Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases
Abstract
:1. Introduction
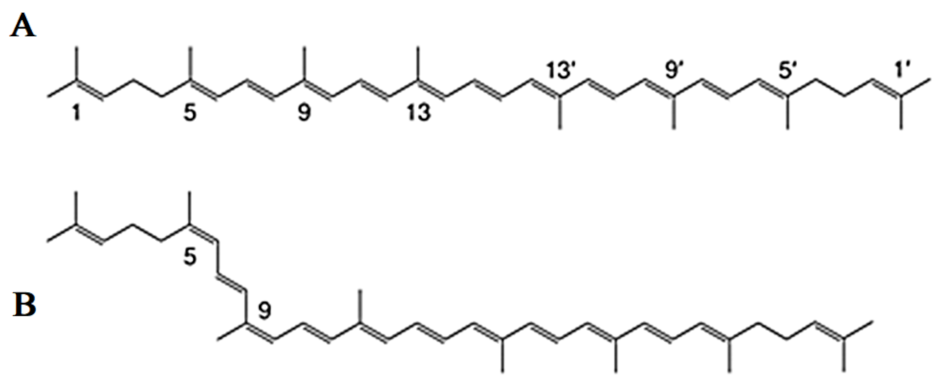
2. Discovery, Chemical Structure, Properties, Biosynthesis and Physiological Role of Lycopene
3. Lycopene as an Antioxidant
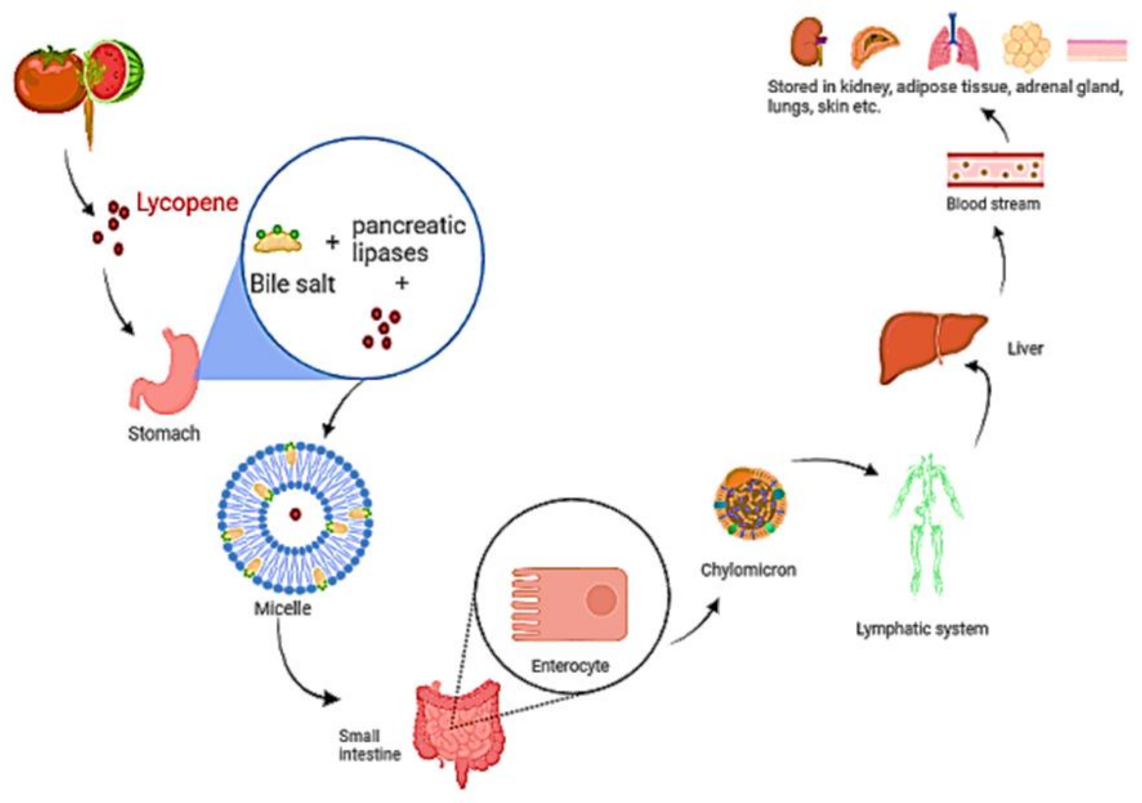
4. Lycopene in Human Health and Diseases
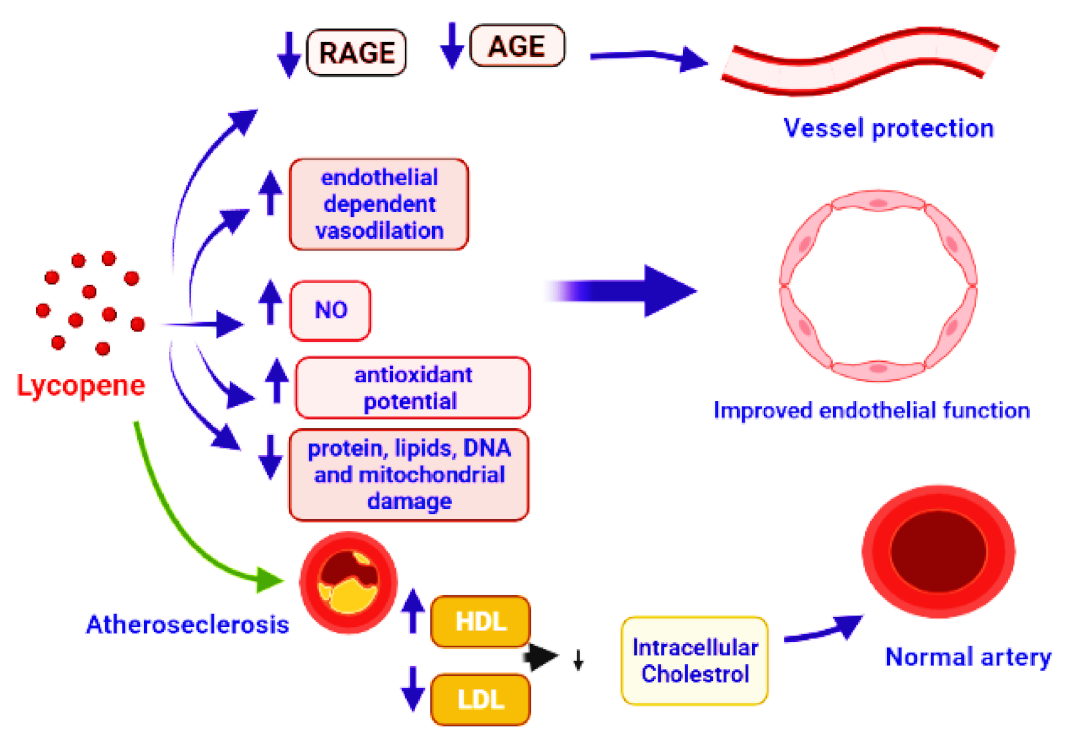
5. Lycopene in Cardiovascular Diseases (CVDs)
5.1. Coronary Artery Disease (CAD) and Lycopene
5.2. Hypertension and Lycopene
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kario, K.; Kagitani, H.; Hayashi, S.; Hanamura, S.; Ozawa, K.; Kanegae, H.A. Japan nationwide web-based survey of patient preference for renal denervation for hypertension treatment. Hypertens. Res. 2021, 17, 1–9. [Google Scholar] [CrossRef]
- Abdalla, A.A. Knowledge, attitude and practice towards therapeutic lifestyle changes in the management of hypertension in Khartoum State. Cardiovasc. J. Afr. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Couch, S.C.; Saelens, B.E.; Khoury, P.R.; Dart, K.B.; Hinn, K.; Mitsnefes, M.M.; Daniels, S.R.; Urbina, E.M. Dietary approaches to stop hypertension dietary intervention improves blood pressure and vascular health in youth with elevated blood pressure. Hypertension 2021, 77, 241–251. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Ma, H.; Yin, H.; Wang, P.; Bai, B.; Guo, L.; Geng, Q. Associations between depression, nutrition, and outcomes among individuals with coronary artery disease. Nutrition 2021, 86, 111157. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Schwartz, S.J. Lycopene: Chemical and biological properties. Food Technol. 1999, 53, 38–45. [Google Scholar]
- Mehta, D.N. Lycopene: Structure, pharmacokinetics and role in oral cancer precancerous lesions. J. Res. Adv. Dent. 2012, 1, 44–49. [Google Scholar]
- Heber, D.; Lu, Q.Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food sources, biological activities, and human health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef]
- Hedayati, N.; Oskouei, Z.; Tabeshpour, J.; Naeini, M.B. Berberine and lycopene as alternative or add-on therapy to metformin and statins, a review. Eur. J. Pharmacol. 2021, 913, 174590. [Google Scholar] [CrossRef]
- John, J.H.; Ziebland, S.; Yudkin, P.; Roe, L.S.; Neil, H.A.; Oxford, F.; Vegetable Study, G. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: A randomised controlled trial. Lancet 2002, 359, 1969–1974. [Google Scholar] [CrossRef]
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017, 61, 1601009. [Google Scholar] [CrossRef] [PubMed]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef]
- Kong, K.W.; Khoo, H.E.; Prasad, K.N.; Ismail, A.; Tan, C.P.; Rajab, N.F. Revealing the power of the natural red pigment lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapio, S.; Little, M.P.; Kaiser, J.C.; Impens, N.; Hamada, N.; Georgakilas, A.G.; Simar, D.; Salomaa, S. Ionizing radiation-induced circulatory and metabolic diseases. Environ. Int. 2021, 146, 106235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Tambunan, R.Z.; Rusmarilin, H.; Kaban, J. Antioxidant activity of tomato juice rich in lycopene antioxidant as degenerative chemopreventive agents using citrus aurantifolia juice as a preservative. IOP Conf. Ser. Earth Environ. Sci. 2018, 205, 012035. [Google Scholar] [CrossRef]
- Zeng, J.; Zhao, J.; Dong, B.; Cai, X.; Jiang, J.; Xue, R.; Liu, C. Lycopene protects against pressure overload-induced cardiac hypertrophy by attenuating oxidative stress. J. Nutr. Biochem. 2019, 66, 70–78. [Google Scholar] [CrossRef]
- Goralczyk, R.; Siler, U. The role of lycopene in health and disease. Phytochem. Health Dis. 2004, 285–309. [Google Scholar]
- Palozza, P.; Catalano, A.; Simone, R.; Cittadini, A. Lycopene as a guardian of redox signalling. Acta Biochim. Pol. 2012, 59, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelkel, M.; Schumacher, M.; Dicato, M.; Diederich, M. Antioxidant and anti-proliferative properties of lycopene. Free. Radic. Res. 2011, 45, 925–940. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kováčik, A.; Tušimová, E.; Paál, D.; Mackovich, A.; Alimov, J.; Lukáč, N. Antioxidant efficiency of lycopene on oxidative stress-induced damage in bovine spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lima, T.R.; Martins, P.C.; Guerra, P.H.; Santos Silva, D.A. Muscular strength and cardiovascular risk factors in adults: A systematic review. Physician Sportsmed. 2021, 49, 18–30. [Google Scholar] [CrossRef]
- Lind, L.; Ingelsson, M.; Sundstrom, J.; Ärnlöv, J. Impact of risk factors for major cardiovascular diseases: A comparison of life-time observational and Mendelian randomisation findings. Open Heart 2021, 8, e001735. [Google Scholar] [CrossRef]
- Blaum, C.; Brunner, F.J.; Kröger, F.; Braetz, J.; Lorenz, T.; Goßling, A.; Ojeda, F.; Koester, L.; Karakas, M.; Zeller, T.; et al. Modifiable lifestyle risk factors and C-reactive protein in patients with coronary artery disease: Implications for an anti-inflammatory treatment target population. Eur. J. Prev. Cardiol. 2021, 28, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Niaz, S.; Latif, J.; Hussain, S. Serum resistin: A possible link between inflammation, hypertension and coronary artery disease. Pak. J. Med Sci. 2019, 35, 641–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konukoglu, D.; Uzun, H. Endothelial dysfunction and hypertension. Hypertension: From basic research to clinical practice. Adv. Exp. Med. Biol. 2016, 956, 511–540. [Google Scholar]
- Escobar, E. Hypertension and coronary heart disease. J. Hum. Hypertens. 2002, 16, S61–S63. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Waqar, A.B.; Yan, H.; Wang, Y.; Liang, J.; Fan, J. Renovascular hypertension aggravates atherosclerosis in cholesterol-fed rabbits. J. Vasc. Res. 2019, 56, 28–38. [Google Scholar] [CrossRef]
- Han, G.M.; Liu, P. Higher serum lycopene is associated with reduced prevalence of hypertension in overweight or obese adults. Eur. J. Integr. Med. 2017, 13, 34–40. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and vascular health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Aggarwal, S. Lycopene in oral diseases. Guident 2012, 5, 73–74. [Google Scholar]
- Trumbo, P.R. Are there adverse effects of lycopene exposure? J. Nutr. 2005, 135, 2060S–2061S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elinder, L.S.; Hadell, K.; Johansson, J.; Mølgaard, J.; Holme, I.; Olsson, A.G.; Walldius, G. Probucol treatment decreases serum concentrations of diet derived antioxidants. Arter. Thromb. Vasc. Biol. 1995, 15, 1057–1063. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Chasse, G.A.; Mak, M.L.; Deretey, E.; Farkas, I.; Torday, L.L.; Papp, J.G.; Sarma, D.S.; Agarwal, A.; Chakravarthi, S.; Agarwal, S.; et al. An ab initio computational study on selected lycopene isomers. J. Mol. Struct. Theochem. 2001, 571, 27–37. [Google Scholar] [CrossRef]
- Guo, W.H.; Tu, C.Y.; Hu, C.H. Cis-trans isomerizations of β-carotene and lycopene: A theoretical study. J. Phys. Chem. B. 2008, 112, 12158–12167. [Google Scholar] [CrossRef]
- Honda, M.; Murakami, K.; Ichihashi, K.; Takada, W.; Goto, M. Enriched (Z)-lycopene in Tomato Extract via Co-Extraction of Tomatoes and Foodstuffs Containing Z-isomerization-accelerating Compounds. Catalysts 2021, 11, 462. [Google Scholar] [CrossRef]
- Schierle, J.; Bretzel, W.; Bühler, I.; Faccin, N.; Hess, D.; Steiner, K.; Schüep, W. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997, 59, 459–465. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Karabelas, A.J. Natural origin lycopene and its “green” downstream processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.A.; Mei, Z.; Hassan, F.U.; Saeed, M.; Alagawany, M.; Shar, A.H.; Rajput, I.R. Lycopene: A natural antioxidant for prevention of heat-induced oxidative stress in poultry. World’s Poult. Sci. J. 2018, 74, 89–100. [Google Scholar] [CrossRef]
- Suwanaruang, T. Analyzing lycopene content in fruits. Agric. Agric. Sci. Procedia 2016, 11, 46–48. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M. Carotenoids in Foods. In Harvestplus Handbook for Carotenoid Analysis; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2004; p. 2. [Google Scholar]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; pp. 1–45. [Google Scholar]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef] [Green Version]
- Islamian, J.P.; Mehrali, H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: An overview. Cell J. 2015, 16, 386. [Google Scholar]
- Shi, J.; Le Maguer, M.; Bryan, M. Lycopene from tomatoes. Funct. Foods Biochem. Process. Asp. 2002, 2, 135–167. [Google Scholar]
- Fernandes, R.F.; Maia, L.F.; Couri, M.R.; Costa, L.A.S.; de Oliveira, L.F.C. Raman spectroscopy as a tool in differentiating conjugated polyenes from synthetic and natural sources. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 434–441. [Google Scholar] [CrossRef]
- Roldán-Gutiérrez, J.M.; Dolores Luque de Castro, M. Lycopene: The need for better methods for characterization and determination. Trends Anal. Chem. 2007, 26, 163–170. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An Update on the Health Effects of Tomato Lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [Green Version]
- Petyaev, I.M. Lycopene deficiency in ageing and cardiovascular disease. Oxidative Med. Cell. Longev. 2016, 3218605. [Google Scholar] [CrossRef] [Green Version]
- Dhuique-Mayer, C.; Servent, A.; Descalzo, A.; Mouquet-Rivier, C.; Amiot, M.J.; Achir, N. Culinary practices mimicking a polysaccharide-rich recipe enhance the bioaccessibility of fat-soluble micronutrients. Food Chem. 2016, 210, 182–188. [Google Scholar] [CrossRef]
- Marshall, J.H. Production of Secondary Metabolites from Acetyl Co-A Precursors in Bacterial and Fungal Hosts. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2004. [Google Scholar]
- Thulasiram, H.V.; Poulter, C.D. Farnesyl diphosphate synthase: The art of compromise between substrate selectivity and stereoselectivity. J. Am. Chem. Soc. 2006, 128, 15819–15823. [Google Scholar] [CrossRef] [Green Version]
- Shahbani, Z.H.; Akbari, N.K.; Samoudi, M.; Omid, Y.N.; Abou Alhasanirad, S.; Safari, A.; Hosseini, F.; Hajhosseini, R. Effect of concomitant lycopene biosynthesis on CoQ10 accumulation in transformed Escherichia coli strains. Iran. J. Biotechnol. 2009, 7, 224–232. [Google Scholar]
- Hong, J.; Park, S.H.; Kim, S.; Kim, S.W.; Hahn, J.S. Efficient production of lycopene in Saccharomyces cerevisiae by enzyme engineering and increasing membrane flexibility and NAPDH production. Appl. Microbiol. Biotechnol. 2019, 103, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Siddiqui, M.W.; Tlili, I.; Montefusco, A.; Piro, G.; Hdider, C.; Lenucci, M.S. When color really matters: Horticultural performance and functional quality of high-lycopene tomatoes. Crit. Rev. Plant Sci. 2018, 37, 15–53. [Google Scholar] [CrossRef]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.Y.; Katz, N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar] [CrossRef]
- Liu, C.; Lian, F.; Smith, D.E.; Russell, R.M.; Wang, X.D. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003, 63, 3138–3144. [Google Scholar] [PubMed]
- Stahl, W.; Sies, H. Uptake of lycopene and its geometrical isomers is greater from heat-processed than from unprocessed tomato juice in humans. J. Nutr. 1992, 122, 2161–2166. [Google Scholar] [CrossRef]
- Re, R.; Fraser, P.D.; Long, M.; Bramley, P.M.; Rice-Evans, C. Isomerization of lycopene in the gastric milieu. Biochem. Biophys. Res. Commun. 2001, 281, 576–581. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Harrison, E.H. Intestinal absorption and metabolism of carotenoids: Insights from cell culture. Arch. Biochem. Biophys. 2004, 430, 77–88. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of carotenoid intestinal absorption: Where do we stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapelli-Brahm, P.; Margier, M.; Desmarchelier, C.; Halimi, C.; Nowicki, M.; Borel, P.; Meléndez-Martínez, A.J.; Reboul, E. Comparison of the bioavailability and intestinal absorption sites of phytoene, phytofluene, lycopene and β-carotene. Food Chem. 2019, 300, 125232. [Google Scholar] [CrossRef]
- During, A.; Dawson, H.D.; Harrison, E.H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in caco-2 cells treated with ezetimibe. J. Nutr. 2005, 135, 2305–2312. [Google Scholar] [CrossRef]
- Moussa, M.; Landrier, J.; Reboul, E.; Ghiringhelli, O.; Comera, C.; Collet, X.; Borel, P. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class B type I but not Niemann-Pick C1-like 1. J. Nutr. 2008, 138, 1432–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arballo, J.; Amengual, J.; Erdman, J.W. Lycopene: A critical review of digestion, absorption, metabolism, and excretion. Antioxidants 2021, 10, 342. [Google Scholar] [CrossRef]
- Clinton, S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998, 56, 35–51. [Google Scholar] [CrossRef]
- Przybylska, S. Lycopene—A bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Boileau, P.; Krishnan, S.G.; Tinsi, L.; Walch, G.; Coste, J.S.; Molé, D. Tuberosity malposition and migration: Reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J. Shoulder Elb. Surg. 2002, 11, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Gouin, J.P.; Glaser, R.; Malarkey, W.B.; Beversdorf, D.; Kiecolt-Glaser, J. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 2012, 31, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, P.; Matthews, A.; Coyne, I. Stress and stressors in the clinical environment: A comparative study of fourth-year student nurses and newly qualified general nurses in Ireland. J. Clin. Nurs. 2013, 22, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gu, C.; Chen, D.; Yu, B.; He, J. Oxidative stress-induced diseases and tea polyphenols. Oncotarget 2017, 8, 81649–81661. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [Green Version]
- Karadas, F.; Erdoğan, S.; Kor, D.; Oto, G.; Uluman, M. The effects of different types of antioxidants (Se, vitamin E and carotenoids) in broiler diets on the growth performance, skin pigmentation and liver and plasma antioxidant concentrations. Braz. J. Poult. Sci. 2016, 18, 101–116. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Antioxidants as modulators of arsenic-induced oxidative stress tolerance in plants: An overview. J. Hazard. Mater. 2021, 127891. [Google Scholar] [CrossRef]
- Amarowicz, R. Natural antioxidants as a subject of research. Eur. J. Lipid Sci. Technol. 2009, 111, 1053–1056. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.E.; Catalano, A.; Mele, M.C. Tomato lycopene and lung cancer prevention: From experimental to human studies. Cancers 2011, 3, 2333–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuorro, A.; Lavecchia, R.; Medici, F.; Piga, L. Enzyme-assisted production of tomato seed oil enriched with lycopene from tomato pomace. Food Bioprocess Technol. 2013, 6, 3499–3509. [Google Scholar] [CrossRef]
- Pennathur, S.; Maitra, D.; Byun, J.; Sliskovic, I.; Abdulhamid, I.; Saed, G.M.; Abu-Soud, H.M. Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid. Free Radic. Biol. Med. 2010, 49, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative stress and DNA damage: Implications in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef]
- Gonfloni, S.; Maiani, E.; Di Bartolomeo, C.; Diederich, M.; Cesareni, G. Oxidative stress, DNA damage, and c-Abl signaling: At the crossroad in neurodegenerative diseases? Int. J. Cell Biol. 2012, 2012, 683097. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Agarwal, A.; Ong, C.; Prashast, P. Lycopene and male infertility. Asian J. Androl. 2014, 16, 420–425. [Google Scholar]
- Müller, L.; Goupy, P.; Fröhlich, K.; Dangles, O.; Caris-Veyrat, C.; Böhm, V. Comparative study on antioxidant activity of lycopene (Z)-isomers in different assays. J. Agric. Food Chem. 2011, 59, 4504–4511. [Google Scholar] [CrossRef]
- Luo, C.; Wu, X.G. Lycopene enhances antioxidant enzyme activities and immunity function in N-Methyl-N′-nitro-N-nitrosoguanidine–induced gastric cancer rats. Int. J. Mol. Sci. 2011, 12, 3340–3351. [Google Scholar] [CrossRef] [Green Version]
- Campos, K.K.; de Oliveira Ramos, C.; Martins, T.L.; de Paula Costa, G.; Talvani, A.; Garcia, C.C.; Oliveira, L.A.; Cangussu, S.D.; Costa, D.C.; Bezerra, F.S. Lycopene mitigates pulmonary emphysema induced by cigarette smoke in a murine model. J. Nutr. Biochem. 2019, 65, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Xu, F.; Yu, K.; Yu, H.; Wang, P.; Song, M.; Xiu, C.; Li, Y. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J. Funct. Foods 2017, 39, 215–224. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Ali, S.; Sahin, N. Tomato powder supplementation activates nrf-2 via erk/akt signalling pathway and attenuates heat stress-related responses in quails. Anim. Feed. Sci. Technol. 2011, 165, 230–237. [Google Scholar] [CrossRef]
- Otsuki, A.; Yamamoto, M. Cis-element architecture of Nrf2–sMaf heterodimer binding sites and its relation to diseases. Arch. Pharmacal Res. 2020, 43, 275–285. [Google Scholar] [CrossRef]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of β-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 2018, 32, 255–264. [Google Scholar] [PubMed] [Green Version]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Hedayati, N.; Naeini, M.B.; Nezami, A.; Hosseinzadeh, H.; Hayes, A.W.; Hosseini, S.; Imenshahidi, M.; Karimi, G. Protective effect of lycopene against chemical and natural toxins: A review. BioFactors 2019, 45, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Karaca, A.; Yilmaz, S.; Kaya, E.; Altun, S. The effect of lycopene on hepatotoxicity of aflatoxin B1 in rats. Arch. Physiol. Biochem. 2019, 127, 429–436. [Google Scholar] [CrossRef]
- Park, H.-A.; Stumpf, A.; Broman, K.; Jansen, J.; Dunn, T.; Scott, M.; Crowe-White, K.M. Role of lycopene in mitochondrial protection during differential levels of oxidative stress in primary cortical neurons. Brain Disord. 2021, 3, 100016. [Google Scholar] [CrossRef]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef]
- Ural, M.Ş. Chlorpyrifos-induced changes in oxidant/antioxidant status and haematological parameters of Cyprinus carpio carpio: Ameliorative effect of lycopene. Chemosphere 2013, 90, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Francisco-Marquez, M. Reactions of OOH radical with β-carotene, lycopene, and torulene: Hydrogen atom transfer and adduct formation mechanisms. J. Phys. Chem. B 2009, 113, 11338–11345. [Google Scholar] [CrossRef]
- Sgherri, C.; Pérez-López, U.; Pinzino, C. Antioxidant Properties of Food Products Containing Lycopene are Increased by the Presence of Chlorophyll. In Lycopene: Food Sources, Potential Role in Human Health and Antioxidant Effects; Bailey, J.R., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; pp. 39–90. [Google Scholar]
- Santos Sánchez, N.; Salas-Coronado, R.; Villanueva, C.; Hernandez-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Intechopen: London, UK, 2019. [Google Scholar]
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef]
- Ahmed, T.A.I.; Ibrahim, A.T.A. Protective role of lycopene and vitamin E against diazinon-induced biochemical changes in Oreochromis niloticus. Afr. J. Environ. Sci. Technol. 2015, 9, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Palozza, P. Prooxidant actions of carotenoids in biologic systems. Nutr. Rev. 1998, 56, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ascenso, A.; Pedrosa, T.; Pinho, S.; Pinho, F.; De Oliveira, J.M.; Marques, H.C.; Santos, C. The effect of lycopene preexposure on UV-B-irradiated human keratinocytes. Oxidative Med. Cell. Longev. 2016, 2016, 8214631. [Google Scholar] [CrossRef]
- Zhang, F.F.; Morioka, N.; Kitamura, T.; Fujii, S.; Miyauchi, K.; Nakamura, Y.; Hisaoka-Nakashima, K.; Nakata, Y. Lycopene ameliorates neuropathic pain by upregulating spinal astrocytic connexin 43 expression. Life Sci. 2016, 155, 116–122. [Google Scholar] [CrossRef]
- Sahin, I.; Bilir, B.; Ali, S.; Sahin, K.; Kucuk, O. Soy isoflavones in integrative oncology: Increased efficacy and decreased toxicity of cancer therapy. Integr. Cancer Ther. 2019, 18, 1534735419835310. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Campos, K.K.D.; Araújo, G.R.; Martins, T.L.; Bandeira, A.C.B.; Costa, G.d.P.; Talvani, A.; Bezerra, F.S. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J. Nutr. Biochem. 2017, 48, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascenso, A.; Pedrosa, T.; Pinho, S.; Pinho, F.; Oliveira, J.; Cabral, H.; Marques, H.O.; Simões, S.; Santos, C. The Effect of UV-B Irradiation on Human Keratinocytes after Lycopene Exposure. Carr. Mediat. Dermal Deliv. Prev. Treat. Ski. Disord. 2012, 1001, 221. [Google Scholar]
- Aust, O.; Ale-Agha, N.; Zhang, L.; Wollersen, H.; Sies, H.; Stahl, W. Lycopene oxidation product enhances gap junctional communication. Food Chem. Toxicol. 2003, 41, 1399. [Google Scholar] [CrossRef]
- Mein, J.R.; Lian, F.; Wang, X.-D. Biological activity of lycopene metabolites: Implications for cancer prevention. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef]
- Li, W.; Jiang, B.; Cao, X.; Xie, Y.; Huang, T. Protective effect of lycopene on fluoride-induced ameloblasts apoptosis and dental fluorosis through oxidative stress-mediated Caspase pathways. Chem.-Biol. Interact. 2017, 261, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.S.; Wu, W.B.; Fang, J.Y.; Chen, D.F.; Chen, B.H.; Huang, C.C.; Chen, Y.T.; Hung, C.F. Lycopene inhibits PDGF-BB-induced signaling and migration in human dermal fibroblasts through interaction with PDGF-BB. Life Sci. 2007, 81, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yang, F. The effects of lycopene supplementation on serum insulin-like growth factor 1 (IGF-1) levels and cardiovascular disease: A dose-response meta-analysis of clinical trials. Complementary Ther. Med. 2021, 56, 102632. [Google Scholar] [CrossRef]
- Chen, J.; O’Donoghue, A.; Deng, Y.F.; Zhang, B.; Kent, F.; O’Hare, T. The effect of lycopene on the PI3K/Akt signalling pathway in prostate cancer. Anti-Cancer Agents Med. Chem. 2014, 14, 800–805. [Google Scholar] [CrossRef]
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B.; et al. Lycopene Protects Against Spontaneous Ovarian Cancer Formation in Laying Hens. J. Cancer Prev. 2018, 23, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Aydemir, G.; Kasiri, Y.; Bartók, E.-M.; Birta, E.; Fröhlich, K.; Böhm, V.; Mihaly, J.; Rühl, R. Lycopene supplementation restores vitamin A deficiency in mice and possesses thereby partial pro-vitamin A activity transmitted via RAR signaling. Mol. Nutr. Food Res. 2016, 60, 2413–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboubakr, M.; Elshafae, S.M.; Abdelhiee, E.Y.; Fadl, S.E.; Soliman, A.; Abdelkader, A.; Abdel-Daim, M.M.; Bayoumi, K.A.; Baty, R.S.; Elgendy, E.; et al. Antioxidant and Anti-Inflammatory Potential of Thymoquinone and Lycopene Mitigate the Chlorpyrifos-Induced Toxic Neuropathy. Pharmaceuticals 2021, 14, 940. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Zhou, X.; Pan, W.; Shi, Y.; Wang, M.; Zhang, X.; Qi, D.; Li, L.; Ma, K.; et al. Depression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammation. J. Neuroimmunol. 2016, 298, 1–8. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Lucas, R.; Mihály, J.; Lowe, G.M.; Graham, D.L.; Szklenar, M.; Szegedi, A.; Rühl, R. Reduced carotenoid and retinoid concentrations and altered lycopene isomer ratio in plasma of atopic dermatitis patients. Nutrients 2018, 10, 1390. [Google Scholar] [CrossRef] [Green Version]
- Shah, H.; Mahajan, S.R. Screening of topical gel containing lycopene and 1417 dexamethasone against UV radiation induced photoaging in mice. Biomed. Aging Pathol. 2014, 4, 303–308. [Google Scholar] [CrossRef]
- Palozza, P.; Colangelo, M.; Simone, R.; Catalano, A.; Boninsegna, A.; Lanza, P.; Monego, G.; Ranelletti, F.O. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Li, Q.; Lin, T. Lycopene attenuates Staphylococcus aureus-induced inflammation via inhibiting α-hemolysin expression. Microbes Infect. 2021, 23, 104853. [Google Scholar] [CrossRef]
- Martínez, A.; Melendez-Martínez, A.J. Lycopene, oxidative cleavage derivatives and antiradical activity. Comput. Theor. Chem. 2016, 1077, 92–98. [Google Scholar] [CrossRef]
- Soleymaninejad, M.; Joursaraei, S.G.; Feizi, F.; Jafari Anarkooli, I. The effects of lycopene and insulin on histological changes and the expression level of Bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J. Diabetes Res. 2017, 2017, 4650939. [Google Scholar] [CrossRef] [Green Version]
- Tabrez, S.; Al-Shali, K.Z.; Ahmad, S. Lycopene powers the inhibition of glycation-induced diabetic nephropathy: A novel approach to halt the AGE-RAGE axis menace. Biofactors 2015, 41, 372–381. [Google Scholar] [CrossRef]
- Greish, S.M.; Kader, G.S.A.; Abdelaziz, E.Z.; Eltamany, D.A.; Sallam, H.S.; Abogresha, N.M. Lycopene is superior to moringa in improving fertility markers in diet-induced obesity male rats. Saudi J. Biol. Sci. 2021, 28, 2956–2963. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Kumar, A. Lycopene protects against memory impairment and mito-oxidative damage induced by colchicine in rats: An evidence of nitric oxide signaling. Eur. J. Pharmacol. 2013, 721, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A.; Kamboj, S.S. Lycopene prevents 3-nitropropionic acid-induced mitochondrial oxidative stress and dysfunctions in nervous system. Neurochem. Int. 2010, 57, 579–587. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Wang, J.; Li, Y.; Liu, W.; Xia, J. Lycopene supplementation protects vascular dementia gerbils against the impairment of learning and memory. Folia Neuropathol. 2021, 59, 161–173. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, C.; Wang, R.; Gao, X.; Hao, C.; Liu, C. A combination of lycopene and human amniotic epithelial cells can ameliorate cognitive deficits and suppress neuroinflammatory signaling by choroid plexus in Alzheimer’s disease rat. J. Nutr. Biochem. 2021, 88, 108558. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.K.; Chopra, K. Lycopene abrogates Aβ (1–42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J. Nutr. Biochem. 2015, 26, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ratto, F.; Franchini, F.; Musicco, M.; Caruso, G.; Di Santo, S.G. A narrative review on the potential of tomato and lycopene for the prevention of Alzheimer’s disease and other dementias. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Ning, W.J.; Lv, R.J.; Xu, N.; Hou, X.Y.; Shen, C.; Guo, Y.L.; Fan, Z.Y.; Cao, N.; Liu, X.P. Lycopene-Loaded Microemulsion Regulates Neurogenesis in Rats with Aβ-Induced Alzheimer’s Disease Rats Based on the Wnt/β-catenin Pathway. Neural Plast. 2021, 2021. [Google Scholar] [CrossRef]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective Effect of Lycopene on Oxidative Stress and Cognitive Decline in Rotenone Induced Model of Parkinson’s Disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef]
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015, 599, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Man, H.B.; Bi, W.P. Protective effect of lycopene in a mouse model of Parkinson’s disease via reducing oxidative stress and apoptosis. Anal. Quant. Cytopathol. Histopathol. 2018, 40, 253–258. [Google Scholar]
- Ardawi, M.-S.M.; Badawoud, M.H.; Hassan, S.M.; Rouzi, A.A.; Ardawi, J.M.; AlNosani, N.M.; Qari, M.H.; Mousa, S.A. Lycopene treatment against loss of bone mass, microarchitecture and strength in relation to regulatory mechanisms in a postmenopausal osteoporosis model. Bone 2016, 83, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.S.; Shao, M.L.; Sun, Z.; Chen, S.M.; Hu, Y.J.; Wang, H.T.; Wei, T.K.; Li, X.S.; Zheng, H.X. Lycopene ameliorates diabetic osteoporosis via anti-inflammatory, anti-oxidation, and increasing Osteoprotegerin/RANKL expression ratio. J. Funct. Foods 2021, 83, 104539. [Google Scholar] [CrossRef]
- Whelton, S.P.; McEvoy, J.W.; Shaw, L.; Psaty, B.M.; Lima, J.A.C.; Budoff, M.; Nasir, K.; Szklo, M.; Blumenthal, R.S.; Blaha, M.J. Association of Normal Systolic Blood Pressure Level with Cardiovascular Disease in the Absence of Risk Factors. JAMA Cardiol. 2020, 5, 1011–1018. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Ohukainen, P.; Virtanen, J.K.; Ala-Korpela, M. Vexed causal inferences in nutritional epidemiology—Call for genetic help. Int. J. Epidemiology 2021, in press. [Google Scholar] [CrossRef]
- Shixian, Q.; Dai, Y.; Kakuda, Y.; Shi, J.; Mittal, G.; Yeung, D.; Jiang, Y. Synergistic antioxidative effects of lycopene with other bioactive compounds. Food Rev. Int. 2005, 21, 295–311. [Google Scholar] [CrossRef]
- Castro, I.A.; Moraes Barros, S.B.; Lanfer Marquez, U.M.; Motizuki, M.; Higashi Sawada, T.C. Optimization of the antioxidant capacity of a mixture of carotenoids and α-tocopherol in the development of a nutritional supplement. Food Res. Int. 2005, 38, 861–866. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily watermelon consumption decreases plasma sVCAM-1 levels in overweight and obese postmenopausal women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef]
- Casas, R.; Castro-Barquero, S.; Estruch, R.; Sacanella, E. Nutrition and cardiovascular health. Int. J. Mol. sciences 2018, 19, 3988. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xu, J.; Liu, Y.; Zhao, X.; Deng, X.; Guo, L.; Gu, J. A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck). J. Exp. Bot. 2007, 58, 4161–4171. [Google Scholar] [CrossRef]
- Dhar, I.; Caspar-Bell, G.; Prasad, K. Role of Advanced Glycation End Products and Its Receptors in the Pathogenesis of Cigarette Smoke-Induced Cardiovascular Disease. Int. J. Angiol. 2015, 24, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Sanajou, D.; Haghjo, A.G.; Argani, H.; Aslani, S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. Eur. J. Pharmacol. 2018, 833, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wautier, M.-P.; Guillausseau, P.-J.; Wautier, J.-L. Activation of the receptor for advanced glycation end products and consequences on health. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 305–309. [Google Scholar] [CrossRef]
- Kellow, N.; Coughlan, M.T. Effect of diet-derived advanced glycation end products on inflammation. Nutr. Rev. 2015, 73, 737–759. [Google Scholar] [CrossRef]
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef]
- Treggiari, D.; Dalbeni, A.; Meneguzzi, A.; Delva, P.; Fava, C.; Molesini, B.; Pandolfini, T.; Minuz, P. Lycopene inhibits endothelial cells migration induced by vascular endothelial growth factor A increasing nitric oxide bioavailability. J. Funct. Foods 2018, 42, 312–318. [Google Scholar] [CrossRef]
- Watters, J.L.; A Satia, J.; Kupper, L.L.; A Swenberg, J.; Schroeder, J.C.; Switzer, B.R.; Florin, T.A.; Fryer, G.E.; Miyoshi, T.; Weitzman, M.; et al. Associations of Antioxidant Nutrients and Oxidative DNA Damage in Healthy African-American and White Adults. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1428–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scolastici, C.; de Lima, R.A.; Barbisan, L.F.; Ferreira, A.; Ribeiro, D.; Salvadori, D. Antigenotoxicity and antimutagenicity of lycopene in HepG2 cell line evaluated by the comet assay and micronucleus test. Toxicol. Vitr. 2008, 22, 510–514. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Ma, Y.; Qu, Y.; Lu, X.; Luo, H. Effects of dietary lycopene supplementation on plasma lipid profile, lipid peroxidation and antioxidant defense system in feedlot Bamei lamb. Asian-Australas. J. Anim. Sci. 2015, 28, 958. [Google Scholar] [CrossRef]
- Fachinello, M.R.; Gasparino, E.; Partyka, A.V.; de Souza Khatlab, A.; Castilha, L.D.; Huepa, L.M.; Ferreira, L.F.; Pozza, P.C. Dietary lycopene alters the expression of antioxidant enzymes and modulates the blood lipid profile of pigs. Anim. Prod. Sci. 2020, 60, 806–814. [Google Scholar] [CrossRef]
- Tinkler, J.H.; Böhm, F.; Schalch, W.; Truscott, T. Dietary carotenoids protect human cells from damage. J. Photochem. Photobiol. B Biol. 1994, 26, 283–285. [Google Scholar] [CrossRef]
- Riso, P.; Visioli, F.; Erba, D.; Testolin, G.; Porrini, M. Lycopene and vitamin C concentrations increase in plasma and lymphocytes after tomato intake. Effects on cellular antioxidant protection. Eur. J. Clin. Nutr. 2004, 58, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Riso, P.; Visioli, F.; Grande, S.; Guarnieri, S.; Gardana, C.; Simonetti, P.; Porrini, M. Effect of a tomato-based drink on markers of inflammation, immunomodulation, and oxidative stress. J. Agric. Food Chem. 2006, 54, 2563–2566. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qu, Q.; Kakuda, Y.; Xue, S.J.; Jiang, Y.; Koide, S.; Shim, Y.-Y. Investigation of the antioxidant and synergistic activity of lycopene and other natural antioxidants using LAME and AMVN model systems. J. Food Compos. Anal. 2007, 20, 603–608. [Google Scholar] [CrossRef]
- Stahl, W.; Junghans, A.; de Boer, B.; Driomina, E.S.; Briviba, K.; Sies, H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: Synergistic effects of lycopene and lutein. FEBS Lett. 1998, 427, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gaur, S. Lycopene: Chemistry, Biosynthesis, Health Benefits and Nutraceutical Applications. In Plant-Derived Bioactives; Springer: Singapore, 2020; pp. 251–263. [Google Scholar]
- Misra, R.; Mangi, S.; Joshi, S.; Mittal, S.; Gupta, S.K.; Pandey, R.M. LycoRed as an alternative to hormone replacement therapy in lowering serum lipids and oxidative stress markers: A randomized controlled clinical trial. J. Obstet. Gynaecol. Res. 2006, 32, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Periago, M.J.; Böhm, V.; Berruezo, G.R. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br. J. Nutr. 2008, 99, 137–146. [Google Scholar] [CrossRef]
- Wong, N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 2014, 11, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Shin, S.; Park, H.B.; Chang, H.J.; Arsanjani, R.; Min, J.K.; Kim, Y.J.; Lee, B.K.; Choi, J.H.; Hong, G.R.; Chung, N. Impact of intensive LDL cholesterol lowering on coronary artery atherosclerosis progression: A serial CT angiography study. JACC Cardiovasc. Imaging 2017, 10, 437–446. [Google Scholar] [CrossRef]
- Asanuma, T.; Higashikuni, Y.; Yamashita, H.; Nagai, R.; Hisada, T.; Sugiura, S. Discordance of the Areas of Peak Wall Shear Stress and Tissue Stress in Coronary Artery Plaques as Revealed by Fluid-Structure Interaction Finite Element Analysis. Int. Heart J. 2013, 54, 54–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusis, A.J.; Mar, R.; Pajukanta, P. Genetics of atherosclerosis. Annu. Rev. Genomics Hum. Genet. 2004, 5, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Sanz, J.; Moreno, P.; Fuster, V. The year in atherothrombosis. J. Am. Coll. Cardiol. 2013, 62, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Ghattas, A.; Griffiths, H.R.; Devitt, A.; Lip, G.Y.; Shantsila, E. Monocytes in coronary artery disease and atherosclerosis: Where are we now? J. Am. Coll. Cardiol. 2013, 62, 1541–1551. [Google Scholar] [CrossRef] [Green Version]
- Battes, L.C.; Cheng, J.M.; Oemrawsingh, R.M.; Boersma, E.; Garcia-Garcia, H.M.; De Boer, S.P.; Buljubasic, N.; Van Mieghem, N.A.; Regar, E.; Van Geuns, R.-J.; et al. Circulating cytokines in relation to the extent and composition of coronary atherosclerosis: Results from the ATHEROREMO-IVUS study. Atherosclerosis 2014, 236, 18–24. [Google Scholar] [CrossRef]
- Kleemann, R.; Zadelaar, S.; Kooistra, T. Cytokines and atherosclerosis: A comprehensive review of studies in mice. Cardiovasc. Res. 2008, 79, 360–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabas, I. Macrophage death and defective infammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010, 10, 36–46. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arter. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [Green Version]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of Atherosclerosis Plaque Progression. Heart Lung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Doyle, B.; Caplice, N. Plaque Neovascularization and Antiangiogenic Therapy for Atherosclerosis. J. Am. Coll. Cardiol. 2007, 49, 2073–2080. [Google Scholar] [CrossRef] [Green Version]
- Mehilli, J.; Presbitero, P. Coronary artery disease and acute coronary syndrome in women. Heart 2020, 106, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Libby, P.; Gersh, B.; Yellon, D.; Böhm, M.; Lopaschuk, G.; Opie, L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 2014, 383, 1933–1943. [Google Scholar] [CrossRef] [Green Version]
- Fox, K.M.; Ferrari, R. Heart rate: A forgotten link in coronary artery disease? Nat. Rev. Cardiol. 2011, 8, 369–379. [Google Scholar] [CrossRef]
- Panza, J.A.; Holly, T.A.; Asch, F.M.; She, L.; Pellikka, P.A.; Velazquez, E.J.; Lee, K.L.; Borges-Neto, S.; Farsky, P.S.; Jones, R.H.; et al. Inducible Myocardial Ischemia and Outcomes in Patients with Coronary Artery Disease and Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2013, 61, 1860–1870. [Google Scholar] [CrossRef] [Green Version]
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views 2017, 18, 109. [Google Scholar] [CrossRef]
- Neaton, J.; Wentworth, D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and difference by age for 316.099 white man. Multiple Risk Factor Intervention Trial Research Group. Arch. Intern. Med. 1992, 152, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Chang, C.C.; Hadley, T. Genetic risk stratification: A paradigm shift in prevention of coronary artery disease. JACC Basic Transl. Sci. 2021, 6, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Bos, M.M.; de Vries, L.; Rensen, P.C.; van Dijk, K.W.; Blauw, G.J.; van Heemst, D.; Noordam, R. Apolipoprotein E genotype, lifestyle and coronary artery disease: Gene-environment interaction analyses in the UK Biobank population. Atherosclerosis 2021. [Google Scholar] [CrossRef] [PubMed]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- Ra, L.M.; Franch, A.A.; Gil-Campos, M.; Trabazo, R.L.; Moreno Villares, J.M.; Suarez, M.V.; Giner, P.M.C.; Nutrition Committee of the Spanish Association of Pediatrics. Childhood obesity. Recommendations of the nutrition committee of the Spanish association of pediatrics. Part, I. Prevention. Early detection. Role of the pediatrician. An. Pediatr. 2006, 65, 607–615. [Google Scholar]
- Li-Ping, C.H.; Shu-Ying, H.E.; Zheng, H.; Yu-Lu, D.A. Effects and mechanisms of lycopene on the proliferation of vascular smooth muscle cells. Chin. J. Nat. Med. 2010, 8, 218–222. [Google Scholar]
- Kim, J.-W.; Covel, N.; Guess, P.; Rekow, E.; Zhang, Y. Concerns of Hydrothermal Degradation in CAD/CAM Zirconia. J. Dent. Res. 2010, 89, 91–95. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.S.; Tong, Y.; Ling, L.; Chen, D.; Sun, H.J.; and Zhou, H. NLRP3 gene deletion attenuates angiotensin ii-induced phenotypic transformation of vascular smooth muscle cells and vascular remodeling. Cell. Physiol. Biochem. 2017, 44, 2269–2280. [Google Scholar] [CrossRef]
- Chen, S.; Huang, L.; Hu, C. Antioxidative reaction of carotenes against peroxidation of fatty acids initiated by nitrogen dioxide: A theoretical study. J. Phys. Chem. B 2015, 119, 9640–9650. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-M.; Hung, C.-F.; Tseng, Y.-L.; Chen, B.-H.; Jian, J.-S.; Wu, W.-B. Lycopene binds PDGF-BB and inhibits PDGF-BB-induced intracellular signaling transduction pathway in rat smooth muscle cells. Biochem. Pharmacol. 2007, 74, 54–63. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. 2015, 214, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Sung, H.J.; Eskin, S.G.; Sakurai, Y.; Yee, A.; Kataoka, N.; McIntire, L.V. Oxidative stress produced with cell migration increases synthetic phenotype of vascular smooth muscle cells. Ann. Biomed. Eng. 2005, 33, 1546–1554. [Google Scholar] [CrossRef]
- Bae, J.W.; Bae, J.-S. Barrier protective effects of lycopene in human endothelial cells. Inflamm. Res. 2011, 60, 751–758. [Google Scholar] [CrossRef]
- Tang, X.; Yang, X.; Peng, Y.; Lin, J. Protective Effects of Lycopene against H2O2-Induced Oxidative Injury and Apoptosis in Human Endothelial Cells. Cardiovasc. Drugs Ther. 2009, 23, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Klipstein-Grobusch, K.; Launer, L.J.; Geleijnse, J.M.; Boeing, H.; Hofman, A.; Witteman, J.C.M. Serum carotenoids and atherosclerosis The Rotterdam Study. Atherosclerosis 2000, 148, 49–56. [Google Scholar] [CrossRef]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Salonen, J.T. Lycopene, atherosclerosis, and coronary heart disease. Exp. Biol. Med. 2002, 227, 900–907. [Google Scholar] [CrossRef]
- Hu, M.-Y.; Li, Y.-L.; Jiang, C.-H.; Liu, Z.-Q.; Qu, S.-L.; Huang, Y.-M. Comparison of lycopene and fluvastatin effects on atherosclerosis induced by a high-fat diet in rabbits. Nutrition 2008, 24, 1030–1038. [Google Scholar] [CrossRef]
- Rissanen, T.H.; Voutilainen, S.; Nyyssönen, K.; Salonen, R.; Kaplan, G.A.; Salonen, J.T. Serum lycopene concentrations and carotid atherosclerosis: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2003, 77, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Engelhard, Y.N.; Gazer, B.; Paran, E.; Sheva, B. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am. Heart J. 2006, 151, 100. [Google Scholar] [CrossRef]
- Ranjbar, F.; Akbarzade, F.; Khaneshi, M. The study oftype A personality in essential Hypertensionpatient. Med. J. Kordestan Univ. Med. Sci. 2006, 11, 26–31. [Google Scholar]
- Kooshki, A.; Yaghoubifar, M.A.; Behnam, V.H.R. Relation between drinking water and blood pressure. Sabzevar J. Med. Sci. 2004, 10, 23–28. [Google Scholar]
- Kooshki, A.; Mohajeri, N.; Movahedi, A. Prevalence of CVD Risk Factors related to diet in Patients Referring to Modarres Hospital in Tehran in 1999. Sabzevar J. Med. Sci. 2004, 10, 17–22. [Google Scholar]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L. Montezano AC. Oxidative stress: A unifying paradigm in hypertension. Can. J. Cardiol. 2020, 36, 659–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.I.; Ungvari, Z. Role of mitochondrial oxidative stress in hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2013, 305, H1417–H1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, M.; Wilcox, C.S. Oxidative stress in hypertension: Role of the kidney. Antioxid. Redox Signal. 2014, 20, 74–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prysyazhna, O.; Wolhuter, K.; Switzer, C.; Santos, C.; Yang, X.; Lynham, S.; Shah, A.M.; Eaton, P.; Burgoyne, J.R. Blood pressure–lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation 2019, 140, 126–137. [Google Scholar] [CrossRef]
- Navar, L.G.; Kobori, H.; Prieto, M.C.; Gonzalez-Villalobos, R.A. Intratubular Renin-Angiotensin System in Hypertension. Hypertension 2011, 57, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Whitworth, J.A. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003, 21, 1983–1992. [Google Scholar]
- Assersen, K.B.; Sumners, C.; Steckelings, U.M. The Renin-Angiotensin System in Hypertension, a Constantly Renewing Classic: Focus on the Angiotensin AT2-Receptor. Can. J. Cardiol. 2020, 36, 683–693. [Google Scholar] [CrossRef]
- Hasan, A.; Paray, B.A.; Hussain, A.; Qadir, F.A.; Attar, F.; Aziz, F.M.; Sharifi, M.; Derakhshankhah, H.; Rasti, B.; Mehrabi, M.; et al. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2021, 39, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Santos, P.C.J.; Krieger, J.; Pereira, A.C. Renin-angiotensin system, hypertension, and chronic kidney disease: Pharmacogenetic implications. J. Pharmacol. Sci. 2012, 120, 77–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Li, X.; Xu, J. Lycopene Supplement and Blood Pressure: An Updated Meta-Analysis of Intervention Trials. Nutrients 2013, 5, 3696–3712. [Google Scholar] [CrossRef] [PubMed]
- Paran, E.; Novack, V.; Engelhard, Y.N.; Hazan-Halevy, I. The Effects of Natural Antioxidants from Tomato Extract in Treated but Uncontrolled Hypertensive Patients. Cardiovasc. Drugs Ther. 2008, 23, 145–151. [Google Scholar] [CrossRef]
- Aman, U.; Vaibhav, P.; Balaraman, R. Tomato lycopene attenuates myocardial infarction induced by isoproterenol: Electrocardiographic, biochemical and anti-apoptotic study. Asian Pac. J. Trop. Biomed. 2012, 2, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Wong, Z.W.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of the protective role of natural products on isoproterenol-induced myocardial infarction: A review. Biomed. Pharmacother. 2017, 94, 1145–1166. [Google Scholar] [CrossRef]
- Yeo, H.Y.; Kim, O.Y.; Lim, H.H.; Kim, J.Y.; Lee, J.H. Association of serum lycopene and brachial-ankle pulse wave velocity with metabolic syndrome. Metabolism 2011, 60, 537–543. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, Y.-S.; Ahn, S.; Chun, Y.H.; Lim, K.S. The Usefulness of Procalcitonin and C-Reactive Protein as Early Diagnostic Markers of Bacteremia in Cancer Patients with Febrile Neutropenia. Cancer Res. Treat. 2011, 43, 176–180. [Google Scholar] [CrossRef]



| Diseases | Issue | Dose of Lycopene | Subjected Time | Role/Activity of Lycopene | References |
|---|---|---|---|---|---|
| Cancer | Loss of gap junctional communication (GJC) | 4–8 mg | 3–12 months | Suppression of carcinogen formation. | [92] |
| 60 mg | 9 weeks | GJC is boosted by a lycopene oxidation product. | [66,122] | ||
| 50 mg/kg | 5 to 7 days | Metabolite of lycopene, can enhance connexln 43, which is linked to GJC. | [116,123] | ||
| Apoptosis | 10, 40, 120 mg/kg | 9 weeks | Lycopene triggers apoptosis in cells. | [124] | |
| Melanoma | 10 mg/kg 15 mg/day | 5 weeks 12 weeks | Lycopene inhibits melanoma development. | [121,125] | |
| Mammary and endometrial cancer | 76 to 154 mg | 14 days | Lycopene inhibits the insulin-like growth factor 1 (IGF-1R) signaling pathway. | [125,126,127] | |
| Prostate and colon cancer | 10–30 mg | 3 to 5 days | Inhibit Ras signaling. | [26,128] | |
| ovarian cancer | 20–40 mg/kg | 18 weeks | Downregulation of STAT3 reduces tumor development. | [129] | |
| Lung cancer | 5 mg/kg | 16 weeks | Inhibits lung cancer. | [87] | |
| Oral cancer | 5 mg/kg | 16 weeks | ROS scavenger. | [87] | |
| Vitamin A deficiency | 10–2400 mg | 90 days | Upregulate signaling pathways | [130] | |
| Inflammatory Diseases | Brain tissue inflammation | 50, 100, 150 mg/kg | 24 weeks | Lycopene boosts antioxidant gene expression, inflammatory mediators. | [17,119] |
| 60 mg/kg | 7 days | Lycopene helps to prevent inflammation by lowering the levels of plasma interleukin (IL)-6 and TNF. | [131,132] | ||
| Skin Diseases | Photodamage by UV-B | 10 mg/kg | 5 weeks | Lycopene inhibits epidermal ornithine decarboxylase. Prevents DNA damage. | [121,133] |
| Atopic dermatitis (AD) | 100 mg | 7 days | The activation of nuclear hormone receptor signaling pathways. | [134]. | |
| Photo aging | 2.5–10 µM | 24 h | Lycopene gel provided superior photoaging protection. | [135] | |
| Cardiovascular diseases | 2 mg/day | 12–20 weeks | Lycopene reduces atherosclerotic plaques. | [136,137] | |
| Bacterial infection | 50, 100, 150 mg/kg | 24 weeks | Lycopene stimulates immune response. | [119,138] | |
| Age-related macular degeneration | 4 mg/kg/day | 10 weeks | Lycopene is capable to quench singlet oxygen in the eye. | [139] | |
| Diabetes | 10 mg/kg | 5 weeks | Enhanced antioxidant enzymes, suppresses RAGE expression, increased NF-B expression. | [140,141] | |
| Infertility | 4–8 mg | 3–12 months | Boosted antioxidants, reduced lipid peroxidation, decreased DNA damage in spermatozoa. | [92,142] | |
| Neurobehavioral deficits and poorer cognition | 10 mg/kg | 15 days | Increased cognition, increased brain’s antioxidant, nitric oxide pathways are inhibited. | [143,144,145,146] | |
| Alzheimer disease | 30 mg | 5 weeks | Reduced mitochondrial dysfunction, reduced inflammatory cytokine. | [147,148,149] | |
| Parkinson disease | 15, 30, 45 mg/kg/day | 12 weeks | Induction of ROS and neurobehavioral deficits. | [150,151,152] | |
| Bone diseases | 50 mg/kg/day | 10 weeks | Regulation of metabolism, osteoclast differentiation, osteoblasts upregulation. | [153,154,155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. https://doi.org/10.3390/antiox11020232
Bin-Jumah MN, Nadeem MS, Gilani SJ, Mubeen B, Ullah I, Alzarea SI, Ghoneim MM, Alshehri S, Al-Abbasi FA, Kazmi I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants. 2022; 11(2):232. https://doi.org/10.3390/antiox11020232
Chicago/Turabian StyleBin-Jumah, May Nasser, Muhammad Shahid Nadeem, Sadaf Jamal Gilani, Bismillah Mubeen, Inam Ullah, Sami I. Alzarea, Mohammed M. Ghoneim, Sultan Alshehri, Fahad A. Al-Abbasi, and Imran Kazmi. 2022. "Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases" Antioxidants 11, no. 2: 232. https://doi.org/10.3390/antiox11020232
APA StyleBin-Jumah, M. N., Nadeem, M. S., Gilani, S. J., Mubeen, B., Ullah, I., Alzarea, S. I., Ghoneim, M. M., Alshehri, S., Al-Abbasi, F. A., & Kazmi, I. (2022). Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants, 11(2), 232. https://doi.org/10.3390/antiox11020232






