Quercetin Improves Pulmonary Function and Prevents Emphysema Caused by Exposure to Cigarette Smoke in Male Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cigarette Smoke Exposure Protocol
2.3. Pulmonary Function
2.4. Blood Collection
2.5. Collection and Analysis of Bronchoalveolar Lavage Fluid (BALF)
2.6. Lung Collection
2.7. Immunoenzymatic Assay for Inflammatory Mediators
2.8. Biomarkers of Oxidative Stress and Antioxidant Defense
2.9. Stereological and Morphometric Analysis
2.10. Statistical Analysis
3. Results
3.1. Analysis of Pulmonary Function in the Experimental Groups
3.2. Hematological Data
3.3. Analysis of Quercetin Administration and Cigarette Smoke Exposure on Cell Recruitment to BALF
3.4. Inflammatory Cytokine Levels in Pulmonary Homogenate
3.5. Biomarkers Analysis of Oxidative Damage and Antioxidant Defense
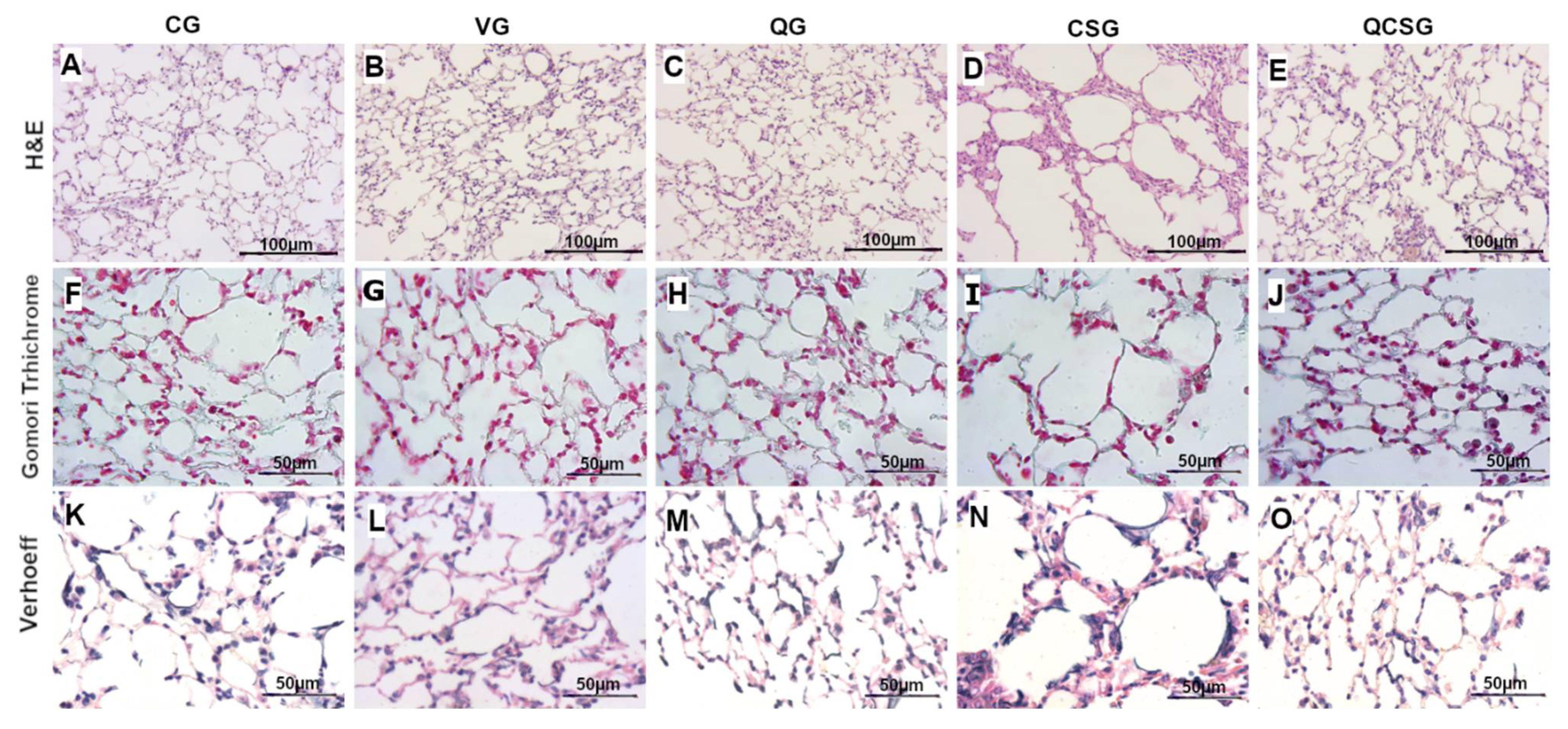
3.6. Morphometric Analyses of the Lung Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Day, W.L. Healthy Lungs for All—Global Initiative for Chronic Obstructive Lung Disease—GOLD. Available online: https://goldcopd.org/world-lung-day-2019-healthy-lungs-for-all (accessed on 14 July 2021).
- Agusti, A.; Hogg, J.C. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019, 381, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Poh, T.Y.; Mac Aogain, M.; Chan, A.K.; Yii, A.C.; Yong, V.F.; Tiew, P.Y.; Koh, M.S.; Chotirmall, S.H. Understanding COPD-overlap syndromes. Expert Rev. Respir. Med. 2017, 11, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Quaderi, S.A.; Hurst, J.R. The unmet global burden of COPD. Glob. Health Epidemiol. Genom. 2018, 3, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.D. COPD and the response of the lung to tobacco smoke exposure. Pulm. Pharmacol. Ther. 2010, 23, 376–383. [Google Scholar] [CrossRef]
- Alexander, L.E.C.; Shin, S.; Hwang, J.H. Inflammatory diseases of the lung induced by conventional cigarette smoke: A review. Chest 2015, 148, 1307–1322. [Google Scholar] [CrossRef]
- Kim, J.W.; Zhou, Z.; Yun, H.; Park, S.; Choi, S.J.; Lee, S.H.; Lim, C.W.; Lee, K.; Kim, B. Cigarette smoking differentially regulates inflammatory responses in a mouse model of nonalcoholic steatohepatitis depending on exposure time point. Food Chem. Toxicol. 2020, 135, 110930. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, W.; Ma, C.; Wang, J.; Gao, X.; Wei, L. Macrophages Inhibit Ciliary Protein Levels by Secreting BMP-2 Leading to Airway Epithelial Remodeling Under Cigarette Smoke Exposure. Front. Mol. Biosci 2021, 8, 663987. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Mutlu, G.M.; Cai, H. More to Explore: Further Definition of Risk Factors for COPD—Differential Gender Difference, Modest Elevation in PM2. 5, and e-Cigarette Use. Front. Physiol. 2021, 12, 423. [Google Scholar] [CrossRef]
- Kazi, A.A.; Subba Reddy, B.V.; Ravithej Singh, L. Synthetic approaches to FDA approved drugs for asthma and COPD from 1969 to 2020. Bioorg. Med. Chem. 2021, 41, 116212. [Google Scholar] [CrossRef]
- Lo Bello, F.; Hansbro, P.M.; Donovan, C.; Coppolino, I.; Mumby, S.; Adcock, I.M.; Caramori, G. New drugs under development for COPD. Expert Opin. Emerg. Drugs 2020, 25, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Matera, M.G.; Cazzola, M.; Page, C. Prospects for COPD treatment. Curr. Opin. Pharmacol. 2021, 56, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Chattoraj, S.S.; Chattoraj, A.; Burgess, J.R.; Curtis, J.L.; Martinez, F.J.; Zick, S.; Hershenson, M.B.; et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir. Res. 2010, 11, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.L.; Olivier, Y.; Trouillas, P. Free radical scavenging by natural polyphenols: Atom versus electron transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.P.; Reddy, H.; Caivano, M.; Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000, 351, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Li, S.; Xin, Q.; Yuan, M.; Li, H.; Song, X.; Gao, H.; Pervaiz, N.; Sun, X.; et al. Quercetin Inhibits the Migration and Invasion of HCCLM3 Cells by Suppressing the Expression of p-Akt1, Matrix Metalloproteinase (MMP) MMP-2, and MMP-9. Med. Sci. Monit. 2018, 24, 2583–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva Araujo, N.P.; de Matos, N.A.; Leticia Antunes Mota, S.; Farias de Souza, A.B.; Dantas Cangussu, S.; Cunha Alvim de Menezes, R.; Silva Bezerra, F. Quercetin Attenuates Acute Lung Injury Caused by Cigarette Smoke Both In Vitro and In Vivo. COPD 2020, 17, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Farazuddin, M.; Mishra, R.; Jing, Y.; Srivastava, V.; Comstock, A.T.; Sajjan, U.S. Quercetin prevents rhinovirus-induced progression of lung disease in mice with COPD phenotype. PLoS ONE 2018, 13, e0199612. [Google Scholar] [CrossRef]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, K.K.D.; de Oliveira Ramos, C.; Martins, T.L.; Costa, G.P.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Cangussu, S.D.; Costa, D.C.; Bezerra, F.S. Lycopene mitigates pulmonary emphysema induced by cigarette smoke in a murine model. J. Nutr. Biochem. 2019, 65, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Machado-Junior, P.A.; Araujo, N.P.S.; Souza, A.B.F.; Castro, T.F.; Oliveira, M.; Costa, G.P.; Matos, N.A.; Vieira, P.M.A.; Talvani, A.; Bezerra, F.S.; et al. Protective Effects of Quercetin on Livers from Mice Exposed to Long-Term Cigarette Smoke. Biomed Res. Int. 2020, 2020, 2196207. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.O.; Campos, K.K.D.; Costa, G.P.; Cangussú, S.D.; Talvani, A.; Bezerra, S.F. Taurine treatment decreases inflammation and oxidative stress in lungs of adult mice exposed to cigarette smoke. Regul. Toxicol. Pharmacol. 2018, 98, 50–57. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mandarim-de-Lacerda, C.A. Stereological tools in biomedical research. An. Acad. Bras. Ciências 2003, 75, 469–486. [Google Scholar] [CrossRef]
- Valenca, S.S.; Castro, P.; Pimenta, W.A.; Lanzetti, M.; Silva, S.V.; Barja-Fidalgo, C.; Koatz, V.L.; Porto, L.C. Light cigarette smoke-induced emphysema and NFkappaB activation in mouse lung. Int. J. Exp. Pathol. 2006, 87, 373–381. [Google Scholar] [CrossRef]
- Churg, A.; Wang, R.D.; Tai, H.; Wang, X.; Xie, C.; Wright, J.L. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am. J. Respir. Crit. Care Med. 2004, 170, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Skoczynski, S.; Krzyzak, D.; Studnicka, A.; Ogonowski, M.; Tobiczyk, E.; Brozek, G.; Pierzchala, W.; Barczyk, A. Chronic Obstructive Pulmonary Disease and Platelet Count. Adv. Exp. Med. Biol. 2019, 1160, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Mandal, A.K. Conjugation of para-benzoquinone of Cigarette Smoke with Human Hemoglobin Leads to Unstable Tetramer and Reduced Cooperative Oxygen Binding. J. Am. Soc. Mass Spectrom. 2018, 29, 2048–2058. [Google Scholar] [CrossRef] [PubMed]
- Kogel, U.; Wong, E.T.; Szostak, J.; Tan, W.T.; Lucci, F.; Leroy, P.; Titz, B.; Xiang, Y.; Low, T.; Wong, S.K.; et al. Impact of whole-body versus nose-only inhalation exposure systems on systemic, respiratory, and cardiovascular endpoints in a 2-month cigarette smoke exposure study in the ApoE (-/-) mouse model. J. Appl. Toxicol. 2021, 41, 1598–1619. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Hattori, N.; Haruta, Y.; Sugiyama, A.; Iwamoto, H.; Ishikawa, N.; Fujitaka, K.; Murai, H.; Tanaka, J.; Kohno, N. Effect of increasing respiratory rate on airway resistance and reactance in COPD patients. Respirology 2015, 20, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Akata, K.; van Eeden, S.F. Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, L.E.; Lourenço, J.D.; Silva, K.R.; Santana, F.P.R.; Kohler, J.B.; Moreira, A.R.; Velosa, A.P.P.; Prado, C.M.; Vieira, R.P.; Aun, M.V. Th17/Treg imbalance in COPD development: Suppressors of cytokine signaling and signal transducers and activators of transcription proteins. Sci. Rep. 2020, 10, 15287. [Google Scholar] [CrossRef]
- Starkey, M.R.; Plank, M.W.; Casolari, P.; Papi, A.; Pavlidis, S.; Guo, Y.; Cameron, G.J.M.; Haw, T.J.; Tam, A.; Obiedat, M.; et al. IL-22 and its receptors are increased in human and experimental COPD and contribute to pathogenesis. Eur. Respir. J. 2019, 54, 1800174. [Google Scholar] [CrossRef]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Kubysheva, N.; Boldina, M.; Eliseeva, T.; Soodaeva, S.; Klimanov, I.; Khaletskaya, A.; Bayrasheva, V.; Solovyev, V.; Villa-Vargas, L.A.; Ramirez-Salinas, M.A.; et al. Relationship of Serum Levels of IL-17, IL-18, TNF-alpha, and Lung Function Parameters in Patients with COPD, Asthma-COPD Overlap, and Bronchial Asthma. Mediat. Inflamm. 2020, 2020, 4652898. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.T.; Cervilha, D.A.d.B.; Lourenço, J.D.; Gonçalves, N.G.; Volpini, R.A.; Caldini, E.G.; Landman, G.; Lin, C.J.; Velosa, A.P.P.; Teodoro, W.P.R. Th17/Treg imbalance in COPD progression: A temporal analysis using a CS-induced model. PLoS ONE 2019, 14, e0209351. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, J.F. IL-22 Plays a Critical Role in Maintaining Epithelial Integrity During Pulmonary Infection. Front. Immunol. 2020, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. The cytokine network in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2009, 41, 631–638. [Google Scholar] [CrossRef]
- Veith, C.; Drent, M.; Bast, A.; van Schooten, F.; Boots, A. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol. Appl. Pharmacol. 2017, 336, 40–48. [Google Scholar] [CrossRef]
- Numazawa, S.; Yoshida, T. Nrf2-dependent gene expressions: A molecular toxicological aspect. J. Toxicol. Sci. 2004, 29, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, D.; Thimmulappa, R.; Navas-Acien, A.; Sandford, A.; Elliott, M.; Singh, A.; Chen, L.; Zhuang, X.; Hogg, J.; Pare, P. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am. J. Respir. Crit. Care Med. 2008, 178, 592–604. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Rial, S.; Girón-Martínez, Á.; Peces-Barba, G.J.A.D.B. Animal models of chronic obstructive pulmonary disease. Arch. Bronconeumol. (Engl. Ed.) 2015, 51, 121–127. [Google Scholar] [CrossRef]
- Shu, J.; Li, D.; Ouyang, H.; Huang, J.; Long, Z.; Liang, Z.; Chen, Y.; Chen, Y.; Zheng, Q.; Kuang, M.; et al. Comparison and evaluation of two different methods to establish the cigarette smoke exposure mouse model of COPD. Sci. Rep. 2017, 7, 15454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peh, H.Y.; Tan, W.D.; Chan, T.K.; Pow, C.W.; Foster, P.S.; Wong, W.F. Vitamin E isoform γ-tocotrienol protects against emphysema in cigarette smoke-induced COPD. Free Radic. Biol. Med. 2017, 110, 332–344. [Google Scholar] [CrossRef]
- Posso, S.V.; Quesnot, N.; Moraes, J.A.; Brito-Gitirana, L.; Kennedy-Feitosa, E.; Barroso, M.V.; Porto, L.C.; Lanzetti, M.; Valença, S.S. AT-RVD1 repairs mouse lung after cigarette smoke-induced emphysema via downregulation of oxidative stress by NRF2/KEAP1 pathway. Int. Immunopharmacol. 2018, 56, 330–338. [Google Scholar] [CrossRef]
- Yang, Y.; Di, T.; Zhang, Z.; Liu, J.; Fu, C.; Wu, Y.; Bian, T. Dynamic evolution of emphysema and airway remodeling in two mouse models of COPD. BMC Pulm. Med. 2021, 21, 134. [Google Scholar] [CrossRef]
- Tanner, L.; Single, A.B. Animal Models Reflecting Chronic Obstructive Pulmonary Disease and Related Respiratory Disorders: Translating Pre-Clinical Data into Clinical Relevance. J. Innate Immun. 2020, 12, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Smith, D.; Chokshi, K.; Ezegbunam, W.; Charoenpong, P.; Foley, K.; Cargill, A.; Geraghty, P. Animal Models of Chronic Obstructive Pulmonary Disease. In COPD An Update in Pathogenesis and Clinical Management; IntechOpen Limited: London, UK, 2018; ISBN 978-953-51-3783-2. [Google Scholar] [CrossRef] [Green Version]

| Nutrients (g) | Standard Diet |
|---|---|
| Carbohydrates | 657 |
| Protein | 193 |
| Fat | 80 |
| Fiber | 10 |
| Mineral mix a | 50 |
| Vitamin mix a | 10 |
| Energy density, kcal | 4220 |
| CG | VG | QG | CSG | QCSG | |
|---|---|---|---|---|---|
| RR (breaths/min) | 163.0 ± 6.23 | 165.0 ± 13.97 | 162.8± 9.87 | 181.8 ± 1.94 a,b,c | 163.7± 2.58 d |
| VT (mL) | 0.302 ± 0.04 | 0.339 ± 0.03 | 0.340 ± 0.09 | 0.321 ± 0.04 | 0.341 ± 0.04 |
| MV (mL/min) | 49.38 ± 7.94 | 55.07 ± 8.29 | 55.07 ± 13.06 | 58.31 ± 6.91 | 55.65 ± 6.97 |
| CG | VG | QG | CSG | QCSG | |
|---|---|---|---|---|---|
| Erythrocytes (×106/mm3) | 7.99 ± 0.28 | 7.45 ± 0.29 | 7.53 ± 0.55 | 12.55 ± 1.28 a,b,c | 11.98 ± 0.95 a,b,c |
| Hemoglobin (g/dL) | 13.26 ± 0.56 | 11.91 ± 0.29 | 12.89 ± 0.56 | 19.09 ± 1.73 a,b,c | 19.16 ± 2.07 a,b,c |
| Hematocrit (%) | 36.61 ± 0.93 | 33.33 ± 0.42 | 34.64 ± 1.72 | 51.47 ± 5.90 a,b,c | 44.86 ± 3.81 a,b,c,d |
| CG | VG | QG | CSG | QCSG | |
|---|---|---|---|---|---|
| Total leucocytes (×103/mL) | 4.87 ± 1.02 | 4.12 ± 0.52 | 4.17 ± 0.70 | 25.17± 3.48 a,b,c | 13.13 ± 2.29 a,b,c,d |
| Macrophages (×103/mL) | 4.84 ± 1.00 | 4.10 ± 0.52 | 4.15 ± 0.71 | 11.10 ± 2.13 a,b,c | 8.02 ± 1.47 a,b,c,d |
| Lymphocytes (×103/mL) | 0.015 (0.00 ± 0.052) | 0.00 (0.00 ± 0.052) | 0.00 (0.00 ± 0.040) | 1.36 (0.86 ± 2.12) a,b,c | 0.90 (0.69 ± 1.06) c |
| Neutrophils (×103/mL) | 0.00 (0.00 ± 0.00) | 0.00 (0.00 ± 0.00) | 0.00 (0.00 ± 0.00) | 12.33 (8.84 ± 16.62) a,b,c | 4.19 (3.53 ± 5.30) |
| CG | VG | QG | CSG | QCSG | |
|---|---|---|---|---|---|
| IL-10 (pg/mL) | 340.8 ± 136.81 | 287.4 ± 105.33 | 374.4 ± 92.11 | 631.6 ± 224.83 a,b,c | 370.6 ± 59.20 d |
| IL-13 (pg/mL) | 95.6 ± 23.46 | 104.4 ± 44.9 | 91.8 ± 30.21 | 240.4 ± 108.8 a,b,c | 135.6 ± 33,69 d |
| IL-17 (pg/mL) | 109.8 ± 27.09 | 159.6 ± 26.17 | 133.4 ± 13.58 | 264.2 ± 89.39 a,b,c | 385.8 ± 173.8 a,b |
| IL-22 (pg/mL) | 598.2 ± 166.15 | 649.8 ± 152.25 | 666.2 ± 257.44 | 918.8 ± 140.27 a | 547.2 ± 44.64 d |
| CG | VG | QG | CSG | QCSG | |
|---|---|---|---|---|---|
| SOD (U/mg protein) | 30.54 ± 4.72 | 28.03 ± 8.79 | 27.26 ± 10.04 | 18.28 ± 4.94 a | 32.57 ± 3.69 d |
| CAT (U/mg protein) | 0.77 ± 0.29 | 0.68 ± 0.22 | 0.67 ± 0.30 | 0.32 ± 0.13 a | 0.77 ± 0.23 d |
| GSH/GSSG ratio | 2.94 (2.68–3.84) | 2.61 (2.51–2.94) | 2.74 (2.53–2.94) | 1.75 (1.39–2.04) a,c | 1.90 (1.80–1.92) a |
| MPO (U/mg protein) | 0.21 ± 0.05 | 0.23 ± 0.04 | 0.23 ± 0.03 | 0.56 ± 0.29 a,b,c | 0.20 ± 0.03 d |
| TBARS (nmol/mg protein) | 0.76 ± 0.11 | 0.94 ± 0.30 | 0.78 ± 0.17 | 1.68 ± 0.68 a,b,c | 1.04 ± 0.28 d |
| Protein carbonyl (nmol/mg protein) | 6.36 ± 0.55 | 6.98 ± 2.71 | 7.74 ± 1.32 | 14.98 ± 2.10 a,b,c | 7.38 ± 1.65 d |
| CG | VG | QG | CSG | QCSG | |
|---|---|---|---|---|---|
| Vv [a] (%) | 60.00 (57.50–60.94) | 55.31 (53.75–59.38) | 57.50 (56.25–60.94) | 66.25 (64.69–70.31) a,b,c | 55.00 (53.13–58.44) d |
| Vv [sa] (%) | 41.56 (39.38–44.06) | 45.31 (44.38–47.19) | 42.81 (41.56–44.69) | 33.75 (29.69–35.31) a,b,c | 46.56 (41.88–48.44) d |
| Vv [col] (%) | 25.16 (23.20–30.47) | 25.47 (24.53–29.69) | 29.38 (21.25–34.53) | 7.97 (6.50–9.30) a,b,c | 23.60 (19.69–24.69) |
| Vv [ela] (%) | 18.13 (16.02–20.16) | 17.50 (16.17–18.60) | 16.10 (14.69–20.39) | 12.19 (11.72–12.74) a,b,c | 15.94 (13.83–16.33) |
| Lm (µm) | 127.6 ± 9.98 | 140.7 ± 9.72 | 121.2 ± 8.47 | 257.9 ± 69.61 a,b,c | 140.1 ± 6.88 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, N.P.d.S.; de Matos, N.A.; Oliveira, M.; de Souza, A.B.F.; Castro, T.d.F.; Machado-Júnior, P.A.; de Souza, D.M.S.; Talvani, A.; Cangussú, S.D.; de Menezes, R.C.A.; et al. Quercetin Improves Pulmonary Function and Prevents Emphysema Caused by Exposure to Cigarette Smoke in Male Mice. Antioxidants 2022, 11, 181. https://doi.org/10.3390/antiox11020181
Araújo NPdS, de Matos NA, Oliveira M, de Souza ABF, Castro TdF, Machado-Júnior PA, de Souza DMS, Talvani A, Cangussú SD, de Menezes RCA, et al. Quercetin Improves Pulmonary Function and Prevents Emphysema Caused by Exposure to Cigarette Smoke in Male Mice. Antioxidants. 2022; 11(2):181. https://doi.org/10.3390/antiox11020181
Chicago/Turabian StyleAraújo, Natália Pereira da Silva, Natália Alves de Matos, Michel Oliveira, Ana Beatriz Farias de Souza, Thalles de Freitas Castro, Pedro Alves Machado-Júnior, Débora Maria Soares de Souza, André Talvani, Sílvia Dantas Cangussú, Rodrigo Cunha Alvim de Menezes, and et al. 2022. "Quercetin Improves Pulmonary Function and Prevents Emphysema Caused by Exposure to Cigarette Smoke in Male Mice" Antioxidants 11, no. 2: 181. https://doi.org/10.3390/antiox11020181
APA StyleAraújo, N. P. d. S., de Matos, N. A., Oliveira, M., de Souza, A. B. F., Castro, T. d. F., Machado-Júnior, P. A., de Souza, D. M. S., Talvani, A., Cangussú, S. D., de Menezes, R. C. A., & Bezerra, F. S. (2022). Quercetin Improves Pulmonary Function and Prevents Emphysema Caused by Exposure to Cigarette Smoke in Male Mice. Antioxidants, 11(2), 181. https://doi.org/10.3390/antiox11020181







