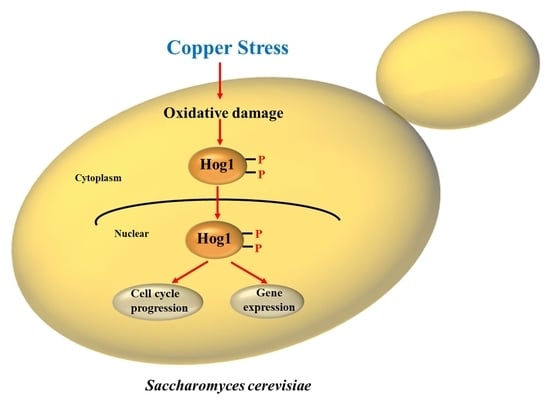
Involvement of the High-Osmolarity Glycerol Pathway of Saccharomyces Cerevisiae in Protection against Copper Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains, Media, and Cultures
2.2. Cell Growth Analysis and Spot Susceptibility Assay
2.3. Analysis of Oxidative Damage and Antioxidant Enzyme Activity
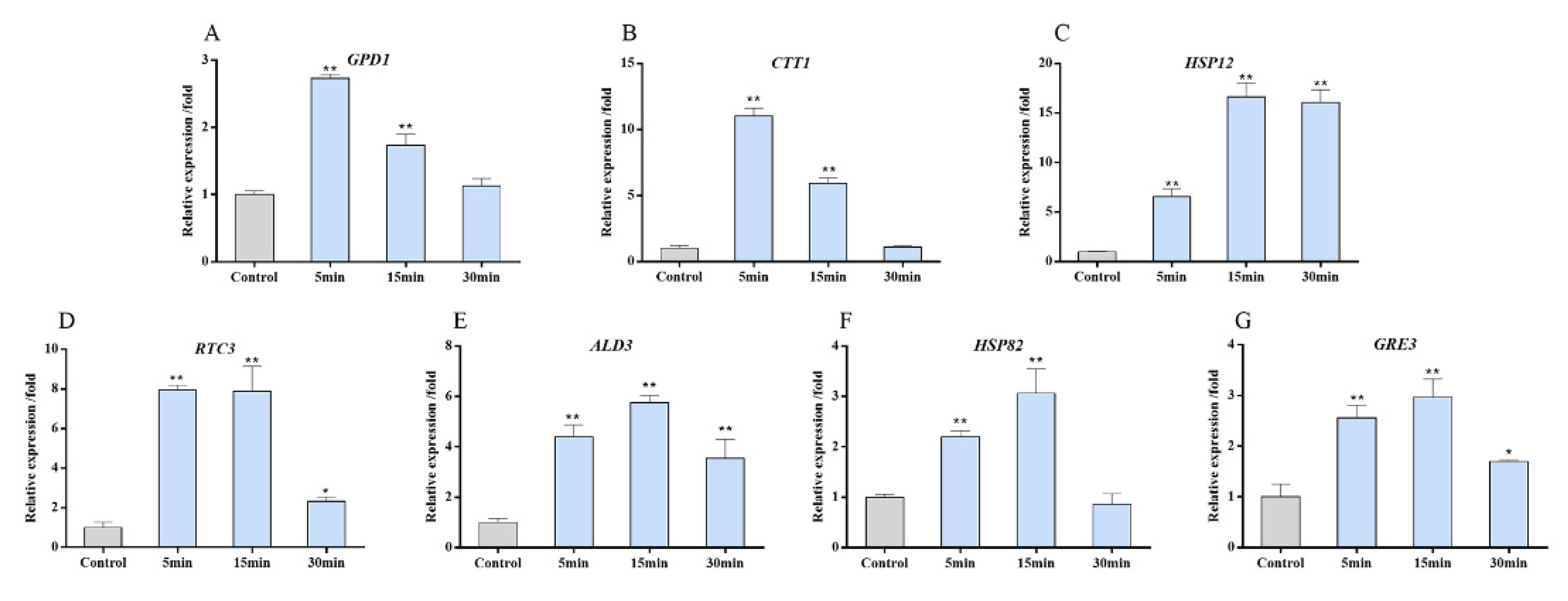
2.4. Gene Expression Confirmation by Quantitative Real-Time PCR
2.5. Subcellular Localization Analysis
2.6. Western Blotting
2.7. Cell Cycle Analysis
2.8. Statistical Analysis
3. Results
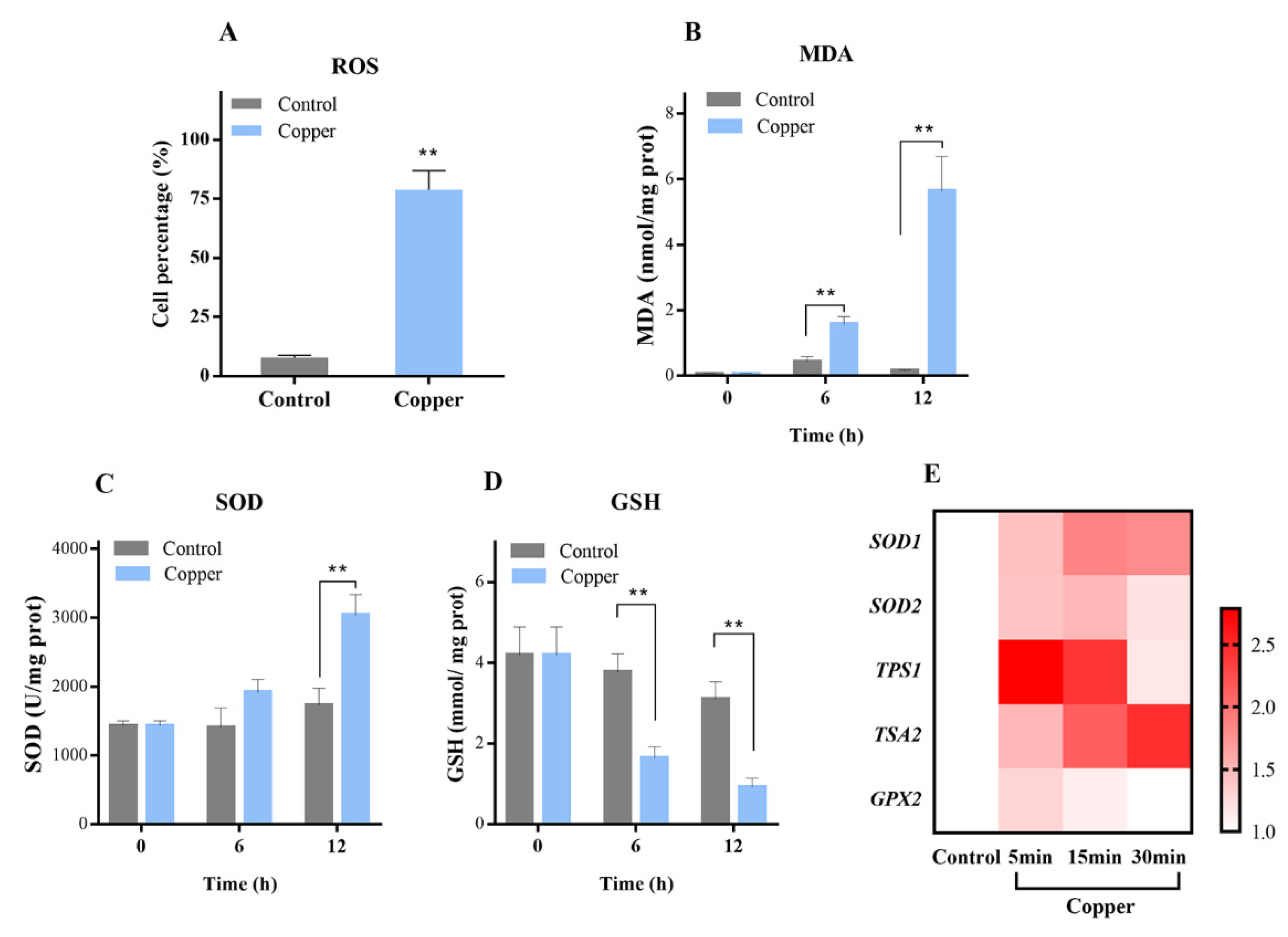
3.1. Copper Distinctly Induced Oxidative Stress
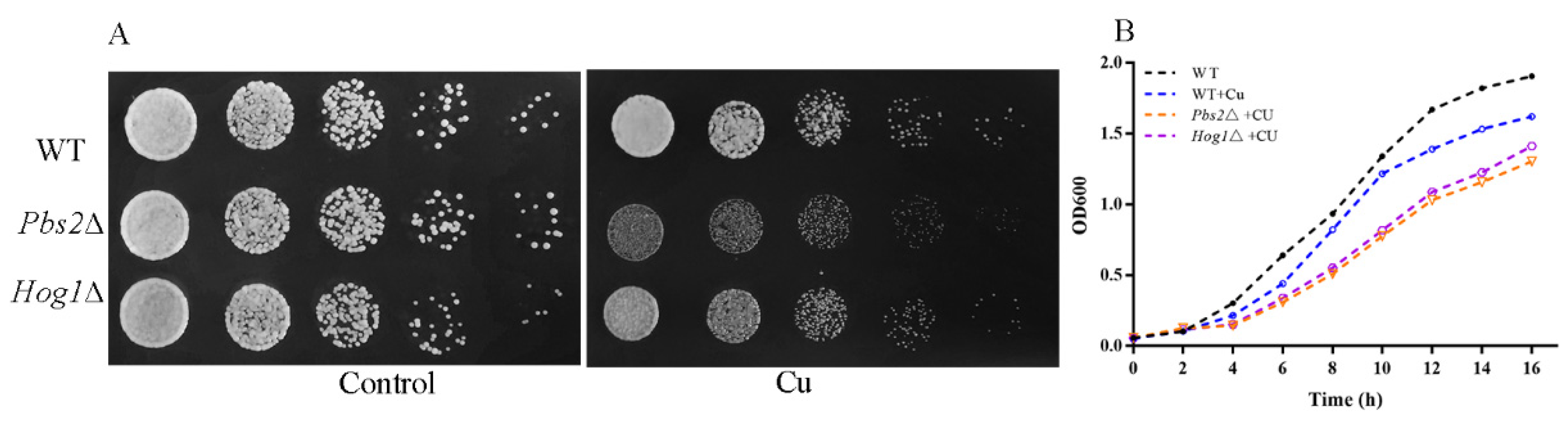
3.2. HOG Pathway Activation under Copper Exposure
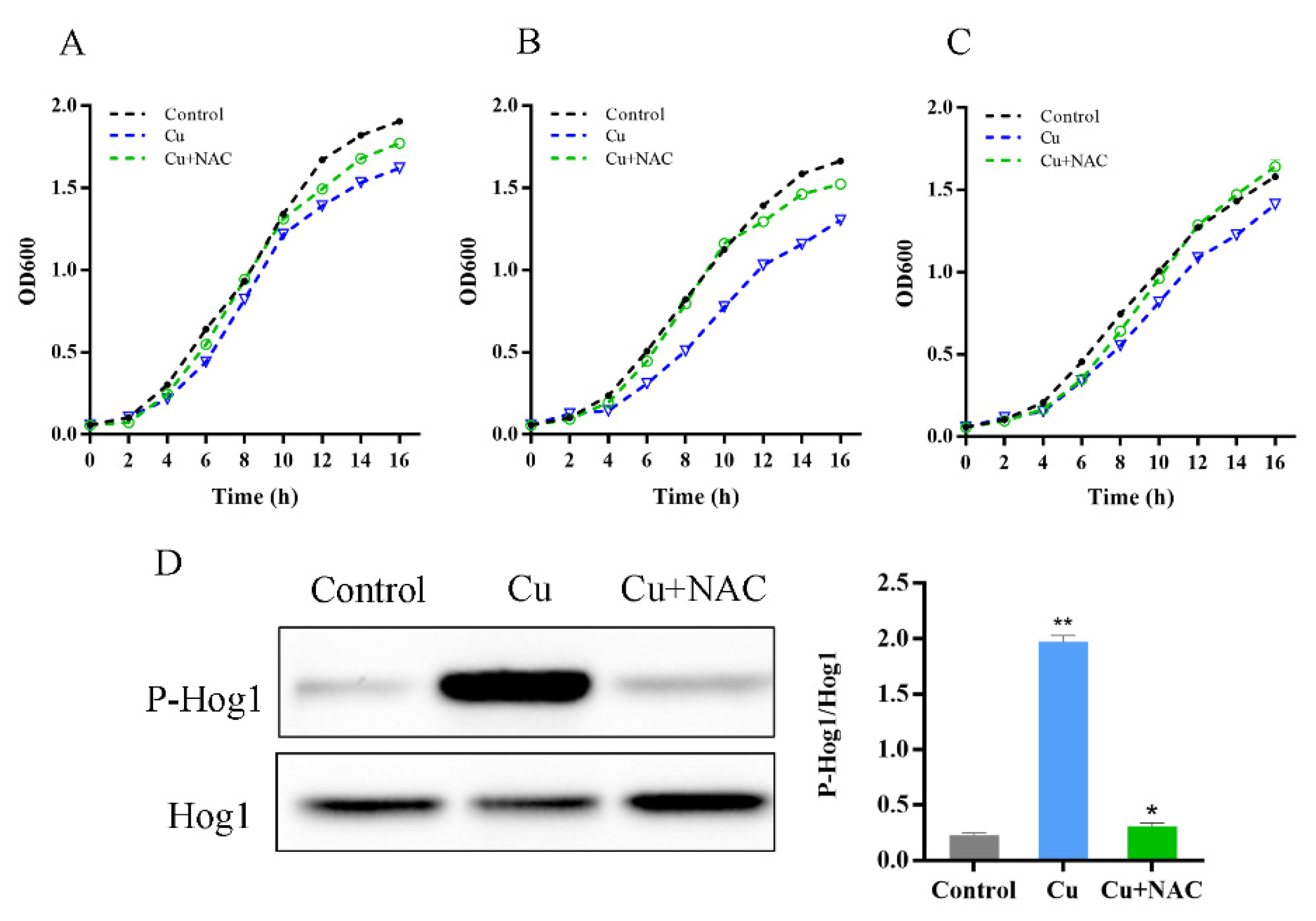
3.3. Oxidative Stress Is Necessary for Hog1 Activation by Copper
3.4. Hog1 Is Partially Translocated to the Nucleus under Copper Exposure
3.5. Target Genes Expression of the HOG Pathway after Copper Exposure
3.6. Hog1 Is Partially Involved in Mediation of Cell-Cycle Delay upon Copper Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirchman, P.A.; Botta, G. Copper supplementation increases yeast life span under conditions requiring respiratory metabolism. Mech. Ageing Dev. 2007, 128, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in fungal virulence. FEMS Microbiol. Rev. 2018, 42, fux050. [Google Scholar] [CrossRef] [Green Version]
- Dong, K.; Addinall, S.G.; Lydall, D.; Rutherford, J.C. The Yeast Copper Response Is Regulated by DNA Damage. Mol. Cell. Biol. 2013, 33, 4041–4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, C.; Carvalho, A.; Gaivão, I.; Lima-Brito, J. Evaluation of copper-induced DNA damage in Vitis vinifera L. using Comet-FISH. Environ. Sci. Pollut. Res. 2021, 28, 6600–6610. [Google Scholar] [CrossRef]
- Udom, N.; Chansongkrow, P.; Charoensawan, V.; Auesukaree, C. Coordination of the cell wall integrity and highosmolarity glycerol pathways in response to ethanol stress in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2019, 85, e00551-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, R.; Zhou, P.; Wu, C.; Liu, L.; Jing, W. Sml1 Inhibits the DNA Repair Activity of Rev1 in Saccharomyces cerevisiae during Oxidative Stress. Appl. Environ. Microbiol. 2020, 86, e02838-19. [Google Scholar] [CrossRef]
- Lucena, R.M.; Dolz-Edo, L.; Brul, S.; de Morais, M.A.; Smits, G. Extreme low cytosolic ph is a signal for cell survival in acid stressed yeast. Genes 2020, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- De Nadal, E.; Ammerer, G.; Posas, F. Controlling gene expression in response to stress. Nat. Rev. Genet. 2011, 12, 833–845. [Google Scholar] [CrossRef]
- De Lucena, R.M.; Elsztein, C.; Simões, D.A.; De Morais, M.A. Participation of CWI, HOG and Calcineurin pathways in the tolerance of Saccharomyces cerevisiae to low pH by inorganic acid. J. Appl. Microbiol. 2012, 113, 629–640. [Google Scholar] [CrossRef]
- Rodriguez-Pena, M.; Garcia, R.; Nombela, C.; Arroyo, J. The high-osmolarity glycerol (HOG) and cell wall integrity (CWI) signalling pathways interplay: A yeast dialogue between MAPK routes. Yeast 2010, 27, 495–502. [Google Scholar] [CrossRef]
- Guaragnella, N.; Stirpe, M.; Marzulli, D.; Mazzoni, C.; Giannattasio, S. Acid stress triggers resistance to acetic acid-induced regulated cell death through Hog1 activation which requires RTG2 in yeast. Oxid. Med. Cell. Longev. 2019, 2019, 4651062. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Cao, C.; Zhang, L.; Lin, W.; Xia, J.; Xu, H.; Zhang, Y. Cadmium-induced activation of high osmolarity glycerol pathway through its Sln1 branch is dependent on the MAP kinase kinase kinase Ssk2, but not its paralog Ssk22, in budding yeast. FEMS Yeast Res. 2014, 14, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Nasution, O.; Lee, Y.M.; Kim, E.; Lee, Y.; Kim, W.; Choi, W. Overexpression of OLE1 enhances stress tolerance and constitutively activates the MAPK HOG pathway in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Duch, A.; De Nadal, E.; Posas, F. The p38 and Hog1 SAPKs control cell cycle progression in response to environmental stresses. FEBS Lett. 2012, 586, 2925–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nadal, E.; Posas, F. Osmostress-induced gene expression—A model to understand how stress-activated protein kinases (SAPKs) regulate transcription. FEBS J. 2015, 282, 3275–3285. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, E.; An, J.; Lee, Y.; Choi, E.; Choi, W.; Moon, E.; Kim, W. Dissection of the HOG pathway activated by hydrogen peroxide in Saccharomyces cerevisiae. Environ. Microbiol. 2017, 19, 584–597. [Google Scholar] [CrossRef]
- Bilsland, E.; Molin, C.; Swaminathan, S.; Ramne, A.; Sunnerhagen, P. Rck1 and Rck2 MAPKAP kinases and the HOG pathway are required for oxidative stress resistance. Mol. Microbiol. 2004, 53, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Sunyer-Figueres, M.; Mas, A.; Beltran, G.; Torija, M.J. Protective effects of melatonin on saccharomyces cerevisiae under ethanol stress. Antioxidants 2021, 10, 1735. [Google Scholar] [CrossRef]
- Martins, T.S.; Pereira, C.; Canadell, D.; Vilaça, R.; Teixeira, V.; Moradas-Ferreira, P.; de Nadal, E.; Posas, F.; Costa, V. The Hog1p kinase regulates Aft1p transcription factor to control iron accumulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, S.; Wang, J.; Liu, Y.; Deng, Y. Roles of high osmolarity glycerol and cell wall integrity pathways in cadmium toxicity in saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 6169. [Google Scholar] [CrossRef]
- Shanmuganathan, A.; Avery, S.V.; Willetts, S.A.; Houghton, J.E. Copper-induced oxidative stress in Saccharomyces cerevisiae targets enzymes of the glycolytic pathway. FEBS Lett. 2004, 556, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Avery, S.V. Metal toxicity in yeasts and the role of oxidative stress. Adv. Appl. Microbiol. 2001, 49, 111–142. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; He, C. A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol. 2017, 11, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.K.W.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F.S. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef] [Green Version]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The response to heat shock and oxidative stress in saccharomyces cerevisiae. Genetics 2012, 190, 1157–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Harvey, L.M.; McNeil, B. Oxidative stress in industrial fungi. Crit. Rev. Biotechnol. 2009, 29, 199–213. [Google Scholar] [CrossRef]
- Zimdars, S.; Schrage, L.; Sommer, S.; Schieber, A.; Weber, F. Influence of Glutathione on Yeast Fermentation Efficiency under Copper Stress. J. Agric. Food Chem. 2019, 67, 10913–10920. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Eteshola, E.O.U.; Haupt, D.A.; Koos, S.I.; Siemer, L.A.; Morris, D.L. The role of metal ion binding in the antioxidant mechanisms of reduced and oxidized glutathione in metal-mediated oxidative DNA damage. Metallomics 2020, 12, 79–91. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Du, J.; Zhan, J. Effect of trehalose in resistance of wine yeast to copper stress. Sci. Agric. Sin. 2011, 44, 4867–4873. [Google Scholar]
- Condeles, A.L.; Gomes, F.; de Oliveira, M.A.; Netto, L.E.S.; Junior, J.C.T. Thiol peroxidases as major regulators of intracellular levels of peroxynitrite in live saccharomyces cerevisiae cells. Antioxidants 2020, 9, 434. [Google Scholar] [CrossRef]
- Hao, N.; Behar, M.; Parnell, S.C.; Torres, M.P.; Borchers, C.H.; Elston, T.C.C.; Dohlman, H.G. A Systems-Biology Analysis of Feedback Inhibition in the Sho1 Osmotic-Stress-Response Pathway. Curr. Biol. 2007, 17, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Sellers-Moya, Á.; Nuévalos, M.; Molina, M.; Martín, H. Clotrimazole-induced oxidative stress triggers novel yeast pkc1-independent cell wall integrity mapk pathway circuitry. J. Fungi 2021, 7, 647. [Google Scholar] [CrossRef]
- Haghnazari, E.; Heyer, W.D. The Hog1 MAP kinase pathway and the Mec1 DNA damage checkpoint pathway independently control the cellular responses to hydrogen peroxide. DNA Repair 2004, 3, 769–776. [Google Scholar] [CrossRef]
- Capaldi, A.P.; Kaplan, T.; Liu, Y.; Habib, N.; Regev, A.; Friedman, N.; O’shea, E.K. Structure and function of a transcriptional network activated by the MAPK Hog1. Nat. Genet. 2008, 40, 1300–1306. [Google Scholar] [CrossRef]
- Saito, H.; Posas, F. Response to hyperosmotic stress. Genetics 2012, 192, 289–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Novo, A.; Jiménez, J.; Clotet, J.; Nadal-Ribelles, M.; Cavero, S.; de Nadal, E.; Posas, F. Hog1 Targets Whi5 and Msa1 Transcription Factors to Downregulate Cyclin Expression upon Stress. Mol. Cell. Biol. 2015, 35, 1606–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, C.; Tesker, M.; Melamed-Kadosh, D.; Engelberg, D.; Admon, A. Hog1-induced transcription of RTC3 and HSP12 is robust and occurs in cells lacking Msn2, Msn4, Hot1 and Sko1. PLoS ONE 2020, 15, e0237540. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, T.; Zhuo, J.; Zhan, C.; Zhang, F.; Linhardt, R.J.; Bai, Z.; Yang, Y. MAPK/HOG signaling pathway induced stress-responsive damage repair is a mechanism for Pichia pastoris to survive from hyperosmotic stress. J. Chem. Technol. Biotechnol. 2021, 96, 412–422. [Google Scholar] [CrossRef]
- Bonny, A.R.; Kochanowski, K.; Diether, M.; El-Samad, H. Stress-induced growth rate reduction restricts metabolic resource utilization to modulate osmo-adaptation time. Cell Rep. 2021, 34, 108854. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Vanoni, M.; Cusimano, V.; Busti, S.; Marano, F.; Manes, C.; Alberghina, L. Whi5 phosphorylation embedded in the G 1 /S network dynamically controls critical cell size and cell fate. Nat. Commun. 2016, 7, 11372. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, S.; Jiménez, J.; Viganò, M.; Duch, A.; Queralt, E.; de Nadal, E.; Posas, F. Hog1 activation delays mitotic exit via phosphorylation of Net1. Proc. Natl. Acad. Sci. USA 2020, 117, 8924–8933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leech, C.M.; Flynn, M.J.; Arsenault, H.E.; Ou, J.; Liu, H.; Zhu, L.J.; Benanti, J.A. The coordinate actions of calcineurin and Hog1 mediate the stress response through multiple nodes of the cell cycle network. PLoS Genet. 2020, 16, e1008600. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Queralt, E.; Posas, F.; de Nadal, E. The regulation of Net1/Cdc14 by the Hog1 MAPK upon osmostress unravels a new mechanism regulating mitosis. Cell Cycle 2020, 19, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, J.; Zhu, G.; Yao, R.; Chen, X.; Liu, L. CgHog1-Mediated CgRds2 Phosphorylation Alters Glycerophospholipid Composition To Coordinate Osmotic Stress in Candida glabrata. Appl. Environ. Microbiol. 2019, 85, e02822-18. [Google Scholar] [CrossRef] [Green Version]
- Escoté, X.; Zapater, M.; Clotet, J.; Posas, F. Hog1 mediates cell-cycle arrest in G1 phase by the dual targeting of Sic1. Nat. Cell Biol. 2004, 6, 997–1002. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Li, R.; Han, B.; You, Y.; Huang, W.; Du, G.; Zhan, J. Involvement of the High-Osmolarity Glycerol Pathway of Saccharomyces Cerevisiae in Protection against Copper Toxicity. Antioxidants 2022, 11, 200. https://doi.org/10.3390/antiox11020200
Ren M, Li R, Han B, You Y, Huang W, Du G, Zhan J. Involvement of the High-Osmolarity Glycerol Pathway of Saccharomyces Cerevisiae in Protection against Copper Toxicity. Antioxidants. 2022; 11(2):200. https://doi.org/10.3390/antiox11020200
Chicago/Turabian StyleRen, Mengmeng, Ruilong Li, Bin Han, Yilin You, Weidong Huang, Gang Du, and Jicheng Zhan. 2022. "Involvement of the High-Osmolarity Glycerol Pathway of Saccharomyces Cerevisiae in Protection against Copper Toxicity" Antioxidants 11, no. 2: 200. https://doi.org/10.3390/antiox11020200
APA StyleRen, M., Li, R., Han, B., You, Y., Huang, W., Du, G., & Zhan, J. (2022). Involvement of the High-Osmolarity Glycerol Pathway of Saccharomyces Cerevisiae in Protection against Copper Toxicity. Antioxidants, 11(2), 200. https://doi.org/10.3390/antiox11020200