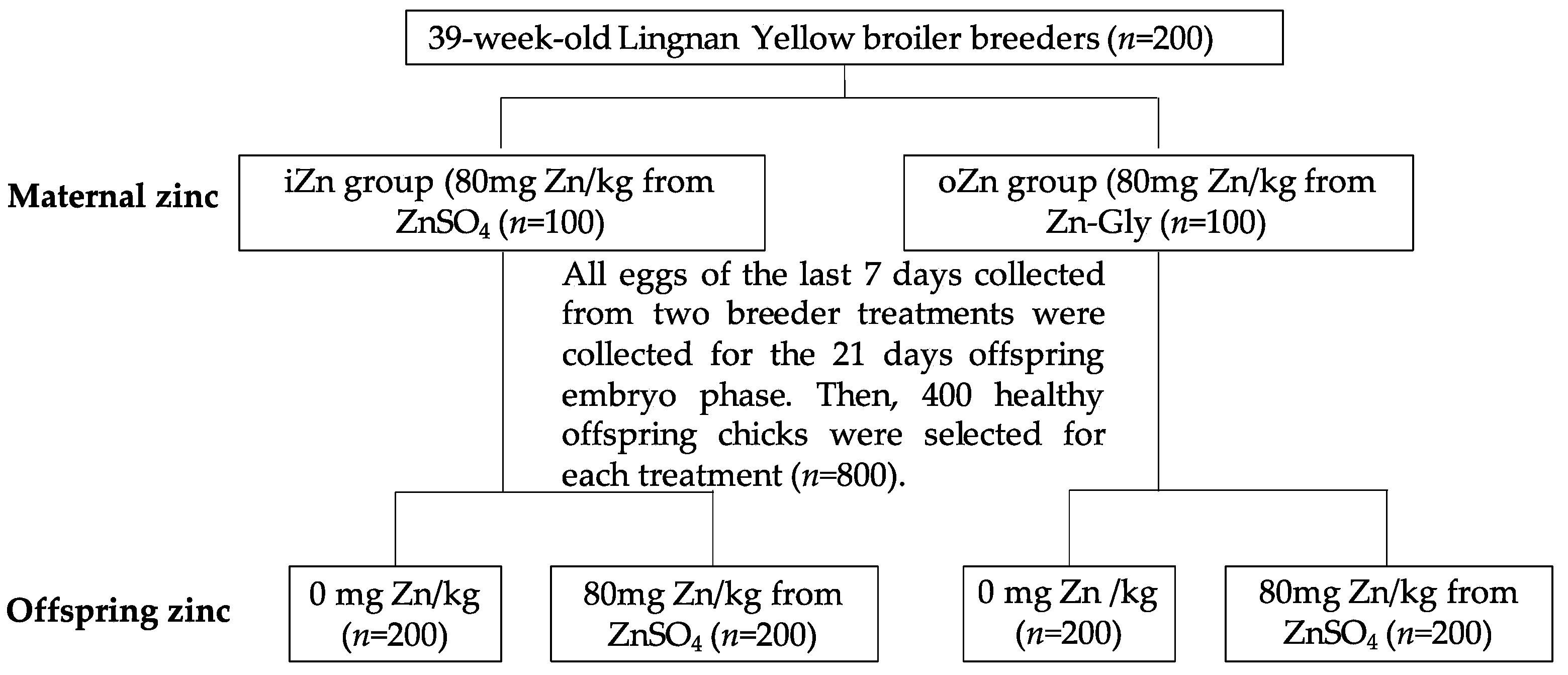
2.1. Experimental Design, Birds, and Diets
2.2. Measurement of Growth Performance and Sample Collection
2.3. Zn Concentration Determination
2.4. Antioxidant Status Analysis
2.5. Chemical and Cytokine Secretions Analyses
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Zn Concentrations
3.3. Antioxidant Status in Serum
3.4. Antioxidant Status in Liver
3.5. Antioxidant Status in Muscle
3.6. Cytokine Secretion
3.7. Immunoglobulin Content
3.8. Stress Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwiecien, M.; Winiarska-Mieczan, A.; Milczarek, A.; Klebaniuk, R. Biological Response of Broiler Chickens to Decreasing Dietary Inclusion Levels of Zinc Glycine Chelate. Biol. Trace Elem. Res. 2017, 175, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.L.; Olin, K.L.; Fraga, C.G.; Keen, C.L. Oxidant defense systems in testes from zinc-deficient rats. Proc. Soc. Exp. Biol. Med. 1996, 213, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, A.H.; Prasad, A.S. Zinc and immune function: The biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998, 68, 447s–463s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muiruri, H.K.; Harrison, P.C. Effect of Roost Temperature on Performance of Chickens in Hot Ambient Environments. Poult. Sci. 1991, 70, 2253–2258. [Google Scholar] [CrossRef]
- Tao, S.; Monteiro, A.P.A.; Thompson, I.M.; Hayen, M.J.; Dahl, G.E. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J. Dairy Sci. 2012, 95, 7128–7136. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.W.; Li, W.X.; Lu, L.; Zhang, L.Y.; Ji, C.; Lin, X.; Liu, H.C.; Odle, J.; Luo, X.G. Impact of maternal heat stress in conjunction with dietary zinc supplementation on hatchability, embryonic development, and growth performance in offspring broilers. Poult. Sci. 2017, 96, 2351–2359. [Google Scholar] [CrossRef]
- Gao, J.; Lv, Z.P.; Li, C.W.; Yue, Y.S.; Zhao, X.; Wang, F.L.; Guo, Y.M. Maternal Zinc Supplementation Enhanced Skeletal Muscle Development Through Increasing Protein Synthesis and Inhibiting Protein Degradation of Their Offspring. Biol. Trace Elem. Res. 2014, 162, 309–316. [Google Scholar] [CrossRef]
- Li, C.W.; Guo, S.S.; Gao, J.; Guo, Y.M.; Du, E.C.; Lv, Z.P.; Zhang, B.B. Maternal high-zinc diet attenuates intestinal inflammation by reducing DNA methylation and elevating H3K9 acetylation in the A20 promoter of offspring chicks. J. Nutr. Biochem. 2015, 26, 173–183. [Google Scholar] [CrossRef]
- Virden, W.S.; Yeatman, J.B.; Barber, S.J.; Zumwalt, C.D.; Ward, T.L.; Johnson, A.B.; Kidd, M.T. Hen mineral nutrition impacts progeny livability. J. Appl. Poult. Res. 2003, 12, 411–416. [Google Scholar] [CrossRef]
- Oestreicher, P.; Cousins, R.J. Copper and zinc absorption in the rat: Mechanism of mutual antagonism. J. Nutr. 1985, 115, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Star, L.; vanderKlis, J.D.; Rapp, C.; Ward, T.L. Bioavailability of organic and inorganic zinc sources in male broilers. Poult. Sci. 2012, 91, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Lu, L.; Wang, R.L.; Lei, H.L.; Li, S.F.; Zhang, L.Y.; Luo, X.G. Effects of supplemental zinc source and level on antioxidant ability and fat metabolism-related enzymes of broilers. Poult. Sci. 2015, 94, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Virden, W.S.; Yeatman, J.B.; Barber, S.J.; Willeford, K.O.; Ward, T.L.; Fakler, T.M.; Wideman, R.F.; Kidd, M.T. Immune system and cardiac functions of progeny chicks from dams fed diets differing in zinc and manganese level and source. Poult. Sci. 2004, 83, 344–351. [Google Scholar] [CrossRef]
- Additives, E.P.; Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Durjava, M.F.; Kouba, M. Safety of the feed additive consisting of manganese chelates of lysine and glutamic acid for all animal species (Zinpro Animal Nutrition). EFSA J. 2021, 19, e06454. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.X.; Xiao, X.; Wang, J.S.; Wang, Q.; Li, K.X.; Guo, T.Y.; Zhan, X.A. Effects of Zinc Glycinate on Productive and Reproductive Performance, Zinc Concentration and Antioxidant Status in Broiler Breeders. Biol. Trace Elem. Res. 2017, 178, 320–326. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.S.; Wang, Q.; Li, K.X.; Guo, T.Y.; Xiao, X.; Wang, Y.X.; Zhan, X.A. Effects of Maternal Zinc Glycine on Mortality, Zinc Concentration, and Antioxidant Status in a Developing Embryo and 1-Day-Old Chick. Biol. Trace Elem. Res. 2018, 181, 323–330. [Google Scholar] [CrossRef]
- Sun, Q.J.; Guo, Y.M.; Ma, S.D.; Yuan, J.M.; An, S.Y.; Li, J.H. Dietary Mineral Sources Altered Lipid and Antioxidant Profiles in Broiler Breeders and Posthatch Growth of Their Offsprings. Biol. Trace Elem. Res. 2012, 145, 318–324. [Google Scholar] [CrossRef]
- Zhao, J.M.; Shirley, R.B.; Dibner, J.J.; Wedekind, K.J.; Yan, F.; Fisher, P.; Hampton, T.R.; Evans, J.L.; Vazquez-Anon, M. Superior growth performance in broiler chicks fed chelated compared to inorganic zinc in presence of elevated dietary copper. J. Anim. Sci. Biotechnol. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Jahanian, R.; Rasouli, E. Effects of dietary substitution of zinc-methionine for inorganic zinc sources on growth performance, tissue zinc accumulation and some blood parameters in broiler chicks. J. Anim. Physiol. Anim. Nutr. 2015, 99, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.M.; Lee, H.R.; Jo, C.; Lee, S.K.; Lee, B.D. Effect of dietary zinc proteinate supplementation on growth performance, and skin and meat quality of male and female broiler chicks. Brit. Poult. Sci. 2012, 53, 116–124. [Google Scholar] [CrossRef]
- Jahanian, R.; Moghaddam, H.N.; Rezaei, A. Improved broiler chick performance by dietary supplementation of organic zinc sources. Asian-Austral. J. Anim. 2008, 21, 1348–1354. [Google Scholar] [CrossRef]
- Park, S.Y.; Birkhold, S.G.; Kubena, L.F.; Nisbet, D.J.; Ricke, S.C. Review on the role of dietary zinc in poultry nutrition, immunity, and reproduction. Biol. Trace Elem. Res. 2004, 101, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Rutz, F.; Anciuti, M.A.; Rech, J.L.; Zauk, N.H.F. Influence of graded levels of organic zinc on growth performance and carcass traits of broilers. J. Appl. Poult. Res. 2007, 16, 219–225. [Google Scholar] [CrossRef]
- Blamberg, D.L.; Blackwood, U.B.; Supplee, W.C.; Combs, G.F. Effect of Zinc Deficiency in Hens on Hatchability and Embryonic Development. Proc. Soc. Exp. Biol. Med. 1960, 104, 217–220. [Google Scholar] [CrossRef]
- Kienholz, E.W.; Turk, D.E.; Sunde, M.L.; Hoekstra, W.G. Effects of zinc deficiency in the diets of hens’. J. Nutr. 1961, 75, 211–221. [Google Scholar] [CrossRef]
- Hudson, B.P.; Dozier, W.A.; Wilson, J.L.; Sander, J.E.; Ward, T.L. Reproductive performance and immune status of caged broiler breeder hens provided diets supplemented with either inorganic or organic sources of zinc from hatching to 65 wk of age. J. Appl. Poult. Res. 2004, 13, 349–359. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, L.; Wang, R.L.; Xi, L.; Luo, X.G.; Liu, B. Effects of zinc source and phytate on zinc absorption by in situ ligated intestinal loops of broilers. Poult. Sci. 2010, 89, 2157–2165. [Google Scholar] [CrossRef]
- Yair, R.; Uni, Z. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult. Sci. 2011, 90, 1523–1531. [Google Scholar] [CrossRef]
- Jiang, D.; Yan, S. Effects of Cd, Zn, or Pb Stress in Populus alba berolinensis on the Antioxidant, Detoxifying, and Digestive Enzymes of Lymantria dispar. Environ. Entomol. 2018, 47, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Kucuk, O. Zinc in a combination with magnesium helps reducing negative effects of heat stress in quails. Biol. Trace Elem. Res. 2008, 123, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Ruram, A.A.; Singh, S.S.; Devi, S.B.; Devi, T.I.; Singh, W.G. Lipid peroxidation and antioxidant vitamins in urolithasis. Indian J. Clin. Biochem. 2007, 22, 128–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, T.M.; Bettger, W.J. The Physiological-Role of Zinc as an Antioxidant. Free Radic. Biol. Med. 1990, 8, 281–291. [Google Scholar] [CrossRef]
- Taylor, C.G.; McCutchon, T.L.; Boermans, H.J.; DiSilvestro, R.A.; Bray, T.M. Comparison of Zn and vitamin E for protection against hyperoxia-induced lung damage. Free Radic. Biol. Med. 1997, 22, 543–550. [Google Scholar] [CrossRef]
- Sidhu, P.; Garg, M.L.; Dhawan, D.K. Protective effects of zinc on oxidative stress enzymes in liver of protein-deficient rats. Drug Chem. Toxicol. 2005, 28, 211–230. [Google Scholar] [CrossRef]
- Shaheen, A.A.; Elfattah, A.A.A. Effect of Dietary Zinc on Lipid-Peroxidation, Glutathione, Protein Thiols Levels and Superoxide-Dismutase Activity in Rat-Tissues. Int. J. Biochem. Cell B 1995, 27, 89–95. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of Dietary Zinc Oxide Nanoparticles on Growth Performance and Antioxidative Status in Broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef]
- Wellinghausen, N.; Rink, L. The significance of zinc for leukocyte biology. J. Leukoc. Biol. 1998, 64, 571–577. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Muzzioli, M.; Giacconi, R. Zinc and immunoresistance to infection in aging: New biological tools. Trends Pharmacol. Sci. 2000, 21, 205–208. [Google Scholar] [CrossRef]
- Feng, J.; Ma, W.Q.; Niu, H.H.; Wu, X.M.; Wang, Y.; Feng, J. Effects of Zinc Glycine Chelate on Growth, Hematological, and Immunological Characteristics in Broilers. Biol. Trace Elem. Res. 2010, 133, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Blanchard, R.K.; Cousins, R.J. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc. Natl. Acad. Sci. USA 2006, 103, 1699–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, P.C.S.; Sena-Evangelista, K.C.M.; Paiva, M.S.M.D.; Ferreira, D.Q.C.; Ururahy, M.A.G.; Rezende, A.A.; Abdalla, D.S.P.; Pedrosa, L.F.C. The beneficial effects of rosuvastatin are independent of zinc supplementation in patients with atherosclerosis. J. Trace Elem. Med. Biol. 2014, 28, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Rishi, P.; Kaur, P.; Virdi, J.S.; Shukla, G.; Koul, A. Amelioratory effects of zinc supplementation on Salmonella-induced hepatic damage in the murine model. Dig. Dis. Sci. 2008, 53, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Cousins, R.J.; Leinart, A.S. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J. 1988, 2, 2884–2890. [Google Scholar] [CrossRef] [Green Version]
- Burns, R.B. Antibody production suppressed in the domestic fowl (Gallus domesticus) by zinc deficiency. Avian Pathol. 1983, 12, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Beach, R.S.; Gershwin, M.E.; Makishima, R.K.; Hurley, L.S. Impaired Immunological Ontogeny in Postnatal Zinc Deprivation. J. Nutr. 1980, 110, 805–815. [Google Scholar] [CrossRef]
- Fraker, P.J.; King, L.E.; Laakko, T.; Vollmer, T.L. The dynamic link between the integrity of the immune system and zinc status. J. Nutr. 2000, 130, 1399s–1406s. [Google Scholar] [CrossRef] [Green Version]
- Wellinghausen, N.; Kirchner, H.; Rink, L. The immunobiology of zinc. Immunol. Today 1997, 18, 519–521. [Google Scholar] [CrossRef]
- Garriga, C.; Hunter, R.R.; Amat, C.; Planas, J.M.; Mitchell, M.A.; Moreto, M. Heat stress increases apical glucose transport in the chicken jejunum. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 290, R195–R201. [Google Scholar] [CrossRef]
- Wang, W.C.; Yan, F.F.; Hu, J.Y.; Amen, O.A.; Cheng, H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018, 96, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | |
|---|---|
| Maize | 64.6 |
| Soybean meal | 25.0 |
| CaHPO4 | 1.8 |
| Limestone | 7.0 |
| Salt | 0.3 |
| DL-methionine | 0.3 |
| Premix 1 | 1.0 |
| Nutrient Composition | |
| ME 2 (MJ/kg) | 11.21 |
| Crude protein | 16.11 |
| Calcium | 3.04 |
| Total phosphorus | 0.63 |
| Lysine | 0.80 |
| Met + Cys | 0.82 |
| Zn (mg/kg) | 24 |
| Item | Starter Period 1 to 21d | Grower Period 22 to 42d | Finisher Period 43 to 60d |
|---|---|---|---|
| Maize | 59.0 | 63.6 | 65.5 |
| Soybean meal | 36.0 | 31.0 | 29.0 |
| Soybean oil | 1.0 | 1.4 | 2.0 |
| CaHPO4 | 1.5 | 1.2 | 1.0 |
| Limestone | 1.2 | 1.5 | 1.2 |
| Salt | 0.3 | 0.3 | 0.3 |
| Premix 1 | 1.0 | 1.0 | 1.0 |
| Total | 100 | 100 | 100 |
| Nutrient levels | |||
| ME 2 (MJ/kg) | 11.88 | 12.18 | 12.43 |
| Crude protein | 20.52 | 18.68 | 17.95 |
| Lysine | 1.21 | 1.05 | 1.02 |
| Methionine | 0.49 | 0.41 | 0.42 |
| Met + Cys | 0.81 | 0.73 | 0.71 |
| Calcium | 0.92 | 0.93 | 0.76 |
| Total phosphorus | 0.64 | 0.57 | 0.53 |
| Zn (mg/kg) | 28.0 | 26.6 | 26.0 |
| Maternal Zinc | Offspring Zinc (mg/kg) | ADG (g) | ADFI (g) | F/G | Mortality (%) |
|---|---|---|---|---|---|
| ZnSO4 1 | 0 | 30.62 ± 0.22 | 76.15 ± 1.32 | 2.53 ± 0.04 | 10.00 ± 0.00 a |
| 80 | 31.40 ± 0.27 | 73.22 ± 0.63 | 2.49 ± 0.01 | 2.67 ± 1.15 c | |
| Zn–Gly 1 | 0 | 31.33 ± 0.16 | 73.98 ± 3.71 | 2.49 ± 0.03 | 4.00 ± 0.00 b |
| 80 | 32.11 ± 0.08 | 73.46 ± 1.72 | 2.49 ± 0.14 | 2.00 ± 0.00 c | |
| ZnSO4 2 | 31.01 ± 0.48 b | 74.68 ± 1.86 | 2.51 ± 0.03 | 6.33 ± 4.08 | |
| Zn–Gly 2 | 31.72 ± 0.45 a | 73.72 ± 2.60 | 2.48 ± 0.09 | 3.00 ± 1.09 | |
| 0 3 | 30.97 ± 0.43 b | 75.07 ± 2.76 | 2.51 ± 0.04 | 7.00 ± 3.29 | |
| 80 3 | 31.76 ± 0.43 a | 73.34 ± 1.16 | 2.49 ± 0.09 | 2.33 ± 0.81 | |
| p-value | Maternal zinc | <0.001 | 0.464 | 0.600 | <0.001 |
| Offspring zinc | <0.001 | 0.204 | 0.663 | <0.001 | |
| Maternal zinc × offspring zinc | 0.988 | 0.364 | 0.649 | <0.001 |
| Maternal Zinc | Offspring Zinc (mg/kg) | 21d | 60d | ||||
|---|---|---|---|---|---|---|---|
| Serum (μmol/L) | Liver (µg/g) | Muscle (µg/g) | Serum (μmol/L) | Liver (µg/g) | Muscle (µg/g) | ||
| ZnSO4 1 | 0 | 40.29 ± 3.71 | 44.86 ± 3.79 | 38.77 ± 7.70 | 27.37 ± 1.07 | 52.36 ± 2.03 | 20.46 ± 3.28 b |
| 80 | 44.84 ± 2.36 | 47.16 ± 2.87 | 49.84 ± 4.91 | 31.08 ± 0.99 | 57.25 ± 2.26 | 34.08 ± 4.89 a | |
| Zn–Gly 1 | 0 41.44 ± 2.95 45.92 ± 4.51 46.73 ± 2.22 29.90 ± 2.09 55.74 ± 2.59 32.30 ± 3.02 a | 41.44 ± 2.95 | 45.92 ± 4.51 | 46.73 ± 2.22 | 29.90 ± 2.09 | 55.74 ± 2.59 | 32.30 ± 3.02 a |
| 80 | 46.46 ± 2.73 | 55.48 ± 0.98 | 54.55 ± 6.09 | 36.46 ± 2.51 | 61.46 ± 2.38 | 35.28 ± 2.36 a | |
| ZnSO4 2 | 43.02 ± 3.64 | 46.17 ± 3.23 b | 44.30 ± 8.38 | 28.85 ± 2.16 b | 54.46 ± 3.26 b | 27.27 ± 8.34 b | |
| Zn–Gly 2 | 43.32 ± 3.72 | 50.02 ± 6.05 a | 50.64 ± 5.96 | 33.18 ± 4.11 a | 58.60 ± 3.84 a | 33.79 ± 2.96 a | |
| 0 3 | 40.93 ± 3.15 b | 45.47 ± 3.91 b | 43.32 ± 6.54 b | 28.38 ± 1.95 b | 53.81 ± 2.75 b | 26.38 ± 7.07 b | |
| 80 3 | 45.38 ± 2.45 a | 50.73 ± 4.92 a | 52.53 ± 5.84 a | 33.77 ± 3.37 a | 59.35 ± 3.09 a | 34.68 ± 3.52 a | |
| p-value | Maternal zinc | 0.347 | 0.029 | 0.056 | <0.001 | 0.016 | 0.013 |
| Offspring zinc | 0.005 | 0.009 | 0.009 | <0.001 | 0.002 | 0.004 | |
| Maternal zinc × offspring zinc | 0.873 | 0.078 | 0.591 | 0.104 | 0.756 | 0.031 | |
| Maternal Zinc | Offspring Zinc (mg/kg) | T-AOC (U/mg Prot) | MDA (nmol/mg Prot) | T-SOD (U/mg Prot) | CuZn-SOD (U/mg Prot) |
|---|---|---|---|---|---|
| 21d | |||||
| ZnSO4 1 | 0 | 8.14 ± 0.57 | 7.91 ± 0.25 a | 516.6 ± 17.43 | 262.0 ± 8.87 |
| 80 | 11.69 ± 0.75 | 5.46 ± 0.24 c | 571.8 ± 7.09 | 271.1 ± 11.41 | |
| Zn–Gly 1 | 0 | 8.47 ± 0.23 | 6.00 ± 0.43 b | 568.1 ± 32.66 | 265.7 ± 9.58 |
| 80 | 13.26 ± 1.07 | 5.15 ± 0.35 c | 600.9 ± 30.53 | 296.2 ± 16.67 | |
| ZnSO4 2 | 9.72 ± 1.97 b | 6.51 ± 1.33 | 537.3 ± 31.69 b | 267.8 ± 11.08 b | |
| Zn–Gly 2 | 10.27 ± 2.55 a | 5.61 ± 0.59 | 583.4 ± 34.92 a | 284.5 ± 20.78 a | |
| 0 3 | 8.31 ± 0.45 b | 6.58 ± 0.99 | 548.3 ± 37.48 b | 264.1 ± 8.89 b | |
| 80 3 | 12.36 ± 1.17 a | 5.27 ± 0.34 | 592.1 ± 28.82 a | 284.5 ± 19.05 a | |
| p-value | Maternal zinc | 0.012 | <0.001 | 0.004 | 0.016 |
| Offspring zinc | <0.001 | <0.001 | 0.002 | 0.002 | |
| Maternal zinc × offspring zinc | 0.077 | <0.001 | 0.374 | 0.064 | |
| 60d | |||||
| ZnSO4 1 | 0 | 10.12 ± 1.07 c | 7.03 ± 0.32 a | 427.0 ± 4.64 c | 316. 7 ± 8.38 |
| 80 | 12.07 ± 0.74 b | 6.90 ± 0.61 a | 615.8 ± 13.91 a | 331.0 ± 7.9 | |
| Zn–Gly 1 | 0 | 10.82 ± 0.85 c | 6.99 ± 0.76 a | 569.9 ± 36.59 b | 322.0 ± 9.67 |
| 80 | 13.84 ± 1.18 a | 5.80 ± 0.33 b | 630.7 ± 19.40 a | 345.2 ± 19.76 | |
| ZnSO4 2 | 11.26 ± 1.31 b | 6.97 ± 0.46 a | 559.2 ± 91.91 | 323.9 ± 10.77 | |
| Zn–Gly 2 | 12.16 ± 1.85 a | 6.24 ± 0.78 b | 610.5 ± 38.63 | 337.5 ± 20.06 | |
| 0 3 | 10.47 ± 0.98 b | 7.01 ± 0.50 a | 498.5 ± 81.69 | 319.0 ± 8.63 b | |
| 80 3 | 12.72 ± 1.24 a | 6.29 ± 0.73 b | 622.7 ± 17.71 | 339.5 ± 17.07 a | |
| p-value | Maternal zinc | 0.009 | 0.046 | <0.001 | 0.0187 |
| Offspring zinc | <0.001 | 0.025 | <0.001 | 0.019 | |
| Maternal zinc × offspring zinc | 0.217 | 0.061 | <0.001 | 0.538 | |
| Maternal Zinc | Offspring Zinc (mg/kg) | T-AOC (U/mg Prot) | MDA (nmol/mg Prot) | T-SOD (U/mg Prot) | CuZn-SOD (U/mg Prot) | MT (ng/g) |
|---|---|---|---|---|---|---|
| 21d | ||||||
| ZnSO4 1 | 0 | 0.97 ± 0.08 | 0.75 ± 0.01 a | 383.0 ± 15.76 | 260.0 ± 5.21 | 34.15 ± 2.36 |
| 80 | 1.15 ± 0.10 | 0.56 ± 0.03 bc | 431.7 ± 13.42 | 277.0 ± 3.84 | 42.96 ± 2.32 | |
| Zn–Gly 1 | 0 | 1.05 ± 0.07 | 0.58 ± 0.03 b | 413.2 ± 15.54 | 265.3 ± 4.98 | 36.38 ± 1.81 |
| 80 | 1.19 ± 0.09 | 0.52 ± 0.02 c | 464.9 ± 16.92 | 287.5 ± 8.10 | 46.44 ± 2.23 | |
| ZnSO4 2 | 1.05 ± 0.13 | 0.68 ± 0.10 | 411.4 ± 28.61 b | 267.2 ± 10.06 b | 38.82 ± 5.07 b | |
| Zn–Gly 2 | 1.15 ± 0.11 | 0.55 ± 0.04 | 433. 9 ± 30.71 a | 276.4 ± 13.30 a | 41.02 ± 5.56 a | |
| 0 3 | 1.00 ± 0.09 b | 0.67 ± 0.09 | 399.5 ± 21.65 b | 262.9 ± 5.53 b | 35.19 ± 2.35 b | |
| 80 3 | 1.17 ± 0.09 a | 0.54 ± 0.03 | 443.8 ± 21.78 a | 283.6 ± 8.46 a | 44.35 ± 2.82 a | |
| p-value | Maternal zinc | 0.128 | <0.001 | <0.001 | 0.020 | 0.002 |
| Offspring zinc | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Maternal zinc × offspring zinc | 0.601 | <0.001 | 0.824 | 0.403 | 0.451 | |
| 60d | ||||||
| ZnSO4 1 | 0 | 1.03 ± 0.10 c | 0.80 ± 0.05 | 442.0 ± 12.45 c | 303.0 ± 1.37 c | 33.07 ± 1.21 |
| 80 | 1.21 ± 0.09 b | 0.69 ± 0.05 | 554.3 ± 37.62 ab | 335.6 ± 2.57 b | 37.51 ± 1.75 | |
| Zn–Gly 1 | 0 | 1.06 ± 0.11 c | 0.72 ± 0.07 | 530.6 ± 26.56 b | 303.5 ± 5.51 c | 34.62 ± 1.84 |
| 80 | 1.59 ± 0.05 a | 0.65 ± 0.09 | 563.0 ± 8.89 a | 355.8 ± 6.40 a | 41.51 ± 1.58 | |
| ZnSO4 2 | 1.14 ± 0.13 | 0.76 ± 0.08 | 498.1 ± 63.99 | 317.0 ± 17.54 | 35.92 ± 2.69 b | |
| Zn–Gly 2 | 1.37 ± 0.28 | 0.68 ± 0.08 | 546.8 ± 25.37 | 320. 9 ± 26.71 | 37.84 ± 3.93 a | |
| 0 3 | 1.05 ± 0.10 | 0.77 ± 0.07 a | 478.0 ± 49.25 | 303.3 ± 4.19 | 34.02 ± 1.76 b | |
| 80 3 | 1.41 ± 0.21 | 0.67 ± 0.07 b | 558.0 ± 28.51 | 345.7 ± 11.86 | 39.26 ± 2.62 a | |
| p-value | Maternal zinc | <0.001 | 0.162 | <0.001 | 0.001 | <0.001 |
| Offspring zinc | <0.001 | 0.036 | <0.001 | <0.001 | <0.001 | |
| Maternal zinc × offspring zinc | <0.001 | 0.552 | <0.001 | 0.001 | 0.065 | |
| Maternal Zinc | Offspring Zinc (mg/kg) | T-AOC (U/mg Prot) | MDA (nmol/mg Prot) | T-SOD (U/mg Prot) | CuZn-SOD (U/mg Prot) |
|---|---|---|---|---|---|
| 21d | |||||
| ZnSO4 1 | 0 | 0.09 ± 0.008 b | 0.47 ± 0.02 a | 86.42 ± 2.31 c | 52.59 ± 4.21 b |
| 80 | 0.11 ± 0.009 a | 0.31 ± 0.01 c | 103.1 ± 6.58 b | 62.98 ± 2.95 a | |
| Zn–Gly 1 | 0 | 0.11 ± 0.001 a | 0.35 ± 0.03 b | 87.68 ± 6.18 c | 52.77 ± 1.39 b |
| 80 | 0.11 ± 0.01 a | 0.24 ± 0.017 d | 111.6 ± 8.44 a | 65.45 ± 3.74 a | |
| ZnSO4 2 | 0.097 ± 0.01 b | 0.38 ± 0.09 a | 95.51 ± 9.98 | 56.92 ± 6.44 | |
| Zn–Gly 2 | 0.111 ± 0.007 a | 0.30 ± 0.06 b | 101.3 ± 14.28 | 59.11 ± 7.15 | |
| 0 3 | 0.10 ± 0.011 b | 0.40 ± 0.07 a | 87.11 ± 4.66 b | 52.67 ± 3.11 b | |
| 80 3 | 0.11 ± 0.009 a | 0.27 ± 0.03 b | 107.9 ± 8.61 a | 64.33 ± 3.48 a | |
| p-value | Maternal zinc | 0.012 | <0.001 | 0.085 | 0.345 |
| Offspring zinc | 0.012 | <0.001 | <0.001 | <0.001 | |
| Maternal zinc × offspring zinc | 0.135 | 0.031 | 0.194 | 0.412 | |
| 60d | |||||
| ZnSO4 1 | 0 | 0.055 ± 0.004 d | 0.37 ± 0.007 a | 83.77 ± 7.37 | 50.55 ± 4.58 |
| 80 | 0.078 ± 0.005 b | 0.22 ± 0.036 c | 86.03 ± 3.94 | 51.60 ± 3.00 | |
| Zn–Gly 1 | 0 | 0.068 ± 0.001 c | 0.32 ± 0.009 b | 84.39 ± 2.79 | 50.37 ± 4.41 |
| 80 | 0.086 ± 0.006 a | 0.20 ± 0.024 c | 86.05 ± 4.60 | 55.10 ± 2.75 | |
| ZnSO4 2 | 0.067 ± 0.013 b | 0.31 ± 0.08 a | 85.18 ± 5.08 | 50.88 ± 3.78 | |
| Zn–Gly 2 | 0.076 ± 0.010 a | 0.26 ± 0.06 b | 86.30 ± 3.83 | 52.83 ± 4.30 | |
| 0 3 | 0.06 ± 0.007 b | 0.35 ± 0.026 a | 84.12 ± 4.70 | 50.45 ± 4.28 | |
| 80 3 | 0.08 ± 0.007 a | 0.22 ± 0.029 b | 87.00 ± 3.92 | 53.35 ± 3.29 | |
| p-value | Maternal zinc | 0.002 | 0.014 | 0.552 | 0.283 |
| Offspring zinc | <0.001 | <0.001 | 0.209 | 0.099 | |
| Maternal zinc × offspring zinc | 0.390 | 0.207 | 0.739 | 0.330 | |
| Maternal Zinc | Offspring Zinc (mg/kg) | IL-1 (μg/L) | IL-2 (μg/L) | TNF-α (ng/L) |
|---|---|---|---|---|
| 21d | ||||
| ZnSO4 1 | 0 | 0.11 ± 0.01 | 0.13 ± 0.01 | 37.68 ± 1.20 |
| 80 | 0.10 ± 0.01 | 0.13 ± 0.02 | 36.63 ± 2.24 | |
| Zn–Gly 1 | 0 | 0.11 ± 0.01 | 0.13 ± 0.02 | 36.75 ± 2.20 |
| 80 | 0.10 ± 0.01 | 0.14 ± 0.01 | 36.57 ± 0.75 | |
| ZnSO4 2 | 0.11 ± 0.01 | 0.13 ± 0.01 | 37.15 ± 1.84 | |
| Zn–Gly 2 | 0.10 ± 0.01 | 0.14 ± 0.02 | 36.66 ± 1.61 | |
| 0 3 | 0.11 ± 0.01 | 0.13 ± 0.02 | 37.22 ± 1.80 | |
| 80 3 | 0.10 ± 0.01 | 0.14 ± 0.01 | 36.60 ± 1.63 | |
| p-value | Maternal zinc | 0.829 | 0.279 | 0.374 |
| Offspring zinc | 0.314 | 0.293 | 0.266 | |
| Maternal zinc × offspring zinc | 0.846 | 0.568 | 0.429 | |
| 60d | ||||
| ZnSO4 1 | 0 | 0.17 ± 0.03 a | 0.15 ± 0.02 | 48.62 ± 6.06 a |
| 80 | 0.13 ± 0.02 b | 0.16 ± 0.03 | 40.30 ± 2.11 b | |
| Zn–Gly 1 | 0 | 0.14 ± 0.02 b | 0.15 ± 0.02 | 41.60 ± 3.23 b |
| 80 | 0.13 ± 0.01 b | 0.17 ± 0.02 | 38.70 ± 1.85 b | |
| ZnSO4 2 | 0.15 ± 0.03 a | 0.15 ± 0.02 | 44.46 ± 6.14 a | |
| Zn–Gly 2 | 0.13 ± 0.02 b | 0.16 ± 0.02 | 40.07 ± 2.93 b | |
| 0 3 | 0.15 ± 0.03 a | 0.15 ± 0.02 | 45.29 ± 6.00 a | |
| 80 3 | 0.13 ± 0.01 b | 0.16 ± 0.03 | 39.50 ± 2.10 b | |
| p-value | Maternal zinc | 0.006 | 0.255 | 0.001 |
| Offspring zinc | 0.001 | 0.063 | <0.001 | |
| Maternal zinc × offspring zinc | 0.012 | 0.741 | 0.030 | |
| Maternal Zinc | Offspring Zinc (mg/kg) | IgG (μg/mL) | IgA (μg/mL) | IgM (μg/mL) |
|---|---|---|---|---|
| 21d | ||||
| ZnSO4 1 | 0 | 0.82 ± 0.08 | 0.45 ± 0.01 b | 6.68 ± 0.54 b |
| 80 | 0.88 ± 0.11 | 0.47 ± 0.02 a | 7.39 ± 0.95 ab | |
| Zn–Gly 1 | 0 | 0.83 ± 0.10 | 0.46 ± 0.01 b | 6.86 ± 0.39 ab |
| 80 | 0.89 ± 0.09 | 0.47 ± 0.02 a | 7.69 ± 1.14 a | |
| ZnSO4 2 | 0.85 ± 0.10 | 0.46 ± 0.02 | 7.03 ± 0.83 b | |
| Zn–Gly 2 | 0.86 ± 0.09 | 0.46 ± 0.01 | 7.27 ± 0.93 a | |
| 0 3 | 0.83 ± 0.09 | 0.45 ± 0.01 b | 6.77 ± 0.47 | |
| 80 3 | 0.88 ± 0.10 | 0.47 ± 0.02 a | 7.54 ± 1.03 | |
| p-value | maternal zinc | 0.830 | 0.234 | 0.357 |
| Offspring zinc | 0.072 | <0.001 | 0.005 | |
| Maternal zinc × offspring zinc | 0.989 | 0.508 | 0.817 | |
| 60d | ||||
| ZnSO4 1 | 0 | 0.92 ± 0.13 b | 0.46 ± 0.01 | 6.17 ± 0.48 c |
| 80 | 1.01 ± 0.12 ab | 0.46 ± 0.01 | 7.42 ± 0.76 a | |
| Zn–Gly 1 | 0 | 0.96 ± 0.09 ab | 0.46 ± 0.02 | 6.83 ± 0.43 b |
| 80 | 1.05 ± 0.13 a | 0.47 ± 0.01 | 7.71 ± 0.66 a | |
| ZnSO4 2 | 0.97 ± 0.13 | 0.46 ± 0.01 | 6.80 ± 0.89 b | |
| Zn–Gly 2 | 1.01 ± 0.12 | 0.46 ± 0.02 | 7.29 ± 0.71 a | |
| 0 3 | 0.94 ± 0.11 b | 0.46 ± 0.01 | 6.48 ± 0.56 b | |
| 80 3 | 1.03 ± 0.12 a | 0.47 ± 0.02 | 7.56 ± 0.71 a | |
| p-value | maternal zinc | 0.274 | 0.452 | 0.020 |
| Offspring zinc | 0.032 | 0.084 | <0.001 | |
| Maternal zinc × offspring zinc | 0.988 | 0.887 | 0.337 | |
| Maternal Zinc | Offspring Zinc (mg/kg) | 21d | 60d | ||||
|---|---|---|---|---|---|---|---|
| CORT (μg/L) | HSP70 (μg/L) | CK (μg/L) | CORT (μg/L) | HSP70 (μg/L) | CK (μg/L) | ||
| ZnSO4 1 | 0 | 419.5 ± 47.44 | 0.19 ± 0.02 | 3.16 ± 0.43 | 553.7 ± 62.68 a | 0.25 ± 0.02 b | 3.87 ± 0.52 a |
| 80 | 383.3 ± 36.50 | 0.21 ± 0.01 | 2.99 ± 0.35 | 457.5 ± 38.99 b | 0.26 ± 0.03 b | 3.60 ± 0.28 ab | |
| Zn–Gly 1 | 0 | 405.2 ± 55.05 | 0.21 ± 0.02 | 3.08 ± 0.38 | 473.9 ± 54.37 b | 0.26 ± 0.03 b | 3.62 ± 0.35 ab |
| 80 | 363.5 ± 28.53 | 0.21 ± 0.03 | 2.93 ± 0.26 | 448.5 ± 39.66 b | 0.31 ± 0.03 a | 3.43 ± 0.34 b | |
| ZnSO4 2 | 401.4 ± 45.20 | 0.20 ± 0.02 | 3.07 ± 0.39 | 505.6 ± 70.82 | 0.26 ± 0.02 | 3.73 ± 0.43 | |
| Zn–Gly 2 | 384.4 ± 47.74 | 0.21 ± 0.02 | 3.00 ± 0.33 | 460.5 ± 47.64 | 0.28 ± 0.043 | 3.53 ± 0.35 | |
| 0 3 | 412.4 ± 50.56 a | 0.20 ± 0.02 | 3.12 ± 0.40 | 515.9 ± 70.40 | 0.25 ± 0.03 | 3.75 ± 0.45 | |
| 80 3 | 373.4 ± 33.46 b | 0.21 ± 0.02 | 2.96 ± 0.30 | 453.0 ± 38.56 | 0.28 ± 0.03 | 3.52 ± 0.32 | |
| p-value | Maternal zinc | 0.219 | 0.162 | 0.536 | 0.009 | 0.009 | 0.101 |
| Offspring zinc | 0.007 | 0.076 | 0.175 | 0.001 | 0.002 | 0.065 | |
| Maternal zinc × offspring zinc | 0.842 | 0.221 | 0.931 | 0.033 | 0.021 | 0.742 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, L.; Xu, Y.; Ding, X.; Wang, Y.; Fu, A.; Zhan, X. Impact of Maternal and Offspring Dietary Zn Supplementation on Growth Performance and Antioxidant and Immune Function of Offspring Broilers. Antioxidants 2022, 11, 2456. https://doi.org/10.3390/antiox11122456
Wang Y, Zhang L, Xu Y, Ding X, Wang Y, Fu A, Zhan X. Impact of Maternal and Offspring Dietary Zn Supplementation on Growth Performance and Antioxidant and Immune Function of Offspring Broilers. Antioxidants. 2022; 11(12):2456. https://doi.org/10.3390/antiox11122456
Chicago/Turabian StyleWang, Yuanyuan, Ling Zhang, Yibin Xu, Xiaoqing Ding, Yongxia Wang, Aikun Fu, and Xiuan Zhan. 2022. "Impact of Maternal and Offspring Dietary Zn Supplementation on Growth Performance and Antioxidant and Immune Function of Offspring Broilers" Antioxidants 11, no. 12: 2456. https://doi.org/10.3390/antiox11122456





