Endoplasmic Reticulum Stress in Chemotherapy-Induced Peripheral Neuropathy: Emerging Role of Phytochemicals
Abstract
:1. Introduction
2. Chemotherapy Induces Peripheral Neuropathy
3. CIPN Impairs Quality of Life
3.1. Sensory Changes
3.2. Falls and Gait Changes
4. Molecular Mechanisms Underlying CIPN
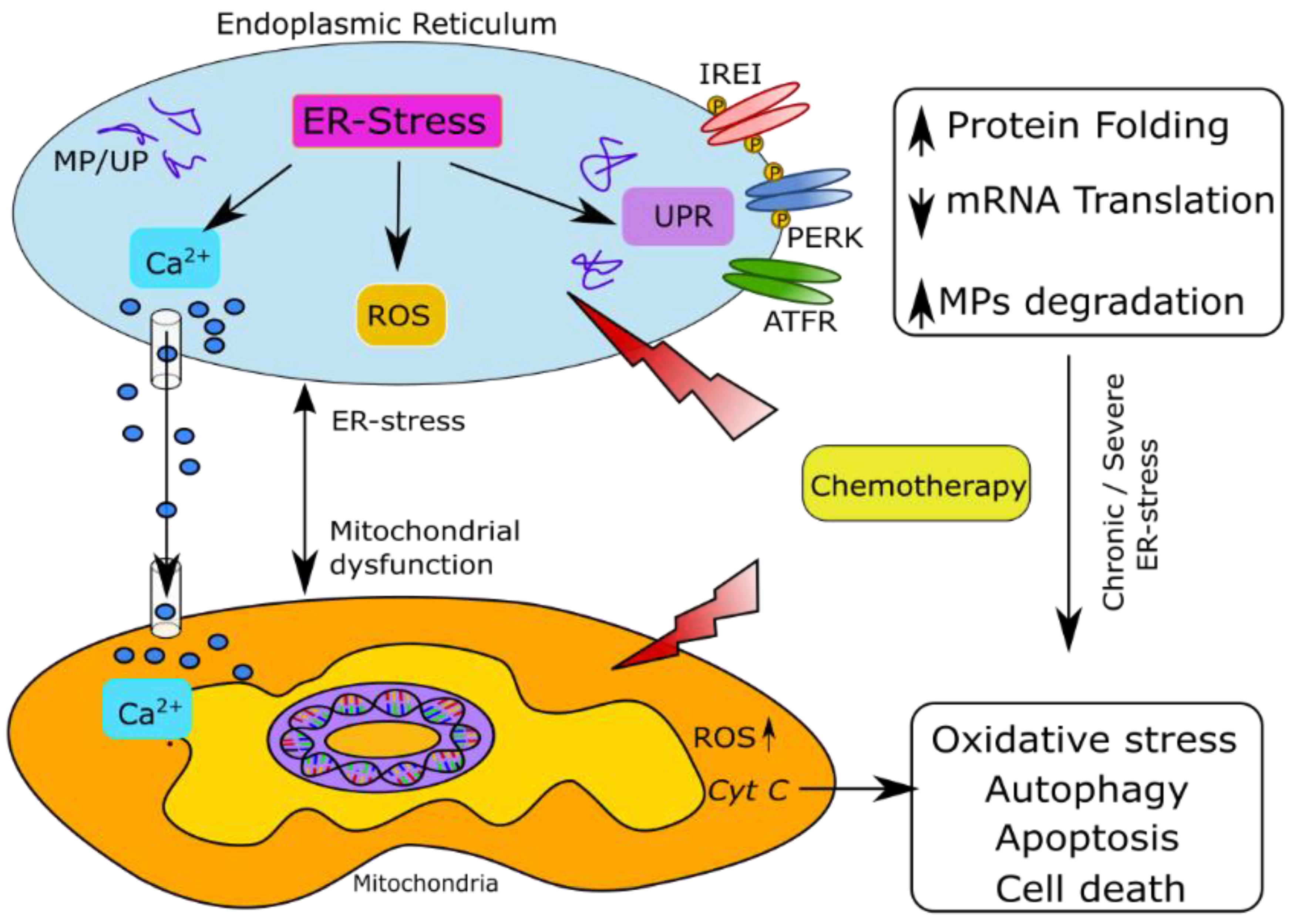
5. Endoplasmic Reticulum Stress as a Contributor to CIPN
5.1. ER Structure and Function
5.2. Factors Regulating Oxidative Homeostasis in Axons under ER Stress
5.2.1. Misfolded Protein Response
5.2.2. Mitochondrial Calcium Signaling
5.2.3. Glutathione Imbalance
5.2.4. Autophagy
5.2.5. Bioenergetic Imbalance
6. Preclinical Evidence of ER Stress in CIPN
7. ER Stress in Disorders of the Nervous System
8. Current Therapy and Phytochemicals Targeting ER Stress for CIPN
8.1. Duloxetine
8.2. Other Drugs under Study for CIPN
8.3. Phytochemicals to Target ER Stress in CIPN
9. Phytochemicals Targeting ER Stress in Nervous System Disorders
9.1. Resveratrol (3,5,4′-Trihydroxy-Trans-Stilbene)
9.2. Berberine (9,10-Dimethoxy-5,6-Dihydro-2H-7λ5-[1,3] Dioxolo[4,5-g]Isoquinolino[3,2-a]Isoquinolin-7-Ylium)
9.3. Curcumin(1E,6E)-1,7-bis (4-Hydroxy-3-Methoxyphenyl)-1,6-Heptadiene-3,5-Dione)
9.4. Epigallocatechin (Epigallocatechin-3-Gallate, EGCG)
9.5. Ginsenoside Rb1 (C54H92O23)
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer disease |
| AIF | Apoptosis inducing factor |
| AMPK | AMP-activated protein kinase |
| ART | Antiretroviral treatment |
| ATF6α | Activating transcription factor 6α |
| ATP | Adenosine triphosphate |
| BACE1 | Beta-Secretase 1 |
| Bax | BCL-2 associated X |
| BBB | Blood brain barrier |
| Bcl-2 | B-cell lymphoma 2 |
| BiP | Binding immunoglobulin protein |
| Ca2+ | Calcium ion |
| CHOP | CAAT/enhancer-binding protein homologous protein |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| CMTD | Charcot–Marie–Tooth disease |
| CNS | Central nervous system |
| EGCG | Epigallocatechin gallate |
| eIF2α | Eukaryotic translation initiation factor 2α |
| ER | Endoplasmic Reticulum |
| ERAD | Endoplasmic reticulum-associated protein degradation |
| ERK | Extracellular signal-regulated kinase |
| ERO1 | Endoplasmic reticulum oxidoreductase 1 |
| ETC | Electron transport chain |
| GRP78 | Glucose-regulating protein78 |
| GSH | Glutathione |
| GSK3β | Glycogen synthase kinase-3β |
| GSSG | Oxidized glutathione |
| HIV-1 | Human immunodeficiency virus 1 |
| HO-1 | Heme oxygenase-1 |
| Hsp70 | Heat shock protein 70 family |
| IRE1α | Inositol requiring enzyme 1α |
| JNK | c-Jun N-terminal kinase |
| K+ | Potassium ion |
| MAM | Mitochondria-associated membranes |
| MAPK | Mitogen-activated protein kinase |
| MP | Misfolded protein |
| MTI | Microtubule inhibitors |
| Na+ | Sodium ion |
| NAC | N-acetyl cysteine |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| NLRP3 | Nod-like receptor protein 3 |
| NMDA | N-methyl-D-aspartate |
| PBA | 4-phenylbutyric acid |
| PD | Parkinson’s disease |
| PDI | Protein disulphide isomerase |
| PERK | Protein kinase RNA-activated-like ER kinase |
| POCD | Postoperative cognitive dysfunction |
| p-STAT3 | Phosphorylated signal transducer and activator of transcription 3 |
| RER | Rough endoplasmic reticulum |
| ROS | Reactive oxygen species |
| SAH | Subarachnoid hemorrhage |
| SCD | Sickle cell disease |
| SER | Smooth endoplasmic reticulum |
| SIRT1 | Silent mating type information regulation 2 homolog |
| SOD | Superoxide dismutase |
| TLR4 | Toll-like receptor |
| TNF-α | Tumor necrosis factor-α |
| TRPC6 | Transient receptor potential cation channel subfamily C member 6 |
| TXNIP | Thioredoxin-interacting protein |
| UP | Unfolded protein |
| UPR | Unfolded protein response |
| XBP1 | X-box binding protein 1 |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.L.C.; de Brito, B.B.; da Silva, F.A.F.; Botelho, A.; de Melo, F.F. Nephrotoxicity in cancer treatment: An overview. World J. Clin. Oncol. 2020, 11, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.L.; Nohria, A. Prevention of Chemotherapy Induced Cardiomyopathy. Curr. Heart Fail. Rep. 2017, 14, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H. Chemotherapy-induced pulmonary toxicity in lung cancer patients. J. Thorac. Oncol. 2010, 5, 1313–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafieemehr, H.; Maleki Behzad, M.; Azandeh, S.; Farshchi, N.; Ghasemi Dehcheshmeh, M.; Saki, N. Chemo/radiotherapy-Induced Bone Marrow Niche Alterations. Cancer Investig. 2021, 39, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Caro, G.; Fortuna, M.C.; Pigliacelli, F.; D’Arino, A.; Carlesimo, M. Prevention and Treatment of Chemotherapy-Induced Alopecia. Dermatol. Pract. Concept. 2020, 10, e2020074. [Google Scholar] [CrossRef] [PubMed]
- Sougiannis, A.T.; VanderVeen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G712–G719. [Google Scholar] [CrossRef]
- Zajaczkowska, R.; Kocot-Kepska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [Green Version]
- Waimey, K.E.; Smith, B.M.; Confino, R.; Jeruss, J.S.; Pavone, M.E. Understanding Fertility in Young Female Cancer Patients. J. Women’s Health 2015, 24, 812. [Google Scholar] [CrossRef]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Carozzi, V.; Canta, A.; Chiorazzi, A. Corrigendum to “Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms?” [Neuroscience Letters 596 (2015) 90–107]. Neurosci. Lett. 2015, 596, 108. [Google Scholar] [CrossRef]
- Ewertz, M.; Qvortrup, C.; Eckhoff, L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 2015, 54, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Marmiroli, P.; Cavaletti, G.; Kalofonos, H.P. Epothilone-induced peripheral neuropathy: A review of current knowledge. J. Pain Symptom Manag. 2011, 42, 931–940. [Google Scholar] [CrossRef]
- Yamamoto, S.; Egashira, N. Pathological Mechanisms of Bortezomib-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2021, 22, 888. [Google Scholar] [CrossRef] [PubMed]
- Mezo, M.; Castaneda, C.; Weiss, L.; Minton, N.; Hirsch, D.; Renz, C.; Arunagiri, U.; Brown, B.; Gambini, D.; Peng, M.; et al. Peripheral Neuropathy (PN) with Immunomodulatory Drugs in Patients with Multiple Myeloma (MM). Blood 2016, 128, 5677. [Google Scholar] [CrossRef]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy (CIPN): Where are we now? Pain 2019, 160 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Hu, S.; Huang, K.M.; Adams, E.J.; Loprinzi, C.L.; Lustberg, M.B. Recent Developments of Novel Pharmacologic Therapeutics for Prevention of Chemotherapy-Induced Peripheral Neuropathy. Clin. Cancer Res. 2019, 25, 6295–6301. [Google Scholar] [CrossRef]
- Li, Y.; Lustberg, M.B.; Hu, S. Emerging Pharmacological and Non-Pharmacological Therapeutics for Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. Cancers 2021, 13, 766. [Google Scholar] [CrossRef]
- Doyle, T.M.; Salvemini, D. Mini-Review: Mitochondrial dysfunction and chemotherapy-induced neuropathic pain. Neurosci. Lett. 2021, 760, 136087. [Google Scholar] [CrossRef]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2020, 11, 626687. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; Dworkin, R.H.; Finnerup, N.B.; Mohile, N.A. Painful chemotherapy-induced peripheral neuropathy: Lack of treatment efficacy or the wrong clinical trial methodology? Pain 2017, 158, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; Fan, L.; Magnuson, A.; Mustian, K.; Peppone, L.; Heckler, C.; Hopkins, J.; Tejani, M.; Morrow, G.R.; Mohile, S.G. Falls and functional impairments in cancer survivors with chemotherapy-induced peripheral neuropathy (CIPN): A University of Rochester CCOP study. Support. Care Cancer 2013, 21, 2059–2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, S.L.; Barton, D.L.; Qin, R.; Wos, E.J.; Sloan, J.A.; Liu, H.S.; Aaronson, N.K.; Satele, D.V.; Mattar, B.I.; Green, N.B.; et al. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support. Care Cancer 2012, 20, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Bhaskar, A. Chemotherapy-induced peripheral neuropathic pain. Bja Educ. 2016, 16, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Kolb, N.; Smith, A.; Singleton, J.; Beck, S.; Stoddard, G.; Brown, S.; Mooney, K. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. 2016, 73, 860–866. [Google Scholar] [CrossRef] [Green Version]
- Park, S.B.; Krishnan, A.V.; Lin, C.S.; Goldstein, D.; Friedlander, M.; Kiernan, M.C. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr. Med. Chem. 2008, 15, 3081–3094. [Google Scholar] [CrossRef]
- Windebank, A.J.; Grisold, W. Chemotherapy-induced neuropathy. J. Peripher. Nerv. Syst. 2008, 13, 27–46. [Google Scholar] [CrossRef]
- Wolf, S.; Barton, D.; Kottschade, L.; Grothey, A.; Loprinzi, C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur. J. Cancer 2008, 44, 1507–1515. [Google Scholar] [CrossRef]
- Wang, A.B.; Housley, S.N.; Flores, A.M.; Kircher, S.M.; Perreault, E.J.; Cope, T.C. A review of movement disorders in chemotherapy-induced neurotoxicity. J. Neuroeng. Rehabil. 2021, 18, 16. [Google Scholar] [CrossRef]
- Han, Y.; Smith, M. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front. Pharmacol. 2013, 4, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Overcash, J. Prediction of Falls in Older Adults With Cancer: A Preliminary Study. Oncol. Nurs. Forum 2007, 34, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Monfort, S.M.; Pan, X.; Patrick, R.; Ramaswamy, B.; Wesolowski, R.; Naughton, M.J.; Loprinzi, C.L.; Chaudhari, A.M.W.; Lustberg, M.B. Gait, balance, and patient-reported outcomes during taxane-based chemotherapy in early-stage breast cancer patients. Breast Cancer Res. Treat. 2017, 164, 69–77. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Horak, F.; Jacobs, P.G.; Trubowitz, P.; Dieckmann, N.F.; Stoyles, S.; Faithfull, S. Falls, Functioning, and Disability Among Women With Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy. J. Clin. Oncol. 2017, 35, 2604–2612. [Google Scholar] [CrossRef] [Green Version]
- Tofthagen, C.; Overcash, J.; Kip, K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support. Care Cancer 2012, 20, 583–589. [Google Scholar] [CrossRef]
- Marshall, T.F.; Zipp, G.P.; Battaglia, F.; Moss, R.; Bryan, S. Chemotherapy-induced-peripheral neuropathy, gait and fall risk in older adults following cancer treatment. J. Cancer Res. Pract. 2017, 4, 134–138. [Google Scholar] [CrossRef]
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity-focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Sagi, V.; Mittal, A.; Tran, H.; Gupta, K. Pain in sickle cell disease: Current and potential translational therapies. Transl. Res. 2021, 234, 141–158. [Google Scholar] [CrossRef]
- Kohli, D.R.; Li, Y.; Khasabov, S.G.; Gupta, P.; Kehl, L.J.; Ericson, M.E.; Nguyen, J.; Gupta, V.; Hebbel, R.P.; Simone, D.A.; et al. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: Modulation by cannabinoids. Blood 2010, 116, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Kiven, S.; Wang, Y.; Aich, A.; Argueta, D.A.; Lei, J.; Sagi, V.; Tennakoon, M.; Bedros, S.J.; Lambrecht, N.; Gupta, K. Spatiotemporal Alterations in Gait in Humanized Transgenic Sickle Mice. Front. Immunol. 2020, 11, 561947. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Li, Y.; Segal, R.A. A Mechanistic Understanding of Axon Degeneration in Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2017, 11, 481. [Google Scholar] [CrossRef]
- Nicolini, G.; Monfrini, M.; Scuteri, A. Axonal Transport Impairment in Chemotherapy-Induced Peripheral Neuropathy. Toxics 2015, 3, 322–341. [Google Scholar] [CrossRef] [Green Version]
- Aromolaran, K.A.; Goldstein, P.A. Ion channels and neuronal hyperexcitability in chemotherapy-induced peripheral neuropathy; cause and effect? Mol. Pain 2017, 13, 1744806917714693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotto, S.; Ferrari, S.; Sorio, M.; Benedetti, F.; Tridente, G.; Cavallaro, T.; Gajofatto, A.; Monaco, S. Brentuximab vedotin: Axonal microtubule’s Apollyon. Blood Cancer J. 2015, 5, e343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousuf, M.S.; Maguire, A.D.; Simmen, T.; Kerr, B.J. Endoplasmic reticulum-mitochondria interplay in chronic pain: The calcium connection. Mol. Pain 2020, 16, 1744806920946889. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, A.; Flatters, S.J.L. Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. Int. Rev. Neurobiol. 2019, 145, 83–126. [Google Scholar]
- Eldridge, S.; Guo, L.; Hamre, J., 3rd. A Comparative Review of Chemotherapy-Induced Peripheral Neuropathy in In Vivo and In Vitro Models. Toxicol. Pathol. 2020, 48, 190–201. [Google Scholar] [CrossRef]
- Luarte, A.; Cornejo, V.H.; Bertin, F.; Gallardo, J.; Couve, A. The axonal endoplasmic reticulum: One organelle-many functions in development, maintenance, and plasticity. Dev. Neurobiol. 2018, 78, 181–208. [Google Scholar] [CrossRef]
- Tsukita, S.; Ishikawa, H. Three-dimensional distributionof smooth endoplasmic reticulum in myelinated axons. Microscopy 1976, 25, 141–149. [Google Scholar]
- Karagas, N.E.; Venkatachalam, K. Roles for the Endoplasmic Reticulum in Regulation of Neuronal Calcium Homeostasis. Cells 2019, 8, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasaki, M. Axonal endoplasmic reticulum is very narrow. J. Cell Sci. 2018, 131, jcs210450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, C.; Canovas, J.; Fresno, J.; Couve, E.; Court, F.A.; Couve, A. Axons provide the secretory machinery for trafficking of voltage-gated sodium channels in peripheral nerve. Proc. Natl. Acad. Sci. USA 2016, 113, 1823–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, O.A.; Couve, A. The endoplasmic reticulum and protein trafficking in dendrites and axons. Trends Cell Biol. 2011, 21, 219–227. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [Green Version]
- Sonda, S.; Pendin, D.; Daga, A. ER Morphology in the Pathogenesis of Hereditary Spastic Paraplegia. Cells 2021, 10, 2870. [Google Scholar] [CrossRef] [PubMed]
- Handley, M.T.; Mégarbané, A.; Meynert, A.M.; Brown, S.; Freyer, E.; Taylor, M.S.; Jackson, I.J.; Aligianis, I.A. Loss of ALDH 18A1 function is associated with a cellular lipid droplet phenotype suggesting a link between autosomal recessive cutis laxa type 3A and Warburg Micro syndrome. Mol. Genet. Genom. Med. 2014, 2, 319–325. [Google Scholar] [CrossRef]
- Larrea, D.; Pera, M.; Gonnelli, A.; Quintana–Cabrera, R.; Akman, H.O.; Guardia-Laguarta, C.; Velasco, K.R.; Area-Gomez, E.; Dal Bello, F.; De Stefani, D. MFN2 mutations in Charcot–Marie–Tooth disease alter mitochondria-associated ER membrane function but do not impair bioenergetics. Hum. Mol. Genet. 2019, 28, 1782–1800. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, D.; Berenguer-Escuder, C.; Bellet, M.E.; Scheibner, D.; Bohler, J.; Massart, F.; Rapaport, D.; Skupin, A.; Fouquier d’Herouel, A.; Sharma, M.; et al. Mutations in RHOT1 Disrupt Endoplasmic Reticulum-Mitochondria Contact Sites Interfering with Calcium Homeostasis and Mitochondrial Dynamics in Parkinson’s Disease. Antioxid. Redox Signal. 2019, 31, 1213–1234. [Google Scholar] [CrossRef] [Green Version]
- Zatti, G.; Burgo, A.; Giacomello, M.; Barbiero, L.; Ghidoni, R.; Sinigaglia, G.; Florean, C.; Bagnoli, S.; Binetti, G.; Sorbi, S.; et al. Presenilin mutations linked to familial Alzheimer’s disease reduce endoplasmic reticulum and Golgi apparatus calcium levels. Cell Calcium 2006, 39, 539–550. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, J.; Wang, Y.H.; Hang, C.H. ROS-Mediated Mitochondrial Dysfunction and ER Stress Contribute to Compression-Induced Neuronal Injury. Neuroscience 2019, 416, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, L.; Selzer, M.E.; Hu, Y. Neuronal endoplasmic reticulum stress in axon injury and neurodegeneration. Ann. Neurol. 2013, 74, 768–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y. Axon injury induced endoplasmic reticulum stress and neurodegeneration. Neural Regen. Res. 2016, 11, 1557–1559. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell. Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindholm, D.; Korhonen, L.; Eriksson, O.; Koks, S. Recent Insights into the Role of Unfolded Protein Response in ER Stress in Health and Disease. Front. Cell Dev. Biol. 2017, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Rao, R.V.; Bredesen, D.E. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr. Opin. Cell Biol. 2004, 16, 653–662. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Raeisossadati, R.; Ferrari, M.F.R. Mitochondria-ER Tethering in Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2020, 1–14. [Google Scholar] [CrossRef]
- Bravo, R.; Gutierrez, T.; Paredes, F.; Gatica, D.; Rodriguez, A.E.; Pedrozo, Z.; Chiong, M.; Parra, V.; Quest, A.F.; Rothermel, B.A.; et al. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int. J. Biochem. Cell Biol. 2012, 44, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, W.C.; Shastri, M.D.; Eri, R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int. J. Mol. Sci. 2017, 18, 771. [Google Scholar] [CrossRef] [PubMed]
- Bertero, E.; Maack, C. Calcium Signaling and Reactive Oxygen Species in Mitochondria. Circ. Res. 2018, 122, 1460–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Baxter, P.S.; Hardingham, G.E. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, K.; Nakaki, T. Impaired glutathione synthesis in neurodegeneration. Int. J. Mol. Sci. 2013, 14, 21021–21044. [Google Scholar] [CrossRef] [Green Version]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic Reticulum Stress and Associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Arikkath, J.; Yang, L.; Guo, M.L.; Periyasamy, P.; Buch, S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy 2016, 12, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Stavoe, A.K.H.; Holzbaur, E.L.F. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol. 2019, 35, 477–500. [Google Scholar] [CrossRef] [PubMed]
- Kongara, S.; Karantza, V. The interplay between autophagy and ROS in tumorigenesis. Front. Oncol. 2012, 2, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.H.; Shim, J.H.; Kim, K.H.; Ha, J.Y.; Han, J.Y. Neuronal autophagy and neurodegenerative diseases. Exp. Mol. Med. 2012, 44, 89–98. [Google Scholar] [CrossRef]
- Inceoglu, B.; Bettaieb, A.; da Silva, C.A.T.; Lee, K.S.S.; Haj, F.G.; Hammock, B.D. Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc. Natl. Acad. Sci. USA 2015, 112, 9082–9087. [Google Scholar] [CrossRef] [Green Version]
- Gherardi, G.; Monticelli, H.; Rizzuto, R.; Mammucari, C. The Mitochondrial Ca(2+) Uptake and the Fine-Tuning of Aerobic Metabolism. Front. Physiol. 2020, 11, 554904. [Google Scholar] [CrossRef]
- Yong, J.; Bischof, H.; Burgstaller, S.; Siirin, M.; Murphy, A.; Malli, R.; Kaufman, R.J. Mitochondria supply ATP to the ER through a mechanism antagonized by cytosolic Ca2+. Elife 2019, 8, e49682. [Google Scholar] [CrossRef]
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase 3, NF-kappaB, ATF-6 pathways and activating Nrf2, Akt pathways. Neurotoxicology 2020, 81, 137–146. [Google Scholar] [CrossRef]
- Zhu, J.; Carozzi, V.A.; Reed, N.; Mi, R.; Marmiroli, P.; Cavaletti, G.; Hoke, A. Ethoxyquin provides neuroprotection against cisplatin-induced neurotoxicity. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Chine, V.B.; Au, N.P.B.; Kumar, G.; Ma, C.H.E. Targeting axon integrity to prevent chemotherapy-induced peripheral neuropathy. Mol. Neurobiol. 2019, 56, 3244–3259. [Google Scholar] [CrossRef]
- Chine, V.B.; Au, N.P.B.; Ma, C.H.E. Therapeutic benefits of maintaining mitochondrial integrity and calcium homeostasis by forced expression of Hsp27 in chemotherapy-induced peripheral neuropathy. Neurobiol. Dis. 2019, 130, 104492. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Tan, S.K.; Lieu, C.H.; Jung, H.K. Involvement of endoplasmic reticulum in paclitaxel-induced apoptosis. J. Cell. Biochem. 2008, 104, 1509–1523. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Thorne, R.; Zhang, X.D.; Hersey, P. Involvement of endoplasmic reticulum stress in Docetaxel-induced JNK-dependent apoptosis of human melanoma. Apoptosis 2008, 13, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Tanimukai, H.; Kanayama, D.; Omi, T.; Takeda, M.; Kudo, T. Paclitaxel induces neurotoxicity through endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2013, 437, 151–155. [Google Scholar] [CrossRef]
- Ozturk, Z.; O’Kane, C.J.; Perez-Moreno, J.J. Axonal Endoplasmic Reticulum Dynamics and Its Roles in Neurodegeneration. Front. Neurosci. 2020, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.D.; Hinder, L.M.; Sakowski, S.A.; Feldman, E.L. ER stress in diabetic peripheral neuropathy: A new therapeutic target. Antioxid. Redox Signal. 2014, 21, 621–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Shang, Y.; Yan, Z.; Li, H.; Wang, Z.; Li, Z.; Liu, Z. Pim1 kinase provides protection against high glucose-induced stress and apoptosis in cultured dorsal root ganglion neurons. Neurosci. Res. 2021, 169, 9–16. [Google Scholar] [CrossRef]
- Amruth, G.; Praveen-Kumar, S.; Nataraju, B.; Nagaraja, B.S. HIV Associated Sensory Neuropathy. J. Clin. Diagn. Res. 2014, 8, Mc4–Mc7. [Google Scholar]
- Nooka, S.; Ghorpade, A. HIV-1-associated inflammation and antiretroviral therapy regulate astrocyte endoplasmic reticulum stress responses. Cell Death Discov. 2017, 3, 17061. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Paul, J.; Wang, Y.; Gupta, M.; Vang, D.; Thompson, S.; Jha, R.; Nguyen, J.; Valverde, Y.; Lamarre, Y.; et al. Heme Causes Pain in Sickle Mice via Toll-Like Receptor 4-Mediated Reactive Oxygen Species- and Endoplasmic Reticulum Stress-Induced Glial Activation. Antioxid. Redox Signal. 2021, 34, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, G.; Rajput, S.; Gupta, K.; Simone, D.A. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain 2015, 156, 722–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, H.; Mittal, A.; Sagi, V.; Luk, K.; Nguyen, A.; Gupta, M.; Nguyen, J.; Lamarre, Y.; Lei, J.; Guedes, A.; et al. Mast Cells Induce Blood Brain Barrier Damage in SCD by Causing Endoplasmic Reticulum Stress in the Endothelium. Front. Cell. Neurosci. 2019, 13, 56. [Google Scholar] [CrossRef]
- Ballas, S.K.; Darbari, D.S. Neuropathy, neuropathic pain, and sickle cell disease. Am. J. Hematol. 2013, 88, 927–929. [Google Scholar] [CrossRef] [Green Version]
- McCray, B.A.; Scherer, S.S. Axonal Charcot-Marie-Tooth Disease: From Common Pathogenic Mechanisms to Emerging Treatment Opportunities. Neurotherapeutics 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Mallinckrodt, C.; Lu, Y.; Demitrack, M.A. Duloxetine in the treatment of major depressive disorder: A double-blind clinical trial. J. Clin. Psychiatry 2002, 63, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershman, D.L.; Unger, J.M.; Crew, K.D.; Minasian, L.M.; Awad, D.; Moinpour, C.M.; Hansen, L.; Lew, D.L.; Greenlee, H.; Fehrenbacher, L. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J. Clin. Oncol. 2013, 31, 2627. [Google Scholar] [CrossRef] [Green Version]
- Loven, D.; Levavi, H.; Sabach, G.; Zart, R.; Andras, M.; Fishman, A.; Karmon, Y.; Levi, T.; Dabby, R.; Gadoth, N. Long-term glutamate supplementation failed to protect against peripheral neurotoxicity of paclitaxel. Eur. J. Cancer Care 2009, 18, 78–83. [Google Scholar] [CrossRef]
- Stubblefield, M.; Vahdat, L.; Balmaceda, C.; Troxel, A.; Hesdorffer, C.; Gooch, C. Glutamine as a neuroprotective agent in high-dose paclitaxel-induced peripheral neuropathy: A clinical and electrophysiologic study. Clin. Oncol. 2005, 17, 271–276. [Google Scholar] [CrossRef]
- Strasser, F.; Demmer, R.; Böhme, C.; Schmitz, S.F.H.; Thuerlimann, B.; Cerny, T.; Gillessen, S. Prevention of docetaxel-or paclitaxel-associated taste alterations in cancer patients with oral glutamine. a randomized, placebo-controlled, double-blind study. Oncologist 2008, 13, 337–346. [Google Scholar] [CrossRef]
- Kottschade, L.A.; Sloan, J.A.; Mazurczak, M.A.; Johnson, D.B.; Murphy, B.P.; Rowland, K.M.; Smith, D.A.; Berg, A.R.; Stella, P.J.; Loprinzi, C.L. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: Results of a randomized phase III clinical trial. Support. Care Cancer 2011, 19, 1769–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Jones, D.; Palmer, J.L.; Forman, A.; Dakhil, S.R.; Velasco, M.R.; Weiss, M.; Gilman, P.; Mills, G.; Noga, S.J. Oral alpha-lipoic acid to prevent chemotherapy-induced peripheral neuropathy: A randomized, double-blind, placebo-controlled trial. Support. Care Cancer 2014, 22, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Schloss, J.M.; Colosimo, M.; Airey, C.; Masci, P.; Linnane, A.W.; Vitetta, L. A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Support. Care Cancer 2017, 25, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Boyette-Davis, J.; Dougherty, P.M. Protection against oxaliplatin-induced mechanical hyperalgesia and intraepidermal nerve fiber loss by minocycline. Exp. Neurol. 2011, 229, 353–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao-Ying, Q.L.; Kavelaars, A.; Krukowski, K.; Huo, X.J.; Zhou, W.; Price, T.J.; Cleeland, C.; Heijnen, C.J. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS ONE 2014, 9, e100701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, L.; Patte-Mensah, C.; Taleb, O.; Mensah-Nyagan, A.G. Cellular and functional evidence for a protective action of neurosteroids against vincristine chemotherapy-induced painful neuropathy. Cell. Mol. Life Sci. 2010, 67, 3017–3034. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.; Patte-Mensah, C.; Taleb, O.; Mensah-Nyagan, A.G. Allopregnanolone prevents and suppresses oxaliplatin-evoked painful neuropathy: Multi-parametric assessment and direct evidence. Pain 2011, 152, 170–181. [Google Scholar] [CrossRef]
- Muthuraman, A.; Singh, N. Attenuating effect of hydroalcoholic extract of Acorus calamus in vincristine-induced painful neuropathy in rats. J. Nat. Med. 2011, 65, 480–487. [Google Scholar] [CrossRef]
- Rahn, E.; Makriyannis, A.; Hohmann, A. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br. J. Pharmacol. 2007, 152, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Jaggi, A.S.; Singh, N. Exploring the potential effect of Ocimum sanctum in vincristine-induced neuropathic pain in rats. J. Brachial Plex. Peripher. Nerve Inj. 2010, 5, e3–e11. [Google Scholar] [CrossRef] [Green Version]
- Namvaran-Abbas-Abad, A.; Tavakkoli, F. Antinociceptive effect of salvia extract on cisplatin-induced hyperalgesia in mice. Neurophysiology 2012, 43, 452–458. [Google Scholar] [CrossRef]
- Shabani, M.; Nazeri, M.; Parsania, S.; Razavinasab, M.; Zangiabadi, N.; Esmaeilpour, K.; Abareghi, F. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology 2012, 33, 1314–1321. [Google Scholar] [CrossRef]
- Ushio, S.; Egashira, N.; Sada, H.; Kawashiri, T.; Shirahama, M.; Masuguchi, K.; Oishi, R. Goshajinkigan reduces oxaliplatin-induced peripheral neuropathy without affecting anti-tumour efficacy in rodents. Eur. J. Cancer 2012, 48, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.A.; Andoh, T.; Ogura, K.; Hayakawa, Y.; Saiki, I.; Kuraishi, Y. Herbal Medicine Goshajinkigan Prevents Paclitaxel-Induced Mechanical Allodynia without Impairing Antitumor Activity of Paclitaxel. Evid. Based Complement. Alternat. Med. 2013, 2013, 849754. [Google Scholar] [CrossRef] [Green Version]
- Ahn, B.S.; Kim, S.K.; Kim, H.N.; Lee, J.H.; Lee, J.H.; Hwang, D.S.; Bae, H.; Min, B.I.; Kim, S.K. Gyejigachulbu-Tang Relieves Oxaliplatin-Induced Neuropathic Cold and Mechanical Hypersensitivity in Rats via the Suppression of Spinal Glial Activation. Evid. Based Complement. Alternat. Med. 2014, 2014, 436482. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Shima, T.; Nagira, K.; Ieki, M.; Nakamura, T.; Aono, Y.; Kuraishi, Y.; Arai, T.; Saito, S. Herbal medicine Shakuyaku-kanzo-to reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur. J. Pain 2009, 13, 22–27. [Google Scholar] [CrossRef]
- Oveissi, V.; Ram, M.; Bahramsoltani, R.; Ebrahimi, F.; Rahimi, R.; Naseri, R.; Belwal, T.; Devkota, H.P.; Abbasabadi, Z.; Farzaei, M.H. Medicinal plants and their isolated phytochemicals for the management of chemotherapy-induced neuropathy: Therapeutic targets and clinical perspective. Daru-J. Pharm. Sci. 2019, 27, 389–406. [Google Scholar] [CrossRef]
- Kawashiri, T.; Inoue, M.; Mori, K.; Kobayashi, D.; Mine, K.; Ushio, S.; Kudamatsu, H.; Uchida, M.; Egashira, N.; Shimazoe, T. Preclinical and Clinical Evidence of Therapeutic Agents for Paclitaxel-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2021, 22, 8733. [Google Scholar] [CrossRef]
- Semis, H.S.; Kandemir, F.M.; Kaynar, O.; Dogan, T.; Arikan, S.M. The protective effects of hesperidin against paclitaxel-induced peripheral neuropathy in rats. Life Sci. 2021, 287, 120104. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverria, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sanchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.-W.; Choi, K.-C. Potential Roles of Iridoid Glycosides and Their Underlying Mechanisms against Diverse Cancer Growth and Metastasis: Do They Have an Inhibitory Effect on Cancer Progression? Nutrients 2021, 13, 2974. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Uta, D.; Kato, M.; Toume, K.; Komatsu, K.; Kuraishi, Y. Prophylactic Administration of Aucubin Inhibits Paclitaxel-Induced Mechanical Allodynia via the Inhibition of Endoplasmic Reticulum Stress in Peripheral Schwann Cells. Biol. Pharm. Bull. 2017, 40, 473–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Xia, X.; Rui, Y.; Zhang, Z.; Qin, L.; Han, S.; Wan, Z. The combination of 1alpha,25dihydroxyvitaminD3 with resveratrol improves neuronal degeneration by regulating endoplasmic reticulum stress, insulin signaling and inhibiting tau hyperphosphorylation in SH-SY5Y cells. Food Chem. Toxicol. 2016, 93, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, H.H.; Zakaria, S.S.; Elbatsh, M.M.; Tahoon, N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem. Biol. Interact. 2016, 251, 10–16. [Google Scholar] [CrossRef]
- Yoon, D.H.; Kwon, O.Y.; Mang, J.Y.; Jung, M.J.; Kim, D.Y.; Park, Y.K.; Heo, T.H.; Kim, S.J. Protective potential of resveratrol against oxidative stress and apoptosis in Batten disease lymphoblast cells. Biochem. Biophys. Res. Commun. 2011, 414, 49–52. [Google Scholar] [CrossRef]
- Moraes, D.S.; Moreira, D.C.; Andrade, J.M.O.; Santos, S.H.S. Sirtuins, brain and cognition: A review of resveratrol effects. IBRO Rep. 2020, 9, 46–51. [Google Scholar] [CrossRef]
- Wang, B.; Ge, S.; Xiong, W.; Xue, Z. Effects of resveratrol pretreatment on endoplasmic reticulum stress and cognitive function after surgery in aged mice. BMC Anesthesiol. 2018, 18, 141. [Google Scholar] [CrossRef]
- Pan, P.T.; Lin, H.Y.; Chuang, C.W.; Wang, P.K.; Wan, H.C.; Lee, M.C.; Kao, M.C. Resveratrol alleviates nuclear factor-kappaB-mediated neuroinflammation in vasculitic peripheral neuropathy induced by ischaemia-reperfusion via suppressing endoplasmic reticulum stress. Clin. Exp. Pharmacol. Physiol. 2019, 46, 770–779. [Google Scholar] [CrossRef]
- Tian, X.S.; Xu, H.; He, X.J.; Li, Y.; He, B.; Zhao, D. Endoplasmic reticulum stress mediates cortical neuron apoptosis after experimental subarachnoid hemorrhage in rats. Int. J. Clin. Exp. Pathol. 2020, 13, 1569–1577. [Google Scholar]
- Xie, Y.K.; Zhou, X.; Yuan, H.T.; Qiu, J.; Xin, D.Q.; Chu, X.L.; Wang, D.C.; Wang, Z. Resveratrol reduces brain injury after subarachnoid hemorrhage by inhibiting oxidative stress and endoplasmic reticulum stress. Neural Regen. Res. 2019, 14, 1734–1742. [Google Scholar] [PubMed]
- Guo, D.; Xie, J.; Zhao, J.; Huang, T.; Guo, X.; Song, J. Resveratrol protects early brain injury after subarachnoid hemorrhage by activating autophagy and inhibiting apoptosis mediated by the Akt/mTOR pathway. Neuroreport 2018, 29, 368–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Agrawal, M.; Dore, S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem. Neurosci. 2013, 4, 1151–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, M.C.; Connell, B.J.; Saleh, T.M. Resveratrol Preconditioning Induces Cellular Stress Proteins and Is Mediated Via Nmda and Estrogen Receptors. Neuroscience 2010, 166, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis Vulgaris and Berberine: An Update Review. Phytother. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Q.; Wen, B.; Wu, N.; He, B.; Chen, J. Berberine Reduces Abeta42 Deposition and Tau Hyperphosphorylation via Ameliorating Endoplasmic Reticulum Stress. Front. Pharmacol. 2021, 12, 640758. [Google Scholar] [CrossRef]
- Liang, Y.; Ye, C.; Chen, Y.; Chen, Y.; Diao, S.; Huang, M. Berberine Improves Behavioral and Cognitive Deficits in a Mouse Model of Alzheimer’s Disease via Regulation of beta-Amyloid Production and Endoplasmic Reticulum Stress. ACS Chem. Neurosci. 2021, 12, 1894–1904. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, X.C.; Xu, Y.M.; Luo, N.C.; Luo, S.; Hao, X.Y.; Cheng, S.Y.; Fang, J.S.; Wang, Q.; Zhang, S.J.; et al. Berberine Improves Diabetic Encephalopathy Through the SIRT1/ER Stress Pathway in db/db Mice. Rejuvenation Res. 2018, 21, 200–209. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, A.; Zirak, M.R.; Wallace Hayes, A.; Reiter, R.; Karimi, G. Curcumin and its analogues protect from endoplasmic reticulum stress: Mechanisms and pathways. Pharmacol. Res. 2019, 146, 104335. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, S.H.; Cho, J.; Kim, J.O.; Yoon, J.H.; Park, E. Exercise and Curcumin in Combination Improves Cognitive Function and Attenuates ER Stress in Diabetic Rats. Nutrients 2020, 12, 1309. [Google Scholar] [CrossRef]
- Fridman, V.; Saporta, M.A. Mechanisms and Treatments in Demyelinating CMT. Neurotherapeutics 2021, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Mekahli, D.; Bultynck, G.; Parys, J.B.; De Smedt, H.; Missiaen, L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a004317. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Fan, Y.X.; Sun, H.Y.; Chen, L.Y.; Man, X. Curcumin inhibits endoplasmic reticulum stress induced by cerebral ischemia-reperfusion injury in rats. Exp. Ther. Med. 2017, 14, 4047–4052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, Y.; Pehlivan, D.; Wiszniewski, W.; Beck, C.R.; Snipes, G.J.; Lupski, J.R.; Khajavi, M. Curcumin facilitates a transitory cellular stress response in Trembler-J mice. Hum. Mol. Genet. 2013, 22, 4698–4705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh Brahma, N.; Sharmila SSrivastava Rakesh, K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, K.; Liu, M.; Zhong, X.; Yao, W.; Xiao, Q.; Wen, Q.; Yang, B.; Wei, M. Epigallocatechin Gallate Reduces Amyloid beta-Induced Neurotoxicity via Inhibiting Endoplasmic Reticulum Stress-Mediated Apoptosis. Mol. Nutr. Food Res. 2018, 62, e1700890. [Google Scholar] [CrossRef]
- Yao, C.Y.; Zhang, J.C.; Liu, G.P.; Chen, F.; Lin, Y. Neuroprotection by (-)-epigallocatechin-3-gallate in a rat model of stroke is mediated through inhibition of endoplasmic reticulum stress. Mol. Med. Rep. 2014, 9, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Holczer, M.; Besze, B.; Zambo, V.; Csala, M.; Banhegyi, G.; Kapuy, O. Epigallocatechin-3-Gallate (EGCG) Promotes Autophagy-Dependent Survival via Influencing the Balance of mTOR-AMPK Pathways upon Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2018, 2018, 6721530. [Google Scholar] [CrossRef] [Green Version]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.O.; Lee, J.; Cho, J.Y. Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Gu, W.; Liu, Y.; Zhang, M. Ginsenoside Rb1 protects hippocampal neurons from high glucose-induced neurotoxicity by inhibiting GSK3beta-mediated CHOP induction. Mol. Med. Rep. 2014, 9, 1434–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhang, H.; Gu, W.; Liu, Y.; Zhang, M. Neuroprotective effects of ginsenoside Rb1 on high glucose-induced neurotoxicity in primary cultured rat hippocampal neurons. PLoS ONE 2013, 8, e79399. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ma, Q.; Fan, C.; Chen, X.; Zhang, H.; Tang, M. Ginsenosides Rb1 and Rg1 Protect Primary Cultured Astrocytes against Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury via Improving Mitochondrial Function. Int. J. Mol. Sci. 2019, 20, 6086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.P.; Jeong, H.G. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol. Appl. Pharmacol. 2010, 242, 18–28. [Google Scholar] [CrossRef]
| Medicinal Herbs and Formulas | Chemotherapeutic Agent | Preclinical Outcomes | References |
|---|---|---|---|
| Acorus calamus | Vincristine | Attenuated vincristine-induced thermal and mechanical hyperalgesia, biochemical and histopathological changes via its anti-oxidative, anti-inflammatory, neuroprotective, and calcium inhibitory actions. | [118] |
| Cannabinoids | Vincristine | Inhibited vincristine-induced mechanical allodynia through activation of CB1 and CB2 receptors at the spinal cord level. | [119] |
| Ocimum sanctum L | Vincristine | Attenuated vincristine-induced neuropathic pain and decreased oxidative stress and calcium levels. | [120] |
| Salvia officinalis (Sage) | Cisplatin | Anti-nociceptive effect on cisplatin-induced hyperalgesia in mice comparable to morphine injections. | [121] |
| Walnut | Cisplatin | Walnut consumption improved memory and motor abilities in cisplatin-treated rats. | [122] |
| Jesengsingi-Hwan (Goshajinkigan in Japanese) | Oxaliplatin | Relieved the oxaliplatin-induced cold hyperalgesia and mechanical allodynia without affecting the anti-tumor activity of oxaliplatin. | [123] |
| Paclitaxel | Markedly prevented paclitaxel-induced mechanical allodynia. | [124] | |
| Gyejigachulbu-tang | Oxaliplatin | Relieved oxaliplatin-induced cold and mechanical hyperalgesia possibly by suppression of spinal glial activation. | [125] |
| Jakyakgamcho-Tang | Paclitaxel | Significantly improved the paclitaxel-induced allodynia and hyperalgesia. | [126] |
| Phytochemical | Major Source | ER Stress Regulators | Disorder | References |
|---|---|---|---|---|
| Hesperidin | Citrus aurantium | PERK↓ IRE1↓ ATF6↓ GRP78↓ CHOP↓ | Chemotherapy Induced peripheral neuropathy | [129] |
| Aucubin | Plantaginis semen | CHOP↓ | Chemotherapy Induced peripheral neuropathy | [132] |
| Resveratrol | Polygonum cuspidatum | GRP78↓ CHOP↓ XBP1↓ p-eIF2α↓ PERK↓ | Alzheimer’s disease | [134] |
| GRP78↓ CHOP↓ | Parkinson’s disease | [135] | ||
| GRP78↓ | Batten disease | [136] | ||
| XBP1↓ PERK↓ IRE1↓ | Post-Operative Cognitive Dysfunction | [138] | ||
| IRE1↓ | Neuropathy | [139] | ||
| GRP78↓ | Cerebral ischemia | [143] | ||
| Berberine | Berberis vulgaris, Berberis aristata | PERK↓, eIF2α↓ | Alzheimer’s disease | [147] |
| PERK↓, IRE1α↓, eIF-2α↓, PDI↓, CHOP↓ | Diabetic Encephalopathy | [148] | ||
| Curcumin | Curcuma longa | BiP↓ CHOP↓ | Diabetes-related cognitive dysfunction | [150] |
| BiP↓ CHOP↓ | Charcot-Marie-Tooth disease | [155] | ||
| Epigallocatechin-3-gallate | Camellia sinensis | GRP78↓ CHOP↓ | Alzheimer’s disease | [157] |
| GRP78↓ CHOP↓ | Cerebral ischemia | [158] | ||
| Ginsenoside Rb1 | Panax ginseng | PERK↓, CHOP↓ | high glucose-treated hippocampal neurons | [161] |
| PERK↓, CHOP↓ GSK3β↓ | Formaldehyde induced neurotoxicity | [162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goel, Y.; Fouda, R.; Gupta, K. Endoplasmic Reticulum Stress in Chemotherapy-Induced Peripheral Neuropathy: Emerging Role of Phytochemicals. Antioxidants 2022, 11, 265. https://doi.org/10.3390/antiox11020265
Goel Y, Fouda R, Gupta K. Endoplasmic Reticulum Stress in Chemotherapy-Induced Peripheral Neuropathy: Emerging Role of Phytochemicals. Antioxidants. 2022; 11(2):265. https://doi.org/10.3390/antiox11020265
Chicago/Turabian StyleGoel, Yugal, Raghda Fouda, and Kalpna Gupta. 2022. "Endoplasmic Reticulum Stress in Chemotherapy-Induced Peripheral Neuropathy: Emerging Role of Phytochemicals" Antioxidants 11, no. 2: 265. https://doi.org/10.3390/antiox11020265
APA StyleGoel, Y., Fouda, R., & Gupta, K. (2022). Endoplasmic Reticulum Stress in Chemotherapy-Induced Peripheral Neuropathy: Emerging Role of Phytochemicals. Antioxidants, 11(2), 265. https://doi.org/10.3390/antiox11020265






