Optimization of Ultrasound-Assisted Extraction and Encapsulation of Antioxidants from Orange Peels in Alginate-Chitosan Microparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material
2.3. Ultrasound-Assisted Extraction
2.4. Central Composite Design
2.5. Determination of Total Antioxidant Content
2.6. Phenolic Profile of the Extract
2.7. DPPH Assay
2.8. Encapsulation of Orange Peel Extract in Alginate-Chitosan Microparticles
2.9. Characterization of Microparticles
2.9.1. Determination of Shape and Size of Alginate-Chitosan Particles
2.9.2. Encapsulation Efficiency
2.9.3. FTIR Analysis
2.9.4. Determination of Swelling Ability of Alginate-Chitosan Microparticles
2.9.5. In Vitro Release of Antioxidants from Alginate-Chitosan Microparticles
In Vitro release
2.9.6. Release Kinetics of Antioxidants from Microparticles
2.9.7. Determination of the Antioxidant Activity of the Extract after Encapsulation
2.10. Statistical Analysis
3. Results
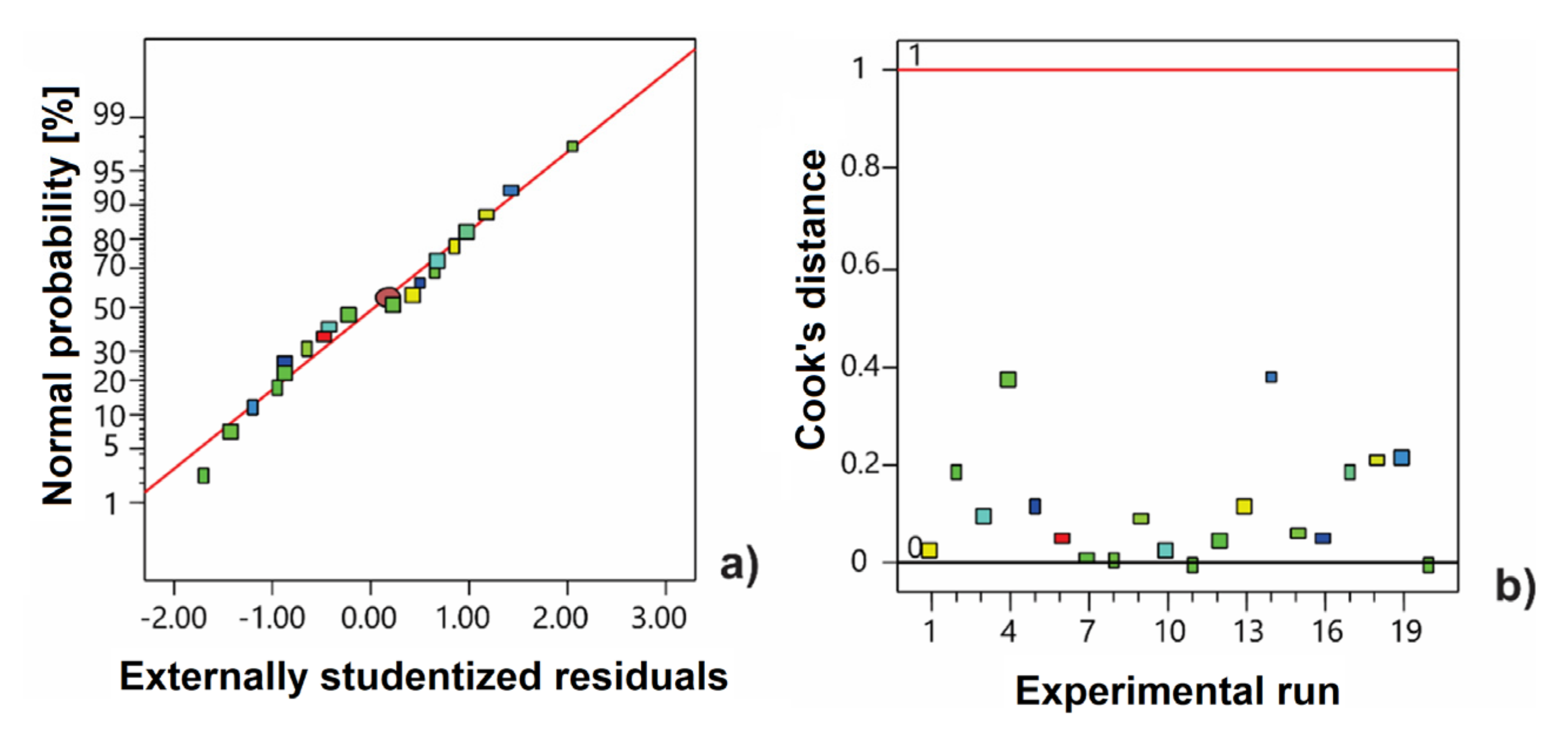
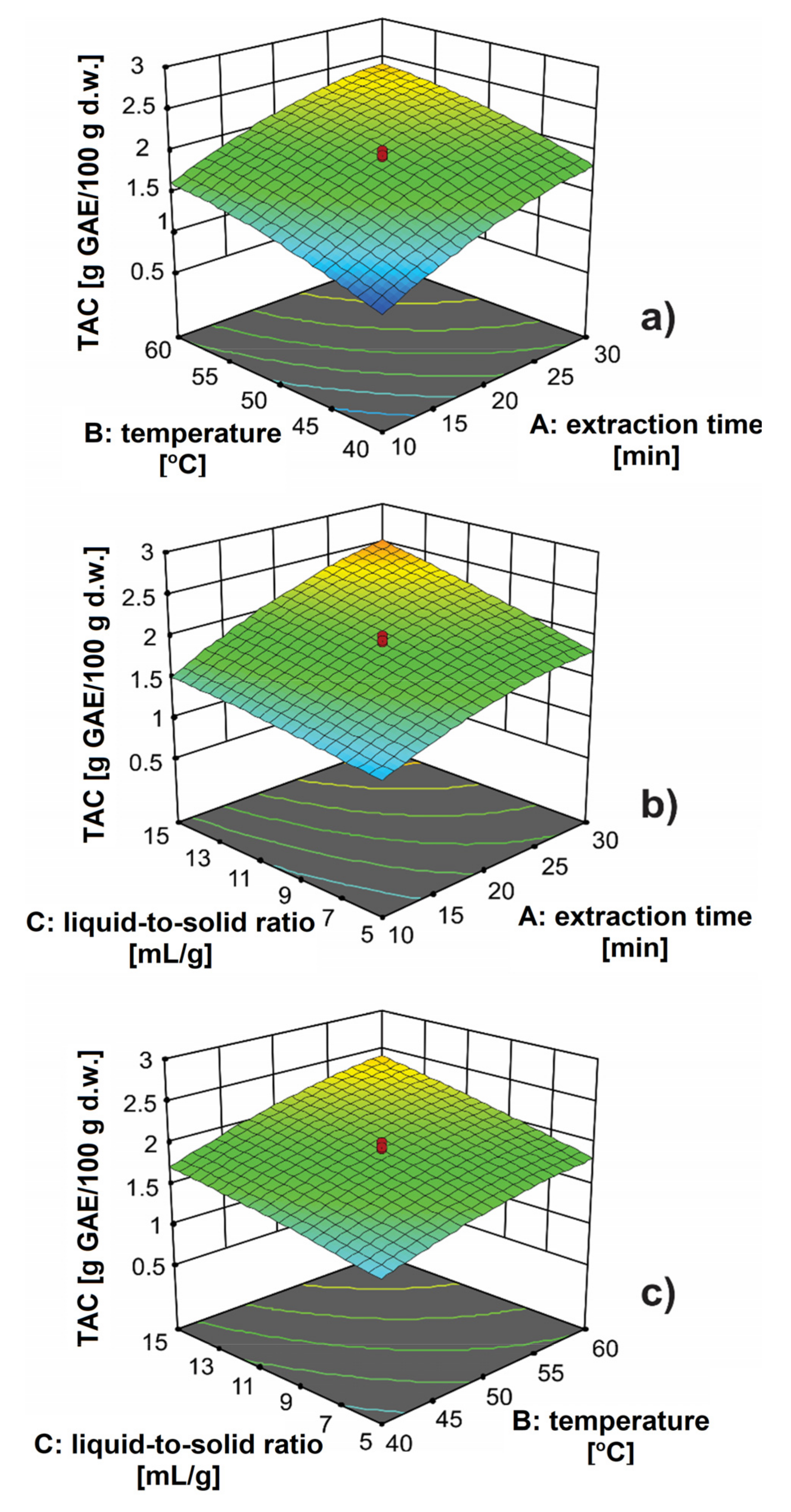
3.1. Development of Extraction Procedure of Antioxidants from Orange Peels
3.2. Characterization of Orange Peel Extract
3.3. Characterization Analysis of Microparticles
3.3.1. Microparticle Shape and Size
3.3.2. Encapsulation Efficiency
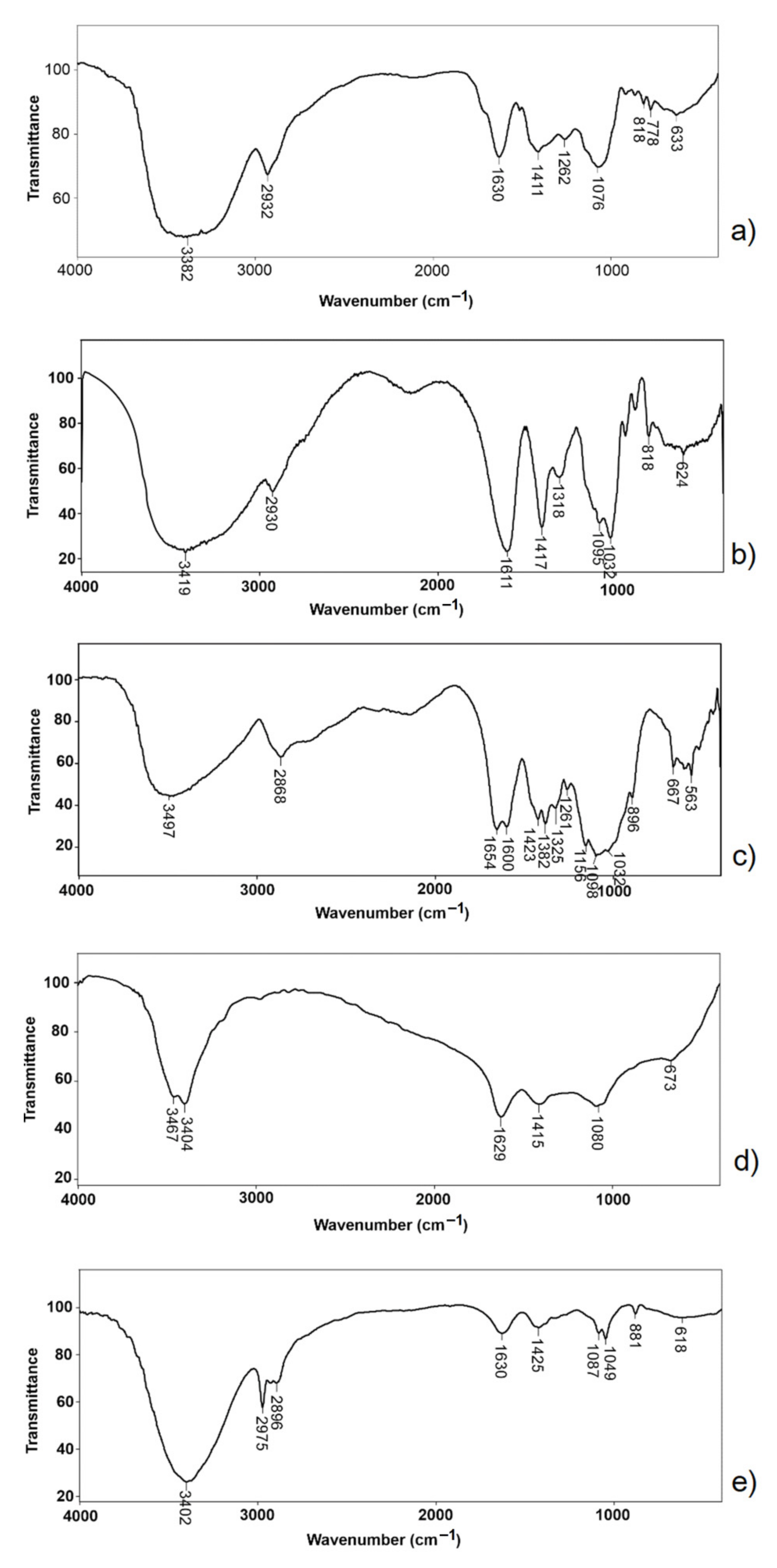
3.3.3. FTIR Analysis
3.3.4. Swelling Study
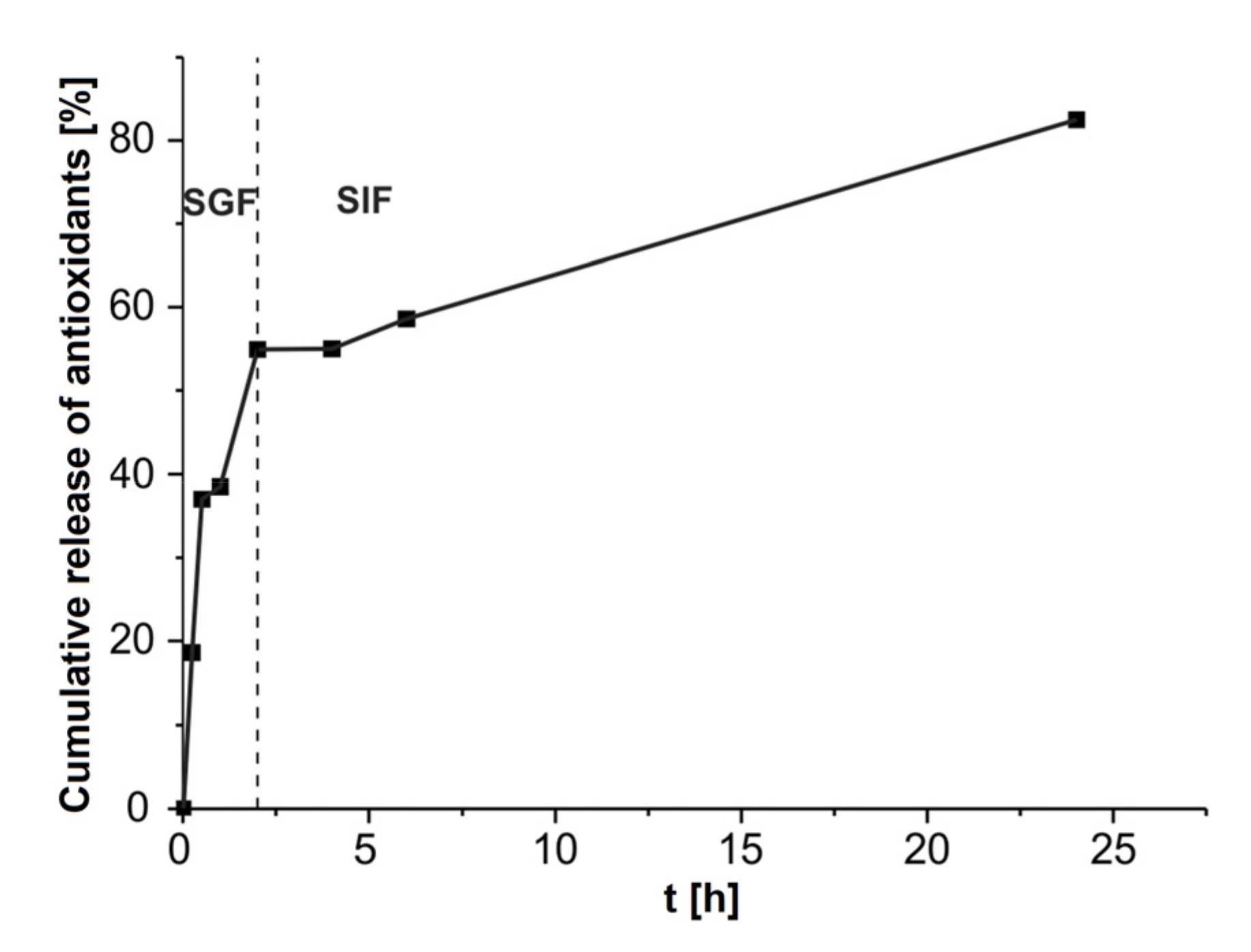
3.3.5. In Vitro Study of Antioxidant Release in Simulated Gastrointestinal Fluids
3.3.6. Antioxidant Activity of Encapsulated Alginate-Chitosan Microparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murador, D.C.; Salafia, F.; Zoccali, M.; Martins, P.L.; Ferreira, A.G.; Dugo, P.; Mondello, P.; de Rosso, V.V.; Giuffrida, D. Green extraction approaches for carotenoids and esters: Characterization of native composition from orange peel. Antioxidants 2019, 8, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagha-Benamrouche, S.; Madani, K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crop. Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- Haya, S.; Bentahar, F.; Trari, M. Optimization of polyphenols extraction from orange peel. J. Food Meas. Charact. 2019, 13, 614–621. [Google Scholar] [CrossRef]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and ultrasound-assisted extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaiswal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; Barbosa, P.D.P.M.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of phenolic compounds from citrus by-products using pressurized liquids―An application to orange peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Li, L.; Aghdam, M.S.; Wei, X.; Liu, J.; Xu, Y.; Luo, Z. Elevated CO2 delayed the chlorophyll degradation and anthocyanin accumulation in postharvest strawberry fruit. Food Chem. 2019, 285, 163–170. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, X.Y.; Gan, R.Y.; Zheng, J.; Li, Y.; Zhang, J.J.; Xu, D.P.; Li, H.B. Optimization of ultrasound-assisted extraction of antioxidant polyphenols from the seed coats of red sword bean (Canavalia gladiate (Jacq.) DC.). Antioxidants 2019, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Donati, I.; Paoletti, S. Material properties of alginates In Alginates: Biology and Applications; Springer: Berlin, Heidelberg, 2009; pp. 1–53. [Google Scholar]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Zhou, P.; Luo, Z.; Li, X.; Gao, L. Development of the pH responsive chitosan-alginate based microgel for encapsulation of Jughans regia L. polyphenols under simulated gastrointestinal digestion in vitro. Carbohydr. Polym. 2020, 250, 116917. [Google Scholar] [CrossRef] [PubMed]
- El-Messery, T.M.; El-Said, M.M.; Shahein, N.M.; El-Din, H.M.F.; Farrag, A. Functional yoghurt supplemented with extract orange peel encapsulated using coacervation technique. Pak. J. Biol. Sci. PJBS 2019, 22, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasani, O.; Javadian, S.R. Effect of encapsulated bitter orange peel extract and BHT on the quality of common carp fillet during refrigerated storage. Int. J. Food Eng. 2016, 12, 303–310. [Google Scholar] [CrossRef]
- Dehghan, B.; Esmaeilzadeh Kenari, R.; Raftani Amiri, Z. Nano-encapsulation of orange peel essential oil in native gums (Lepidium sativum and Lepidium perfoliatum): Improving oxidative stability of soybean oil. J. Food Process. Preserv. 2020, 44, e14889. [Google Scholar] [CrossRef]
- Kovac, M. (Ed.) Yugoslav Pharmacopoeia 2000; Savezni Zvod za Zaštitu i Unapređenje Zdravlja: Belgrade, Serbia, 2001. (In Serbian) [Google Scholar]
- Savic Gajic, I.; Savic, I.; Boskov, I.; Žerajić, S.; Markovic, I.; Gajic, D. Optimization of ultrasound-assisted extraction of phenolic compounds from black locust (Robiniae pseudoacaciae) flowers and comparison with conventional methods. Antioxidants 2019, 8, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef]
- Chan, E.S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Martinez, À.; Riu, J.; Rius, F.X. Lack of fit in linear regression considering errors in both axes. Chemom. Intell. Lab. Syst. 2000, 54, 61–73. [Google Scholar] [CrossRef]
- Duba, K.S.; Casazza, A.A.; Mohamed, H.B.; Perego, P.; Fiori, L. Extraction of polyphenols from grape skins and defatted grape seeds using subcritical water: Experiments and modeling. Food Bioprod. Process. 2015, 94, 29–38. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Q.; Ye, X.Q.; Fang, Z.X.; Chen, J.C.; Xu, G.H.; Liu, D.H. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of Satsuma mandarin (Citrus unshiu Marc.) peels. J. Agric. Food Chem. 2008, 56, 5682–5690. [Google Scholar] [CrossRef]
- Nakajima, V.M.; Madeira, J.V., Jr.; Macedo, G.A.; Macedo, J.A. Biotransformation effects on anti lipogenic activity of citrus extracts. Food Chem. 2016, 197, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Gornas, P.; Rudzinska, M.; Raczyk, M.; Misina, I.; Soliven, A.; Lacis, G.; Seglina, D. Impact of species and variety on concentrations of minor lipophilic bioactive compounds in oils recovered from plum kernels. J. Agr. Food Chem. 2016, 64, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Awad, T.S.; Asker, D.H.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Omoba, O.S.; Obafaye, R.O.; Salawu, S.O.; Boligon, A.A.; Athayde, M.L. HPLC-DAD phenolic characterization and antioxidant activities of ripe and unripe sweet orange peels. Antioxidants 2015, 4, 498–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganora, L. Herbal Constituents: Foundations of Phytochemistry: A Holistic Approach for Students and Practitioners of Botanical Medicine; HerbalChem Press: Louisville, CO, USA, 2008; pp. 38–52. [Google Scholar]
- Khorshidian, N.; Mahboubi, A.; Kalantari, N.; Hosseini, H.; Yousefi, M.; Arab, M.; da Crus, A.G.; Mortazavian, A.M.; Mahdavi, F.S. Chitosan-coated alginate microcapsules loaded with herbal galactagogue extract: Formulation optimization and characterization. Iran. J. Pharm. Res. IJPR 2019, 18, 1180. [Google Scholar]
- Moschona, A.; Liakopoulou-Kyriakides, M. Encapsulation of biological active phenolic compounds extracted from wine wastes in alginate-chitosan microbeads. J. Microencapsulation 2018, 35, 229–240. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Pasparakis, G.; Bouropoulos, N. Swelling studies and in vitro release of verapamil from calcium alginate and calcium alginate–chitosan beads. Int. J. Pharmaceutics 2006, 323, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Delivery 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Kelishomi, Z.H.; Goliaei, B.; Mahdavi, H.; Nikoofar, A.; Rahimi, M.; Moosavi-Movahedi, A.A.; Mamashli, F.; Bigdeli, B. Antioxidant activity of low molecular weight alginate produced by thermal treatment. Food Chem. 2016, 196, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polymers 2008, 74, 840–844. [Google Scholar] [CrossRef]


| Factor | Coded Values | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| Actual Values | |||||
| extraction time [min] | 3 | 10 | 20 | 30 | 37 |
| extraction temperature [°C] | 33 | 40 | 50 | 60 | 67 |
| liquid-to-solid ratio [mL/g] | 2 | 5 | 10 | 15 | 18 |
| Std. | Exp. Run | Factor 1 | Factor 2 | Factor 3 | Response Y: TAC [g GAE/100 g d.w.] |
|---|---|---|---|---|---|
| A: Extraction Time [min] | B: Temperature [°C] | C: Liquid-to-Solid Ratio [mL/g] | |||
| 14 | 1 | 20 | 50 | 18 | 2.28 ± 0.06 |
| 7 | 2 | 10 | 60 | 15 | 1.76 ± 0.05 |
| 3 | 3 | 10 | 60 | 5 | 1.41 ± 0.08 |
| 4 | 4 | 30 | 60 | 5 | 1.95 ± 0.08 |
| 9 | 5 | 3 | 50 | 10 | 0.86 ± 0.04 |
| 8 | 6 | 30 | 60 | 15 | 2.79 ± 0.09 |
| 17 | 7 * | 20 | 50 | 10 | 1.89 ± 0.08 |
| 19 | 8 * | 20 | 50 | 10 | 1.96 ± 0.04 |
| 6 | 9 | 30 | 40 | 15 | 2.08 ± 0.02 |
| 13 | 10 | 20 | 50 | 2 | 1.41 ± 0.09 |
| 15 | 11 * | 20 | 50 | 10 | 1.92 ± 0.05 |
| 20 | 12 * | 20 | 50 | 10 | 1.86 ± 0.10 |
| 10 | 13 | 37 | 50 | 10 | 2.28 ± 0.12 |
| 5 | 14 | 10 | 40 | 15 | 1.11 ± 0.09 |
| 16 | 15 * | 20 | 50 | 10 | 2.01 ± 0.11 |
| 1 | 16 | 10 | 40 | 5 | 0.90 ± 0.09 |
| 2 | 17 | 30 | 40 | 5 | 1.52 ± 0.11 |
| 12 | 18 | 20 | 67 | 10 | 2.24 ± 0.12 |
| 11 | 19 | 20 | 33 | 10 | 1.15 ± 0.06 |
| 18 | 20 * | 20 | 50 | 10 | 1.94 ± 0.07 |
| Sum of Squares | Df | Middle Square | F-Value | p-Value | |
|---|---|---|---|---|---|
| Model | 4.780 | 9 | 0.531 | 221.8 | 2.59 × 10−10 |
| A-time | 2.254 | 1 | 2.254 | 941.4 | 3.17 × 10−11 |
| B-temperature | 1.251 | 1 | 1.251 | 522.4 | 5.80 × 10−10 |
| C-liquid-to-solid ratio | 0.858 | 1 | 0.858 | 358.4 | 3.67 × 10−9 |
| AB | 0.000 | 1 | 0.000 | 2.1 × 10−2 | 0.888 |
| AC | 0.088 | 1 | 0.088 | 36.8 | 1.20 × 10−4 |
| BC | 0.022 | 1 | 0.022 | 9.2 | 1.26 × 10−2 |
| A² | 0.233 | 1 | 0.233 | 97.4 | 1.80 × 10−6 |
| B² | 0.099 | 1 | 0.099 | 41.5 | 7.45 × 10−5 |
| C² | 0.013 | 1 | 0.013 | 5.4 | 4.24 × 10−2 |
| Residue | 0.024 | 10 | 0.002 | ||
| Lack of fit | 0.010 | 5 | 0.002 | 0.7 | 0.642 |
| Pure error | 0.014 | 5 | 0.003 | ||
| Total correction | 4.804 | 19 |
| Std. dev. | 0.05 | R² | 0.995 |
| Mean value | 1.77 | Adj. R² | 0.991 |
| C.V. [%] | 2.77 | Pred. R² | 0.979 |
| Adequate Precision | 55.5 | ||
| Phenolic Acid | λ [nm] | tR [min] | Calibration Curve | Concentration [mg/100 g d.w.] |
|---|---|---|---|---|
| gallic acid | 278 | 5.38 | A = 1.320 × 10−5 C + 0.287 (R2 = 0.9995) | 157.08 ± 1.26 |
| chlorogenic acid | 300 | 29.12 | A = 1.601 × 10−5 C + 1.447 (R2 = 0.9987) | 29.60 ± 0.59 |
| caffeic acid | 300 | 31.02 | A = 8.406 × 10−6 C + 0.209 (R2 = 0.9982) | 127.03 ± 1.32 |
| coumaric acid | 300 | 45.98 | A = 5.706 × 10−6 C + 1.241 (R2 = 0.9991) | 26.15 ± 0.89 |
| ferulic acid | 300 | 50.09 | A = 9.320 × 10−6 C − 0.101 (R2 = 0.9989) | 20.91 ± 0.28 |
| sinapic acid | 300 | 51.12 | A = 1.120 × 10−5 C − 0.242 (R2 = 0.9993) | 28.61 ± 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savic, I.M.; Savic Gajic, I.M.; Milovanovic, M.G.; Zerajic, S.; Gajic, D.G. Optimization of Ultrasound-Assisted Extraction and Encapsulation of Antioxidants from Orange Peels in Alginate-Chitosan Microparticles. Antioxidants 2022, 11, 297. https://doi.org/10.3390/antiox11020297
Savic IM, Savic Gajic IM, Milovanovic MG, Zerajic S, Gajic DG. Optimization of Ultrasound-Assisted Extraction and Encapsulation of Antioxidants from Orange Peels in Alginate-Chitosan Microparticles. Antioxidants. 2022; 11(2):297. https://doi.org/10.3390/antiox11020297
Chicago/Turabian StyleSavic, Ivan M., Ivana M. Savic Gajic, Miljana G. Milovanovic, Stanko Zerajic, and Dragoljub G. Gajic. 2022. "Optimization of Ultrasound-Assisted Extraction and Encapsulation of Antioxidants from Orange Peels in Alginate-Chitosan Microparticles" Antioxidants 11, no. 2: 297. https://doi.org/10.3390/antiox11020297
APA StyleSavic, I. M., Savic Gajic, I. M., Milovanovic, M. G., Zerajic, S., & Gajic, D. G. (2022). Optimization of Ultrasound-Assisted Extraction and Encapsulation of Antioxidants from Orange Peels in Alginate-Chitosan Microparticles. Antioxidants, 11(2), 297. https://doi.org/10.3390/antiox11020297








