The Cyclic Nitroxide TEMPOL Ameliorates Oxidative Stress but Not Inflammation in a Cell Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation into Neuronal Phenotype
2.2. Drug Treatments and Cell Harvesting
2.3. Tyrosine Hydroxylase Immunocytochemistry
2.4. Assessment of Cell Viability
2.5. Flow Cytometry
2.6. Protein Analysis using Bicinchoninic Acid Assay
2.7. Enzyme-Linked Immunosorbent Assay
2.8. SDS PAGE and Western Blot
2.9. Gene Expression Analysis
2.10. cDNA Synthesis
2.11. Quantitative Polymerase Chain Reaction
2.12. MitoSOX Red Fluorescence Imaging
2.13. Lipid Peroxidation (Malondialdehyde) Assay
2.14. Statistical Analysis
3. Results
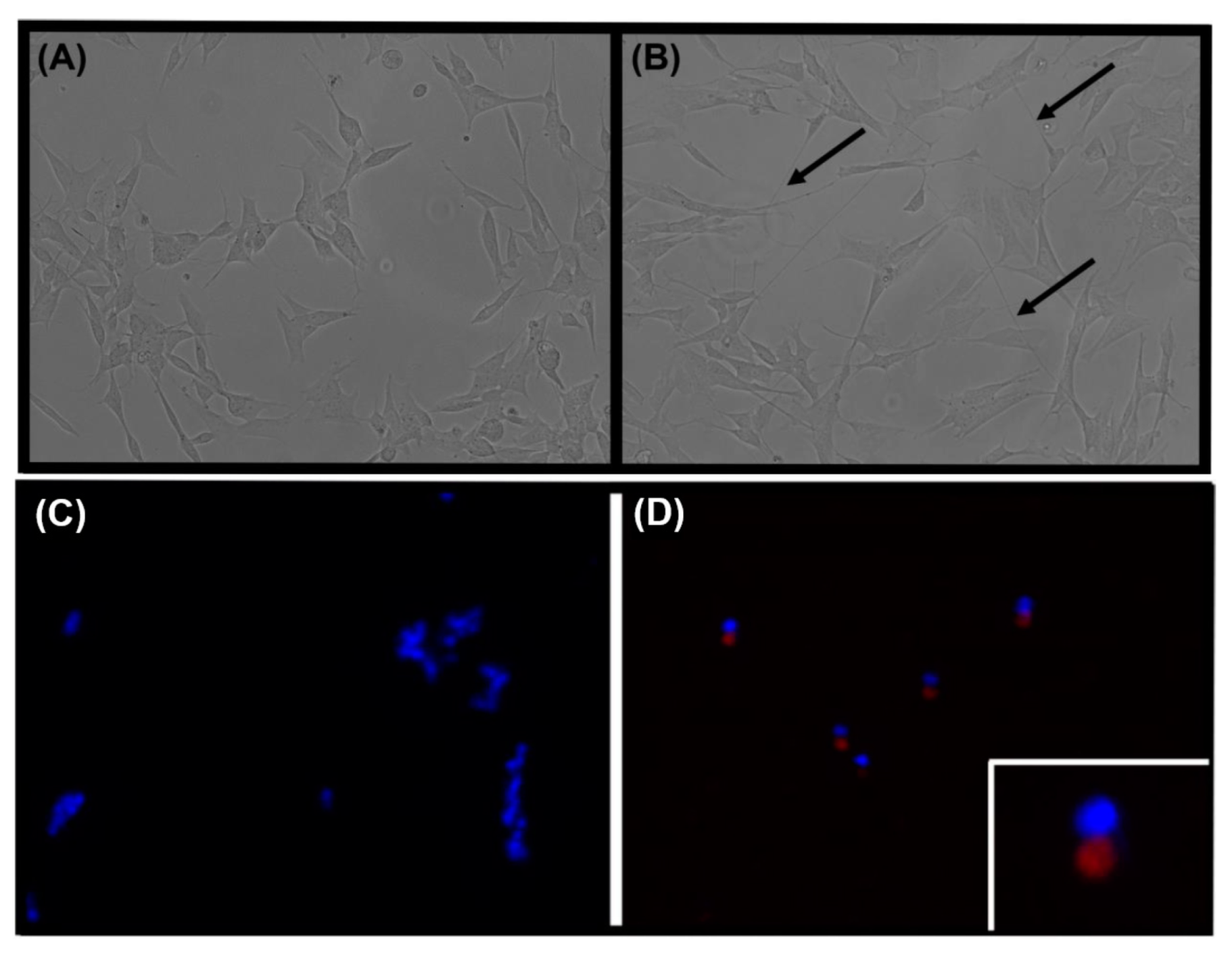
3.1. Characterizing the Cell Model of Parkinson’s Disease
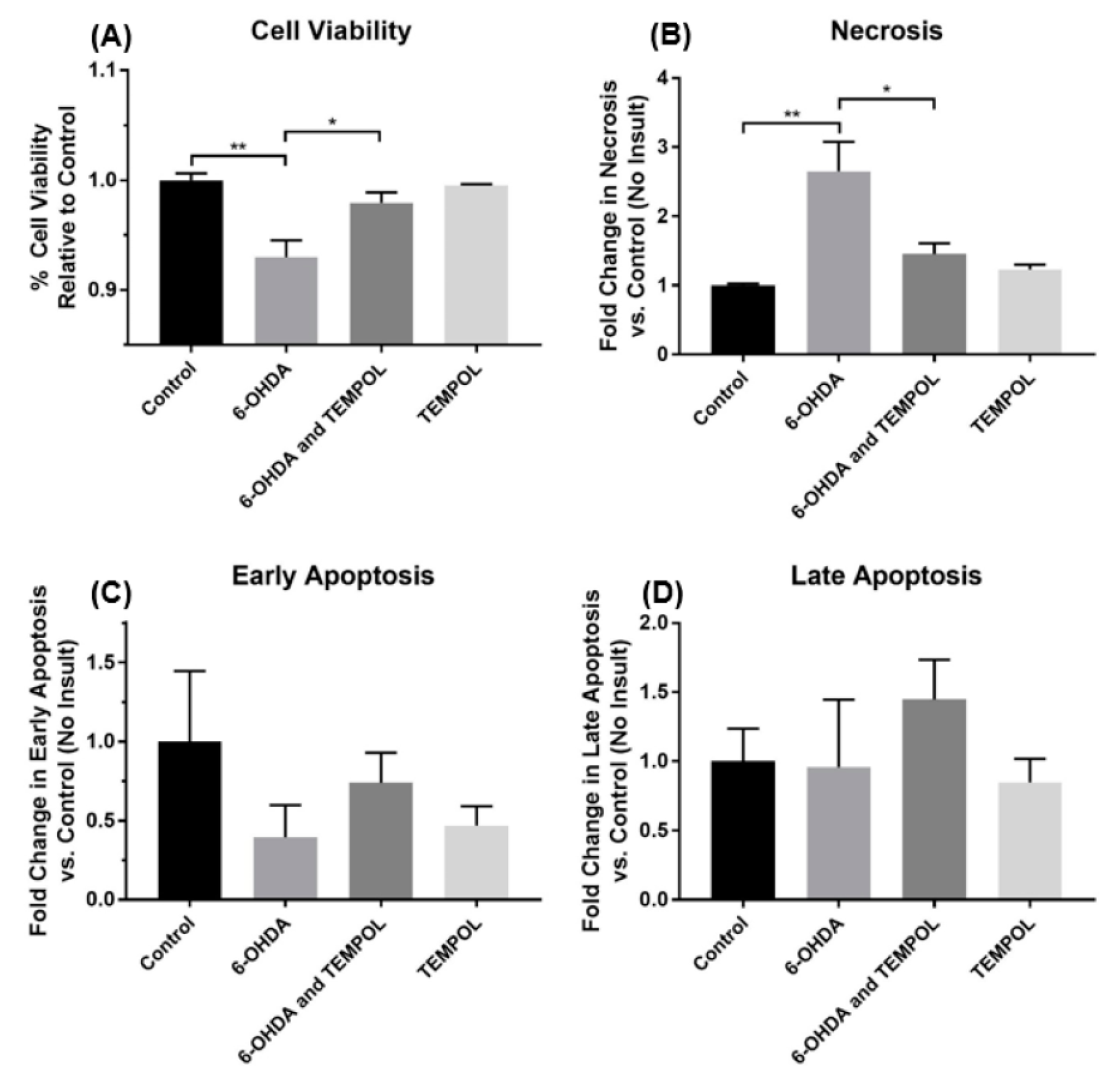
3.2. Assessing Cell Viability on the Absence and Presence of TEMPOL
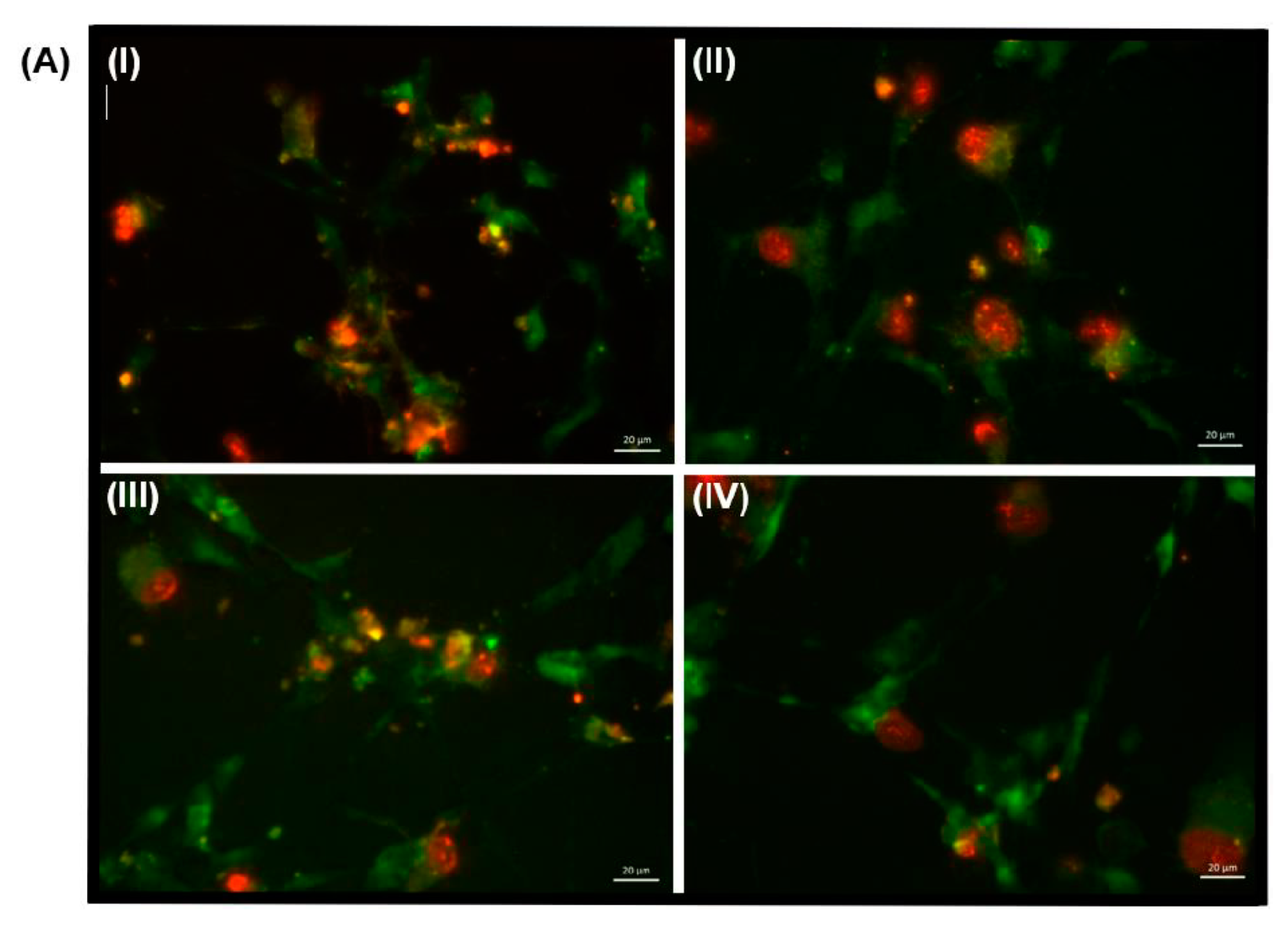
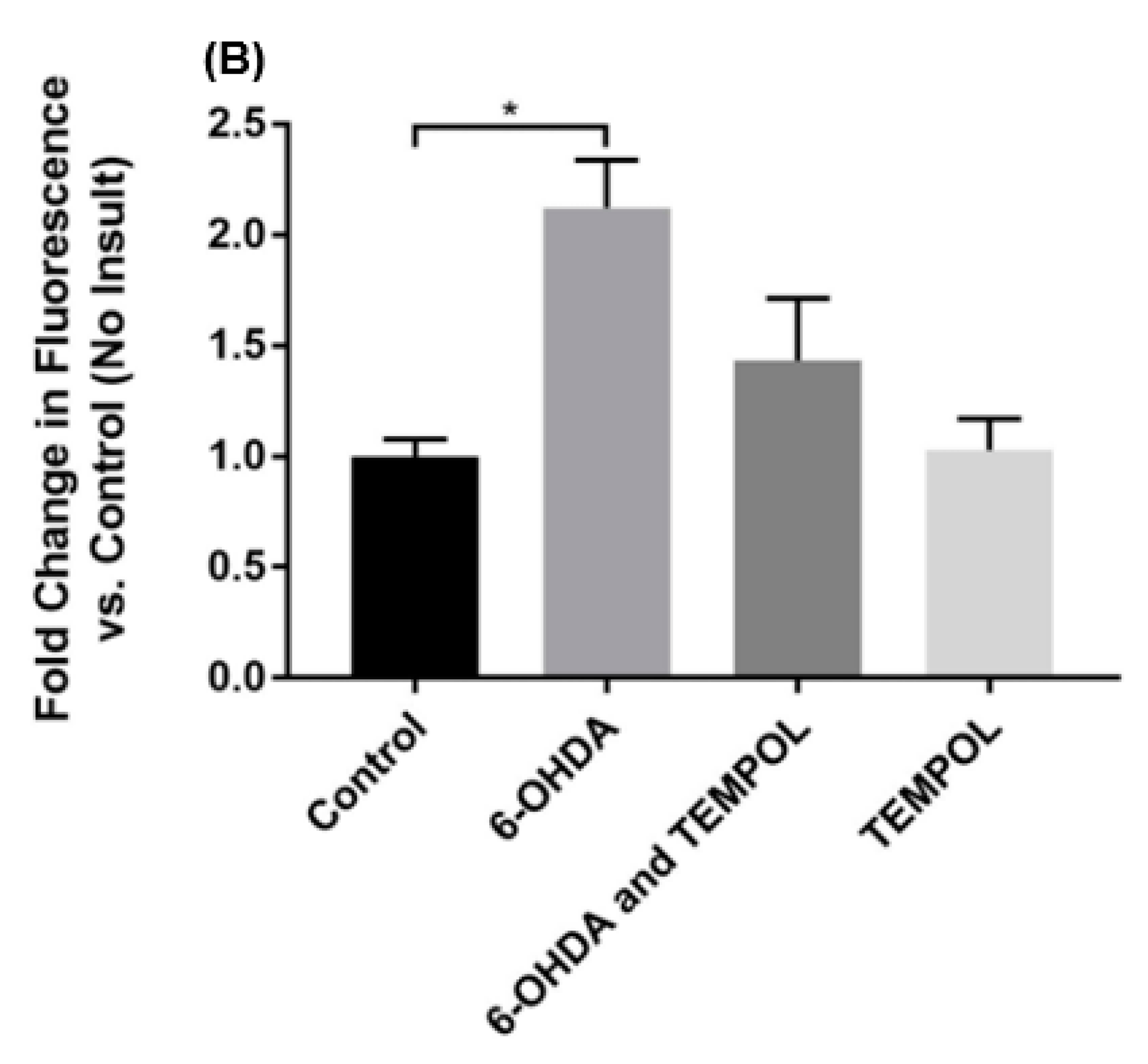
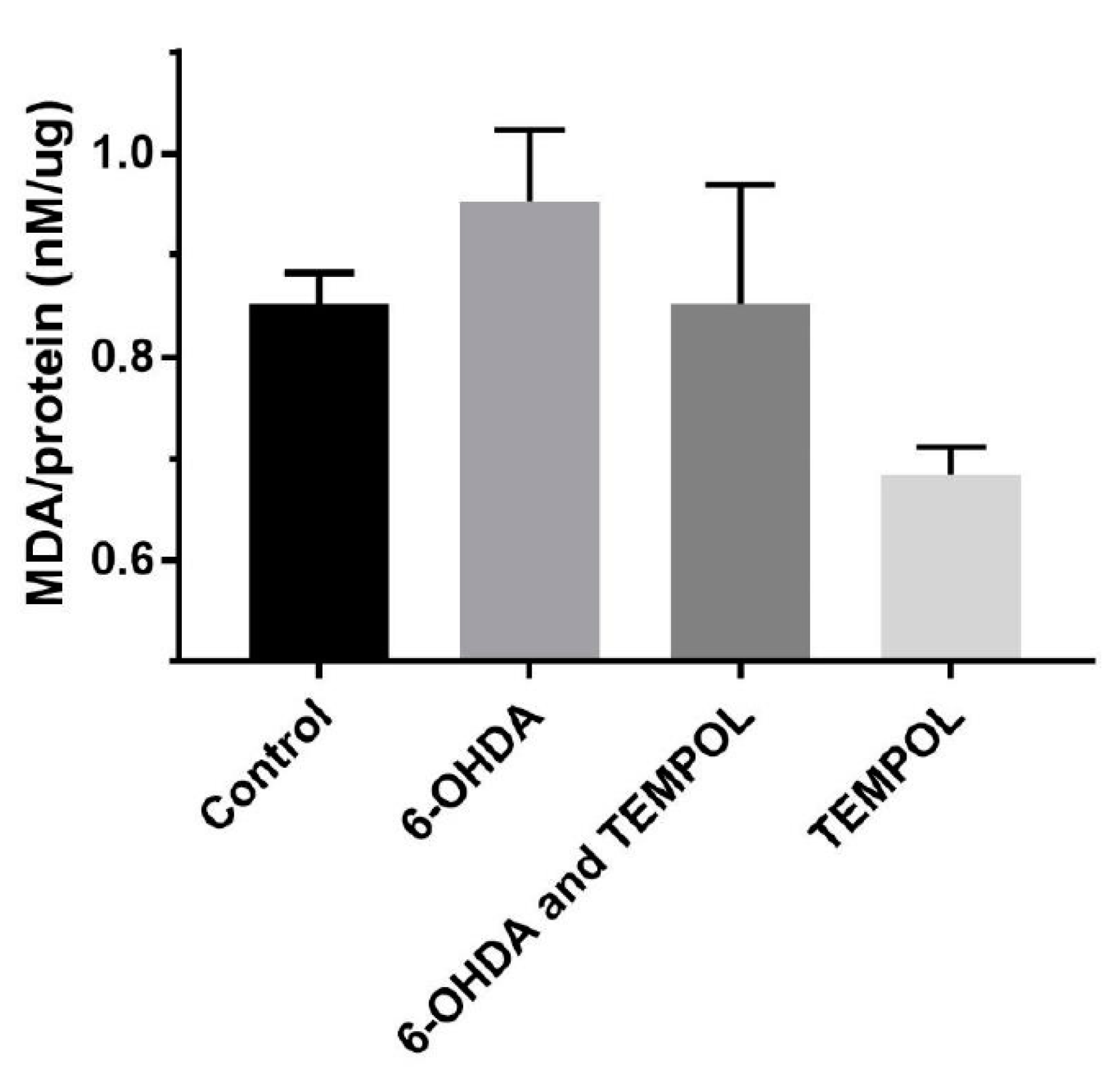
3.3. Assessment of Oxidative Damage to Cellular Lipids
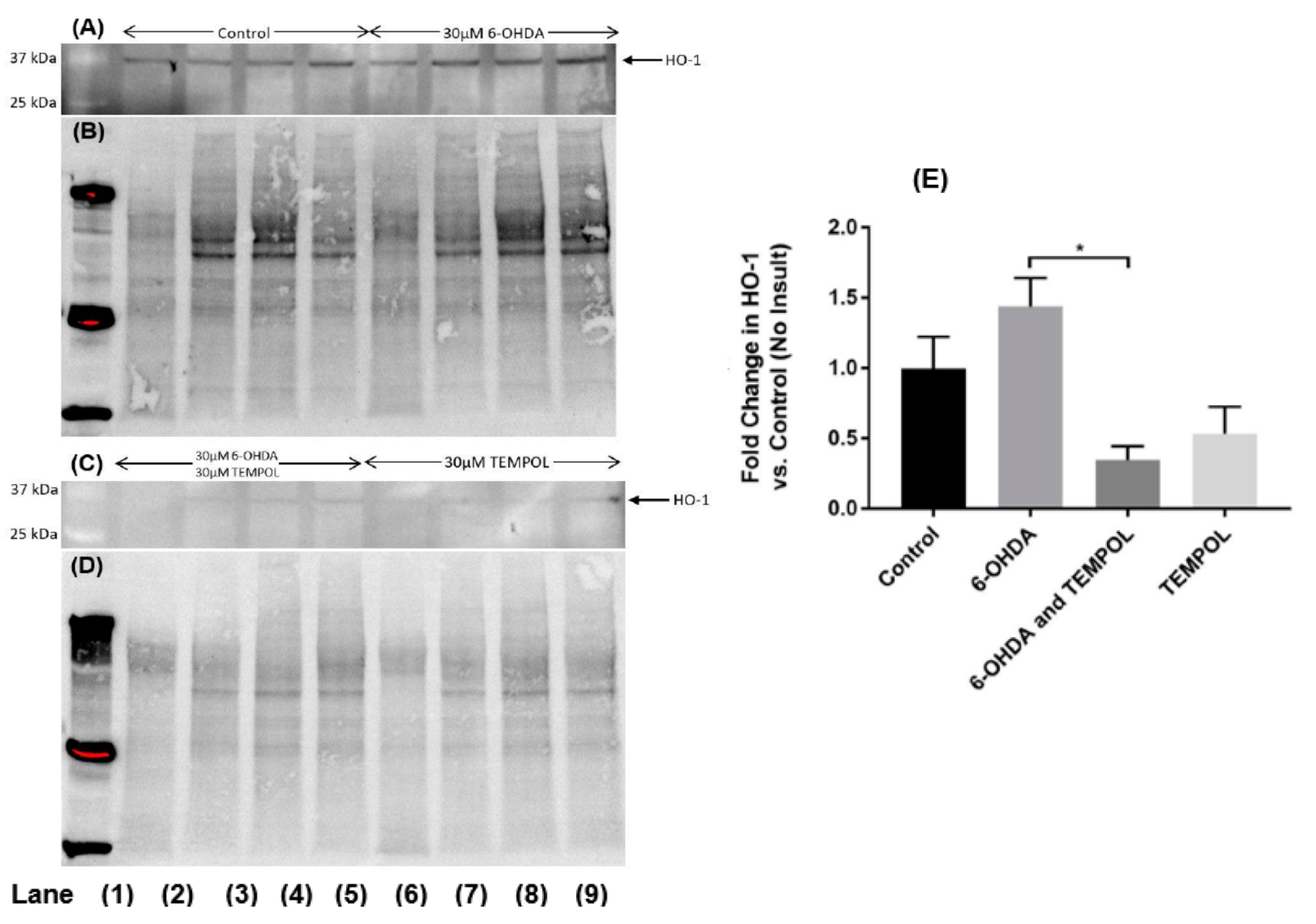
3.4. Cellular Antioxidant Response Element HO-1
3.5. 6-OHDA Elicits Alterations to Selected Genes
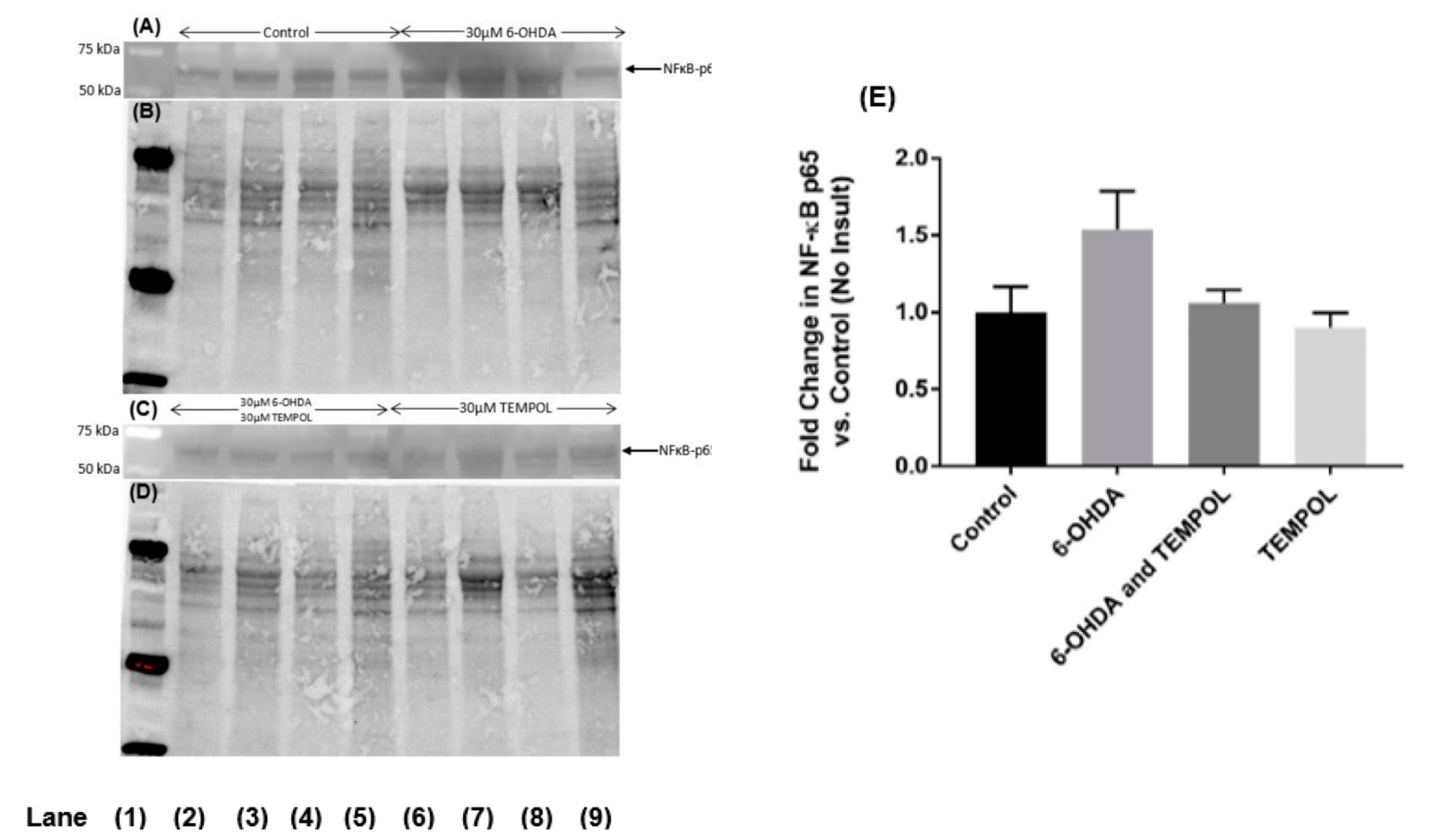
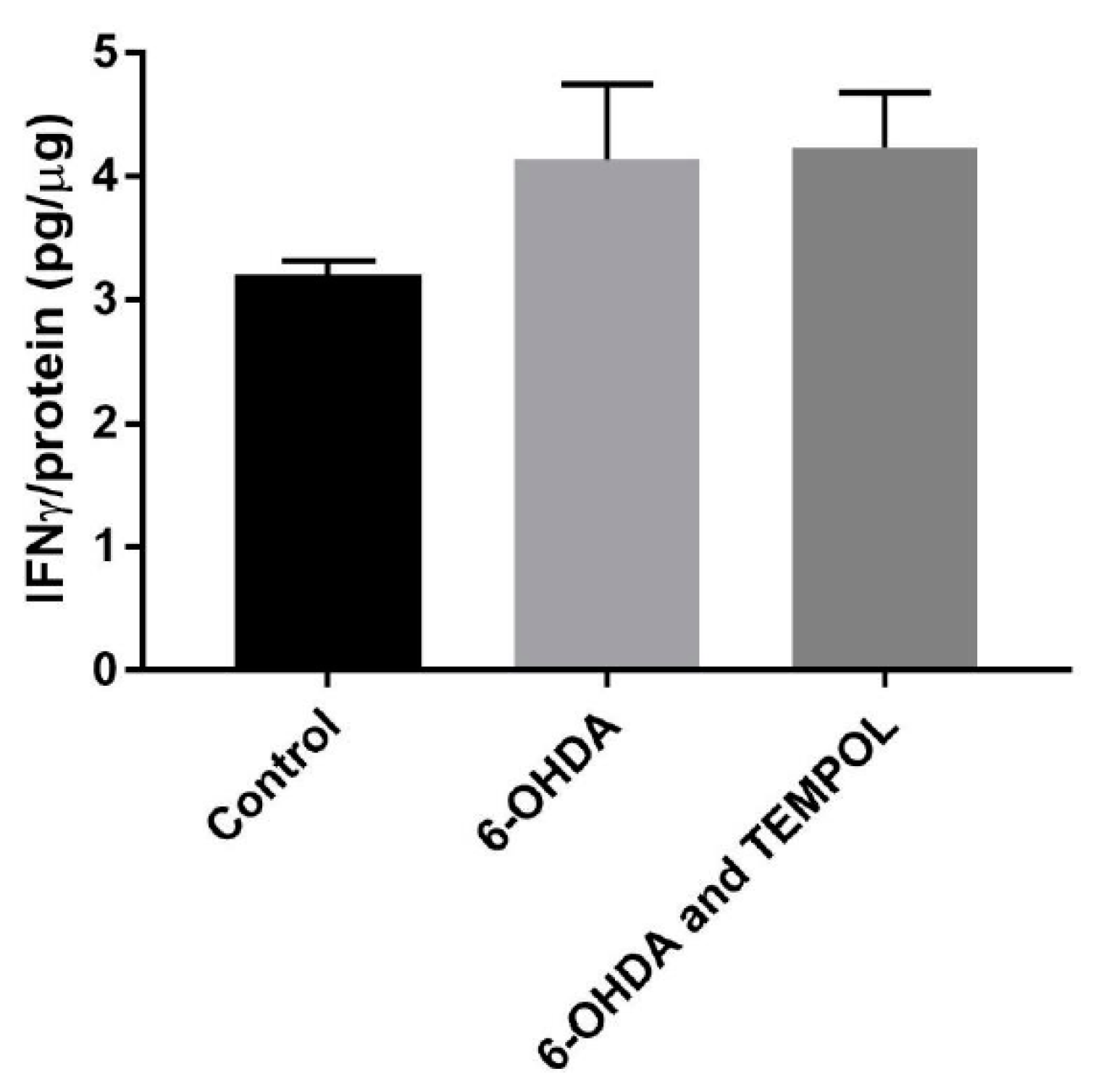
3.6. Assessing Markers of Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy Body in Parkinson’s Disease and Related Neurodegenerative Disorders. Mol. Neurobiol. 2012, 47, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korczyn, A. Drug treatment of Parkinson’s disease. Dialog. Clin. Neurosci. 2004, 6, 315–322. [Google Scholar]
- Davie, C. A review of Parkinson’s disease. Br. Med. Bull. 2008, 86, 109–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell. 2019, 18, e13031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakabeppu, Y.; Tsuchimoto, D.; Yamaguchi, H.; Sakumi, K. Oxidative damage in nucleic acids and Parkinson’s disease. J. Neurosci. Res. 2007, 85, 919–934. [Google Scholar] [CrossRef]
- Bosco, D.; Fowler, D.; Zhang, Q.; Nieva, J.; Powers, E.; Wentworth, P., Jr.; Lerner, R.A.; Kelly, J.W. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate α-synuclein fibrilization. Nat. Chem. Biol. 2006, 2, 346. [Google Scholar] [CrossRef]
- Zeevalk, G.; Razmpour, R.; Bernard, L. Glutathione and Parkinson’s disease: Is this the elephant in the room? Biomed. Pharmacother. 2008, 62, 236–249. [Google Scholar] [CrossRef]
- Venkateshapp, C.; Harish, G.; Mythri, R.; Mahadevan, A.; Srinivas Bharath, M.; Shankar, S. Increased Oxidative Damage and Decreased Antioxidant Function in Aging Human Substantia Nigra Compared to Striatum: Implications for Parkinson’s Disease. Neurochem. Res. 2011, 37, 358–369. [Google Scholar] [CrossRef]
- Trist, B.G.; Davies, K.M.; Cottam, V.; Genoud, S.; Ortega, R.; Roudeau, S.; Carmona, A.; De Silva, K.; Wasinger, V.; Lewis, S.J.G.; et al. Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson’s disease brain. Acta Neuropathol. 2017, 134, 113–127. [Google Scholar] [CrossRef]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. 2003, 53, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O. Role of Oxidative Stress in Parkinson’s Disease. Exp. Neurobiol. 2013, 22, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betarbet, R.; Sherer, T.; Greenamyre, J. Animal models of Parkinson’s disease. Bioessays. 2002, 24, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Palacino, J.; Sagi, D.; Goldberg, M.; Krauss, S.; Motz, C.; Wacker, M.; Klose, J.; Shen, J. Mitochondrial Dysfunction and Oxidative Damage inparkin-deficient Mice. J. Biol. Chem. 2004, 279, 18614–18622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, S.; Wood-Kaczmar, A.; Yao, Z.; Plun-Favreau, H.; Deas, E.; Klupsch, K.; Downward, J.; Latchman, D.S.; Tabrizi, S.J.; Wood, N.W.; et al. PINK1-Associated Parkinson’s Disease Is Caused by Neuronal Vulnerability to Calcium-Induced Cell Death. Mol. Cell. 2009, 33, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Irrcher, I.; Aleyasin, H.; Seifert, E.; Hewitt, S.; Chhabra, S.; Phillips, M.; Lutz, A.K.; Rousseaux, M.W.C.; Bevilacqua, L.; Jahani-Asl, A.; et al. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum. Mol. Genet. 2010, 19, 3734–3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, W.; Boyson, S.; Parks, J. Abnormalities of the electron transport chain in idiopathic parkinson’s disease. Ann. Neurol. 1989, 26, 719–723. [Google Scholar] [CrossRef]
- Bender, A.; Krishnan, K.; Morris, C.; Taylor, G.; Reeve, A.; Perry, R.; Jaros, E.; Hersheson, J.S.; Betts, J.; Klopstock, T.; et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006, 38, 515–517. [Google Scholar] [CrossRef]
- Schapira, A.H.; Holt, I.J.; Sweeney, M.; Harding, A.E.; Jenner, P.; Marsden, C.D. Mitochondrial DNA analysis in Parkinson’s disease. Mov. Disord. 1990, 5, 294–297. [Google Scholar] [CrossRef]
- Bilgic, B.; Pfefferbaum, A.; Rohlfing, T.; Sullivan, E.; Adalsteinsson, E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage 2012, 59, 2625–2635. [Google Scholar] [CrossRef] [Green Version]
- Hare, D.; Double, K. Iron and dopamine: A toxic couple. Brain 2016, 139, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.; Carayon, A.; Javoy-Agid, F.; Agid, Y.; Wells, F.; Daniel, S.E.; Lees, A.J.; Jenner, P.; Marsden, C.D. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 1991, 114, 1953–1975. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Roberts, B.R.; Hare, D.J.; de Jonge, M.D.; Birchall, I.E.; Jenkins, N.L.; Cherny, R.A.; Bush, A.I.; McColl, G. Direct in vivo imaging of ferrous iron dyshomeostasis in ageing Caenorhabditis elegans. Chem. Sci. 2015, 6, 2952–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, A.; Pezzella, A.; Prota, G. New Reaction Pathways of Dopamine under Oxidative Stress Conditions: Nonenzymatic Iron-Assisted Conversion to Norepinephrine and the Neurotoxins 6-Hydroxydopamine and 6,7-Dihydroxytetrahydroisoquinoline. Chem. Res. Toxicol. 1999, 12, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.; Benabid, A.; Sadoul, R.; Verna, J. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef]
- Hernandez-Baltazar, D.; Zavala-Flores, L.M.; Villanueva-Olivo, A. The 6-hydroxydopamine model and parkinsonian pathophysiology: Novel findings in an older model. Neurologia 2017, 32, 533–539. [Google Scholar] [CrossRef]
- Tufekci, K.; Meuwissen, R.; Genc, S.; Genc, K. Inflammation in Parkinson’s Disease. Adv. Protein. Chem. Struct. Biol. 2012, 88, 69–132. [Google Scholar]
- Lull, M.; Block, M. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Flood, P.; Hong, J. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J. Neural. Transm. 2010, 117, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Jeitner, T.; Kalogiannis, M.; Krasnikov, B.; Gomlin, I.; Peltier, M.; Moran, G. Linking Inflammation and Parkinson Disease: Hypochlorous Acid Generates Parkinsonian Poisons. Toxicol. Sci. 2016, 151, 388–402. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, C. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soule, B.; Hyodo, F.; Matsumoto, K.; Simone, N.; Cook, J.; Krishna, M.; Mitchell, J. The chemistry and biology of nitroxide compounds. Free Radic. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pichla, M.; Pulaski, Ł.; Kania, K.D.; Stefaniuk, I.; Cieniek, B.; Pieńkowska, N.; Bartosz, G.; Sadowska-Bartosz, I. Nitroxide Radical-Containing Redox Nanoparticles Protect Neuroblastoma SH-SY5Y Cells against 6-Hydroxydopamine Toxicity. Oxid. Med. Cell. Longev. 2020, 2020, 9260748. [Google Scholar] [CrossRef] [PubMed]
- Cannizzo, B.; Quesada, I.; Militello, R.; Amaya, C.; Miatello, R.; Cruzado, M.; Castro, C. Tempol attenuates atherosclerosis associated with metabolic syndrome via decreases vascular inflammation and NADPH-2 oxidase expression. Free Radic. Res. 2014, 48, 526–533. [Google Scholar] [CrossRef]
- Dornas, W.; Silva, M.; Tavares, R.; de Lima, W.; dos Santos, R.; Pedrosa, M.; Silva, M. Efficacy of the superoxide dismutase mimetic tempol in animal hypertension models. J. Hypertens. 2015, 33, 14–23. [Google Scholar]
- Wanyong, Y.; Zefeng, T.; Xiufeng, X.; Dawei, D.; Xiaoyan, L.; Ying, Z.; Yaogao, F. Tempol alleviates intracerebral hemorrhage-induced brain injury possibly by attenuating nitrative stress. Neuroreport 2015, 26, 842–849. [Google Scholar] [CrossRef]
- Liang, Q.; Smith, A.; Pan, S.; Tyurin, V.; Kagan, V.; Hastings, T.; Schor, N. Neuroprotective effects of TEMPOL in central and peripheral nervous system models of Parkinson’s disease. Biochem. Pharmacol. 2005, 70, 1371–1381. [Google Scholar] [CrossRef]
- Weinberg, A.; Nylander, K.D.; Yan, C.; Ma, L.; Hsia, C.J.C.; Tyurin, V.A.; Kagan, V.E.; Schor, N.F. Prevention of catecholaminergic oxidative toxicity by 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl and its recycling complex with polynitroxylated albumin, TEMPOL/PNA. Brain Res. 2004, 1012, 13–21. [Google Scholar] [CrossRef]
- Perier, C.; Bové, J.; Dehay, B.; Jackson-Lewis, V.; Rabinovitch, P.S.; Przedborski, S.; Vila, M. Apoptosis-inducing factor deficiency sensitizes dopaminergic neurons to parkinsonian neurotoxins. Ann. Neurol. 2010, 68, 184–192. [Google Scholar]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An In Vitro Model for Neuroscience: Differentiation of SH-SY5Y Cells into Cells with Morphological and Biochemical Characteristics of Mature Neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [Green Version]
- Daubner, S.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chami, B.; Jeong, G.; Varda, A.; Maw, A.; Kim, H.; Fong, G.; Simone, M.; Rayner, B.S.; Wang, X.-S.; Dennis, J.M.; et al. The nitroxide 4-methoxy TEMPO inhibits neutrophil-stimualted kinase activation in H9c2 cardiomyocytes. Arch. Biochem. Biophys. 2017, 629, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Bottle, S.E.; Eng, G.; Chami, B.; Witting, P.K. Nitroxides. Chapter 12, Nitroxide Intervention in Oxidative and Free Radical Damage in Biology and Disease. In Nitroxides: Syntehsis, Properties and Applications, 1st ed.; Ouari, O., Gigmes, D., Eds.; The Royal Society of Chemistry: London, UK, 2021; pp. 449–481. [Google Scholar]
- Choi, A.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Seeman, P.; Rajput, A.; Farley, I.; Hornykiewicz, O. Receptor basis for dopaminergic supersensitivity in Parkinson’s disease. Nature 1978, 273, 59–61. [Google Scholar] [CrossRef]
- Ryoo, H.; Pierrotti, D.; Joyce, J. Dopamine D3 receptor is decreased and D2 receptor is elevated in the striatum of Parkinson’s disease. Mov. Disord. 1998, 13, 788–797. [Google Scholar] [CrossRef]
- Simunovic, F.; Yi, M.; Wang, Y.; Macey, L.; Brown, L.; Krichevsky, A.; Andersen, S.L.; Stephens, R.M.; Benes, F.M.; Sonntag, K.C. Gene expression profiling of substantia nigra dopamine neurons: Further insights into Parkinson’s disease pathology. Brain 2008, 132, 1795–1809. [Google Scholar] [CrossRef] [Green Version]
- Bate, C.; Kempster, S.; Last, V.; Williams, A. Interferon-gamma increases neuronal death in response to amyloid-beta1-42. J. Neuroinflamm. 2006, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bissonnette, C.; Klegeris, A.; McGeer, P.; McGeer, E. Interleukin 1α and interleukin 6 protect human neuronal SH-SY5Y cells from oxidative damage. Neurosci. Lett. 2004, 361, 40–43. [Google Scholar] [CrossRef]
- Giordano, S.; Lee, J.; Darley-Usmar, V.; Zhang, J. Distinct Effects of Rotenone, 1-methyl-4-phenylpyridinium and 6-hydroxydopamine on Cellular Bioenergetics and Cell Death. PLoS ONE 2012, 7, e44610. [Google Scholar] [CrossRef]
- Iglesias-González, J.; Sánchez-Iglesias, S.; Méndez-Álvarez, E.; Rose, S.; Hikima, A.; Jenner, P.; Soto-Otero, R. Differential Toxicity of 6-Hydroxydopamine in SH-SY5Y Human Neuroblastoma Cells and Rat Brain Mitochondria: Protective Role of Catalase and Superoxide Dismutase. Neurochem. Res. 2012, 37, 2150–2160. [Google Scholar] [CrossRef]
- Storch, A.; Kaftan, A.; Burkhardt, K.; Schwarz, J. 6-Hydroxydopamine toxicity towards human SH-SY5Y dopaminergic neuroblastoma cells: Independent of mitochondrial energy metabolism. J. Neural. Transm. 2000, 107, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shi, X.; Lu, L.; Jiang, Y.; Liu, B. Stimulus-dependent neuronal cell responses in SH-SY5Y neuroblastoma cells. Mol. Med. Rep. 2016, 13, 2215–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, M.; Bottle, S.; Fairfull-Smith, K.; Malle, E.; Whitelock, J.; Davies, M. Inhibition of myeloperoxidase-mediated hypochlorous acid production by nitroxides. Biochem. J. 2009, 421, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dexter, D.T.; Carter, C.J.; Wells, F.R.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef]
- Ansarin, K.; Khoubnasabjafari, M.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts 2017, 5, 123–127. [Google Scholar] [CrossRef]
- Kato, N.; Yanaka, K.; Hyodo, K.; Homma, K.; Nagase, S.; Nose, T. Stable nitroxide Tempol ameliorates brain injury by inhibiting lipid peroxidation in a rat model of transient focal cerebral ischemia. Brain Res. 2003, 979, 188–193. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A. Heme Oxygenase-1 as a Therapeutic Target in Neurodegenerative Diseases and Brain Infections. Curr. Pharm. Des. 2008, 14, 429–442. [Google Scholar] [CrossRef]
- Fan, J.; Xu, G.; Jiang, T.; Qin, Y. Pharmacologic Induction of Heme Oxygenase-1 Plays a Protective Role in Diabetic Retinopathy in Rats. Invest Opthalmol. Vis. Sci. 2012, 53, 6541. [Google Scholar] [CrossRef] [Green Version]
- Sau, D.; De Biasi, S.; Vitellaro-Zuccarello, L.; Riso, P.; Guarnieri, S.; Porrini, M.; Simeoni, S.; Crippa, V.; Onesto, E.; Palazzolo, I.; et al. Mutation of SOD1 in ALS: A gain of a loss of function. Hum. Mol. Genet. 2007, 16, 1604–1618. [Google Scholar] [CrossRef] [Green Version]
- Rojo, A. Regulation of Cu/Zn-Superoxide Dismutase Expression via the Phosphatidylinositol 3 Kinase/Akt Pathway and Nuclear Factor B. J. Neurosci. 2004, 24, 7324–7334. [Google Scholar] [CrossRef] [Green Version]
- Hisahara, S.; Shimohama, S. Dopamine Receptors and Parkinson’s Disease. Int. J. Med. Chem. 2011, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Mei, C.; Ramos, M.; Iitaka, C.; Borrelli, E. Getting specialized: Presynaptic and postsynaptic dopamine D2 receptors. Curr. Opin. Pharmacol. 2009, 9, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabbani, N.; Negyessy, L.; Lin, R.; Goldman-Rakic, P.; Levenson, R. Interaction with Neuronal Calcium Sensor NCS-1 Mediates Desensitization of the D2 Dopamine Receptor. J. Neurosci. 2002, 22, 8476–8486. [Google Scholar] [CrossRef] [PubMed]
- Celsi, F.; Pizzo, P.; Brini, M.; Leo, S.; Fotino, C.; Pinton, P.; Rizzuto, R. Mitochondria, calcium and cell death: A deadly triad in neurodegeneration. Biochim. Biophys. Acta 2009, 1787, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Zhou, Z.; Zhang, Q.; Sun, Y.; Li, C.; Cheng, C.; Zhong, Z.; Wang, S. Protective Effect of (±) Isoborneol Against 6-OHDA-Induced Apoptosis in SH-SY5Y Cells. Cell Physiol. Biochem. 2007, 20, 1019–1032. [Google Scholar] [CrossRef]
- Dufty, B.; Warner, L.; Hou, S.; Jiang, S.; Gomez-Isla, T.; Leenhouts, K.; Oxford, J.T.; Feany, M.B.; Masliah, E.; Rohn, T.T. Calpain-Cleavage of α-Synuclein. Am. J. Pathol. 2007, 170, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Hunot, S.; Brugg, B.; Ricard, D.; Michel, P.; Muriel, M.; Ruberg, M.; Faucheux, B.A.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF- B is increased in dopaminergic neurons of patients with Parkinson disease. Proc. Nat. Acad. Sci. USA 1997, 94, 7531–7536. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.; Liu, Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2010, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Roy, A.; Liu, X.; Kordower, J.H.; Mufson, E.J.; Hartley, D.M.; Ghosh, S.; Mosley, R.L.; Gendelman, H.E.; Pahan, K. Selective inhibition of NF- B activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2007, 104, 18754–18759. [Google Scholar] [CrossRef] [Green Version]
- Mount, M.; Lira, A.; Grimes, D.; Smith, P.; Faucher, S.; Slack, R.; Anisman, H.; Hayley, S.; Park, D.S. Involvement of Interferon- in Microglial-Mediated Loss of Dopaminergic Neurons. J. Neurosci. 2007, 27, 3328–3337. [Google Scholar] [CrossRef] [Green Version]
- Titze-de-Almeida, S.; Lustosa, C.; Horst, C.; Bel, E.; Titze-de-Almeida, R. Interferon Gamma Potentiates the Injury Caused by MPP(+) on SH-SY5Y Cells, Which is Attenuated by the Nitric Oxide Synthases Inhibition. Neurochem. Res. 2014, 39, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, K.W.; Schuh, A.F.S.; Saute, J.; Townsend, R.; Fricke, D.; Leke, R.; Souza, D.O.; Portela, L.V.; Chaves, M.L.F.; Rieder, C.R.N. Interleukin-6 Serum Levels in Patients with Parkinson’s Disease. Neurochem. Res. 2009, 34, 1401–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xicoy, H.; Wieringa, B.; Martens, G. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishna, A.; Biryukov, M.; Trefois, C.; Antony, P.; Hussong, R.; Lin, J.; Heinäniemi, M.; Glusman, G.; Köglsberger, S.; Boyd, O.; et al. Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for Parkinson’s disease. BMC Genom. 2014, 15, 1154. [Google Scholar] [CrossRef]
- Lin, Y.; Uang, H.; Lin, R.; Chen, I.; Lo, Y. Neuroprotective Effects of Glyceryl Nonivamide against Microglia-Like Cells and 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Human Dopaminergic Neuroblastoma Cells. J. Pharmacol. Exp. Ther. 2007, 323, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Goralska, M.; Holley, B.; McGahan, M. The effects of Tempol on ferritin synthesis and Fe metabolism in lens epithelial cells. Biochim. Biophys. Acta. 2000, 1497, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Emoto, M.; Sasaki, K.; Maeda, K.; Fujii, H.; Sato, S. Synthesis and Evaluation as a Blood–Brain Barrier-Permeable Probe of 7-N-(PROXYL-3-yl-methyl)theophylline. Chem. Pharm. Bull. 2018, 66, 887–891. [Google Scholar] [CrossRef] [Green Version]
- Miyake, M.; Burks, S.R.; Weaver, J.; Tsai, P.; Liu, W.; Bigio, D.; Bauer, K.S.; Liu, K.J.; Rosen, G.M.; Kao, J.P.Y. Comparison of Two Nitroxide Labile Esters for Delivering Electron Paramagnetic Resonance Probes into Mouse Brain. J. Pharm. Sci. 2010, 99, 3594–3600. [Google Scholar] [CrossRef] [Green Version]
- Carroll, R.T.; Bhatia, D.; Geldenhuys, W.; Bhatia, R.; Miladore, N.; Bishayee, A.; Sutariya, V. Brain-targeted delivery of Tempol-loaded nanoparticles for neurological disorders. J. Drug Target. 2010, 18, 665–674. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer | NCBI Reference |
|---|---|---|---|
| SOD1 | GGTGTGGCCGATGTGTCTAT | CACCTTTGCCCAAGTCATCT | NM_000454.4 |
| DRD2S | GGACTCAATAACGCAGACCAGAA | CGGGCAGCCTCCTTTAGT | NM_016574.3 |
| DRD2L | GGACTCAATAACGCAGACCAGAA | GGTGAGTACAGTTGCCCTTTAGT | NM_000795.3 |
| β-actin | TTCCTTCCTGGGCATGGAGT | CCAGGGCAGTGATCTCCTTC | NM_001101.3 |
| Gene | Control | 30 μM 6-OHDA | 30 μM 6-OHDA + 30 μM TEMPOL | 30 μM TEMPOL (Alone) |
|---|---|---|---|---|
| SOD1 | 1.00 ± 0.01 | 0.83 ± 0.06 * | 0.98 ± 0.02 # | 1.01 ± 0.02 |
| DRD2S | 1.00 ± 0.01 | 0.82 ± 0.06 * | 0.96 ± 0.01 | 0.98 ± 0.02 |
| DRD2L | 1.00 ± 0.01 | 0.85 ± 0.07 | 0.99 ± 0.02 | 0.99 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leathem, A.; Simone, M.; Dennis, J.M.; Witting, P.K. The Cyclic Nitroxide TEMPOL Ameliorates Oxidative Stress but Not Inflammation in a Cell Model of Parkinson’s Disease. Antioxidants 2022, 11, 257. https://doi.org/10.3390/antiox11020257
Leathem A, Simone M, Dennis JM, Witting PK. The Cyclic Nitroxide TEMPOL Ameliorates Oxidative Stress but Not Inflammation in a Cell Model of Parkinson’s Disease. Antioxidants. 2022; 11(2):257. https://doi.org/10.3390/antiox11020257
Chicago/Turabian StyleLeathem, Alexander, Martin Simone, Joanne M. Dennis, and Paul K. Witting. 2022. "The Cyclic Nitroxide TEMPOL Ameliorates Oxidative Stress but Not Inflammation in a Cell Model of Parkinson’s Disease" Antioxidants 11, no. 2: 257. https://doi.org/10.3390/antiox11020257
APA StyleLeathem, A., Simone, M., Dennis, J. M., & Witting, P. K. (2022). The Cyclic Nitroxide TEMPOL Ameliorates Oxidative Stress but Not Inflammation in a Cell Model of Parkinson’s Disease. Antioxidants, 11(2), 257. https://doi.org/10.3390/antiox11020257







