Assessment of Gnaphalium viscosum (Kunth) Valorization Prospects: Sustainable Recovery of Antioxidants by Different Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Two-Phase Solvent Extraction
2.4. Soxhlet Extraction
2.5. Supercritical Fluid Extraction (SFE)
2.6. Characterization and Quantification of the Extracts
2.6.1. LC–High-Resolution Accurate Mass analysis (LC–HRAM)
Chromatographic Conditions
Mass Spectrometry Conditions
Quantitative Analysis
2.6.2. Liquid Chromatography with Tandem Mass Spectrometry (LC–MS/MS) Analysis
Chromatographic Conditions
2.6.3. Gas Chromatography (GC) Analysis
Sample Preparation
Chromatographic Conditions
2.6.4. Measurement of Total Phenolic Content
2.6.5. Antioxidant Activity
DPPH Assay
ABTS Assay
3. Results and Discussion
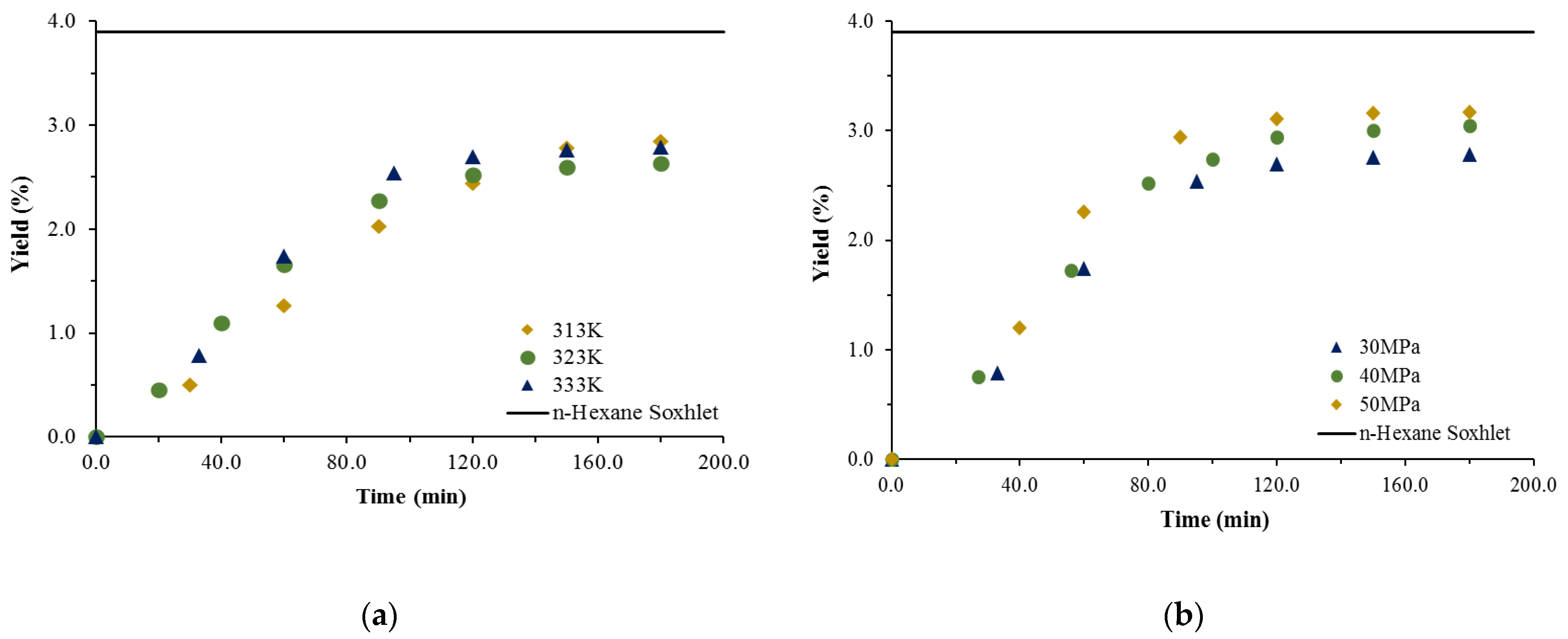
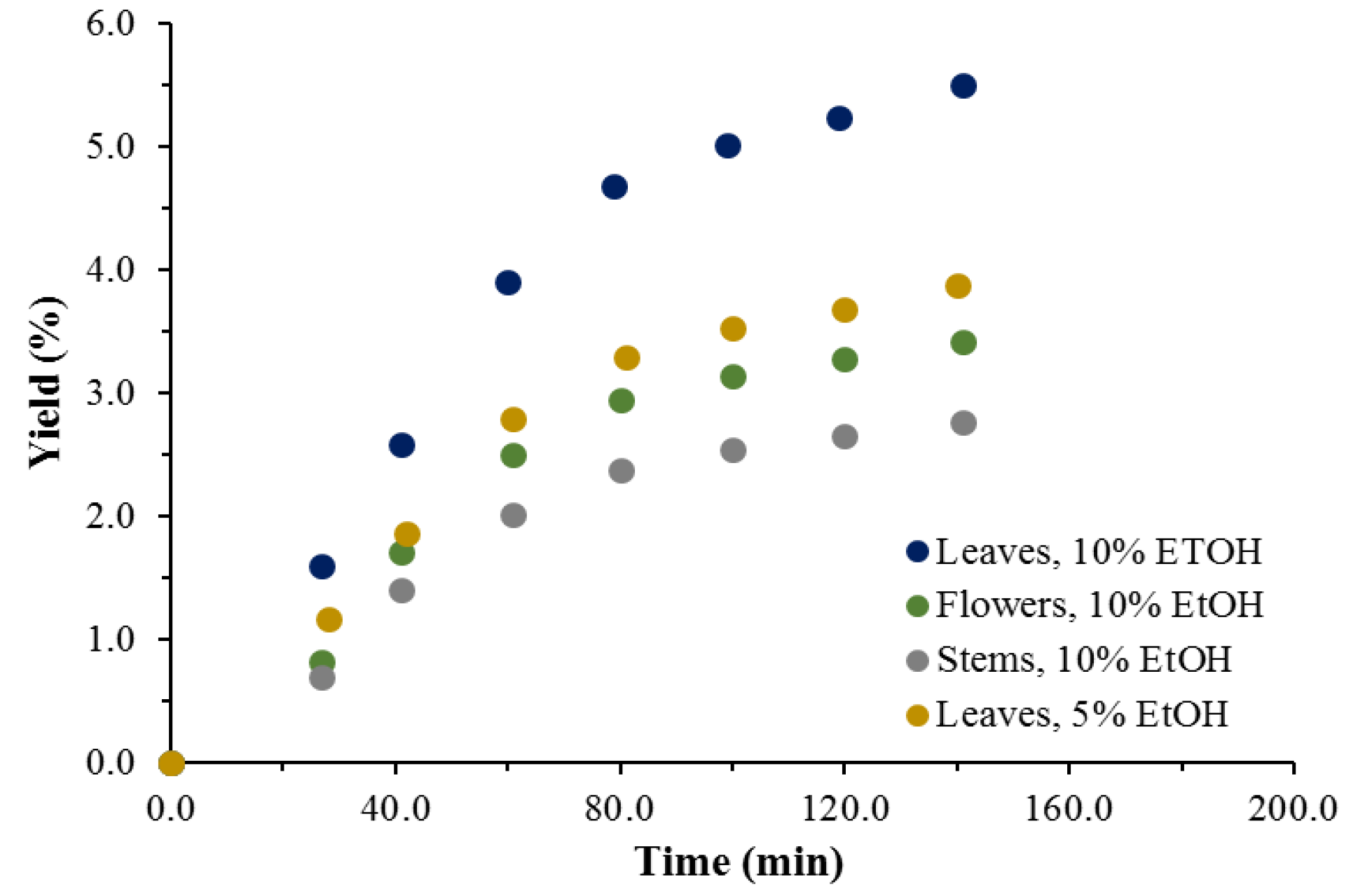
3.1. Extraction Yield
3.2. Phytochemical Analysis
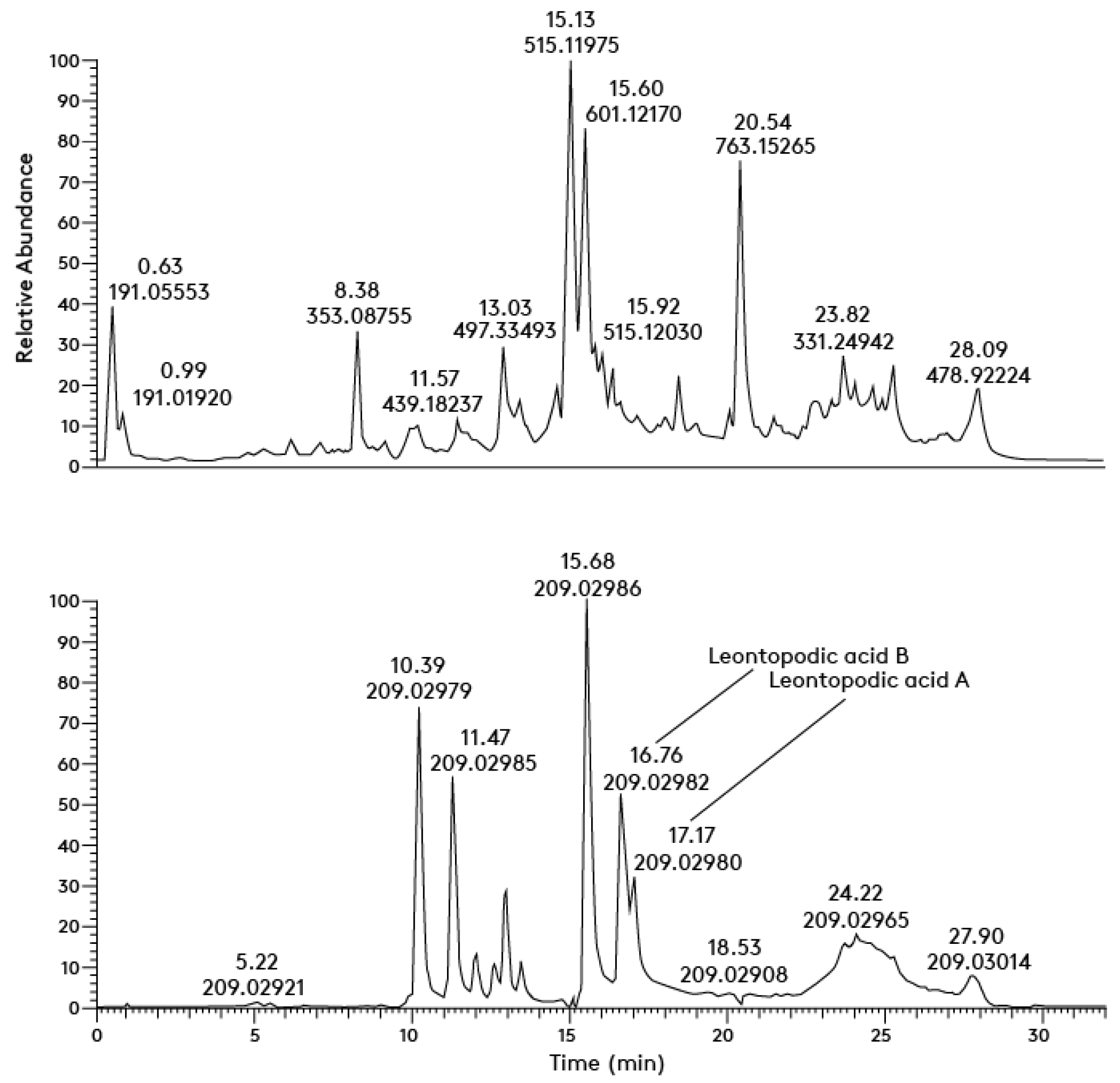
3.2.1. Analysis and Quantification of Antioxidants
3.2.2. Total Phenolic Content (TPC) and Antioxidant Activity (AA)
3.2.3. GC–FID
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.; Wang, W.; Piao, H.; Xu, W.; Shi, H.; Zhao, C. The genus Gnaphalium L. (Compositae): Phytochemical and pharmacological characteristics. Molecules 2013, 18, 8298–8318. [Google Scholar] [CrossRef] [PubMed]
- Quiñonez-Bastidas, G.N.; Navarrete, A. Mexican Plants and Derivates Compounds as Alternative for Inflammatory and Neuropathic Pain Treatment—A Review. Plants 2021, 10, 865. [Google Scholar] [CrossRef]
- Mata, R.; Figueroa, M.; Navarrete, A.; Rivero-Cruz, I. Progress in the Chemistry of Organic Natural Products. In Chemistry and Biology of Selected Mexican Medicinal Plants, 1st ed.; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J.-K., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 108, p. 301. [Google Scholar] [CrossRef]
- Hernández, B.R. Actividad Antimicobacteriana Sobre Mycobacterium Tuberculosis y/o Activadora de Macrófagos de Extractos de Plantas Mexicanas Conocidas. Master’s Thesis, Universidad Autónoma De Nuevo León Facultad De Ciencias Biológicas, San Nicolás de los Garza, Mexico, 2004. Available online: http://eprints.uanl.mx/1587/1/1020150313.PDF (accessed on 3 August 2022).
- Hernández Gómez, K.A. Análisis Fitoquímico y Citotóxico de Extractos de Gnaphalium Viscosum (Kunth) Sobre Líneas Celulares Humanas Malignas de Cérvix (SiHa) y Mama (Mda). Master’s Thesis, Autonomous University of the State of Hidalgo, Pachuca, Mexico, 2018. Available online: http://dgsa.uaeh.edu.mx:8080/bibliotecadigital/handle/231104/2117 (accessed on 3 August 2022).
- Villagomez-Ibarra, J.R.; Sanchez, M.; Espejo, O.; Zuniga-Estrada, A.; Torres-Valencia, J.M.; Joseph-Nathan, P. Discussed the antimicrobial activity of three Mexican Gnaphalium species. Fitoterapia 2001, 72, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, J.L. Check list of the native vascular plants of Mexico (Catálogo de las plantas vasculares nativas de México). Rev. Mex. De Biodivers. 2016, 87, 559–902. [Google Scholar] [CrossRef]
- Ontiveros-Rodríguez, J.C.; Serrano-Contreras, J.I.; Villagomez-Ibarra, J.R.; García-Gutierrez, H.A.; Zepeda-Vallejo, L.G. A semi-targeted NMR-based chemical profiling of retail samples of Mexican gordolobo. J. Pharmaceut. Biomed. 2022, 212, 114651. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, S.M.; Fetzer, D.E.L.; Custodio de Souza, A.R.; Corazza, M.L.; Hamerski, F.; Yankov, D.S.; Stateva, R.P. Valorization by compressed fluids of Arctium lappa seeds and roots as a sustainable source of valuable compounds. J. CO2 Util. 2022, 56, 101821. [Google Scholar] [CrossRef]
- Mišić, D.; Ašanin, R.; Ivanović, J.; Zizovic, I. Investigation of antibacterial activity of supercritical extracts of plants, as well as of extracts obtained by other technological processes on some bacteria isolated from animals. Acta Vet.-Beogr. 2009, 59, 557–568. [Google Scholar]
- Christie, W.W.; Han, X. Preparation of derivatives of fatty acids. In Lipid Analysis, 4th ed.; Christie, W.W., Han, X., Eds.; Woodhed Publishing: Sawston, UK; Elsevier: Cambridge, UK, 2012; pp. 145–158. ISBN 9780955251245. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem. Anal. 2000, 11, 330–338. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS (•+) assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Coelho, J.P.; Filipe, R.M.; Robalo, M.P.; Boyadzhieva, S.S.; Cholakov, G.S.; Stateva, R.P. Supercritical CO2 extraction of spent coffee grounds. Influence of co-solvents and characterization of the extracts. J. Supercrit. Fluid 2020, 161, 104825. [Google Scholar] [CrossRef]
- Cicek, S.S.; Untersulzner, C.; Schwaiger, S.; Zidorn, C. Caffeoyl-D-Glucaric Acid Derivatives in the Genus Gnaphalium (Asteraceae: Gnaphalieae). Rec. Nat. Prod. 2012, 6, 311–315. [Google Scholar]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature Review. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Allen, Y.; Chen, Y.; Chen, C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A Bioactive Phytochemical with Potential Therapeutic Activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, S.; Cervellati, R.; Seger, C.; Ellmerer, E.P.; About, N.; Renimel, I.; Godenir, C.; André, P.; Gafner, F.; Stuppner, H. Leontopodic acid—A novel highly substituted glucaric acid derivative from edelweiss (Leontopodium alpinum Cass.) and its antioxidative and DNA protecting properties. Tetrahedron 2005, 61, 4621–4630. [Google Scholar] [CrossRef]
- Cho, W.K.; Kim, H.-I.; Kim, S.-Y.; Seo, H.H.; Song, J.; Kim, J.; Shin, D.S.; Jo, Y.; Choi, H.; Lee, J.H.; et al. Anti-Aging Effects of Leontopodium alpinum (Edelweiss) Callus Culture Extract through Transcriptome Profiling. Genes 2020, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar] [PubMed]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; Technical Report Series, No. 916 (TRS 916); WHO: Geneva, Switzerland, 2003; pp. 87–88. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_916.pdf (accessed on 3 August 2022).

| Solvent | Extraction Yield (wt%) |
|---|---|
| Leaves | |
| Ethanol | 17.18 ± 0.84 |
| Ethyl Acetate | 7.27 ± 0.36 |
| n-Hexane | 3.99 ± 0.21 |
| Flowers | |
| Ethanol | 12.23 ± 0.56 |
| Ethyl Acetate | 3.96 ± 0.19 |
| n-Hexane | 3.48 ± 0.17 |
| Stems | |
| Ethanol | 8.20 ± 0.38 |
| Ethyl Acetate | 2.88 ± 0.14 |
| n-Hexane | 1.81 ± 0.09 |
| 3-O-Caffeoylquinic (Chlorogenic) Acid | 5-O-Caffeoylquinic (Neo-Chlorogenic) Acid | Glucaric Acid Derivatives | ||
|---|---|---|---|---|
| Leontopodic Acid A | Leontopodic Acid B | |||
| ng/mg | ||||
| Flowers | 4710.0 | 286.3 | 12.9 | 167.2 |
| Leaves | 3366.9 | 198.8 | 8.1 | 141.1 |
| Stems | 2752.8 | 98.7 | 14.1 | 1132.7 |
| Compound | Two-Phase Solvent Technique | Soxhlet EtOH | ||||
|---|---|---|---|---|---|---|
| Flowers | Leaves | Stems | Flowers | Leaves | Stems | |
| ng/mg | ||||||
| Phenolic Acids | ||||||
| Hydroxycinnamic and caffeoylquinic acid derivatives | ||||||
| caffeic acid | 3.15 | 4.28 | 1.95 | 5.99 | 17.21 | 9.24 |
| o-coumaric acid | 2.21 | 5.03 | 4.35 | 10.61 | 16.09 | 4.65 |
| p-coumaric acid | 0.14 | 0.16 | 0.19 | 0.43 | 0.31 | 0.25 |
| m-coumaric acid | 0.05 | 0.81 | 0.18 | 0.34 | 0.39 | 0.89 |
| ferulic acid | 5.04 | 4.77 | 4.62 | 14.01 | 19.23 | 6.11 |
| cinnamic acid | 2.63 | 11.14 | 3.48 | 3.99 | 12.53 | 4.56 |
| 3-O-caffeoylquinic (chlorogenic) acid | 3618.95 | 2116.35 | 1925.44 | 5668.11 | 2421.34 | 2041.15 |
| Hydroxybenzoic acid derivatives | ||||||
| gallic acid | 6.24 | 1.71 | 5.94 | 5.94 | 1.48 | 0.57 |
| vanillic acid | 69.66 | 64.21 | 46.35 | 10.23 | 21.97 | 1.89 |
| ellagic acid | 2.79 | 11.59 | 1.75 | 9.78 | 2.98 | 7.96 |
| gentisic acid | 351.82 | 181.58 | 52.26 | 866.72 | 465.26 | 284.73 |
| protocatechinic acid | 55.46 | 53.48 | 14.09 | 35.58 | 33.44 | 0.23 |
| o-hydroxybenzoic acid | 22.95 | 19.38 | 7.22 | 0.33 | 2.35 | 0.93 |
| m-hydroxybenzoic acid | 4.03 | 4.93 | 0.96 | 7.25 | 6.73 | 4.26 |
| syringic acid | 5.46 | 9.11 | 6.73 | 12.92 | 16.12 | 3.02 |
| 3-OH-4-methoxybenzoic acid | 46.41 | 52.70 | 263.47 | 8.58 | 46.51 | 24.04 |
| Flavonoids | ||||||
| Flavonols | ||||||
| quercetin | 331.41 | 39.93 | 17.41 | 450.27 | 92.48 | 30,96 |
| myrecitrin | 790.80 | 200.78 | 56.72 | 1288.26 | 398.57 | 439.65 |
| myrecitin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| rutin | 4.80 | 1.46 | 0.98 | 5.91 | 38.25 | 90.06 |
| resveratrol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| kaempferol | 798.86 | 82.59 | 13.79 | 1106.91 | 72.32 | 39.68 |
| kaempferol-3-O-glycoside | 45999.92 | 6589.52 | 1207.40 | 76129.67 | 7993.67 | 9827.97 |
| kaempferitin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| fisetin | 7.93 | 2.17 | 1.01 | 21.12 | 2.37 | 2.32 |
| Flavones | ||||||
| luteolin | 6.24 | 1.71 | 0.44 | 5.94 | 1.48 | 0.57 |
| apigenin | 77.97 | 7.22 | 1.02 | 81.78 | 4.47 | 2.39 |
| Flavan-3-ols | ||||||
| catechin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| epicatechin | 0.21 | 0.30 | 0.06 | 1.31 | 0.29 | 0.13 |
| epigallocatechin | 3.74 | n.d. | n.d. | n.d. | n.d. | n.d. |
| epigallocatechin gallate | 0.00 | n.d. | 0.01 | 0.01 | 0.02 | 0.02 |
| epicatechin gallate | 0.19 | 0.22 | 1.91 | 0.22 | 0.12 | 0.16 |
| Flavanones | ||||||
| hisperidin | 2.43 | 0.028 | 0.03 | 0.71 | 0.29 | 0.42 |
| naringenin | 11.02 | 2.08 | 0.58 | 19.91 | 2.52 | 1.95 |
| Proanthocyanidins | ||||||
| procyanidin B1 | 7.14 | 7.15 | 7.14 | 7.17 | 7.13 | 7.14 |
| procyanidin B3 | n.d. | n.d. | 3.19 | n.d. | 4.86 | n.d. |
| Caffeoyl-D-glucaric acid derivatives | ||||||
| Leontopodic acid A | 29.55 | 211.28 | 119.13 | 6.62 | 16.80 | 21.93 |
| Leontopodic acid B | 134.94 | 95.02 | 1011.05 | 2.23 | 71.36 | 4.37 |
| Compound | Flowers | Leaves | Stems | Leaves (5% EtOH) |
|---|---|---|---|---|
| [ng/mg] | ||||
| Phenolic Acids | ||||
| Hydroxycinnamic and caffeoylquinic acid derivatives | ||||
| caffeic acid | 1999.37 | 604.53 | 113.46 | 39.30 |
| o-coumaric acid | 173.29 | 5.59 | 36.91 | 4.95 |
| p-coumaric acid | 1.38 | 0.38 | 0.36 | 0.42 |
| m-coumaric acid | 10.32 | 2.30 | 1.74 | 1.04 |
| ferulic acid | 206.24 | 39.26 | 22.39 | 12.88 |
| cinnamic acid | 9.94 | 21.07 | 4.85 | 15.31 |
| 3-O-caffeoylquinic (chlorogenic) acid | 62.49 | 60.89 | 16.64 | 2.88 |
| Hydroxybenzoic acid derivatives | ||||
| gallic acid | 6.73 | 4.07 | 10.46 | 0.21 |
| vanillic acid | 772.21 | 162.03 | 51.54 | 46.25 |
| ellagic acid | 24.70 | 3.02 | 1.69 | 1.19 |
| gentisic acid | 1091.63 | 184.44 | 49.51 | 14.93 |
| protocatechinic acid | 32.55 | 24.56 | 1.21 | 1.33 |
| o-hydroxybenzoic acid | 174.88 | 41.38 | 17.79 | 8.04 |
| m-hydroxybenzoic acid | 47.90 | 19.33 | 5.39 | 3.99 |
| syringic acid | 160.85 | 50.37 | 33.33 | 17.08 |
| 3-OH-4-methoxybenzoic acid | 726.99 | 138.40 | 48.99 | 45.90 |
| Flavonoids | ||||
| Flavonols | ||||
| quercetin | 3215.37 | 206.14 | 123.49 | 21.76 |
| myrecitrin | 307.14 | 32.38 | 5.62 | 0.95 |
| myrecitin | n.d | n.d | n.d | n.d |
| rutin | 17.33 | 11.49 | 5.13 | 1.98 |
| resveratrol | n.d. | n.d. | n.d. | n.d. |
| kaempferol | 10731.82 | 383.51 | 99.97 | 33.20 |
| kaempferol-3-O-glycoside | 2574.92 | 221.52 | 45.81 | 8.49 |
| kaempferitin | n.d | n.d | 0.01 | n.d |
| fisetin | 30.46 | 3.55 | 0.36 | 0.14 |
| Flavones | ||||
| luteolin | 434.97 | 14.99 | 4.89 | 1.54 |
| apigenin | 15.01 | 0.19 | 0.19 | 0.17 |
| Flavan-3-ols | ||||
| catechin | n.d. | n.d. | n.d. | n.d. |
| epicatechin | 1.70 | 0.01 | n.d. | 0.00 |
| epigallocatechin | 1.35 | 0.11 | 0.01 | 0.02 |
| epigallocatechin gallate | 0.10 | n.d. | n.d. | n.d. |
| epicatechin gallate | 0.12 | 0.02 | 0.01 | 0.01 |
| Flavanones | ||||
| hisperidin | 1.19 | 0.71 | 0.89 | 0.76 |
| naringenin | 3294.13 | 83.91 | 13.42 | 23.06 |
| Proanthocyanidins | ||||
| procyanidin B1 | n.d. | n.d. | n.d. | n.d. |
| procyanidin B3 | n.d. | n.d. | n.d. | n.d. |
| Glucaric acid derivatives | ||||
| Leontopodic acid A | n.d. | n.d. | n.d. | n.d. |
| Leontopodic acid B | n.d. | 34.93 | 0.39 | n.d. |
| Extraction Method | TPC | DPPH | ABTS | ||
|---|---|---|---|---|---|
| Two-Phase Solvent | Quercetin eq. [µg/mg] | Trolox eq. [mM] | IC50 mg Extract | Trolox eq. [mM] | IC50 mg Extract |
| flowers | 130.87 | 12.72 | 1.34 | 9.48 | 2.27 |
| leaves | 95.70 | 9.25 | 2.72 | 6.07 | 4.11 |
| stems | 76.83 | 4.93 | 2.46 | 4.01 | 7.01 |
| Soxhlet ethanol | |||||
| flowers | 113.28 | 6.85 | 0.68 | 5.44 | 1.24 |
| leaves | 69.54 | 3.76 | 0.96 | 3.27 | 2.02 |
| stems | 58.81 | 4.08 | 1.96 | 2.15 | 3.22 |
| scCO2 + 10% ethanol | |||||
| flowers | 162.11 | 1.64 | 3.15 | 2.71 | 36.08 |
| leaves | 36.96 | 0.65 | 18.45 | 1.14 | 10.98 |
| stems | 6.39 | 0.25 | 90.10 | 0.35 | 48.20 |
| Leaves 5% EtOH | 8.05 | 0.22 | 126.42 | 0.55 | 25.93 |
| Fatty Acid | Soxhlet n-Hexane | scCO2, T = 40 °C, p = 40 MPa | scCO2, T = 60 °C, p = 40 MPa | scCO2, T = 60 °C, p = 50 MPa | ||||
|---|---|---|---|---|---|---|---|---|
| % | Str. Dev. | % | Str. Dev. | % | Str. Dev. | % | Str. Dev. | |
| Lauric acid, 12:0 | n.d. | n.d. | 0.40 | 0.05 | n.d | |||
| Myristic acid, 14:0 | 4.68 | 0.48 | 5.67 | 0.97 | 3.04 | 0.40 | 3.19 | 1.2 |
| Palmitic acid, 16:0 | 22.77 | 1.13 | 49.42 | 9.59 | 31.57 | 5.53 | 19.36 | 6.35 |
| Palmitoleic acid, 16:1 | n.d. | n.d. | - | n.d. | 4.46 | 2.62 | ||
| Stearic acid, 18:0 | 13.67 | 1.35 | n.d. | n.d | n.d. | |||
| Linoleic acid, 18:2 | 20.42 | 3.44 | 31.34 | 7.44 | 22.25 | 11.57 | 27.35 | 4.83 |
| Arachidic acid, 20:0 | n.d | n.d. | - | 11.12 | 0.81 | 33.32 | 15.8 | |
| γ-Linolenic acid, 18:3 | 25.48 | 8.38 | n.d. | 22.05 | 6.17 | n.d. | ||
| Behenic acid, 22:0 | 5.42 | 2.20 | 7.15 | 1.79 | 4.83 | 0.94 | 6.52 | 0.7 |
| Lignoceric acid, 24:0 | 7.55 | 3.60 | 6.41 | 1.56 | 4.74 | 1.59 | 5.81 | 1.28 |
| SFA | 54.1 | 68.66 | 55.7 | 68.18 | ||||
| MUFA | n.d. | n.d. | n.d. | 4.46 | ||||
| DUFA | 20.42 | 31.34 | 22.25 | 27.35 | ||||
| PUFA | 25.48 | n.d. | 22.05 | n.d. | ||||
| PUFA:SFA | 0.471 | 0.0 | 0.386 | 0.0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyadzhieva, S.; Coelho, J.A.P.; Errico, M.; Reynel-Avilla, H.E.; Yankov, D.S.; Bonilla-Petriciolet, A.; Stateva, R.P. Assessment of Gnaphalium viscosum (Kunth) Valorization Prospects: Sustainable Recovery of Antioxidants by Different Techniques. Antioxidants 2022, 11, 2495. https://doi.org/10.3390/antiox11122495
Boyadzhieva S, Coelho JAP, Errico M, Reynel-Avilla HE, Yankov DS, Bonilla-Petriciolet A, Stateva RP. Assessment of Gnaphalium viscosum (Kunth) Valorization Prospects: Sustainable Recovery of Antioxidants by Different Techniques. Antioxidants. 2022; 11(12):2495. https://doi.org/10.3390/antiox11122495
Chicago/Turabian StyleBoyadzhieva, Stanislava, Jose A. P. Coelho, Massimiliano Errico, H. Elizabeth Reynel-Avilla, Dragomir S. Yankov, Adrian Bonilla-Petriciolet, and Roumiana P. Stateva. 2022. "Assessment of Gnaphalium viscosum (Kunth) Valorization Prospects: Sustainable Recovery of Antioxidants by Different Techniques" Antioxidants 11, no. 12: 2495. https://doi.org/10.3390/antiox11122495
APA StyleBoyadzhieva, S., Coelho, J. A. P., Errico, M., Reynel-Avilla, H. E., Yankov, D. S., Bonilla-Petriciolet, A., & Stateva, R. P. (2022). Assessment of Gnaphalium viscosum (Kunth) Valorization Prospects: Sustainable Recovery of Antioxidants by Different Techniques. Antioxidants, 11(12), 2495. https://doi.org/10.3390/antiox11122495











