Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products
Abstract
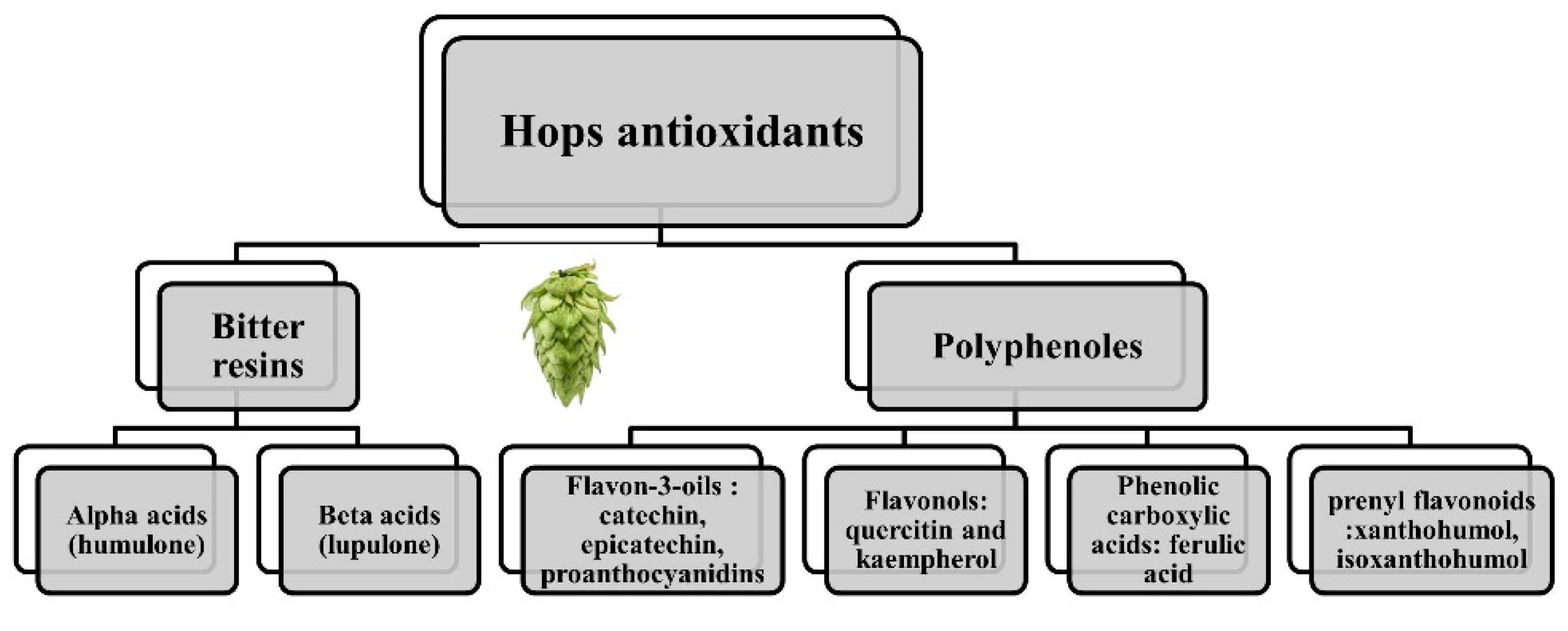
:1. Introduction
2. Bioavailability of the Active Substances
3. Biological Effects
4. Hops Antioxidants and Cancer
5. Hops and the Metabolic Syndrome
5.1. Obesity
5.2. High Density Lipoprotein Cholesterol (HDL-c)
5.3. Triglycerides (TG)
5.4. Glycaemia
6. Safety and Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Koetter, U.; Biendl, M. Hops (Humulus lupulus): A Review of its Historic and Medicinal Uses. HerbalGram 2010, 87, 44–57. [Google Scholar]
- Dimpfel, W.; Suter, A. Sleep improving effects of a single dose administration of a valerian/hops fluid extract—A double blind, randomized, placebo-controlled sleep-EEG study in a parallel design using electrohypnograms. Eur. J. Med. Res. 2008, 13, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzykiewicz, A.; Nowak, A.; Zielonka-Brzezicka, J.; Florkowska, K.; Duchnik, W.; Klimowicz, A. Comparison of antioxidant activity of extracts of hop leaves harvested in different years. Herba Pol. 2019, 65, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.; Lorenzo, J.M. Humulus lupulus L. as a natural source of functional biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Steenackers, B.; De Cooman, L.; De Vos, D. Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: A review. Food Chem. 2015, 172, 742–756. [Google Scholar] [CrossRef]
- Nikolić, D.; van Breemen, R.B. Analytical methods for quantitation of prenylated flavonoids from hops. Curr. Anal. Chem. 2013, 9, 71–85. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Roehrer, S.; Behr, J.; Stork, V.; Ramires, M.; Médard, G.; Frank, O.; Kleigrewe, K.; Hofmann, T.; Minceva, M. Xanthohumol C, a minor bioactive hop compound: Production, purification strategies and antimicrobial test. J. Chromatogr. B 2018, 1095, 39–49. [Google Scholar] [CrossRef]
- Possemiers, S.; Bolca, S.; Grootaert, C.; Heyerick, A.; Decroos, K.; Dhooge, W.; De Keukeleire, D.; Rabot, S.; Verstraete, W.; Van de Wiele, T. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J Nutr. 2006, 136, 1862–1867. [Google Scholar] [CrossRef] [Green Version]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for Human Malignancies: Chemistry, Pharmacokinetics and Molecular Targets. Int. J. Mol. Sci. 2021, 22, 4478. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Wunderlich, S.; Zürcher, A.; Back, W. Enrichment of xanthohumol in the brewing process. Mol. Nutr. Food Res. 2005, 49, 874–881. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Plagmann, L.S.; Yang, L.; Zielke, R.; Gombart, A.F.; Maier, C.S.; Sikora, A.E.; Blakemore, P.R.; Stevens, J.F. Reductive Metabolism of Xanthohumol and 8-Prenylnaringenin by the Intestinal Bacterium Eubacterium ramulus. Mol. Nutr. Food Res. 2019, 63, e1800923. [Google Scholar] [CrossRef]
- Nookandeh, A.; Frank, N.; Steiner, F.; Ellinger, R.; Schneider, B.; Gerhäuser, C.; Becker, H. Xanthohumol metabolites in faeces of rats. Phytochemistry 2004, 65, 561–570. [Google Scholar] [CrossRef]
- Pang, Y.; Nikolic, D.; Zhu, D.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; van Breemen, R.B. Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol. Nutr. Food Res. 2007, 51, 872–879. [Google Scholar] [CrossRef]
- Arczewska, M.; Kamiński, D.M.; Górecka, E.; Pociecha, D.; Rój, E.; Sławińska-Brych, A.; Gagoś, M. The molecular organization of prenylated flavonoid xanthohumol in DPPC multibilayers: X-ray diffraction and FTIR spectroscopic studies. Biochim. Biophys. Acta 2013, 1828, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Wesołowska, O.; Gąsiorowska, J.; Petrus, J.; Czarnik-Matusewicz, B.; Michalak, K. Interaction of prenylated chalcones and flavanones from common hop with phosphatidylcholine model membranes. Biochim. Biophys. Acta 2014, 1838, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Avula, B.; Ganzera, M.; Warnick, J.E.; Feltenstein, M.W.; Sufka, K.J.; Khan, I.A. High-performance liquid chromatographic determination of xanthohumol in rat plasma, urine, and fecal samples. J. Chromatogr. Sci. 2004, 42, 378–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, B.; Poźniak, B.; Popłoński, J.; Bobak, Ł.; Matuszewska, A.; Kwiatkowska, J.; Dziewiszek, W.; Huszcza, E.; Szeląg, A. Pharmacokinetics of xanthohumol in rats of both sexes after oral and intravenous administration of pure xanthohumol and prenylflavonoid extract. Adv. Clin. Exp. Med. 2020, 29, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.; Karnpracha, C.; Reed, R.L.; Choi, J.; Bobe, G.; Christensen, J.M.; Rodriguez-Proteau, R.; Purnell, J.Q.; Stevens, J.F. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 2014, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Cattoor, K.; Remon, J.P.; Boussery, K.; Van Bocxlaer, J.; Bracke, M.; De Keukeleire, D.; Deforce, D.; Heyerick, A. Bioavailability of hop-derived iso-α-acids and reduced derivatives. Food Funct. 2011, 2, 412–422. [Google Scholar] [CrossRef]
- Cattoor, K.O.; Bracke, M.; Deforce, D.; De Keukeleire, D.; Heyerick, A. Transport of hop bitter acids across intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2010, 58, 4132–4140. [Google Scholar] [CrossRef]
- Dupré-Crochet, S.; Erard, M.; Nüβe, O. ROS production in phagocytes: Why, when, and where? J. Leukoc. Biol. 2013, 94, 657–670. [Google Scholar] [CrossRef]
- Pan, L.; Yang, S.; Wang, J.; Xu, M.; Wang, S.; Yi, H. Inducible nitric oxide synthase and systemic lupus erythematosus: A systematic review and meta-analysis. BMC Immunol. 2020, 21, 6. [Google Scholar] [CrossRef] [Green Version]
- Prado, C.M.; Martins, M.A.; Tibério, I.F.L.C. Nitric oxide in asthma physiopathology. ISRN Allergy 2011, 2011, 832560. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.D.R.; Maciel, M.V.D.O.B.; Machado, M.H.; Bazzo, G.C.; de Armas, R.D.; Vitorino, V.B.; Vitali, L.; Block, J.M.; Barreto, P.L.M. Bioactive compounds and antioxidant activities of Brazilian hop (Humulus lupulus L.) extracts. Int. J. Food Sci. Technol. 2020, 55, 340–347. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, X.-H.; Tang, J.; Liu, K. Antioxidant activities of hops (Humulus lupulus) and their products. J. Am. Soc. Brew. Chem. 2007, 65, 116–121. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and in vitro effects of cultivars of humulus lupulus L. Hops on cholinesterase activity and microbial growth. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ćirić, A.; Soković, M.; Calhelha, R.C.; Torija-Isasa, E.; Sánchez-Mata, M.D.C.; Ferreira, I.C.F.R. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crops Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Verzele, M.; Stockx, J.; Fontijn, F.; Anteunis, M. Xanthohumol, a new natural chalkone. Bull. Sociétés Chim. Belg. 1957, 66, 452–475. [Google Scholar] [CrossRef]
- Liu, M.; Yin, H.; Qian, X.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Hops, Inhibits the Viability and Stemness of Doxorubicin-Resistant MCF-7/ADR Cells. Molecules 2016, 22, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legette, L.; Ma, L.; Reed, R.L.; Miranda, C.L.; Christensen, J.M.; Rodriguez-Proteau, R.; Stevens, J.F. Pharmacokinetics of xanthohumol and metabolites in rats after oral and intravenous administration. Mol. Nutr. Food Res. 2012, 56, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possemiers, S.; Heyerick, A.; Robbens, V.; De Keukeleire, D.; Verstraete, W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6288. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.H.; Sun, T.L.; Xiang, D.X.; Wei, S.S.; Li, W.Q. Anticancer activity and mechanism of xanthohumol: A prenylated flavonoid from hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T.L.; Hajirahimkhan, A.; Mbachu, O.; Chen, S.N.; Chadwick, L.; Nikolic, D.; van Breemen, R.B.; Pauli, G.F.; Dietz, B.M. The Multiple Biological Targets of Hops and Bioactive Compounds. Chem. Res. Toxicol. 2019, 32, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Afzal, S.; Jensen, S.A.; Sørensen, J.B.; Henriksen, T.; Weimann, A.; Poulsen, H.E. Oxidative damage to guanine nucleosides following combination chemotherapy with 5-fluorouracil and oxaliplatin. Cancer Chemother. Pharmacol. 2012, 69, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chu, W.; Wei, P.; Liu, Y.; Wei, T. Xanthohumol induces generation of reactive oxygen species and triggers apoptosis through inhibition of mitochondrial electron transfer chain complex I. Free Radic. Biol. Med. 2015, 89, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Festa, M.; Capasso, A.; D’Acunto, C.W.; Masullo, M.; Rossi, A.G.; Pizza, C.; Piacente, S. Xanthohumol induces apoptosis in human malignant glioblastoma cells by increasing reactive oxygen species and activating MAPK pathways. J. Nat. Prod. 2011, 74, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Sun, T.; Du, J.; Zhang, B.; Xiang, D.; Li, W. Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro. Oncol. Rep. 2018, 40, 3213–3222. [Google Scholar] [CrossRef] [PubMed]
- Fouani, L.; Kovacevic, Z.; Richardson, D.R. Targeting Oncogenic Nuclear Factor Kappa B Signaling with Redox-Active Agents for Cancer Treatment. Antioxid. Redox Signal. 2019, 30, 1096–1123. [Google Scholar] [CrossRef]
- Sławińska-Brych, A.; Mizerska-Kowalska, M.; Król, S.K.; Stepulak, A.; Zdzisińska, B. Xanthohumol Impairs the PMA-Driven Invasive Behaviour of Lung Cancer Cell Line A549 and Exerts Anti-EMT Action. Cells 2021, 10, 1484. [Google Scholar] [CrossRef]
- Seliger, J.M.; Misuri, L.; Maser, E.; Hintzpeter, J. The hop-derived compounds xanthohumol, isoxanthohumol and 8-prenylnaringenin are tight-binding inhibitors of human aldo-keto reductases 1B1 and 1B10. J. Enzym. Inhib. Med. Chem. 2018, 33, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Scagliarini, A.; Mathey, A.; Aires, V.; Delmas, D. Xanthohumol, a Prenylated Flavonoid from Hops, Induces DNA Damages in Colorectal Cancer Cells and Sensitizes SW480 Cells to the SN38 Chemotherapeutic Agent. Cells 2020, 9, 932. [Google Scholar] [CrossRef] [Green Version]
- Ambroz, M.; Lnenickova, K.; Matouskova, P.; Skalova, L.; Bousova, I. Antiproliferative Effects of Hop-derived Prenylflavonoids and Their Influence on the Efficacy of Oxaliplatine, 5-fluorouracil and Irinotecan in Human ColorectalC Cells. Nutrients 2019, 11, 879. [Google Scholar] [CrossRef] [Green Version]
- Lněničková, K.; Šadibolová, M.; Matoušková, P.; Szotáková, B.; Skálová, L.; Boušová, I. The Modulation of Phase II Drug-Metabolizing Enzymes in Proliferating and Differentiated CaCo-2 Cells by Hop-Derived Prenylflavonoids. Nutrients 2020, 12, 2138. [Google Scholar] [CrossRef]
- Roehrer, S.; Stork, V.; Ludwig, C.; Minceva, M.; Behr, J. Analyzing bioactive effects of the minor hop compound xanthohumol C on human breast cancer cells using quantitative proteomics. PLoS ONE 2019, 14, e0213469. [Google Scholar] [CrossRef] [PubMed]
- Popłoński, J.; Turlej, E.; Sordon, S.; Tronina, T.; Bartmańska, A.; Wietrzyk, J.; Huszcza, E. Synthesis and Antiproliferative Activity of Minor Hops Prenylflavonoids and New Insights on Prenyl Group Cyclization. Molecules 2018, 23, 776. [Google Scholar] [CrossRef] [Green Version]
- Forino, M.; Pace, S.; Chianese, G.; Santagostini, L.; Werner, M.; Weinigel, C.; Rummler, S.; Fico, G.; Werz, O.; Taglialatela-Scafati, O. Humudifucol and Bioactive Prenylated Polyphenols from Hops (Humulus lupulus cv. “Cascade”). J. Nat. Prod. 2016, 79, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Nickerson, G.B.; Ivancic, M.; Henning, J.; Haunold, A.; Deinzer, M.L. Prenylflavonoid variation in Humulus lupulus: Distribution and taxonomic significance of xanthogalenol and 4’-O-methylxanthohumol. Phytochemistry 2000, 53, 759–775. [Google Scholar] [CrossRef]
- Yasukawa, K.; Takeuchi, M.; Takido, M. Humulon, a bitter in the hop, inhibits tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology 1995, 52, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Lamy, V.; Roussi, S.; Chaabi, M.; Gossé, F.; Lobstein, A.; Raul, F. Lupulone, a hop bitter acid, activates different death pathways involving apoptotic TRAIL-receptors, in human colon tumor cells and in their derived metastatic cells. Apoptosis Int. J. Program Cell Death 2008, 13, 1232–1242. [Google Scholar] [CrossRef]
- Lamy, V.; Bousserouel, S.; Gossé, F.; Minker, C.; Lobstein, A.; Raul, F. Lupulone triggers p38 MAPK-controlled activation of p53 and of the TRAIL receptor apoptotic pathway in human colon cancer-derived metastatic cells. Oncol. Rep. 2011, 26, 109–114. [Google Scholar] [PubMed] [Green Version]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Legette, L.L.; Luna, A.Y.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef]
- Yui, K.; Kiyofuji, A.; Osada, K. Effects of xanthohumol-rich extract from the hop on fatty acid metabolism in rats fed a high-fat diet. J. Oleo Sci. 2014, 63, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Kirkwood, J.S.; Legette, L.L.; Miranda, C.L.; Jiang, Y.; Stevens, J.F. A metabolomics-driven elucidation of the anti-obesity mechanisms of xanthohumol. J. Biol. Chem. 2013, 288, 19000–19013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Bobe, G.; Miranda, C.L.; Lowry, M.B.; Hsu, V.L.; Lohr, C.V.; Wong, C.P.; Jump, D.B.; Robinson, M.M.; Sharpton, T.J.; et al. Tetrahydroxanthohumol, a xanthohumol derivative, attenuates high-fat diet-induced hepatic steatosis by antagonizing PPARγ. eLife 2021, 10, e66398. [Google Scholar] [CrossRef] [PubMed]
- Polvani, S.; Tarocchi, M.; Galli, A. PPARγ and Oxidative Stress: Con(β) Catenating NRF2 and FOXO. PPAR Res. 2012, 2012, 641087. [Google Scholar] [CrossRef] [Green Version]
- Pinto, C.; Duque, A.L.; Rodríguez-Galdón, B.; Cestero, J.J.; Macías, P. Xanthohumol prevents carbon tetrachloride-induced acute liver injury in rats. Food Chem. Toxicol. 2012, 50, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Paraiso, I.L.; Tran, T.Q.; Magana, A.A.; Kundu, P.; Choi, J.; Maier, C.S.; Bobe, G.; Raber, J.; Kioussi, C.; Stevens, J.F. Xanthohumol ameliorates Diet-Induced Liver Dysfunction via Farnesoid X Receptor-Dependent and Independent Signaling. Front. Pharmacol. 2021, 12, 643857. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef]
- Rosso, L.G.; Lhomme, M.; Meroño, T.; Dellepiane, A.; Sorroche, P.; Hedjazi, L.; Zakiev, E.; Sukhorukov, V.; Orekhov, A.; Gasparri, J.; et al. Poor glycemic control in type 2 diabetes enhances functional and compositional alterations of small, dense HDL3c. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2017, 1862, 188–195. [Google Scholar]
- Miura, Y.; Hosono, M.; Oyamada, C.; Odai, H.; Oikawa, S.; Kondo, K. Dietary isohumulones, the bitter components of beer, raise plasma HDL-cholesterol levels and reduce liver cholesterol and triacylglycerol contents similar to PPARalpha activations in C57BL/6 mice. Br. J. Nutr. 2005, 93, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Bouly, M.; Masson, D.; Gross, B.; Jiang, X.C.; Fievet, C.; Castro, G.; Tall, A.R.; Fruchart, J.C.; Staels, B.; Lagrost, L.; et al. Induction of the phospholipid transfer protein gene accounts for the high density lipoprotein enlargement in mice treated with fenofibrate. J. Biol. Chem. 2001, 276, 25841–25847. [Google Scholar] [CrossRef] [Green Version]
- Stevens, J.F.; Miranda, C.L.; Frei, B.; Buhler, D.R. Inhibition of peroxynitrite-mediated LDL oxidation by prenylated flavonoids: The alpha,beta-unsaturated keto functionality of 2’-hydroxychalcones as a novel antioxidant pharmacophore. Chem. Res. Toxicol. 2003, 16, 1277–1286. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Cokkinos, D.V. Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad. Med. J. 2005, 81, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Le, N.A. Postprandial triglycerides, oxidative stress, and inflammation. In Apolipoproteins, Triglycerides and Cholesterol; IntechOpen: London, UK, 2020. [Google Scholar]
- Nozawa, H. Xanthohumol, the chalcone from beer hops (Humulus lupulus L.), is the ligand for farnesoid X receptor and ameliorates lipid and glucose metabolism in KK-A(y) mice. Biochem. Biophys. Res. Commun. 2005, 336, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Yajima, H.; Noguchi, T.; Ikeshima, E.; Shiraki, M.; Kanaya, T.; Tsuboyama-Kasaoka, N.; Ezaki, O.; Oikawa, S.; Kondo, K. Prevention of diet-induced obesity by dietary isomerized hop extract containing isohumulones, in rodents. Int. J. Obes. 2005, 29, 991–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Osada, K. Effect of Dietary Purified Xanthohumol from Hop (Humulus lupulus L.) Pomace on Adipose Tissue Mass, Fasting Blood Glucose Level, and Lipid Metabolism in KK-Ay Mice. J. Oleo Sci. 2017, 66, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Gerloff, A.; Singer, M.V.; Feick, P. Beer and its non-alcoholic compounds: Role in pancreatic exocrine secretion, alcoholic pancreatitis and pancreatic carcinoma. Int. J. Environ. Res. Public. Health 2010, 7, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Kersten, S. Peroxisome proliferator activated receptors and lipoprotein metabolism. PPAR Res. 2008, 132960. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-α as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Obara, K.; Mizutani, M.; Hitomi, Y.; Yajima, H.; Kondo, K. Isohumulones, the bitter component of beer, improve hyperglycemia and decrease body fat in Japanese subjects with prediabetes. Clin. Nutr. 2009, 28, 278–284. [Google Scholar] [CrossRef]
- de Vries, M.A.; Klop, B.; Janssen, H.W.; Njo, T.L.; Westerman, E.M.; Cabezas, M.C. Postprandial inflammation: Targeting glucose and lipids. Adv. Exp. Med. 2014, 824, 161–170. [Google Scholar]
- Piconi, L.; Quagliaro, L.; Assaloni, R.; Da Ros, R.; Maier, A.; Zuodar, G.; Ceriello, A. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab. Res. 2006, 22, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Negrão, R.; Valente, I.; Castela, Â.; Duarte, D.; Guardão, L.; Magalhães, P.J.; Rodrigues, J.A.; Guimarães, J.T.; Gomes, P.; et al. Xanthohumol modulates inflammation, oxidative stress, and angiogenesis in type 1 diabetic rat skin wound healing. J. Nat. Prod. 2013, 76, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yin, H.; Liu, G.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a prenylated chalcone from beer hops, acts as an α-glucosidase inhibitor in vitro. J. Agric. Food Chem. 2014, 62, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, V.; Picchi, V.; Carbone, K. Hop Leaves as an Alternative Source of Health-Active Compounds: Effect of Genotype and Drying Conditions. Plants 2022, 11, 99. [Google Scholar] [CrossRef]
- Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Meyskens, F.L., Jr.; Omenn, G.S.; Valanis, B.; Williams, J.H., Jr. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J. Natl. Cancer Inst. 2004, 96, 1743–1750. [Google Scholar] [CrossRef] [Green Version]
- Butler, L.M.; Huang, J.Y.; Wang, R.; Lee, M.J.; Yang, C.S.; Gao, Y.T.; Yuan, J.M. Urinary biomarkers of catechins and risk of hepatocellular carcinoma in the Shanghai Cohort Study. Am. J. Epidemiol. 2015, 181, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Hops. Available online: https://medlineplus.gov/druginfo/natural/856.html (accessed on 29 December 2021).
- Sławińska-Brych, A.; Król, S.K.; Dmoszyńska-Graniczka, M.; Zdzisińska, B.; Stepulak, A.; Gagoś, M. Xanthohumol inhibits cell cycle progression and proliferation of larynx cancer cells in vitro. Chem. Biol. Interact. 2015, 240, 110–118. [Google Scholar] [CrossRef]
- Yong, W.K.; Ho, Y.F.; Malek, S.N. Xanthohumol induces apoptosis and S phase cell cycle arrest in A549 non-small cell lung cancer cells. Pharmacogn. Mag. 2015, 11, S275–S283. [Google Scholar]
- Vanhoecke, B.W.; Delporte, F.; Van Braeckel, E.; Heyerick, A.; Depypere, H.T.; Nuytinck, M.; De Keukeleire, D.; Bracke, M.E. A safety study of oral tangeretin and xanthohumol administration to laboratory mice. In Vivo 2005, 19, 103–107. [Google Scholar]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Inui, T.; Okumura, K.; Matsui, H.; Hosoya, T.; Kumazawa, S. Effect of harvest time on some in vitro functional properties of hop polyphenols. Food Chem. 2017, 225, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Krofta, K.; Mikyška, A.; Hašková, D. Antioxidant characteristics of hops and hop products. J. Inst. Brew. 2008, 114, 160–166. [Google Scholar] [CrossRef]
- Search in EU Register of Nutrition and Health Claims. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=search (accessed on 29 December 2021).
- Wellness Beers. The Shift Away from Sugary Sports Drinks Has Taken a Surprising Turn. Available online: https://www.theiwsr.com/radius-micro-trend-functional-wellness-beers/ (accessed on 29 December 2021).
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wietstock, P.; Kunz, T.; Shellhammer, T.; Schön, T.; Methner, F.J. Behaviour of antioxidants derived from hops during wort boiling. J. Inst. Brew. 2010, 116, 157–166. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Caro, I.; Blanco, C.; Bodas, R.; Andrés, S.; Giráldez, F.J.; Mateo, J. Effect of the addition of hop (infusion or powder) on the oxidative stability of lean lamb patties during storage. Small Rumin. Res. 2015, 125, 73–80. [Google Scholar] [CrossRef]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef]
- Fernandes, R.; Trindade, M.A.; Lorenzo, J.M.; de Melo, M.P. Assessment of the stability of sheep sausages with the addition of different concentrations of Origanum vulgare extract during storage. Meat Sci. 2018, 137, 244–257. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Khatib, N.; Varidi, M.J.; Mohebbi, M.; Varidi, M.; Hosseini, S. Replacement of nitrite with lupulon-xanthohumol loaded nanoliposome in cooked beef-sausage: Experimental and model based study. J. Food Sci. Technol. 2020, 57, 2629–2639. [Google Scholar] [CrossRef]
- Nionelli, L.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Use of hop extract as antifungal ingredient for bread making and selection of autochthonous resistant starters for sourdough fermentation. Int. J. Food Microbiol. 2018, 266, 173–182. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Biendl, M. Physicochemical and antioxidant properties of biopolymer/candelilla wax emulsion films containing hop extract–A comparative study. Food Hydrocol. 2016, 60, 384–392. [Google Scholar] [CrossRef]
- Hennessy, M. Hops, Fernet and the Next Sriracha? Sensient’s 12 Flavors to Watch in 2014. Available online: https://www.foodnavigator.com/Article/2013/12/18/Hops-fernet-and-the-next-Sriracha-Sensient-s-12-flavors-to-watch-in-2014 (accessed on 29 December 2021).
- Tedesco, I.; Spagnuolo, C.; Bilotto, S.; Izzo, A.A.; Borrelli, F.; Rigano, D.; Russo, M.; Tarricone, F.; Russo, G.L. Antioxidant and Chemopreventive Effect of Aliophen® Formulation Based on Malts and Hops. Antioxidants 2020, 10, 29. [Google Scholar] [CrossRef]
- Mashkour, M.; Maghsoudlou, Y.; Ghorbani, M.; Hadi Solymani, M. Fortification of cherry juice by hops extract. Minerva Biotecnol. 2013, 25, 171–180. [Google Scholar]
- Jelínek, L.; Karabín, M.; Kotlíková, B.; Hudcová, T.; Dostálek, P. Application of a hop by-product in brewing: Reduction in the level of haze-active prolamines and improved antioxidant properties of the beer. J. Inst. Brew. 2014, 120, 99–104. [Google Scholar] [CrossRef]
- Protsenko, L.; Rudyk, R.; Hryniuk, T.; Vlasenko, A.; Protsenko, A.; Ovadenko, O. Beer enrichment with biologically active hop Compounds. Food Technol. 2018, 7, 65–78. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Di Stefano, V.; Delisi, R.; Avellone, G.; Meneguzzo, F.; Pagliaro, M. Beer produced via hydrodynamic cavitation retains higher amounts of xanthohumol and other hops prenylflavonoids. Lwt 2018, 91, 160–167. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zugravu, C.-A.; Bohiltea, R.-E.; Salmen, T.; Pogurschi, E.; Otelea, M.R. Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants 2022, 11, 241. https://doi.org/10.3390/antiox11020241
Zugravu C-A, Bohiltea R-E, Salmen T, Pogurschi E, Otelea MR. Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants. 2022; 11(2):241. https://doi.org/10.3390/antiox11020241
Chicago/Turabian StyleZugravu, Corina-Aurelia, Roxana-Elena Bohiltea, Teodor Salmen, Elena Pogurschi, and Marina Ruxandra Otelea. 2022. "Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products" Antioxidants 11, no. 2: 241. https://doi.org/10.3390/antiox11020241
APA StyleZugravu, C. -A., Bohiltea, R. -E., Salmen, T., Pogurschi, E., & Otelea, M. R. (2022). Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants, 11(2), 241. https://doi.org/10.3390/antiox11020241






